Abstract
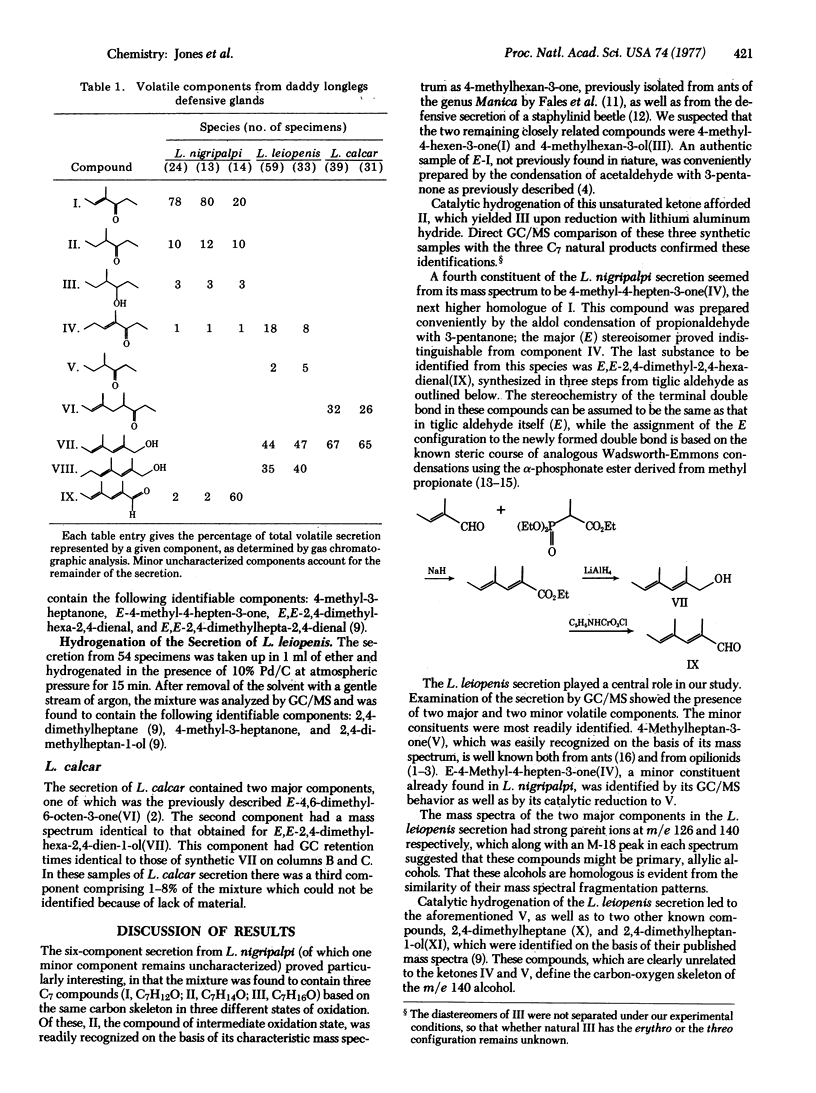
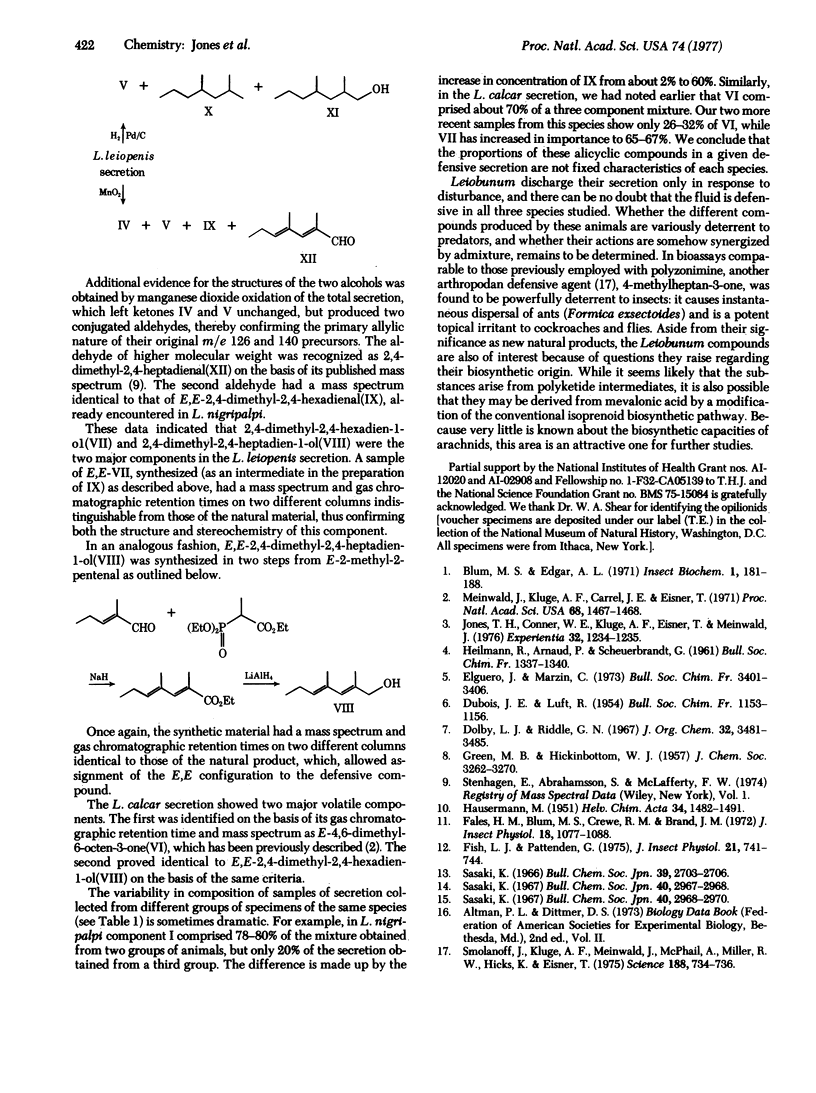
Analyses of the chief volatile constituents of the defensive secretions of three oplionids were carried out. Leiobunum nigripalpi produces three closely related C7 compounds: E-4-methyl-4-hexen-3-one(I), 4-methylhexan-3-one(II), and 4-methylhexan-3-ol(III), along with E-4-methyl-4-hepten-3-one(IV), E,E-2,4-dimethylhexa-2,4-dienal(IX), and a minor, unidentified component. L. leiopenis secretion contains E-4-methyl-4-hepten-3-one(IV), 4-methylheptan-3-one(V), E,E-2,4-dimethylhexa-2,4-dien-1-ol(VII), and E,E-2,4-dimethylhepta-2,4-dien-1-ol(VIII). L. calcar yields chiefly E-4,6-dimethyl-6-octen-3-one(VI) and E,E-2,4-dimethylhexa-2,4-dien-1-ol(VII). Six of these compounds are new natural products. The structures of these compounds, which can be regarded either as polyketide-derived or as modified isoprenoids, raise interesting biosynthetic questions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Meinwald J., Kluge A. F., Carrel J. E., Eisner T. Acyclic ketones in the defensive secretion of a "daddy longlegs" (Leiobunum vittatum). Proc Natl Acad Sci U S A. 1971 Jul;68(7):1467–1468. doi: 10.1073/pnas.68.7.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolanoff J., Kluge A. F., Meinwald J., McPhail A., Miller R. W., Hicks K., Eisner T. Polyzonimine: A novel terpenoid insect repellent produced by a milliped. Science. 1975 May 16;188(4189):734–736. doi: 10.1126/science.1124395. [DOI] [PubMed] [Google Scholar]



