Abstract
Behavioral sensitization has been widely studied in animal models and is theorized to reflect neural modifications associated with human psychostimulant addiction. While the mesolimbic dopaminergic pathway is known to play a role, the neurochemical mechanisms underlying behavioral sensitization remain incompletely understood. In the present study, we conducted the first metabolomics analysis to globally characterize neurochemical differences associated with behavioral sensitization. Methamphetamine-induced sensitization measures were generated by statistically modeling longitudinal activity data for eight inbred strains of mice. Subsequent to behavioral testing, nontargeted liquid and gas chromatography-mass spectrometry profiling was performed on 48 brain samples, yielding 301 metabolite levels per sample after quality control. Association testing between metabolite levels and three primary dimensions of behavioral sensitization (total distance, stereotypy and margin time) showed four robust, significant associations at a stringent metabolome-wide significance threshold (false discovery rate < 0.05). Results implicated homocarnosine, a dipeptide of GABA and histidine, in total distance sensitization, GABA metabolite 4-guanidinobutanoate and pantothenate in stereotypy sensitization, and myo-inositol in margin time sensitization. Secondary analyses indicated that these associations were independent of concurrent methamphetamine levels and, with the exception of the myo-inositol association, suggest a mechanism whereby strain-based genetic variation produces specific baseline neurochemical differences that substantially influence the magnitude of MA-induced sensitization. These findings demonstrate the utility of mouse metabolomics for identifying novel biomarkers, and developing more comprehensive neurochemical models, of psychostimulant sensitization.
Keywords: addiction, sensitization, metabolomics, homocarnosine, myo-inositol, stimulant
INTRODUCTION
Repeated administration of psychostimulants, including methamphetamine (MA), results in a progressive enhancement of the drugs’ behavioral activating effects through a process known as behavioral sensitization (BSn). Observed in both animal models and humans (Pierce & Kalivas, 1997, Strakowski & Sax, 1998), BSn is thought to reflect aspects of the neural adaptations underlying addictive behaviors and motivational states (Robinson & Berridge, 2008, Steketee & Kalivas, 2011, Wise & Bozarth, 1987). Research into the neurochemistry of MA-induced BSn has traditionally focused primarily on dopaminergic neurotransmission. This approach has shown that the psychostimulatory effects of MA are associated with elevated synaptic dopamine (DA) levels due to increased DA release from presynaptic terminals and inhibited reuptake, particularly in mesolimbic neurons projecting from the ventral tegmental area to the nucleus accumbens. Thus, activity-dependent synaptic plasticity and remodeling of the mesolimbic dopaminergic pathway are known to play an important role in psychostimulant sensitization and dependence (Nestler, 2001). However, the neurochemical mechanisms underlying BSn are complex and incompletely understood, extending beyond mesolimbic DA to include GABAergic and glutamatergic neurotransmission (Pierce & Kalivas, 1997, Steketee, 2003), as well as recently identified molecular mediators (e.g., shati and piccolo (Niwa et al., 2008)). While trending toward broader consideration beyond established pathways, no research to date has applied a nontargeted metabolomics approach to comprehensively investigate neurochemical variation associated with BSn.
Metabolomics is an emerging platform that seeks to interrogate the full complement of endogenous small molecules (typically <1500 Da) within a sample in a nontargeted fashion. Thus, instead of limiting focus to specific candidate metabolites or metabolic reaction sets, metabolomics approaches quantitatively assay the entire range of metabolites present in a sample to characterize its overall biochemical state. Although MA sensitization has not yet been investigated using metabolomics, the platform has successfully identified biochemical signatures for other CNS pathologies including schizophrenia, Parkinson’s and motor neuron disease (Bogdanov et al., 2008, Pears et al., 2005, Prabakaran et al., 2004). Thus, metabolomics offers the possibility of comprehensively mapping neurochemical profiles associated with MA-induced BSn, facilitating the identification of novel mechanisms, biomarkers and therapeutic targets.
To date, metabolomics studies of psychiatric disorders have generally focused on readily obtained biomaterials in humans (e.g., urine and serum) (Kaddurah-Daouk & Krishnan, 2009). While human metabolomics research has obvious translational clinical potential, it is also characterized by various limitations including expense, difficulty controlling confounders and inability to access relevant tissue. Mouse metabolomics circumvent many of these limitations, cost-effectively providing control of genetic background and environmental confounders, primary tissues access, and precise behavioral phenotype measurement (Dunn et al., 2011). These advantages, paired with extensive genomic and phenotypic database resources, make mouse metabolomics a valuable research strategy for disorders with robust experimental models (Peters et al., 2007). Following this rationale, we analyzed the relationship between longitudinal measures of MA-induced BSn and 301 metabolite levels quantified via nontargeted liquid and gas chromatography-mass spectrometry of 48 murine brain samples.
MATERIAL AND METHODS
Animals
Male mice aged 7–9 weeks, representing 8 inbred strains chosen to maximize genetic variability (129S1/SvImJ, A/J, C3H/HeJ, C57BL/6J, CAST/EiJ, DBA/2J, NOD/ShiLtJ, PWD/PhJ) (Yang et al., 2007), were obtained from Jackson Laboratory (Bar Harbor, ME) and allowed to acclimate approximately 1week prior to testing. Mice were housed at a maximum of four per cage in an AAALAC-accredited animal facility with food (7012 Teklad LM-485 Mouse/Rat Sterilizable Diet, Harlan Laboratories, Inc., Indianapolis, IN) and water available ad libitum under a 12-h/12-h light/dark cycle (lights on at 0700-h to 1900-h). Testing occurred during light phase. All procedures were carried out in accordance with the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal, 1996) and approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Apparatus
Behavioral testing was conducted using 8 commercially obtained, automated activity monitoring devices, each enclosed in sound- and light-attenuating chambers. Variables considered potentially relevant BSn indicators included: distance traveled (cm), horizontal activity (cm), number of movements, resting time (s), stereotypy count, stereotypy number, stereotypy time (s), margin distance (cm), margin time (s), center time (s) (Supplemental Methods for variable definitions and selection rationale). Variables were collected in 10-min bins during 1-h test sessions via computer-controlled circuitry (AccuScan Instruments, Columbus, OH). Each device’s interior was divided into separate 20×20×30 cm arenas permitting independent and simultaneous measurement of two mice. Sixteen photobeam sensors per axis, spaced 2.5 cm apart along the chambers’ walls, were used to detect movement.
Locomotor Activity Procedure
The behavioral analysis sample included two experimental arms, a 3 mg/kg MA-test group and a control group receiving vehicle-only injections. MA-test and control groups each included 64 mice, 8 per strain per arm (128 total). For both arms, Test-day 1 consisted of two consecutive, 1-h behavioral activity test sessions. A vehicle injection was given immediately prior to Test-day 1 Session 1 for both groups and vehicle or 3 mg/kg MA injection, for control and test groups respectively, was given immediately prior to Test-day 1 Session 2. For Test-days 2–4 a single 1-h test session occurred, immediately preceded by vehicle or 3 mg/kg MA injection, for control and test groups respectively. Test-day 5 exactly replicated the procedure from Test-day 1. Measurements from the initial, vehicle-only sessions on Test-days 1 and 5 were excluded from analysis, resulting in 30 repeated assessments (5 days × 6 bins) per mouse in the behavioral analysis. Boxplots summarizing all activity data for the MA-test group by day, strain and measure, are presented in Supplemental Figures 1 and 2.
After the last activity test session, mice were immediately euthanized by microwave fixation (Muromachi Microwave Fixation System, TMW-4012C, Tokyo). Many neurochemicals undergo rapid post-mortem alterations due to ongoing metabolism, resulting in a distorted characterization of in vivo metabolism. These alterations are minimized by microwave irradiation, which instantaneously denatures all enzymes in the brain, preserving metabolites in the pre-sacrifice state (De Graaf et al., 2009, Ikarashi et al., 1985, Login & Dvorak, 1994). Brain tissue was then extracted by dissection, placed into 2 ml vials, snap-frozen in liquid nitrogen and kept frozen at −80°C (Ultima II, ULT2856-9-A40, Thermo Electron Corporation) prior to shipment to Metabolon. Inc. (Research Triangle Park, NC) for mass spectrometry profiling. Forty-eight MA-test mice were selected for whole brain metabolomics assay (6 randomly selected per strain). Given the study’s aim of characterizing neurochemicals associated with variation in MA-induced BSn, a phenomenon only observable with MA administration, vehicle treated controls were not considered in the metabolomics analysis, but these data have been presented elsewhere (Mcclay et al., 2013).
Drugs
(±)-Methamphetamine (National Institute on Drug Abuse, Rockville, MD) was prepared in 0.9% saline stock solutions sterilized by filtration through 0.2 µm filtration disks. Working MA solutions were injected intraperitoneally at 10 ml/kg body weight. The 3 mg/kg MA test dose has been shown to induce sustained behavioral response, persisting longer than test session duration, in multiple mouse strains (Peachey et al., 1976, Snider et al., 2012, Zhang et al., 2006).
Metabolomics Sample Preparation
Sample preparation was carried out using the automated MicroLab STAR® system (Hamilton Robotics, Reno NV) (Evans et al., 2009, Ohta et al., 2009). Recovery standards were added prior to the first extraction process step for QC purposes. A proprietary (Metabolon) series of organic and aqueous extractions were used to remove the protein fraction while maximizing recovery of small molecules. The resulting extract was divided into liquid chromatography (LC/MS) and gas chromatography (GS/MS) mass spectrometry fractions. Samples were placed briefly on a TuboVap® (Zymark) to remove the organic solvent. Each sample was then frozen and dried under vacuum. Samples were then prepared for either LC/MS or GC/MS. A small aliquot of each experimental sample for a specific matrix was extracted and pooled together as a “client matrix”. Aliquots of client matrix samples were injected throughout the platform day run and served as technical replicates. As such, the variability in the quantitation of all consistently detected biochemicals in the experimental samples was monitored to provide a process variability metric for run-day normalization and quality control.
Liquid chromatography/Mass Spectrometry (LC/MS, LC/MS2)
The LC/MS portion of the platform was based on a Waters Acquity UPLC and a Thermo-Finnigan LTQ mass spectrometer, consisting of an electrospray ionization (ESI) source and linear ion-trap (LIT) mass analyzer. The sample extract was split into two aliquots, dried, then reconstituted in acidic or basic LC-compatible solvents, each of which contain ≥11 injection standards at fixed concentrations. One aliquot was analyzed using acidic positive ion optimized conditions and the other using basic negative ion optimized conditions in two independent injections using separate dedicated columns. Extracts reconstituted in acidic conditions were gradient eluted using water and methanol both containing 0.1% formic acid, while basic extracts, also using water/methanol, contain 6.5mM ammonium bicarbonate. The MS analysis alternated between MS and data-dependent MS2 scans using dynamic exclusion.
The LC/MS accurate mass portion of the platform was based on a Surveyor HPLC and a Thermo-Finnigan LTQ-FT mass spectrometer, which has a linear ion-trap (LIT) frontend and a Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer backend. For ions with counts >2 million, an accurate mass measurement can be performed on both parent ions, as well as fragments. The typical mass error was <5 ppm. Ions with <2 million counts require greater effort to characterize. Fragmentation spectra (MS/MS) were typically generated in data dependent manner, but if necessary targeted MS/MS were employed (i.e., for lower concentration signals). Further details of LC/MS methods can be found elsewhere (Evans et al., 2009).
Gas chromatography /Mass Spectroscopy (GC/MS)
The samples intended for GC/MS analysis were re-dried under vacuum desiccation for a minimum of 24 hours prior to being derivatized under dried nitrogen using bistrimethyl-silyl-triflouroacetamide (BSTFA). The GC column is 5% phenyl and the temperature ramp was from 40° to 300° C in a 16 minute period. Samples were analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization. The instrument was tuned and calibrated for mass resolution and mass accuracy on a daily basis. The information output from the raw data files were automatically extracted as discussed below. Further details regarding GC/MS methods can be found elsewhere (Ohta et al., 2009).
Data Extraction and Compound Identification
Raw MS data files were loaded into a relational database and peaks identified using Metabolon proprietary peak integration software. Biochemicals (and MA) were identified by comparison to library entries of purified standards or recurrent unknown entities, including approximately 1500 commercially available purified standard biochemicals for LC and GC platforms. The combination of chromatographic properties and mass spectra gave an indication of a match to the specific compound or isobaric entity. As previously described, a variety of curation and QC procedures were implemented to ensure accurate and consistent identification of true chemical entities, and to remove those representing system artifacts, misassignments or background noise, and to confirm the consistency of peak identification among the various samples (Evans et al., 2009). Biochemicals with <3-fold greater abundance than background were excluded. A data normalization step was performed to correct variation resulting from instrument inter-day tuning differences to avoid confounding experimental variation with instrument sensitivity. Each compound was corrected in run-day blocks by dividing all samples by that compound’s median (Evans et al., 2009). Thus, while extraction efficiencies, ionization efficiencies and abundance in murine brain are variable across metabolites, they are comparable within metabolites, allowing unbiased comparisons of relative abundance between samples.
Statistical analysis
Linear mixed models were used to model MA-induced BSn effects and generate mouse-specific sensitization measures for MA-test mice (Willett et al., 1998). It was necessary to simultaneously model control and MA-test animal data to accurately estimate sensitization effects by partialling out the effect of protocol habituation (i.e., response to handling and experimental procedures), which was present in both control and MA-test arms, from BSn, which was present only in MA arm. For each phenotype, behavioral activity (Y) for each mouse (i) during each test period (j) was modeled as a function of: (b0) baseline activity level (i.e., intercept); (b1) habituation, modeled as a linear function of study day; (b2) baseline effect of MA administration (i.e., dichotomous indicator of experimental arm); (b3) sensitization to MA exposure, modeled as an interaction between arm and study day; and mouse-level deviations (random effects) from the mean: (u0i) intercept, (u1i) habituation effect, and (u3i) sensitization effect; and a residual (eij). This model is formalized as:
Yij = b0 + b1dayij + b2armi + b3armi × dayij + u0i + u1i + u3i + eij
with random effects specified as orthogonal (Willett et al., 1998).
The term of primary interest was the sensitization random effect—u3i. For each of the 10 behavioral phenotypes this term, quantifying the magnitude of each test mouse’s MA-induced sensitization, was output from the linear mixed model as best linear unbiased predictors (BLUPs) (Robinson, 1991). Sensitivity analyses modeling the effects of habituation and sensitization quadratically yielded equivalent (r>0.95) mouse-level sensitization estimates. Measures of longitudinal change estimated in this fashion reliably increase phenotype signal-to-noise ratio, and thus, statistical power, compared to conventional BSn measurement approaches, such as last minus first MA exposure, or post-sensitization MA challenge in test and control animals (Willett, 1989, Willett et al., 1998). Next, to avoid redundant analysis of multiple variables representing the same underlying sensitization phenomena, we conducted factor analyses to assess dimensionality in the 10 sensitization measure using the standard principle factor method. Several orthogonal rotations were implemented (e.g., varimax, quartimax) and yielded comparable three factor solutions. Additionally, one way ANOVA were used to calculate the proportion of variance explained by strain (i.e., heritability) for all sensitization and metabolite measures.
Primary metabolomics analyses were conducted as a series of bivariate linear regressions, with a separate model estimated for each metabolite-sensitization combination, resulting in 903 total associations (301 metabolites × 3 outcomes). As mice are nested within strains, they are nonindependent, which violates regression assumptions and generally downwardly biases standard errors, increasing Type I errors (Williams, 2000). To account for nonindependence of mice within strains, the Huber/White/Sandwich clustered variance estimator was used to calculate standard errors (Rogers, 1994, Williams, 2000). This method explicitly adjusts for within-cluster (i.e., strain) correlation, allowing unbiased significance testing of clustered data and efficient use of all available information. For each sensitization outcome, false discovery rate (FDR) methods used to adjust significance criterion for multiple testing. As argued previously (Van Den Oord & Sullivan, 2003), we prefer an FDR-based approach to declaring significance because it: (a) balances competing goals of finding true effects versus avoiding false discoveries, (b) provides comparable standards across studies, as it is less affected by number of tests and importantly, (c) is relatively robust to correlated tests (Fernando et al., 2004, Storey, 2003), as were present here. We used an FDR<0.05 threshold for declaring metabolome-wide significance, specifying that, on average, 5% of associations declared significant are expected to be false discoveries. Operationally, FDR levels were controlled using q-values (Storey, 2003).
Additionally, we conducted several sensitivity analyses to test the robustness of the metabolome-wide significant associations. First, to eliminate the possibility that the observed metabolite differences reflect acute MA response, we adjust for levels of locomotor activity observed in the first test session again using cluster-robust standard errors. Second, to eliminate the possibility that metabolome-wide significant associations are driven by outlying mice, we calculate Spearman rank correlations with cluster-robust standard errors. Third, analyses were repeated systematically excluding one strain at a time, to ensure that statistical significance was not driven by a single outlying strain. Only associations satisfying these three sensitivity criteria (p<0.05) were considered robust and included in the final sets of analyses.
Next, multiple regression was used to consider whether the robustly significant metabolite-outcome associations were mediated by concurrent MA level in brain and/or genetic differences between strains. To this end, we compared sensitization variance explained by metabolite level in a bivariate regression model (R2) to marginal sensitization variances explained by metabolite level controlling for MA level (R2M) and strain (R2S), respectively. R2M and R2S were calculated as a partial R2 (Cohen et al., 2003). Substantively, a partial R2 may be interpreted as the additional variance explained by the metabolite, over and above that explained by the putative mediator. Heritability (H2) was calculated and is defined as the proportion of the sensitization-metabolite covariance explained by strain. Lastly, we collapsed metabolite levels into strain means and examined the correlations of these metabolite strain means between the MA-test mice analyzed here and mice sacrificed at baseline (i.e., no MA exposure). This was done to investigate the degree to which associated metabolites reflected baseline strain differences. All formulae are detailed in Supplemental Methods.
RESULTS
As expected, results indicated that for all 10 phenotypes the mean behavioral activating effects of MA significantly sensitized with repeated administration (p<0.05; Supplemental Figure 3). The degree of sensitization significantly varied across individual mice (p<.05; Supplemental Table 1). For each phenotype, measures quantifying mouse-specific MA-induced BSn were extracted from linear mixed models as BLUPs (Robinson, 1991). All BSn measures were approximately normally distributed with no significant outliers/skewness (Supplemental Figure 4). Factor analysis of sensitization measures indicated three substantial factors underlying the measures (explaining 91% cumulative variance). Factor 1 (65% variance) was well-represented by sensitization in total distance (TD) (λ=0.97), Factor 2 (15% variance) by stereotypy number (SN) (λ=0.78), and Factor 3 (11% variance) by margin time (MT) (λ=0.74) (Supplemental Table 2; Supplemental Figure 5). These three BSn measures were used as metabolomics outcomes and raw data used to generate them is presented, by strain, in longitudinal scatterplots with superimposed strain mean trajectories in Figure 1. ANOVA results indicated that these three sensitization measures varied in heritability, with approximately half of sensitization variance due to strain differences for TD (p=6.60E-06, R2=0.46) and SN (p=9.63E-06, R2=0.45), while a nonsignificant proportion of sensitization variance was attributable to strain for MT (p=0.19, R2=0.15). See Supplemental Table 3 for ANOVA results for strain effects on all 10 behavioral indicators.
Figure 1. Longitudinal scatterplots, by strain, of all MA-test mice repeated assessments for behavioral outcomes used in metabolomics analyses.
Mean strain sensitization trajectories are superimposed over scatterplots. A stochastic jitter was used to prevent overlay of points occurring on the same day.
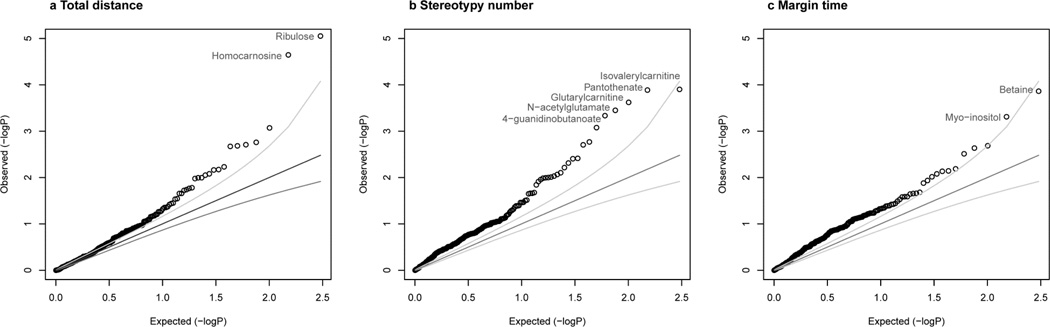
Metabolomics association analyses were conducted as a series of 903 bivariate linear regressions using cluster-robust standard errors, one for each metabolite-outcome combination. Q-Q plots show that associations were generally stronger than expected by chance, with nine associations achieving metabolome-wide significance (q<0.05), 2 for TD, 5 for SN and 2 for MT (Figure 2). Specifically, TD was significantly associated with ribulose and homocarnosine. SN was significantly associated with isovalerylcarnitine, pantothenate, glutarylcarnitine, N-acetylglutamate and 4-guanidinobutanoate. MT was significantly associated with betaine and myo-inositol (Table 1). All results were robust to adjusting for the acute locomotor effects of MA. However, Spearman rank sensitivity analyses indicated that the ribulose-TD, glutarylcarnitine-SN, N-acetylglutamate-SN and betaine-MT associations were driven by outlying mice. Similarly, sensitivity analyses examining the influence of individual strains indicated outlying metabolite levels among 129S1/SvImJ drove the isovalerylcarnitine-SN association. Consequently, these five non-robust associations are excluded from further analysis (Supplemental Figure 6). The four robust associations are visualized in Figure 3. As shown by standardized regression coefficients (β column, Table 1), for each of the robust associations a 1 SD change in metabolite level was associated with 0.35–0.5 SD change in the corresponding BSn measure. Thus, metabolite levels explained 12–25% of sensitization outcome variance in bivariate models (R2 column, Table 1). Among the robustly significant metabolites, ANOVA results indicated high heritability for homocarnosine (p=5.12E-17, R2=0.89), pantothenate (p=1.40E-07, R2=0.66) and 4-guanidinobutanoate (p=1.62E-13, R2=0.83), and nonsignficant heritability for myo-inositol (p=0.17, R2=0.21). Strain ANOVA results for all 301 assayed metabolites, summarized in Supplemental Figure 7, demonstrate significant heritability (p<0.05) for a majority (68.8%) of metabolites, and high heritability (h2>0.70) for substantial minority (13.0%) of metabolites.
Figure 2. Nine neurochemical levels were significantly associated with MA-induced BSn (q<0.05).
Q-Q plots compare observed −log(10) p-values to expected –log(10) p-values under the null distribution for bivariate associations of BSn measures (a Total distance, b Stereotypy number, c Margin time) to 301 neurochemical levels. Each point represents a neurochemical-BSn association p-value and points falling above the null expectation, represented by the dark gray line, are higher than expected by chance. Light gray lines indicate 95% confidence intervals for each p-value rank. All metabolome-wide significant p-values are labeled.
Table 1.
Parameter estimates for metabolome-wide significant (q<0.05) sensitization-neurochemical associations
| Primary analysis | Sensitivity analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adj acute | Spearman rank | ||||||||
| Sensitization outcome | Neurochemical | q | p | beta | R2 | t | p | t | p |
| Total distance | Ribulose | 0.01 | 8.81E-06 | 0.55 | 0.30 | 5.72 | 1.26E-06 | 1.20 | 0.274 |
| Total distance | Homocarnosine | 0.01 | 2.25E-05 | −0.50 | 0.25 | −4.97 | 1.00E-05 | −3.28 | 0.013 |
| Margin time | Betaine | 0.02 | 1.37E-04 | 0.45 | 0.20 | 4.62 | 4.09E-05 | 1.58 | 0.165 |
| Stereotypy number | Isovalerylcarnitine | 0.02 | 1.25E-04 | −0.50 | 0.25 | −6.24 | 1.39E-07 | −2.53 | 0.040 |
| Stereotypy number | Pantothenate | 0.02 | 1.29E-04 | 0.47 | 0.22 | 3.83 | 3.95E-04 | 3.68 | 0.008 |
| Stereotypy number | Glutarylcarnitine | 0.04 | 2.39E-04 | 0.46 | 0.22 | 3.70 | 5.93E-04 | 2.33 | 0.053 |
| Stereotypy number | N-acetylglutamate | 0.05 | 3.53E-04 | −0.25 | 0.06 | −3.98 | 2.48E-04 | −1.03 | 0.338 |
| Stereotypy number | 4-guanidinobutanoate | 0.05 | 4.59E-04 | −0.35 | 0.12 | −3.90 | 3.19E-04 | −3.15 | 0.016 |
| Margin time | Myo-inositol | 0.05 | 4.86E-04 | −0.47 | 0.22 | −2.78 | 7.93E-03 | −2.76 | 0.028 |
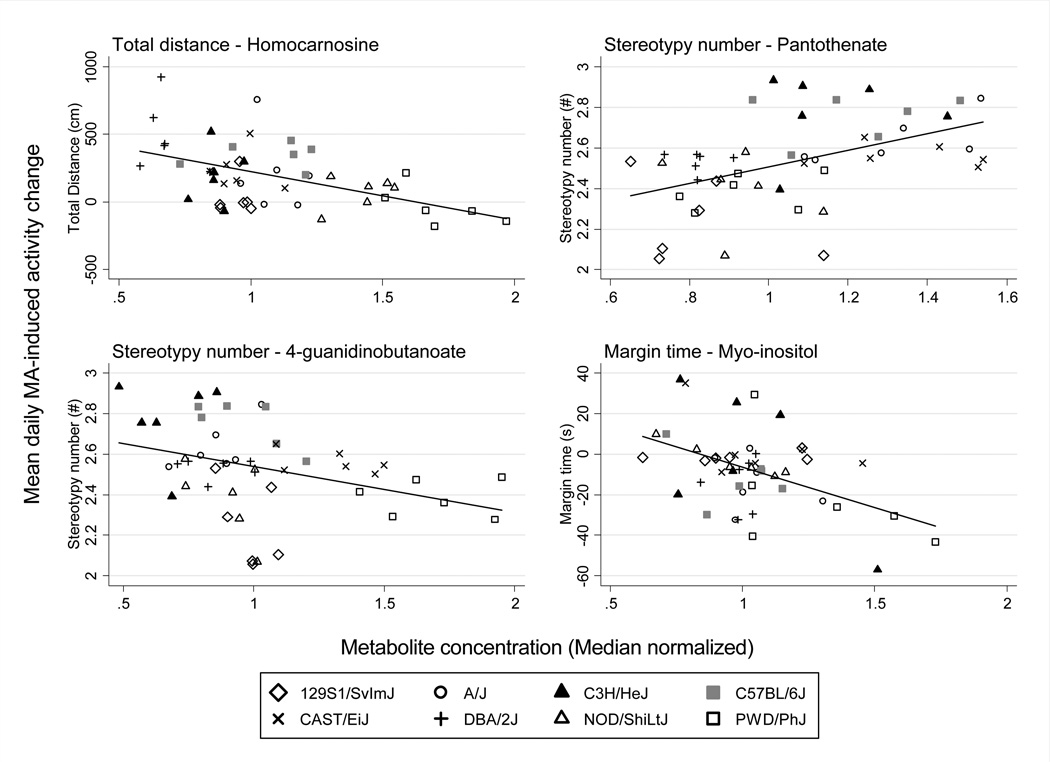
Figure 3. Associations of homocarnosine to TD, pantothenate and 4-guanidinobutanoate to SN, and myo-inositol to MT were robust across the examined inbred strain panel.
Sensitivity analyses indicated that the statistical significance of these associations was robust to outlying mice and the exclusion of any single strain.
Next, to determine whether the observed robustly significant associations were a direct result of concurrent MA level, we adjusted the sensitization-metabolite associations for MA level in brain homogenate at sacrifice. Results indicated that MA level did not significantly mediate associations, with marginal metabolite-sensitization associations remaining highly significant (p<.001) (Figure 4; Supplemental Table 4). Next, we adjusted the metabolite-sensitization associations for strain differences to determine to what extent significant associations were due to genetic differences. Results indicated that associations were generally largely determined by genetic variation between strains, with marginal metabolite effects attenuated to nonsignificant levels when adjusting for strain for homocarnosine-TD, pantothenate-SN and 4-guanidinobutanoate-SN (p>.05; Figure 4). The myo-inositol-MT association was an exception—it was not substantially influenced by strain differences, with its marginal metabolite-sensitization association remaining large and significant (p<.001; Figure 4). These results were consistent with one way ANOVA results showing significant heritability for all implicated metabolites and sensitization outcomes, with the exceptions of myo-inositol and MT.
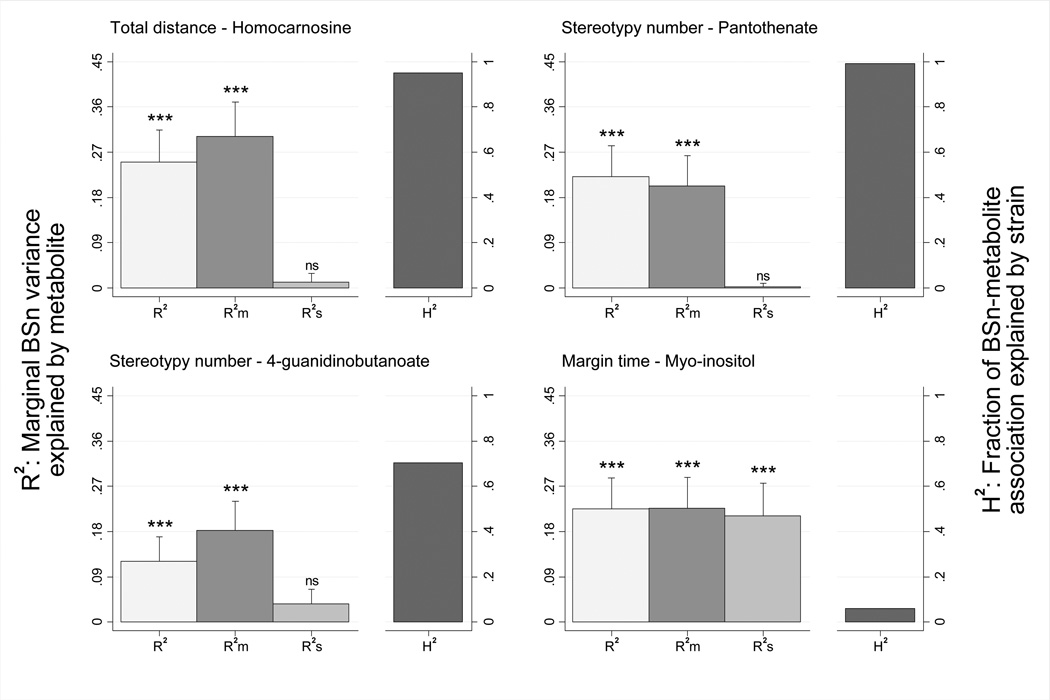
Figure 4. Among robust associations, neurochemical concentrations explained 12–25% of BSn variance and, with the exception of myo-inositol, were largely mediated by genetic differences.
The first columns (light gray) describe unconditional BSn variance explained by the metabolite, with R2 ranging from 0.251 (homocarnosine) to 0.121 (4-guanidinobutanoate) (p<.001; bars represent s.e.m.). The second columns (dark gray) describe marginal BSn variance explained by metabolite adjusting for concurrent MA brain levels (partial R2), with R2m remaining highly significant (p<.001). The third columns (medium gray) represents the metabolite partial R2 adjusting for strain, which was nonsignificant for homocarnosine, pantothenate and 4-guanidinobutanoate (p>.05), but significant for myo-inositol (p<.001). Thus, estimates of the proportion of metabolite-BSn associations explained by strain were high (H2=0.70–0.99) for all associations except myo-inositol (H2=0.06), as shown in the final column (black) of each panel.
Finally, we collapsed metabolite levels into strain means and examined the correlations of the metabolite strain means between the MA-test mice analyzed here and mice sacrificed at baseline (i.e., no MA exposure). The rationale for this analysis is that if, (a) the strain differences explain a large majority of metabolite-sensitization associations, and (b) the correlations of strain metabolite means pre- and post- MA sensitization are high, it can be transitively inferred that, (c) significant results reflect associations between baseline strain neurochemical differences and sensitization. This proved to be the case for metabolites in associations substantially accounted for by strains differences, with homocarnosine (p=0.0001, R2=0.94), pantothenate (p=0.0001, R2=0.94) and 4-guanidinobutanoate (p=0.0003, R2=0.90) all showing a very high degree of correspondence between strain means pre- and post- MA sensitization. A lower, though still substantial, correlation was observed for myo-inositol (p=0.013, R2=0.67), further suggesting a unique mechanism for the myo-inositol-MT association relative to the other robust associations. The full results of pre- and post-sensitization strain mean correlations for all 301 assayed metabolites are summarized in Supplemental Figure 8 and indicate variable correlation strength, but with far more significant associations than expected by chance (61.8% at p<0.05).
DISCUSSION
Here we report the first nontargeted metabolomics analysis to globally characterize neurochemical differences associated with MA-induced BSn. Association testing between sensitization measures and 301 mass spectrometry-derived metabolite levels, measured across eight inbred mouse strains, identified four novel, robust BSn-metabolite associations. Previous research indicates plausible biological mechanisms for several of these associations.
In addition to the primary sensitization metabolomics association analysis, we also analyzed the longitudinal activity data and metabolite battery separately. Results from our activity phenotype modeling yielded several notable findings. First, mean MA-induced BSn was significant across all activity measures and the magnitude of sensitization varied markedly across strains (Supplemental Figure 3; Figure 1). While previous research on strain differences in psychostimulant BSn has yielded mixed findings due to modest statistical power, the strain differences observed here were consistent with the most commonly reported patterns, showing increased BSn in DBA/2J and C57BL/6J, particularly relative to A/J and 129/J strains (Chen et al., 2007, Eisener-Dorman et al., 2011, Mead et al., 2002, Thomsen & Caine, 2011). Next, consistent with findings from BXD recombinant inbred strains (Philip et al., 2010), we found that strain accounted for a significant proportion of sensitization variance (h2=33–50%, p<0.001) for all sensitization phenotypes except margin time (h2=15%, p=0.19) (Supplemental Table 3). Independent analyses of the metabolite battery indicated that heritability ranged widely for the 301 metabolites considered, with most metabolites significantly heritable, many metabolites, including homocarnosine (h2=89%), highly heritable, but 31.2% of metabolites, including myo-inositol, showing nonsignificant heritability (p<0.05) (Supplementary Figure 7).
Results from the primary association analyses of MA-induced activity sensitization (TD, SN and MT) and the 301 brain metabolites indicated four metabolite-sensitization associations were metabolome-wide significant (q<0.05) and satisfied all sensitivity criteria: homocarnosine-TD (β=−0.50, p=2.25E-05), pantothenate-SN (β=0.47, p=1.29E-04), 4-guanidinobutanoate-SN (β=−0.35, p=4.59E-04) and myo-inositol-MT (β=−0.47, p=4.86E-04) (Figure 3). Among these implicated metabolites, the association of homocarnosine to TD provides clear biological rationale, suggesting that strain-based differences in homocarnosine-mediated cortical excitability moderate the magnitude of MA-induced psychomotor sensitization. This interpretation is supported by research showing that homocarnosine, a CNS dipeptide of GABA and histidine, is intimately related to GABAergic function and serves as a potent inhibitory neuromodulator (Petroff et al., 1998b). Brain homocarnosine levels dose-dependently increase in response to antiepileptic drugs including vigabatrin and topiramate, with the degree of homocarnosine increase strongly correlating with seizure control (Petroff et al., 1998a, Petroff et al., 1999). Moreover, homocarnosine has been indirectly implicated in MA-induced sensitization by studies showing that vigabatrin is effective in treating psychostimulant dependence (Brodie et al., 2005, Gerasimov et al., 1999). Vigabatrin is thought to influence psychostimulant addiction through tonically inhibiting both endogenous and psychostimulant-induced extracellular striatal DA release (Dewey et al., 1992, Gerasimov et al., 1999). Additional clinical research has shown that homocarnosine’s cortical inhibitory effects are relevant to several related outcomes, including alcohol dependence and panic disorder (Behar et al., 1999, Goddard et al., 2001).
Homocarnosine’s neural function remains incompletely understood. Although it has been established that hydrolysis of homocarnosine can rapidly liberate GABA in brain, the balance of evidence suggests that homocarnosine most likely exerts inhibitory effects in of itself, without conversion to GABA (Jackson et al., 1994, Petroff, 2002). This is supported by recent studies of homocarnosine function in rat hippocampus indicating that while both homocarnosine and GABA exerted inhibitory effects, homocarnosine showed no effect on membrane potential; its effects were blocked by pretreatment with GABAA inhibitors; and its presence reduced the frequency but not the burst characteristics of induced seizure-like activity (Petroff & Williamson, 2009). Thus, consistent with our findings, it appears that homocarnosine’s inhibitory action does not function through increasing GABA levels, but rather through shifting the balance from intracellular to synaptic locations of neurotransmitter.
The 4-guanidinobutanoate-SN association suggests a related GABAergic mechanism. 4-guanidinobutanoate is a relatively poorly studied metabolite formed by the transfer of the guanidino group from arginine to GABA via transamination (Jansen et al., 2006, Schulze et al., 1998). While research on the neural function of this metabolite is sparse, there is some indication that it exerts GABAergic effects in rodent CNS (Bowery & Brown, 1974). Intriguingly, as with homocarnosine, 4-guanidinobutanoate levels are elevated by vigabtrin treatment (Schulze et al., 1998). These facts, combined with the substantial correlation observed with homocarnosine in the current data (r=0.58; p<0.001), suggest that 4-guanidinobutanoate is plausible biomarker of GABAergic inhibition and perhaps a causal factor in attenuating MA-induced BSn.
The myo-inositol-MT association is also consistent with previous research. Margin time, or thigmotaxis, is a well-established index of anxiety in mice, which has been validated using several anxiogenic and anxiolytic drugs (Simon et al., 1994). Consistent with our results, there is a large body of research in both rodents and humans indicating a negative correlation between CNS myo-inositol levels and anxiety disorders /behaviors (Benjamin et al., 1995, Einat & Belmaker, 2001). This research has demonstrated that chronic myo-inositol administration, but not acute, increases open field activity in rodents and that chronic exposure increases rodent myo-inositol levels in various brain regions, including cortex and hippocampus (Cohen et al., 1997, Einat & Belmaker, 2001). Human postmortem research has shown that reduced myo-inositol levels in frontal cortex are associated with bipolar disorder and suicide, suggesting that endogenous levels are relevant to anxiety-related phenotypes (Shimon et al., 1997). While the precise mechanism by which myo-inositol exerts its anxiolytic effects remains obscure, the metabolite is a key precursor of the phosphoinositide secondary messenger cycle, which is activated following ligand binding with G-protein coupled receptors across several neurotransmitter systems including serotonergic (5-HT1C and 5-HT2) and dopaminergic (D1) receptor types (Kim et al., 2005). Finally, the pantothenate-SN finding is entirely novel, with no notable support from previous research.
Synthesizing all results yields a consistent causal interpretation, with the notable exception of the myo-inositol-MT finding. Thus, the homocarnosine-TD, 4-guanidinobutanoate-SN and pantothenate-SN associations all share several notable features—all of these metabolites and sensitization dimensions are highly heritable in this mouse panel; strain means of the metabolites are virtually perfectly correlated between untreated and MA sensitized mice; and the covariances of metabolites and sensitization measures are highly heritable. Furthermore, analyses controlling for acute MA activity effects and MA brain levels at sacrifice rule out the possibilities that these findings reflect differences in MA metabolism rates or the acute activity-inducing effects of MA. Additionally, none of the significant associations identified here overlap findings from our previous metabolomics analysis of the direct neurochemical effects of MA exposure (Mcclay et al., 2013). Cumulatively, these findings strongly suggest that baseline strain differences in the implicated neurochemicals substantially influence the magnitude MA-induced TD and SN sensitization.
The myo-inositol-MT finding suggests a different, and less certain, causal interpretation. Unlike the other significant associations, myo-inositol and MT were not significantly heritable in this sample, nor was their covariance. Furthermore, the correlation of strain means in untreated and MA sensitized mice indicated substantially less stability than was observed for the other significant metabolites. Thus, this finding is not attributable to strain differences and it is possible that the association may be due to myo-inositol levels differentially shifting as a consequence of repeated MA exposure, rather than baseline neurochemical differences. Given the causal ambiguity of this finding, it may be reasonable to regard it with a heightened degree of skepticism. However, the result is entirely consistent with a large body of previous research showing negative correlations between CNS myo-inositol levels and anxiety traits, including open field measures similar to MT (Einat & Belmaker, 2001, Shimon et al., 1997), supporting its validity as a true discovery.
These results suggest promising directions for future research. Given that the predominant pattern in the significant findings suggests common genetic variation producing baseline neurochemical differences that then substantially predict the magnitude of MA-induced sensitization, future research using a heterogeneous, genetically diverse sample of mice, such as latter generations of the Collaborative Cross (Iraqi et al., 2012), would be advantageous for several reasons. First, the increased genetic diversity would offer greater statistical power and reduce the risk of spurious association (Flint & Eskin, 2012). Second, genomewide genotypes are freely available for the founder strains and hundreds of crosses of the Collaborative Cross panel (Iraqi et al., 2012), enabling inexpensive genetic mapping of both metabolite levels and sensitization variables. Notwithstanding these merits, an awareness of the limits of generalizability between mouse models and humans is warranted given known species differences in potentially relevant neurochemical pathways, such as neuroactive steroid modulation of GABAergic signaling (Nguyen et al., 1995).
Another topic relevant to future research is the use of whole brain homogenate in the current study, the selection of which was influenced by volume requirements for the nontargeted metabolomic assay (Evans et al., 2009, Ohta et al., 2009). While, in principle, volume requirements could have been met through pooling specific regions across mice within strains, implicit in such pooling is the strong assumption of no within-strain variance, which would have precluded investigating heritability and missed the myo-inositol-MT finding, which was driven by within-strain variance. Another, more general limitation of the metabolomics platform is its exclusive focus on changes to the overall metabolite pool. Thus, highly localized effects, such as synaptic release of neurotransmitters, will not be detectable in the tissue homogenate because it is impossible to distinguish between, for example, vesicular and synaptic neurotransmitter molecules. Despite these limitations, the multiple robust signals detected here demonstrate the method’s value as a screening tool. Clearly, this is only the first stage in understanding the role of these metabolites in psychostimulant abuse. Accordingly, we encourage future research into the roles of the implicated metabolites in targeted assays of candidate brain regions.
In conclusion, using nontargeted metabolomic profiling, we identified multiple neurochemical signatures in MA-induced sensitization. Specific results include the finding that relative abundance of two specific GABAergic metabolites, homocarnosine and 4-guanidinobutanoate, attenuate the magnitude of MA psychomotor sensitization. Further, consistent with previous research, we found myo-inositol negatively associated with sensitization of MA’s anxiogenic effects. While the estimated risk of false discoveries was quite low, independent replication and targeted follow-up will be necessary to confirm and elaborate these findings. To facilitate this, we have made p-values from the primary analysis available for download (http://www.people.vcu.edu/~ejvandenoord/) as a resource supporting future replication, follow-up or data integration efforts. It is hoped that this research will encourage further metabolomics applications to identify novel neurochemical mechanisms and potential therapeutic targets in neuropsychiatric disorders.
Supplementary Material
Acknowledgments
This project was supported by National Institute of Health grants R21DA021411 to Dr. van den Oord and K01MH093731 to Dr. Adkins.
References
- Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OAC, Shulman GI, Navarro V, Petrakis IL, Charney DS, Krystal JH. Preliminary evidence of low cortical GABA levels in localized H-1-MR spectra of alcohol-dependent and hepatic encephalopathy patients. American Journal of Psychiatry. 1999;156:952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Levine J, Fux M, Aviv A, Levy D, Belmaker RH. DOUBLE-BLIND, PLACEBO-CONTROLLED, CROSSOVER TRIAL OF INOSITOL TREATMENT FOR PANIC DISORDER. American Journal of Psychiatry. 1995;152:1084–1086. doi: 10.1176/ajp.152.7.1084. [DOI] [PubMed] [Google Scholar]
- Bogdanov M, Matson WR, Wang L, Matson T, Saunders-Pullman R, Bressman SS, Beal MF. Metabolomic profiling to develop blood biomarkers for Parkinson's disease. Brain. 2008;131:389–396. doi: 10.1093/brain/awm304. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Brown DA. DEPOLARIZING ACTIONS OF GAMMA-AMINOBUTYRIC ACID AND RELATED COMPOUNDS ON RAT SUPERIOR CERVICAL GANGLIA IN-VITRO. British Journal of Pharmacology. 1974;50:205–218. doi: 10.1111/j.1476-5381.1974.tb08563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie JD, Figueroa E, Laska EM, Dewey SL. Safety and efficacy of gamma-vinyl GABA (GVG) for the treatment of methamphetamine and/or cocaine addiction. Synapse. 2005;55:122–125. doi: 10.1002/syn.20097. [DOI] [PubMed] [Google Scholar]
- Chen R, Zhang M, Park S, Gnegy ME. C57BL/6J mice show greater amphetamine-induced locomotor activation and dopamine efflux in the striatum than 129S2/SvHsd mice. Pharmacology Biochemistry and Behavior. 2007;87:158–163. doi: 10.1016/j.pbb.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Kotler M, Kaplan Z, Matar MA, Kofman O, Belmaker RH. Inositol has behavioral effects with adaptation after chronic administration. J. Neural Transmission. 1997;104:299–305. doi: 10.1007/BF01273190. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen JB, West SJ, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. Mahwah, N.J.: L. Erlbaum Associates; 2003. [Google Scholar]
- De Graaf RA, Chowdhury GMI, Brown PB, Rothman DL, Behar KL. In situ 3D magnetic resonance metabolic imaging of microwave-irradiated rodent brain: a new tool for metabolomics research. Journal of Neurochemistry. 2009;109:494–501. doi: 10.1111/j.1471-4159.2009.05967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Yu DW, Ferrieri RA, King PT, Macgregor RR, Martin TP, Wolf AP, Volkow ND, Fowler JS, Meller E. GABAERGIC INHIBITION OF ENDOGENOUS DOPAMINE RELEASE MEASURED INVIVO WITH C-11 RACLOPRIDE AND POSITRON EMISSION TOMOGRAPHY. J. Neurosci. 1992;12:3773–3780. doi: 10.1523/JNEUROSCI.12-10-03773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WB, Broadhurst DI, Atherton HJ, Goodacre R, Griffin JL. Systems level studies of mammalian metabolomes: the roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chemical Society Reviews. 2011;40:387–426. doi: 10.1039/b906712b. [DOI] [PubMed] [Google Scholar]
- Einat H, Belmaker RH. The effects of inositol treatment in animal models of psychiatric disorders. Journal of Affective Disorders. 2001;62:113–121. doi: 10.1016/s0165-0327(00)00355-4. [DOI] [PubMed] [Google Scholar]
- Eisener-Dorman AF, Grabowski-Boase L, Tarantino LM. Cocaine locomotor activation, sensitization and place preference in six inbred strains of mice. Behavioral and Brain Functions. 2011;7 doi: 10.1186/1744-9081-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, Nontargeted Ultrahigh Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry Platform for the Identification and Relative Quantification of the Small-Molecule Complement of Biological Systems. Analytical Chemistry. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- Fernando RL, Nettleton D, Southey BR, Dekkers JC, Rothschild MF, Soller M. Controlling the proportion of false positives in multiple dependent tests. Genetics. 2004;166:611–619. doi: 10.1534/genetics.166.1.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Eskin E. Genome-wide association studies in mice. Nature Reviews Genetics. 2012;13:807–817. doi: 10.1038/nrg3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimov MR, Ashby CR, Gardner EL, Mills MJ, Brodie JD, Dewey SL. Gamma-vinyl GABA inhibits methamphetamine, heroin, or ethanol-induced increases in nucleus accumbens dopamine. Synapse. 1999;34:11–19. doi: 10.1002/(SICI)1098-2396(199910)34:1<11::AID-SYN2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OAC, Charney DS, Krystal JH. Reductions in occipital cortex GABA levels in panic disorder detected with H-1-magnetic resonance spectroscopy. Archives of General Psychiatry. 2001;58:556–561. doi: 10.1001/archpsyc.58.6.556. [DOI] [PubMed] [Google Scholar]
- Ikarashi Y, Sasahara T, Maruyama Y. POSTMORTEM CHANGES IN CATECHOLAMINES, INDOLEAMINES, AND THEIR METABOLITES IN RAT-BRAIN REGIONS - PREVENTION WITH 10-KW MICROWAVE IRRADIATION. Journal of Neurochemistry. 1985;45:935–939. doi: 10.1111/j.1471-4159.1985.tb04083.x. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal, R. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- Iraqi FA, Mahajne M, Salaymah Y, Sandovski H, Tayem H, Vered K, Balmer L, Hall M, Manship G, Morahan G, Pettit K, Scholten J, Tweedie K, Wallace A, Weerasekera L, Cleak J, Durrant C, Goodstadt L, Mott R, Yalcin B, Aylor DL, Baric RS, Bell TA, Bendt KM, Brennan J, Brooks JD, Buus RJ, Crowley JJ, Calaway JD, Calaway ME, Cholka A, Darr DB, Didion JP, Dorman A, Everett ET, Ferris MT, Mathes WF, Fu CP, Gooch TJ, Goodson SG, Gralinski LE, Hansen SD, Heise MT, Hoel J, Hua KJ, Kapita MC, Lee S, Lenarcic AB, Liu EY, Liu HD, McMillan L, Magnuson TR, Manly KF, Miller DR, O'Brien DA, Odet F, Pakatci IK, Pan WQ, de Villena FPM, Perou CM, Pomp D, Quackenbush CR, Robinson NN, Sharpless NE, Shaw GD, Spence JS, Sullivan PF, Sun W, Tarantino LM, Valdar W, Wang J, Wang W, Welsh CE, Whitmore A, Wiltshire T, Wright FA, Xie YY, Yun ZN, Zhabotynsky V, Zhang ZJ, Zou F, Powell C, Steigerwalt J, Threadgill DW, Chesler EJ, Churchill GA, Gatti DM, Korstanje R, Svenson KL, Collins FS, Crawford N, Hunter K, Kelada SNP, Peck BCE, Reilly K, Tavarez U, Bottomly D, Hitzeman R, McWeeney SK, Frelinger J, et al. The Genome Architecture of the Collaborative Cross Mouse Genetic Reference Population. Genetics. 2012;190:389-U159. doi: 10.1534/genetics.111.132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MC, Scollard DM, Mack RJ, Lenney JF. LOCALIZATION OF A NOVEL PATHWAY FOR THE LIBERATION OF GABA IN THE HUMAN CNS. Brain Research Bulletin. 1994;33:379–385. doi: 10.1016/0361-9230(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Jansen EEW, Verhoeven NM, Cornelis JA, Schulze A, Senephansiri H, Gupta M, Snead OC, Gibson KM. Increased guanidino species in murine and human succinate semialdehyde dehydrogenase (SSADH) deficiency. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2006;1762:494–498. doi: 10.1016/j.bbadis.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, Krishnan KR. Metabolomics: a global biochemical approach to the study of central nervous system diseases. Neuropsychopharmacology. 2009;34:173–186. doi: 10.1038/npp.2008.174. [DOI] [PubMed] [Google Scholar]
- Kim H, McGrath BM, Silverstone PH. A review of the possible relevance of inositol and the phosphatidylinositol second messenger system (PI-cycle) to psychiatric disorders - focus on magnetic resonance spectroscopy (MRS) studies. Human Psychopharmacology-Clinical and Experimental. 2005;20:309–326. doi: 10.1002/hup.693. [DOI] [PubMed] [Google Scholar]
- Login GR, Dvorak AM. Application of microwave fixation techniques in pathology to neuroscience studies: a review. J Neurosci Methods. 1994;55:173–182. doi: 10.1016/0165-0270(94)90209-7. [DOI] [PubMed] [Google Scholar]
- McClay J, Adkins D, Vunck S, Batman A, Vann R, Clark S, Beardsley P, Oord ECG. Large-scale neurochemical metabolomics analysis identifies multiple compounds associated with methamphetamine exposure. Metabolomics. 2013;9:392–402. doi: 10.1007/s11306-012-0456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Katz JL, Rocha BA. Intravenous cocaine-induced activity in A/J and C57BL/6J mice: behavioral sensitization and conditioned activity. Neuropharmacology. 2002;42:976–986. doi: 10.1016/s0028-3908(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nature Reviews Neuroscience. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nguyen Q, Sapp DW, Vanness PC, Olsen RW. MODULATION OF GABA(A) RECEPTOR-BINDING IN HUMAN BRAIN BY NEUROACTIVE STEROIDS - SPECIES AND BRAIN REGIONAL DIFFERENCES. Synapse. 1995;19:77–87. doi: 10.1002/syn.890190203. [DOI] [PubMed] [Google Scholar]
- Niwa M, Yan Y, Nabeshima T. Genes and molecules that can potentiate or attenuate psychostimulant dependence: relevance of data from animal models to human addiction. Ann N Y Acad Sci. 2008;1141:76–95. doi: 10.1196/annals.1441.024. [DOI] [PubMed] [Google Scholar]
- Ohta T, Masutomi N, Tsutsui N, Sakairi T, Mitchell M, Milburn MV, Ryals JA, Beebe KD, Guo L. Untargeted Metabolomic Profiling as an Evaluative Tool of Fenofibrate-Induced Toxicology in Fischer 344 Male Rats. Toxicologic Pathology. 2009;37:521–535. doi: 10.1177/0192623309336152. [DOI] [PubMed] [Google Scholar]
- Peachey JE, Rogers B, Brien JF, Maclean A, Rogers D. MEASUREMENT OF ACUTE AND CHRONIC BEHAVIORAL-EFFECTS OF METHAMPHETAMINE IN MOUSE. Psychopharmacology. 1976;48:271–275. doi: 10.1007/BF00496860. [DOI] [PubMed] [Google Scholar]
- Pears MR, Cooper JD, Mitchison HM, Mortishire-Smith RJ, Pearce DA, Griffin JL. High resolution (1)H NMR-based metabolomics indicates a neurotransmitter cycling deficit in cerebral tissue from a mouse model of Batten disease. Journal of Biological Chemistry. 2005;280:42508–42514. doi: 10.1074/jbc.M507380200. [DOI] [PubMed] [Google Scholar]
- Peters LL, Robledo RF, Bult CJ, Churchill GA, Paigen BJ, Svenson KL. The mouse as a model for human biology: a resource guide for complex trait analysis. Nature Reviews Genetics. 2007;8:58–69. doi: 10.1038/nrg2025. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Hyder F, Rothman DL, Mattson RH. Human brain GABA and homocarnosine increased after starting topiramate. Neurology. 1998a;50:A312–A312. [Google Scholar]
- Petroff OA, Williamson A. Homocarnosine Enhances Inhibitory Tone in Rat Hippocampus. Annals of Neurology. 2009;66:S14–S14. [Google Scholar]
- Petroff OAC. GABA and glutamate in the human brain. Neuroscientist. 2002;8:562–573. doi: 10.1177/1073858402238515. [DOI] [PubMed] [Google Scholar]
- Petroff OAC, Hyder F, Collins T, Mattson RH, Rothman DL. Acute effects of vigabatrin on brain GABA and homocarnosine in patients with complex partial seizures. Epilepsia. 1999;40:958–964. doi: 10.1111/j.1528-1157.1999.tb00803.x. [DOI] [PubMed] [Google Scholar]
- Petroff OAC, Mattson RH, Behar KL, Hyder F, Rothman DL. Vigabatrin increases human brain homocarnosine and improves seizure control. Annals of Neurology. 1998b;44:948–952. doi: 10.1002/ana.410440614. [DOI] [PubMed] [Google Scholar]
- Philip VM, Duvvuru S, Gomero B, Ansah TA, Blaha CD, Cook MN, Hamre KM, Lariviere WR, Matthews DB, Mittleman G, Goldowitz D, Chesler EJ. High-throughput behavioral phenotyping in the expanded panel of BXD recombinant inbred strains. Genes Brain Behav. 2010;9:129–159. doi: 10.1111/j.1601-183X.2009.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Research Reviews. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JTJ, Griffin JL, Wayland M, Freeman T, Dudbridge F, Lilley KS, Karp NA, Hester S, Tkachev D, Mimmack ML, Yolken RH, Webster MJ, Torrey EF, Bahn S. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Molecular Psychiatry. 2004;9:684–697. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- Robinson GK. That BLUP is a Good Thing: The Estimation of Random Effects. Statistical Science. 1991;6:15–32. [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. B-Biol. Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers W. Regression standard errors in clustered samples. Stata Technical Bulletin. 1994;3:19–23. [Google Scholar]
- Schulze A, Mayatepek E, Frank S, Marescau B, De Deyn PP, Bachert P. Disturbed metabolism of guanidino compounds characterized by elevated excretion of beta-guanidinopropionic acid and gamma-guanidinobutyric acid - An effect of vigabatrin treatment? Journal of Inherited Metabolic Disease. 1998;21:268–271. doi: 10.1023/a:1005376307471. [DOI] [PubMed] [Google Scholar]
- Shimon H, Agam G, Belmaker RH, Hyde TM, Kleinman JE. Reduced frontal cortex inositol levels in postmortem brain of suicide victims and patients with bipolar disorder. American Journal of Psychiatry. 1997;154:1148–1150. doi: 10.1176/ajp.154.8.1148. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. THIGMOTAXIS AS AN INDEX OF ANXIETY IN MICE - INFLUENCE OF DOPAMINERGIC TRANSMISSIONS. Behav. Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Snider SE, Vunck SA, van den Oord E, Adkins DE, McClay JL, Beardsley PM. The glial cell modulators, ibudilast and its amino analog, AV1013, attenuate methamphetamine locomotor activity and its sensitization in mice. European Journal of Pharmacology. 2012;679:75–80. doi: 10.1016/j.ejphar.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Research Reviews. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug Wanting: Behavioral Sensitization and Relapse to Drug-Seeking Behavior. Pharmacological Reviews. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey J. The positive false discovery rate: A Bayesian interpretation and the q-value. Ann Stat. 2003;31:2013–2035. [Google Scholar]
- Strakowski SM, Sax KW. Progressive behavioral response to repeated d-amphetamine challenge: Further evidence for sensitization in humans. Biological Psychiatry. 1998;44:1171–1177. doi: 10.1016/s0006-3223(97)00454-x. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Psychomotor Stimulant Effects of Cocaine in Rats and 15 Mouse Strains. Experimental and Clinical Psychopharmacology. 2011;19:321–341. doi: 10.1037/a0024798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Oord E, Sullivan PF. False discoveries and models for gene discovery. Trends in Genetics. 2003;19:537–542. doi: 10.1016/j.tig.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Willett JB. SOME RESULTS ON RELIABILITY FOR THE LONGITUDINAL MEASUREMENT OF CHANGE - IMPLICATIONS FOR THE DESIGN OF STUDIES OF INDIVIDUAL GROWTH. Educational and Psychological Measurement. 1989;49:587–602. [Google Scholar]
- Willett JB, Singer JD, Martin NC. The design and analysis of longitudinal studies of development and psychopathology in context: Statistical models and methodological recommendations. Development and Psychopathology. 1998;10:395–426. doi: 10.1017/s0954579498001667. [DOI] [PubMed] [Google Scholar]
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A PSYCHOMOTOR STIMULANT THEORY OF ADDICTION. Psychological Review. 1987;94:469–492. [PubMed] [Google Scholar]
- Yang H, Bell TA, Churchill GA, de Villena FPM. On the subspecific origin of the laboratory mouse. Nature Genet. 2007;39:1100–1107. doi: 10.1038/ng2087. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2006;30:1381–1393. doi: 10.1016/j.pnpbp.2006.05.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.