Abstract
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder with a high incidence in the general population. The diagnosis of IBS is mainly based on exclusion of other intestinal conditions through the absence of inflammatory markers and specific antigens. The current pharmacological treatment approaches available focus on reducing symptom severity while often limiting quality of life because of significant side effects. This has led to an effectiveness gap for IBS patients that seek further relief to increase their quality of life. Complementary and alternative medicines (CAM) have been associated with a higher degree of symptom management and quality of life in IBS patients. Over the past decade, a number of important clinical trials have shown that specific herbal therapies (peppermint oil and Iberogast®), hypnotherapy, cognitive behavior therapy, acupuncture, and yoga present with improved treatment outcomes in IBS patients. We propose an integrative approach to treating the diverse symptoms of IBS by combining the benefits of and need for pharmacotherapy with known CAM therapies to provide IBS patients with the best treatment outcome achievable. Initial steps in this direction are already being considered with an increasing number of practitioners recommending CAM therapies to their patients if pharmacotherapy alone does not alleviate symptoms sufficiently.
Keywords: Irritable bowel syndrome, Complementary and alternative medicine, Hypnotherapy, Cognitive behavioral therapy, Herbal therapy, Peppermint
Core tip: Irritable bowel syndrome is a prevalent gastrointestinal disorder that interferes with daily living in 5%-20% of the population. The current review summarizes the most widely used complementary and alternative medicine (CAM) approaches that have proven to be effective and have been endorsed by professional organizations. The review encourages the use of both pharmacotherapy and CAM approaches in an integrative setting to provide the best outcome and quality of life to patients.
INTRODUCTION
Irritable bowel syndrome (IBS) is among the most common gastrointestinal disorders with a prevalence ranging from 5%-20% in the general population worldwide[1,2]. IBS is more commonly diagnosed in women than in men and in people younger than 50 years[1,3,4]. The high prevalence of diagnosis also results in a significant socioeconomic burden through decreased work productivity, increased direct and indirect healthcare costs, and - depending on the severity - a reduction in quality of life for IBS patients and their caregivers[5-8]. The estimated indirect and direct healthcare costs related to IBS in the United States have been steadily increasing and amount to $1.35 billion dollar as of 2003[9]. The worldwide health costs associated with IBS are estimated to exceed $200 billion United States dollars[10]. The International Classification of Diseases (ICD) of the World Health Organization in its latest revision, ICD-10, classifies IBS as a functional digestive disorder with the ICD-10 classification 58.9 with sub-classifications as irritable colon or spastic colon[11]. This classification does not distinguish between the Rome-III criteria and the consensus of many professional medical organizations that have divided IBS into four different subgroups based on the primary symptom presentation as constipation-predominant IBS (IBS-C), diarrhea-predominant IBS (IBS-D), mixed or alternating IBS, and unspecified IBS[2,12]. The diagnosis of IBS is mainly dependent on the absence of pathophysiological and morphological indicators and therefore remains an exclusion diagnosis concentrated on symptom presentation[13]. There have been indications in recent research studies that IBS may be the result of a low-grade inflammatory process within the lower intestinal tract but definitive and validated biochemical markers have not emerged as of yet[14-17]. There also remains a gap in our understanding of the underlying pathophysiology and what causes IBS. A few hypotheses have linked genetic predisposition, post-infectious small bowel bacterial overgrowth, and certain diets with a higher incidence for developing IBS[18-20]. However, a unified understanding of the pathophysiology that may result in a feasible and causal treatment approach has not emerged. In defense of this deficit, similar knowledge gaps exist for a wide range of conditions for which symptomatic treatment to date provides the only therapeutic approach.
Because current pharmacological treatment approaches for IBS are solely based on symptom reduction, many patients remain undertreated and dissatisfied with their quality of life. In addition, many pharmacological treatment approaches are associated with side effects that result in a smaller benefit to the patient in terms of treatment outcomes[18,19]. The treatment also depends on the specific subtype of IBS. While IBS-C patients mainly suffer from abdominal pain because of slow bowel movement and less frequent bowel release, patients with IBS-D suffer from a social stigma due to the frequent bowel release that requires a bathroom in close proximity as well as bloating and increased flatulence[8,19,21,22]. Comorbidities between IBS with depressive and anxiety disorders have been well defined although it remains unknown which of these is the cause and the effect[23-26]. A common treatment for all subtypes of IBS are antidepressants which may indicate that certain depressive and anxiety disorders play a role in the pathophysiology of IBS[25,27-29]. Another emerging field of research is the investigation of the gut-brain axis also referred to as the enteric nervous system (ENS). It has been established that interconnected sensing of afferent and efferent neurons in the ENS influences gut motility based mainly on serotonergic and cholinergic nerve innervations[30,31]. The 2 major serotonin receptors present in the intestinal tract, 5-HT3 and 5-HT4, and a serotonin reuptake transporter are either differently expressed or even present with mutations in certain IBS populations[27,28,32-34]. This correlates well with the current pharmacological treatment approaches of using 5-HT3 receptor antagonists and 5-HT4 receptor agonists in IBS patients to reduce both visceral pain perception and regulate gastrointestinal motility[35-37]. Considering that the neurotransmitter and hormone serotonin is involved in both intestinal motility and mood regulation may serve as an indicator that changes in the ENS neurotransmission are involved in the comorbidity between IBS with depressive and anxiety disorders.
As mentioned, current pharmacological treatment approaches provide limited symptomatic relief to IBS patients. This has resulted in a significant increase in self-medication and the use of complementary and alternative medicines (CAM) by patients and even healthcare providers to bridge the gap and increase quality of life[38-42]. This review will summarize the current knowledge of CAM alone and in conjunction with pharmacological treatments as an integrative approach to manage patients with IBS and improve their quality of life. Although the review is not comprehensive in addressing all aspects of CAM and integrative medical approaches to treating IBS, it is intended to provide practitioners with the most commonly used and most widely recommended CAM approaches that have shown repeated success in clinical trials over the past decades. It is important to point out that pharmacological treatment should not be abandoned by patients and their providers in lieu of CAM approaches but rather an integrative approach considered that provides both maximum relief of symptoms and increased quality of life.
LITERATURE SEARCH
This article reviews current research regarding the most commonly used CAM therapies for IBS in the United States, which are single or combination herbal products, acupuncture, yoga, hypnotherapy, and cognitive behavioral therapy. The literature search covered the period from January 1996 to June 2013 using Medline and PubMed with the search terms “irritable bowel syndrome” in combination with “yoga”, “hypnotherapy”, “cognitive behavioral therapy”, “CBT”, “CAM”, “acupuncture”, “herbal therapy”, and “integrative medicine”. Out of 714 total articles retrieved, 243 were excluded because they were reviews or protocols, 215 were not in English, and 102 were duplicates or did not relate to IBS. A total of 154 articles were selected for inclusion in this review (Figure 1).
Figure 1.
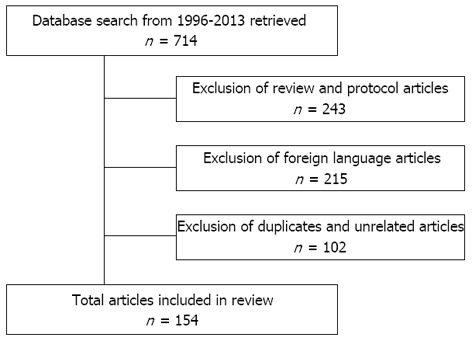
Flow chart illustrating the selection criteria for inclusion of articles.
PHARMACOLOGICAL TREATMENT APPROACHES
The current approach to treating IBS is symptomatic and consists of a regimen of first line pharmacological treatment options often coupled with lifestyle adjustments followed by potential off-label use of a number of other medications that are not specifically indicated for IBS if symptom management is insufficient[2,20,43]. The current leading guidelines have been developed by the Task Force on Irritable Bowel Syndrome of the American College of Gastroenterology (ACG) and the British Society of Gastroenterology[2,12]. Both associations recognize that symptomatic treatment of IBS is associated with a significant placebo effect which has been confirmed in a number of studies as well as in a now well-known unblinded study where patients were told that they were receiving placebo and still showed significant improvements in symptoms[44]. These findings support the hypothesis of an underlying connection between the brain and the gut and the potential interplay of emotions and mood disorders affecting the severity of IBS symptoms.
Considering the increased incidence of comorbid depressive and anxiety disorders in patients with IBS, the use of antidepressants, mainly tricyclic (TCA) and selective serotonin reuptake inhibitor (SSRI) antidepressants, have shown improvements in IBS symptoms and there seems to be an indication that such medication may also provide symptom relief in IBS patients without comorbid psychiatric disorders[45-48]. The effectiveness of low dose TCA and SSRI antidepressants as well as benzodiazepines is yet another indication that the enteric nervous system is influenced by mood and that this in turn affects the innervation by serotonergic and cholinergic neurons in the gastrointestinal (GI) tract[49,50].
The initial step in treating IBS is to consider supportive treatments that may help to alleviate mild gastrointestinal symptoms by increasing fiber and probiotics consumption, regular exercise regimens, and eliminating certain food items that may be linked to an allergic reaction (often lactose intolerance). These supportive treatments can be considered integrative in nature since many IBS patients will remain on a specialized diet even after initiating pharmacotherapy if their symptoms are moderate to severe[40,51,52]. Patients with moderate IBS symptoms often require a first line treatment to reduce symptoms and may also benefit from CAM therapy, especially cognitive behavior therapy (CBT) or hypnotherapy[53-57]. A number of publications indicate that the patient-practitioner relationship can have a significant influence on treatment outcomes[58,59]. Practitioners should try to communicate clearly with patients about the diagnostic process, the potential treatment options, setting realistic goals for outcomes and improvement, and providing an atmosphere that is caring and supportive.
The most commonly used pharmacological interventions for symptomatic relief of moderate to severe IBS constitute prokinetics and antispasmodics for IBS-C patients and opioid agonists, anticholinergics, and 5-HT3 antagonists for relieving IBS-D symptoms[19,20]. Prokinetics are not specific to IBS and increase gastrointestinal motility in general by acting via dopamine and 5-HT3 receptors as antagonists or 5-HT4 receptors as agonists[60,61]. Tegaserod is to date the only Food and Drug Administration approved prokinetic drug specific for the treatment of IBS-C but has been significantly limited in its use due to an increased cardiovascular risk[62,63]. Lubiprostone, a 5-HT4 agonist, has been recently approved to treat IBS-C in women through activation of chlorine channels leading to increased water secretion into the lumen which decreased transit time and associated visceral pain in patients[61]. The common use of 5-HT3 receptor antagonists such as ondansetron and granisetron to reduce visceral pain perception in IBS-D patients has shown some benefits but is also limited by side effects. The best risk-to-benefit ratio has been shown for the 5-HT3 antagonist alosetron to date[36,64,65].
The common use of antispasmodics in both IBS-C and IBS-D patients serves to reduce abdominal pain and cramping but requires close monitoring especially in IBS-C patients because of further slowing of GI motility[66-68]. All of the currently available antispasmodics are not specific to IBS and act as anticholinergics which are associated with a number of side effects including hyposalivation and cardiovascular events[20,69].
The use of opioid agonists to reduce GI motility in IBS-D patients is another off-label use that can help to improve quality of life and reduce pain perception. The most commonly used opioid agonists are diphenoxylate and loperamide although there is a risk for dependence development which needs to be monitored[70,71].
Low-grade inflammation has been observed in some IBS patients, especially those with post-infectious IBS and small intestinal bacterial overgrowth (SIBO) which has led to the use of antibiotics and glucocorticoids[72-74]. An important consideration in the treatment of low-grade inflammatory processes is the choice of agents since the anti-inflammatory effects should remain localized and not influence the systemic immune system. So far, the salicylate derivative mesalazine which is also used to treat Crohn’s disease with a known inflammatory component has shown an increase in quality of life and symptom reduction in IBS-D patients[75]. Glucocorticoids may provide benefits in IBS although this has only been shown in animal models to date and is based on the observation that patients on oral glucocorticoids show a lower incidence of IBS[76,77]. Rifaximin remains the only antibiotic that has been tested in IBS patients and has shown a moderate improvement in GI symptoms and quality of life whereas other antiinfectives such as nystatin and tetracyclines did present with unacceptable systemic side effects and a low responder rate[78-81].
A number of new targets and accompanying drugs are being developed and tested which may provide additional benefits in the treatment of IBS[19,82]. The coming years will show if they are effective and associated with less side effects compared to the currently available pharmacological options.
Aside from the pharmacological treatments which are often limited by significant side effects, up to 50% of patients are self-medicating using herbals and dietary supplements or other CAM approaches to improve their quality of life and reduce IBS symptoms[38,83,84]. Further increases in the use of CAM are a result of underdiagnosed or misdiagnosed IBS since the differential diagnosis of IBS can be complicated and delayed[40,85,86]. This review addresses the current state of CAM use in IBS and how both CAM and conventional therapeutic treatment can be used in synergy as an integrative approach to treating IBS and providing patients with the best possible quality of life.
COMPLEMENTARY AND ALTERNATIVE MEDICINES
The wide variety of CAM approaches includes basic changes in diet and lifestyle such as increased fiber intake or regular exercise as well as specific use of herbal medicines, combination products, mechanical interventions (acupuncture or massage), or behavioral therapy (cognitive behavioral therapy, relaxation techniques, and hypnotherapy)[43,84]. CAM is often used for chronic health conditions either alone or in conjunction with pharmacological treatment options. About half of IBS patients reported using at least one CAM treatment alone or in addition to their prescription medicine[42,87]. A lack of evidence-based clinical trials often limits the available knowledge about the efficacy of CAM in disease conditions, which is why the ACG Task force on IBS reports that CAM have not consistently demonstrated a strong positive outcome[2]. Recent systematic reviews, however, show indications that various CAM modalities may benefit IBS patients and increase their quality of life[42,43,84,88]. Since IBS can present with varying symptoms and severity even with daily or weekly fluctuations within the same patient, the effectiveness of CAM may at times appear inadequate or questionable by patients themselves. The most commonly used CAM interventions that have been evaluated in clinical trials, are dietary changes, use of probiotics, exercise, single herbal extracts, herbal combination products, hypnotherapy, acupuncture, and relaxation techniques. All of these approaches are discussed in more detail.
Diet and lifestyle modifications
Diet modifications are not generally considered CAM and usually are the first step in reducing IBS symptom severity even before pharmacotherapy is initiated (Table 1). However, exclusion diets can often be supplemented with CAM to reduce specific symptoms such as bloating and distension[2,89]. Exclusion diets may benefit patients with a known allergy and those in post-infectious IBS or SIBO[40,51,52] and consists of removing wheat, dairy products, eggs, coffee and caffeinated beverages, yeast, potatoes, and citrus fruits[52]. Despite the reported successes with exclusion diets, they may, at times, be hard to follow thereby resulting in bouts of increased IBS symptom severity. While dietary restrictions may benefit some IBS patients, entirely skipping meals actually has shown to worsen IBS symptoms[52,90].
Table 1.
Clinical trials on diet and exercise interventions for irritable bowel syndrome
| Intervention | Study design | Sample size | Outcome | Ref. |
| Acceptability questionnaire | Anonymous survey | 256 | Most acceptable were tablets (84%), diet and lifestyle changes (82%), yoga (77%); less acceptable were acupuncture (59%) and suppositories (57%) | [40] |
| Food elimination | Open label pilot study | 20 | Significant improvements in stool frequency (P < 0.05), pain (P < 0.05), and IBS-QOL (P < 0.001) | [89] |
| Diet and lifestyle | Cross-sectional study | 1717 | Significant difference between IBS and non-IBS participants in regards to residential type (OR = 1.27) and frequency of meals (OR = 1.69) | [90] |
| Diet and lifestyle | Questionnaire | 983 | BMI was associated with abdominal pain and diarrhea, healthier diet and physical activity were associated with fewer GI symptoms | [91] |
| Diet | 3-way cross-over study | 22 | IBS-D patients showed significant increase in small bowel and mucosal permeability for mannitol and lactulose sugars compared to healthy controls | [92] |
| Diet | Questionnaire | 1978 | Potential for higher lactose intolerance incidence in patients with IBS compared to healthy patients | [93] |
| Diet | Case-control study | 177 | Symptomatic lactose intolerance more frequent in patients with IBS than healthy subjects, but incidence of lactose intolerance not different between groups | [94] |
| Diet | Case-control study | 120 | Lactose intolerance resulted in more frequent self-reported symptoms in patients with IBS-D than controls (P < 0.001, OR = 6.25), IBS-D patients consumed significantly less dairy products (P = 0.04) | [95] |
| Exercise | Randomized, controlled trial | 56 | No difference in quality of life between exercise and usual care groups, exercise group presented with significant less symptoms of constipation after 12 wk intervention | [98] |
| Exercise | Cross-over study | 8 | Gas retention during rest was associated with significant abdominal symptoms in IBS patients (P < 0.01), symptoms improved during exercise (P < 0.05) compared to rest | [99] |
| Exercise | Descriptive comparative study | 89 | Women with IBS report less physical activity (P < 0.05), women with IBS who were physical active reported significantly less symptoms of fatigue (P = 0.003) compared with the ones with IBS who were physically inactive | [100] |
| Yoga | Randomized cross-over study | 25 | Lower functional disability (P = 0.073) and anxiety levels (P = 0.09) in the yoga group compared to the waitlist group, significantly lower GI symptoms (P < 0.01) | [101] |
| Yoga | Randomized parallel design | 21 | Similar reductions in symptoms after 2 mo for yoga and the group receiving loperamide in IBS-D patients | [102] |
IBS: Irritable bowel syndrome; QOL: Quality of life; BMI: Body mass index; IBS-D: Diarrhea-predominant IBS; GI: Gastrointestinal.
Some contribution to intestinal symptoms may come from a diet that is high in fat, carbohydrates, and sugar alcohols. It has been shown that increased fat consumption is linked to increased stool numbers and diarrhea and therefore should be considered as a factor in worsening IBS-D[90,91]. Fructose-rich food and beverage items (soft drinks, baked and packaged goods, cereals) can also aggravate flatulence, abdominal discomfort, and diarrhea and should therefore be monitored in IBS patients[51,52]. Especially poorly absorbed sugar alcohols that are present in diet soft drinks and low carbohydrate foods can exacerbate GI symptoms[51,92]. Together with general restrictions on carbohydrate intake, lactose intolerance and malabsorption appear to be more prevalent in IBS patients. If lactose is not absorbed from the GI tract it is metabolized via the gut bacteria and leads to increased bloating, distension, and diarrhea which can aggravate IBS symptoms[93-95].
Fiber is often recommended as a dietary change to reduce global IBS symptoms but the clinical data to date are less clear. It has been shown that soluble fiber can lower GI symptoms in IBS-C although the data supporting it is highly variable both in the amount of fiber consumed (ranging from 5-30 g/d) and the duration of the trials (3-16 wk)[51,91,96,97]. Fiber, regardless soluble or insoluble, was not able to reduce pain perception in IBS patients and specifically insoluble fiber such as nuts and whole grains may exacerbate IBS symptoms overall[97].
An important lifestyle adjustment that should be recommended to IBS patients is regular exercise. Mild exercise or physical activity has been shown to reduce IBS symptoms and alleviates bloating and gas production in several studies[98,99]. Since regular exercise also helps to increase gastrointestinal motility it is beneficial in IBS-C patients with primary low GI movement and hard stools[100]. As part of exercise, yoga has been investigated due to its low impact on joints and its relatively targeted postures that can help to reduce GI symptoms[101,102]. Pranayama yoga administered twice daily has been shown to increase sympathetic tone and may benefit IBS-D patients that present with decreased sympathetic activity to the same degree than daily loperamide administration in the control group[102].
Herbal medicines and supplements
Aside from diet and lifestyle changes, another commonly used CAM intervention that is often self-administered is the use of herbal supplements either as single herbs or in combination products (Table 2). A few well-designed clinical studies have been performed on a number of such herbal supplements but in general the current knowledge remains limited to make a definitive judgment about their effectiveness in treating IBS symptoms. Many of the commonly used supplements have evolved from folk and traditional applications as remedies for gastrointestinal disorders.
Table 2.
Clinical trials on Herbal medicines and supplements for irritable bowel syndrome
| Intervention | Study design | Sample size | Outcome | Ref. |
| Peppermint oil | Randomized, double-blind, placebo-controlled study | 99 | Peppermint oil (Colpermin®) group showed significant symptom improvement (P < 0.05) compared to placebo group after 1 mo | [104] |
| Peppermint oil | Randomized, placebo-controlled study | 18 | Peppermint oil significantly reduced GI symptoms (P < 0.01) after 3 wk compared to placebo | [106] |
| Peppermint oil | Randomized, double-blind. Placebo-controlled study | 57 | Total IBS severity score was significantly decreased after 4 wk of treatment (P < 0.009) and after 2 mo (P < 0.01) in the peppermint oil group compared to placebo | [108] |
| Peppermint oil | Randomized, double-blind, placebo-controlled study | 90 | Significant reduction in IBS symptoms, no abdominal pain in more patients in the peppermint oil group compared to placebo (P < 0.001), less severe abdominal pain in peppermint oil group (P < 0.05) in peppermint oil group after 2 mo | [109] |
| Peppermint oil | Randomized, double-blind, placebo-controlled study | 65 | Significant reduction in abdominal pain in peppermint oil group compared to placebo group (P < 0.001), but pain score increased 2 wk after completion of trial | [110] |
| Artichoke leaf | Post-marketing surveillance | 279 | Significant reduction (P < 0.05) in overall IBS symptoms after 6 wk of treatment | [113] |
| Artichoke leaf | Post-marketing surveillance in IBS with concomitant dyspepsia | 209 | Significant reduction in Nepean Dyspepsia Index after 2 mo (P < 0.001) and normalization of bowel pattern (P < 0.001) | [114] |
| Turmeric | Partially blinded, randomized, two-dose pilot study | 207 | Reduction in IBS prevalence in both treatment groups (1 or 2 tablets) compared to baseline (P < 0.001) after 2 mo intervention, no significant differences between groups | [116] |
| Curcuma and fumitory | Randomized, double-blind, placebo-controlled study | 106 | No significant differences between curcuma, fumitory, and placebo groups in abdominal pain (P = 0.81) and distension (P = 0.48) after 3 mo | [117] |
| STW5 | Randomized, double-blind, placebo-controlled study in patients with dyspepsia | 137 | Significant decrease in gastrointestinal symptom score between STW5 and placebo (P < 0.001) | [118] |
| STW5 | Randomized, double-blind, placebo-controlled multicenter study in patients with functional dyspepsia | 315 | Significant decrease in gastrointestinal symptom score between STW5 and placebo (P < 0.05) after 2 mo intervention | [119] |
| STW5 | Randomized, double-blind, placebo-controlled multicenter study | 203 | Significant reduction in abdominal pain scores for STW5 (P = 0.009) and STW5-II (P = 0.005) and IBS-SSS (P = 0.001 for STW5 and P = 0.0003 for STW5-II) compared to placebo after 4 wk intervention | [120] |
| Padma Lax | Randomized, double-blind, placebo-controlled pilot study | 61 | Significant improvement in global IBS symptom scores compared to placebo (P < 0.05) following 3 mo intervention | [123] |
| TCM | Randomized, double-blind, placebo-controlled study | 119 | No significant improvements in IBS global symptom score between TCM and placebo group at week 8 (P = 0.38) and week 16 (P = 0.62) | [129] |
IBS: Irritable bowel syndrome; TCM: Traditional chinese medicine; SSS: Symptom severity scale.
The use of peppermint extracts has been studied in a number of clinical trials which evaluated the administration of enteric coated peppermint oil capsules to IBS patients[103-107]. The duration of the trials ranged from 4-8 wk and was not divided into specific IBS subtypes. The trials showed a significant reduction in abdominal pain and severity compared to placebo after 4 wk and a significant increase in quality of life although the effects did not last once peppermint was discontinued[108-110]. The spasmolytic effects of peppermint oil have been well known in folk medicines and mainly been attributed to the presence of mono- and sesquiterpenes[110]. Peppermint oil has also been recommended by the American Academy of Pediatricians as well as received a positive evaluation from the Task force on IBS of the ACG[2,111] although caution is advised for its use in young children due to its side effects of causing respiratory depression and heartburn[111]. Peppermint oil appears to be more potent in exerting the spasmolytic effects than aqueous extractions such as teas since it allows for a more concentrated dose of the proposed active ingredients[112].
Hydroalcoholic extracts from artichoke leaves have also been used to reduce IBS symptoms and evaluated in at least two clinical studies[113,114]. Artichoke has long been used as a digestive aid which aims to reduce bloating, abdominal pain and cramps, as well as reducing both diarrhea and constipation through normalization of GI motility[115]. Both studies were conducted as part of a post-marketing surveillance which may limit the credibility of the results due to limited influence on the study design (no double-blinding, no placebo control) and potential consumer bias. Both trials report a significant improvement in IBS symptoms, specifically in normalizing GI motility and reducing bloating as well as relieving distension and abdominal pain and cramps[113]. Since the change in IBS symptom severity was compared between baseline and follow-up, the results provide limited evidence of the effectiveness compared to placebo or standard pharmacological treatment. Given the high placebo responder rate, artichoke leaf extracts will require additional trials that are more rigorous in terms of study design.
Turmeric, a spice traditionally used in Asian cuisine where it often provides both for taste and color improvement, was evaluated in IBS patients and indicated decreases in IBS symptoms and increased quality of life if given in two different doses of 72 and 144 mg per day over 8 wk[116]. However, this study again lacks a double-blinded and placebo-controlled design which reduces the strength of the presented data. Another double-blind placebo controlled study compared curcuma extract from which turmeric is derived with a placebo and a fumitory extract[117]. Both curcuma and fumitory extracts did not show any significant improvements in abdominal pain and distension compared to the placebo group.
All single herbal supplements discussed have a strong folkloric and traditional background as digestive aids and it is therefore not surprising that to date trials with such herbal supplements are focused on these single extracts.
Combination products of herbal extracts add to the wide range of available supplements used in the self-treatment of IBS. The first combination product which has received some interest from patients and healthcare providers alike is Iberogast®, a mixture of nine herbal plant extracts that was originally mainly used for functional dyspepsia in Germany[118,119]. The product has been on the market for more than 30 years. Iberogast® for use in IBS symptoms was investigated in 208 patients with various IBS subtypes in the United States[120]. This study adheres to the clinical trial standards by utilizing a randomized, double-blind, placebo-controlled study protocol over a 4 wk period. The extract consists of liquid extracts from chamomile flowers, bitter candytuft, angelica root, caraway fruits, milk thistle, lemon balm leaves, greater celandine, licorice root, and peppermint leaves[121]; all of which have been used in folklore and traditional medicines to aid in digestive disorders. The study indicates that Iberogast® significantly improves quality of life and reduces abdominal pain in IBS patients[122] which appears to be mediated through influences on serotonin, acetylcholine, and opioid receptors in the GI tract[121]. Despite the positive outcome, further research is required to support the findings of this study and a few case reports from patients and healthcare providers alike. Iberogast® has been recognized by the ACG task force on IBS as a potential complementary treatment to reduce certain IBS symptoms[2].
A Tibetan preparation of twelve different plant extracts, commonly referred to and marketed as Padma Lax, was tested in IBS-C patients over the course of three months in a randomized and double-blinded observational study and showed significant improvements over a placebo in reducing constipation, abdominal pain, and flatulence[123]. The dose had to be adjusted for some of the patients since they developed loose stools that bordered on diarrhea indicating that this combination herbal product may have significant potential as a laxative. The ingredient list includes the known laxatives rhubarb root, cascara bark, and nux vomica seeds which have been used as strong laxatives in severe constipation in traditional medicines across the world[124]. It has been proposed that some of the herbs exert the laxative effect through activity on cholinergic receptors as antagonists to reduce GI contractility[124,125].
Traditional Chinese medicine (TCM) has provided a range of different treatment approaches over the centuries, among them a number of TCM herbal mixtures that are specifically formulated based on the patients’ symptoms[126]. Such individualized medicine is not uncommon but has the obvious limitation of resisting standardization and fitting the rigorous clinical trial design that is used as a major determinant of effectiveness in Western medicine. Tong Xie Yao Fang (TXYF) is one such TCM that has been studied in a few clinical trials but often adjustments were made to the herbal combination based on the predominant IBS symptom presentation[127-129]. A review of 12 studies with modified TXYF preparations reached the general conclusion that the extracts improved IBS symptoms, in particular abdominal pain, distension, flatulence, and diarrhea[128]. However, the study design and end points were diverse between these studies complicating a direct comparison of outcomes. A more streamlined and standardized approach to TXYF trials is warranted.
Overall, the use of single and combination herbal supplements appears promising but should be approached with caution given the lack of rigorous larger clinical trials. The strongest evidence for the use of herbal medicines is currently available for peppermint oil preparations and the herbal combination product Iberogast®.
Mind-body therapies
Since there is evidence of the involvement of the enteric nervous system or brain-gut axis in the pathophysiology of IBS, the use of mind-body interventions as CAM treatments may provide benefits in relieving symptoms. Mind-body therapies are interventions that primarily “focus on the interactions among the brain, mind, body, and behavior with the intent to use the mind to affect physical functioning and promote health”[130]. Yoga, Tai-chi, meditation, hypnotherapy, deep-breathing exercises, relaxation techniques, and acupuncture all fall under this definition. While yoga, relaxation techniques, and acupuncture are commonly used as CAM therapies, they involve both a physical and psychological component with a focus on influencing physical functions through mechanical interventions. Meditation, hypnotherapy, and CBT do not involve a mechanical component but rather seek to change physiological function through psychological reprogramming entirely[131,132]. Mind-body therapies have been evaluated for their potential application as CAM in IBS. Clinical evidence supporting the use of yoga, relaxation, acupuncture, hypnotherapy, and cognitive behavior therapy is indicating that these CAM interventions show improvement in IBS symptoms and overall quality of life.
Mechanical CAM interventions
Mechanical interventions are based on direct body interventions such as massage, acupuncture, yoga, and physical exercise (Table 3)[131]. While some interventions may not benefit patients with IBS because of the significant physical involvement that may cause a temporary worsening of symptoms, low impact physical exercise such as aerobic training, bike riding, and muscle strengthening have shown benefits in maintaining GI function and reducing flatulence and gas production[90,99,100,133]. One study indicated that strenuous exercise shows an inverse relationship with IBS symptoms[91] while another study pointed to reduction of constipation in IBS-C patients with mild exercise levels[98]. Johannesson and colleagues evaluated the impact of regular exercise on IBS symptoms severity scores compared to a control group and found a significant reduction in symptoms over the course of 12 wk[133]. It is therefore important to emphasize that a patient should start off with low impact and light exercise regimens that are not exhausting or causing increases in abdominal pain or overall IBS symptoms. As such, mild physical exercise may be considered a lifestyle change rather than an actual CAM intervention, but guided assistance may benefit IBS patients who seek counseling and advice on respective exercise regimens for their condition.
Table 3.
Clinical trials on mechanical complementary and alternative medicines interventions for irritable bowel syndrome
| Intervention | Study design | Sample size | Outcome | Ref. |
| Physical activity | Randomized study | 75 | Significant decrease in IBS-SSS between physical activity and placebo group (P = 0.003) | [133] |
| Reflexology | Randomized, single-blind, placebo-controlled study | 34 | No significant difference between foot reflexology and non-reflexology massage group | [134] |
| Acupuncture | Randomized, single-blind, placebo-controlled study | 230 | Acupuncture and sham acupuncture significantly improved IBS-GIS scores compared to waitlist group (P = 0.001), no difference between acupuncture and sham acupuncture during 3 wk intervention | [143] |
| Acupuncture | Randomized, single-blind, placebo-controlled study | 43 | Significant improvements (P = 0.022) in quality of life for both acupuncture and sham acupuncture compared to baseline after 10 intervention sessions (5 wk), no differences between acupuncture and sham acupuncture | [144] |
| Acupuncture/ moxibustion | Randomized, single-blind, placebo-controlled study | 29 | Significant reduction in IBS-SSS in acupuncture/moxibustion group after 4 wk compared to sham acupuncture/moxibustion group (P = 0.01) | [146] |
| Yoga | Observational pilot study (adolescents) | 20 | Decrease in pain frequency (P = 0.031 for 8-11 yr old and P = 0.004 for 12-18 yr old) and pain intensity (P = 0.015 in 8-11 yr old) after 10 yoga sessions compared to baseline, decrease in pain frequency was maintained for 3 mo following intervention (P = 0.004 for 8-11 yr old) | [149] |
IBS: Irritable bowel syndrome; GIS: Global improvement scores; SSS: Symptom severity scale.
To date there is little information about the potential impact of massage and reflexology treatments on IBS symptoms. One study compared the use of foot reflexology massage to non-reflexology foot massage and could not find any significant difference in the small study of 34 patients[134]. However, case reports have indicated that gut-directed massage may relieve specific symptoms such as bloating and chronic constipation although such reports were not specific to IBS patients[135-137]. It therefore remains unknown if reflexology or massage techniques can provide benefits to IBS patients.
A number of trials investigated the effect of gut-directed and general acupuncture on symptom relief in IBS patients. It is well known that acupuncture can affect physiological functions in a number of conditions through regulating various neurotransmitter systems. It has been shown that application of acupuncture in IBS patients targets serotonergic, cholinergic, and glutamatergic pathways, can lower blood cortisol levels related to stress, and can increase the concentration of endogenous opioids to reduce visceral and global pain perception[138-140]. A complicating factor in the study of acupuncture effects are adequate comparison groups. One commonly used comparison group is sham acupuncture which uses needles as well so to suggest to patients that they are being treated when the practitioner does not utilize the specific acupuncture points and only superficially applies needles[141,142]. What appears to be a contributing factor to the effectiveness of acupuncture is the patient-practitioner relationship, especially in IBS patients where an underlying psychological contribution to IBS symptoms can be suspected[58,59]. In one study, a sample of 230 IBS patients were randomly assigned to receive either acupuncture, sham acupuncture, or remain on a waitlist for the duration of the trial[143]. Initially both the true acupuncture and sham acupuncture groups received only sham acupuncture for 3 wk followed by 3 wk of true acupuncture in half of these patients while the other half continued receiving sham acupuncture. Both groups showed significant improvement in global IBS symptoms compared to the waitlist control group, but the patients receiving true acupuncture did not differ from the sham acupuncture group thus complicating interpretation of results related to the actual acupuncture points being used. Other studies using sham acupuncture as a comparator have also found that improvements in quality of life and IBS symptoms did not differ from true acupuncture points thus potentially indicating that acupuncture for IBS is primarily a placebo response[144,145]. When combined with moxibustion, acupuncture has shown significant improvements in IBS symptoms with reduced abdominal pain, gas and bloating being reported in one study including 29 subjects over a 4 wk, eight session intervention[146]. A Cochrane meta-analysis of studies including acupuncture suggests that further studies are warranted to confirm the beneficial effects of such treatments in IBS patients[147].
A mechanical intervention that has been studied in IBS is yoga as a specific form of exercise and focused relaxation technique combined[148]. Yoga consists of different poses accompanied by a specific breathing pattern to focus attention on muscle contraction and relaxation. There are certain poses that can be utilized to focus on GI tract motility and abdominal pain perception. There are only few trials conducted with yoga as the intervention but a number of indicators suggest that yoga may provide relief of IBS symptoms. One study in 22 male patients with confirmed IBS-D compared yoga poses and breathing techniques to conventional treatment over the course of 8 wk[102]. Overall GI symptoms were reduced in both groups at the end of the study but the yogic intervention group showed a higher parasympathetic reactivity leading to improved IBS symptom outcomes whereas the control group presented with increased gastric activity. Another small clinical trial compared the use of yoga in adolescents to a wait list group over the course of 8 wk[101]. In the first 4 wk, the yoga intervention group received instructions on daily yoga practices and continued the poses after the first 4 wk. The control wait list group received yoga intervention after 4 wk for another month at which point both groups were compared. This preliminary study indicates that yoga intervention over the course of a 2 mo period improves GI symptoms in adolescent IBS patients and is well received by youth. Another small yoga intervention study conducted in 20 children aged 8-18 years with IBS or functional abdominal pain indicates that yoga does decrease both pain frequency and pain intensity at the end of intervention and that this effect also persisted for at least another 3 mo after the intervention has ended[149]. In addition, adolescents in this trial reported increased quality of life throughout the intervention and during follow-up. Although not many studies have been conducted using yoga as a CAM intervention, the current data suggests that it may provide benefits in IBS patients by alleviating both pain and GI symptoms.
Psychological CAM interventions
While mechanical interventions can provide IBS symptom relief, compliance can often be an issue as well as limited physical ability to follow the treatment (especially with exercise and yoga). Other mind-body CAM approaches are solely based on psychological interventions with hypnotherapy and cognitive behavior therapy showing the most clinical evidence of effectiveness (Table 4).
Table 4.
Clinical trials on psychological complementary and alternative medicines interventions for irritable bowel syndrome
| Intervention | Study design | Sample size | Outcome | Ref. |
| Hypnotherapy | Pre- and post-assessment | 23 | Normalized hypersensitivity pain threshold in hypersensitive group (P = 0.04) after 12 wk of treatment, no significant change in hyposensitive and normosensitive groups | [154] |
| Hypnotherapy | Randomized controlled trial in children with functional abdominal pain or IBS | 53 | Significant reduction in pain scores in hypnotherapy group (P < 0.001) compared to standard medical therapy at 1-yr after intervention | [159] |
| Hypnotherapy | Questionnaire | 83 | 69% of patients were either satisfied or very satisfied with hypnotherapy following 12 wk intervention, overall improvement in quality of life and GI symptoms | [160] |
| Hypnotherapy | Randomized, placebo-controlled study | 138 in two studies (90 and 48) | Significant reduction in IBS symptoms in hypnotherapy groups (P < 0.05) compared to supportive therapy after 3 mo of intervention | [161] |
| Hypnotherapy | Randomized, placebo-controlled study | 90 | Significant improvement in overall IBS symptoms in gut-directed hypnotherapy and medical treatment group compared to medical treatment group alone (P = 0.046) after 12 wk; improvement remained up to 12 mo after intervention in hypnotherapy group (P = 0.004) compared to medical treatment alone | [162] |
| Hypnotherapy | Pre- and post-assessment | 75 | Group hypnotherapy decreased symptom severity significantly (P < 0.01) at 3, 6, and 12 mo post-intervention | [163] |
| Hypnotherapy | Retrospective analysis | 208 | Significantly higher use of hypnotherapy (P < 0.001) by initial responders vs non-responders at 2-7 yr follow-up, in total 87% of participants reported hypnotherapy to be useful | [164] |
| Cognitive behavior therapy | Randomized-comparator-controlled study in patients with functional bowel disorders | 431 | CBT was more effective than education (P = 0.0001) and desipramine was more effective than placebo (P = 0.01) after 12 wk of treatment as assessed by treatment satisfaction | [170] |
| Cognitive behavior therapy | Randomized, placebo-controlled study in patients with functional bowel disorders | 397 | No significant differences between treatment arms for desipramine, cognitive behavior therapy, and placebo groups | [171] |
| Cognitive behavior therapy and mindfulness training | Randomized controlled trial | 195 | Internet-delivered cognitive behavior therapy resulted in adequate relief of IBS symptoms that was significant compared to internet-delivered stress management at 6 mo follow-up (P = 0.004) | [173] |
| Cognitive behavior therapy | Randomized controlled trial | 149 | Significant reduction in symptom severity scores in CBT plus mebeverine group compared to mebeverine alone at post-treatment and 3, 6, and 12 mo follow-up (regression P = 0.001) | [174] |
| Psychotherapy [cognitive behavior therapy] | Randomized controlled trial | 50 | Rome-II scores significantly decreased (P = 0.001) in patients receiving CBT in conjunction with standard medical care compared to standard medical care alone after 2 mo intervention | [175] |
| Cognitive behavior therapy | Randomized controlled trial | 28 | Psychosocial functioning was significantly improved (P = 0.004) in patients receiving CBT in addition to standard medical care compare to standard medical care alone at 3 mo follow-up | [176] |
| Cognitive behavior therapy | Randomized controlled trial | 76 | Cognitive behavior therapy presented with significant improvements compared to stress management and attention control groups in reducing visceral sensitivity (P < 0.0001) compared to baseline at 3 mo follow-up | [177] |
| Cognitive behavior therapy | Randomized, placebo-controlled study | 85 | Internet delivered CBT reduced several IBS symptom parameters (total pain, diarrhea, bloating primary symptoms all P < 0.001) after 10 wk of intervention compared to discussion board control group; quality of life and visceral sensitivity were also significantly improved (P < 0.001) after 3 mo follow up | [179] |
| Mindfulness training | Randomized controlled trial | 75 | Women in mindfulness training group showed significant reduction (P = 0.006) in IBS symptom severity compared to support control group after 8 wk of intervention which remained significant at 3 mo follow-up (P = 0.001) | [180] |
| Cognitive behavior therapy | Retrospective analysis | 75 | Long-term follow-up after 15-18 mo of original intervention resulted in lasting significant reductions in visceral sensitivity (P < 0.05), increase in quality of life (P < 0.05), and gastrointestinal symptoms (P < 0.05) | [181] |
IBS: Irritable bowel syndrome; CBT: Cognitive behavior therapy; GI: Gastrointestinal.
Hypnotherapy is based on initiating a suggestive state for the patient similar to sedation but without loss of consciousness that allows for heightened senses and control over body functions affecting mood, pain perception, cardiovascular responses, and gastrointestinal motility[150,151]. Hypnosis and hypnotherapy have been used for various applications foremost for the treatment of acute and chronic pain conditions but also to improve mood or change certain undesirable behaviors[152,153]. It has been shown that gut-directed hypnotherapy can alleviate IBS symptoms comparable to current pharmacological treatment approaches[154-156]. Several clinical studies and meta-analysis indicate that 8-12 weekly hypnotherapy sessions can improve pain, GI motility, mood (improving depressive and anxiety disorders), and overall quality of life of IBS patients significantly even in the absence of pharmacological treatment[157-161]. Interestingly, in a number of studies during follow-up the beneficial effects of hypnotherapy remained for at least 10 mo even if patients did not continue therapy[55,155,162-164]. A Cochrane database review examined available studies and found a positive effect associated with hypnotherapy although the effect size could not be determined due to the heterogeneity of study designs and the relatively small sample sizes[54]. Although there is clinical evidence for the use of hypnotherapy in treating IBS symptoms, further research utilizing a homogenous study design needs to be emphasized to support its benefits.
In contrast to hypnosis where the patient is being influenced and subjected to a subconscious suggestive state that influences physiological processes, CBT takes a more direct approach in influencing the conscious awareness of IBS symptoms and attempts to enable the patient to overcome the negative attitudes they may have towards their chronic condition[165-167]. Similar to hypnosis, patients are enabled to change a negatively perceived situation into a positive outlook which then affects the actual symptoms. CBT has been shown to be effective in improving symptoms in a number of chronic disorders such as obesity, chronic pain, insomnia, or depressive disorders[166,168,169]. While hypnosis can be utilized to directly affect perception of symptoms, CBT is more geared towards enabling patients themselves to change their behavior and thought processes about their condition. In IBS patients, symptom aggravation may often occur when patients worry about the condition and focus their thoughts around them[170,171]. CBT has been shown to improve both the quality of life and reduce symptom severity in IBS patients, especially in regards to pain perception and comorbid depressive and anxiety disorders[170,172,173]. Comparing the use of CBT as a CAM approach to treating IBS with conventional pharmacological treatment indicates that it does not only improve the direct IBS symptoms such as pain and GI dysmotility, but in addition also benefits mood and coping with the condition[174-177]. Although the effects of CBT lasted past the intervention period in some of the trials, the beneficial effects seemed to wane over time which may indicate a long-term supportive treatment with CBT at least in frequent intervals following the initial treatment period. Currently, an ongoing irritable bowel syndrome outcome study evaluates the long-term effects of self-versus clinician-administered CBT on IBS symptoms and the economic benefit of this intervention[178].
Closely related to CBT as a CAM intervention is mindfulness exercises that promote acceptance instead of control of IBS symptoms[179,180]. This technique is often delivered in conjunction with CBT and is more patient-centered to increase quality of life through coping mechanisms. In conjunction with CBT, mindfulness exercises have shown a reduction in IBS symptoms beyond the intervention period up to 3 years[173,181].
INTEGRATIVE APPROACHES AND CLINICAL IMPLICATIONS TO TREATING IBS
Most of the clinical trials that have been discussed focus on the comparison between CAM approaches and standard care with pharmacological therapies in treating IBS symptoms. This separation often creates a painful choice for patients that need the immediate relief with medication but also seek long-term alleviation of the symptoms through a pro-active approach. Integrating both conventional pharmacological care and CAM treatments to provide the best symptom relief and highest quality of life to IBS patients should therefore be considered by healthcare providers. A number of clinical trials - although small in sample sizes and number - have shown that a combination of pharmacotherapy with a CAM treatment is superior to either treatment alone. As long as the CAM treatment does not interfere or interact with the pharmacological treatment, both can coexist in the treatment of IBS symptoms.
A study evaluating the use and cost of CAM by patients with functional bowel disorders (including IBS patients) reported a number of interesting results[85]. The most common types of CAM were ginger, massage therapy, and yoga with a median yearly cost of $200, which was considered significantly less compared to standard pharmacotherapy. Furthermore, the use of CAM was associated with female gender, higher education, and comorbid anxiety disorders. Although most of the patients using CAM (35% of 1012 participating patients) were not referred by their healthcare provider, those who received a recommendation from their primary care physician followed the advice. This indicates the important role of the healthcare provider as a mediator and facilitator for patients to seek CAM treatments. Several of the CAM approaches discussed in this review have been recommended or given at least positive consideration by current professional organizations (ACG and the British Society for Gastroenterology) to be considered in the treatment of IBS in addition to pharmacotherapy[2,12]. Another important point that the study reveals is that CAM use is not based on dissatisfaction with pharmacotherapy but seems to be rather linked to higher comorbidity with depressive and anxiety disorders as well as somatization of their condition[85]. CAM users in this study also showed a higher symptom severity on the IBS-SSS as well as gastrointestinal distension. The authors of the study conclude that CAM use can benefit patients with various functional bowel disorders and should be made more widely available potentially through providing insurance coverage. Medical professionals should recommend CAM approaches such as hypnotherapy, yoga, cognitive behavior therapy, or herbal supplements that have shown benefits to IBS patients that do not experience adequate symptom relief with pharmacotherapy alone.
In a study that evaluated a gap in effectiveness between current treatment approaches and treatment outcomes conducted in the United Kingdom, 32% of general practitioners reported an effectiveness gap for IBS patients in their practice[86]. The most common reasons given for the effectiveness gap were lack of effective treatments, adverse effects of current treatments, and unacceptability of available treatments by patients. These findings are supported by other studies which indicate a lack of treatment effectiveness in a significant percentage of IBS patients[40,43,182]. The use of CAM has been shown in randomized controlled trials as well as in systematic reviews to decrease or close the effectiveness gap thereby increasing quality of life and treatment outcomes for IBS patients. The authors of this study conclude that there is a discrepancy between available evidence for the effectiveness of CAM in various chronic conditions and its recommendation by current practice guidelines which often omit such approaches. In addition, patients appear to be more favorable towards trying CAM approaches than practitioners thereby most referrals are initiated by patients themselves or without including the healthcare provider when considering CAM treatments in addition to conventional care[86,183,184].
The integrative medical approach has gained significant traction in the last decade with the growth of the Consortium of Academic Health Centers for Integrative Medicine[185] to which many well respected United States institutions belong (Yale University, Stanford University, University of California, Johns Hopkins University, among others). Integrative medicine as a subdivision of medical practice is seeking to emphasize on patient-centered care and improve wellness and quality of life rather than limiting the treatment outcome to a specific disorder. The definition of integrative medicine remains somewhat elusive to date ranging from simply adding CAM treatments to conventional care to a more holistic healthcare approach overall. While the conventional approach to treating IBS is primarily founded on evidence-based clinical trials and extensive knowledge, the CAM approach to treating IBS also claims a long tradition of use and an increasing body of research supporting the benefits of CAM treatments in IBS. The debate over integrative medicine continues with defenders of conventional medicine indicating that such a definition should not exist because treatments with proven benefits will eventually become the standard of care no matter where they originated from. However, proponents of integrative medicine argue that excluding CAM or preventing patients from seeking CAM treatments in addition to conventional care will result in reduced quality of life and worse treatment outcomes. As mentioned above, this has been supported by various studies. It has been argued that even if a CAM treatment is not supported by sufficient evidence-based clinical trials, as long as it does not cause adverse effects or interfere with conventional therapy it should not be denied to patients seeking such treatments. Instead, physicians and healthcare providers should seek training or knowledge in integrative medicine to best support their patients. This applies especially to chronic conditions such as IBS that present with a high placebo response and where a number of CAM treatments - herbal therapies, probiotics, dietary changes, acupuncture, yoga, hypnotherapy, and cognitive behavior therapy - have shown benefits.
Footnotes
P- Reviewer: Hussain S S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
References
- 1.Rey E, Talley NJ. Irritable bowel syndrome: novel views on the epidemiology and potential risk factors. Dig Liver Dis. 2009;41:772–780. doi: 10.1016/j.dld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1–S35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ, Zinsmeister AR, Melton LJ. Irritable bowel syndrome in a community: symptom subgroups, risk factors, and health care utilization. Am J Epidemiol. 1995;142:76–83. doi: 10.1093/oxfordjournals.aje.a117548. [DOI] [PubMed] [Google Scholar]
- 4.Thompson WG, Irvine EJ, Pare P, Ferrazzi S, Rance L. Functional gastrointestinal disorders in Canada: first population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci. 2002;47:225–235. doi: 10.1023/a:1013208713670. [DOI] [PubMed] [Google Scholar]
- 5.Lacy BE, Weiser K, Noddin L, Robertson DJ, Crowell MD, Parratt-Engstrom C, Grau MV. Irritable bowel syndrome: patients’ attitudes, concerns and level of knowledge. Aliment Pharmacol Ther. 2007;25:1329–1341. doi: 10.1111/j.1365-2036.2007.03328.x. [DOI] [PubMed] [Google Scholar]
- 6.Hungin AP, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21:1365–1375. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 7.Drossman DA, Morris CB, Schneck S, Hu YJ, Norton NJ, Norton WF, Weinland SR, Dalton C, Leserman J, Bangdiwala SI. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol. 2009;43:541–550. doi: 10.1097/MCG.0b013e318189a7f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ringel Y, Williams RE, Kalilani L, Cook SF. Prevalence, characteristics, and impact of bloating symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:68–72; quiz 3. doi: 10.1016/j.cgh.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Inadomi JM, Fennerty MB, Bjorkman D. Systematic review: the economic impact of irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18:671–682. doi: 10.1046/j.1365-2036.2003.t01-1-01736.x. [DOI] [PubMed] [Google Scholar]
- 10.McFarland LV. State-of-the-art of irritable bowel syndrome and inflammatory bowel disease research in 2008. World J Gastroenterol. 2008;14:2625–2629. doi: 10.3748/wjg.14.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th revision. Geneve: World Health Organization; 2007. [Google Scholar]
- 12.Jones J, Boorman J, Cann P, Forbes A, Gomborone J, Heaton K, Hungin P, Kumar D, Libby G, Spiller R, et al. British Society of Gastroenterology guidelines for the management of the irritable bowel syndrome. Gut. 2000;47 Suppl 2:ii1–i19. doi: 10.1136/gut.47.suppl_2.ii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drossman DA, Douglas A. Rome III: The Functional Gastrointestinal Disorder. 3rd ed. McLean: Degnon Associates, Inc; 2006. p. 1048. [Google Scholar]
- 14.Coëffier M, Gloro R, Boukhettala N, Aziz M, Lecleire S, Vandaele N, Antonietti M, Savoye G, Bôle-Feysot C, Déchelotte P, et al. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am J Gastroenterol. 2010;105:1181–1188. doi: 10.1038/ajg.2009.700. [DOI] [PubMed] [Google Scholar]
- 15.Rösch W, Liebregts T, Gundermann KJ, Vinson B, Holtmann G. Phytotherapy for functional dyspepsia: a review of the clinical evidence for the herbal preparation STW 5. Phytomedicine. 2006;13 Suppl 5:114–121. doi: 10.1016/j.phymed.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Macsharry J, O’Mahony L, Fanning A, Bairead E, Sherlock G, Tiesman J, Fulmer A, Kiely B, Dinan TG, Shanahan F, et al. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1467–1476. doi: 10.1080/00365520802276127. [DOI] [PubMed] [Google Scholar]
- 17.Scully P, McKernan DP, Keohane J, Groeger D, Shanahan F, Dinan TG, Quigley EM. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am J Gastroenterol. 2010;105:2235–2243. doi: 10.1038/ajg.2010.159. [DOI] [PubMed] [Google Scholar]
- 18.Katiraei P, Bultron G. Need for a comprehensive medical approach to the neuro-immuno-gastroenterology of irritable bowel syndrome. World J Gastroenterol. 2011;17:2791–2800. doi: 10.3748/wjg.v17.i23.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundmann O, Yoon SL, Moshiree B. Current developments for the diagnosis and treatment of irritable bowel syndrome. Curr Pharm Des. 2010;16:3638–3645. doi: 10.2174/138161210794079227. [DOI] [PubMed] [Google Scholar]
- 20.Grundmann O, Yoon SL. Irritable bowel syndrome: epidemiology, diagnosis and treatment: an update for health-care practitioners. J Gastroenterol Hepatol. 2010;25:691–699. doi: 10.1111/j.1440-1746.2009.06120.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones MP, Keefer L, Bratten J, Taft TH, Crowell MD, Levy R, Palsson O. Development and initial validation of a measure of perceived stigma in irritable bowel syndrome. Psychol Health Med. 2009;14:367–374. doi: 10.1080/13548500902865956. [DOI] [PubMed] [Google Scholar]
- 22.Tang YR, Yang WW, Liang ML, Xu XY, Wang MF, Lin L. Age-related symptom and life quality changes in women with irritable bowel syndrome. World J Gastroenterol. 2012;18:7175–7183. doi: 10.3748/wjg.v18.i48.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathew P, Bhatia SJ. Pathogenesis and management of irritable bowel syndrome. Trop Gastroenterol. 2009;30:19–25. [PubMed] [Google Scholar]
- 24.Blanchard EB, Lackner JM, Jaccard J, Rowell D, Carosella AM, Powell C, Sanders K, Krasner S, Kuhn E. The role of stress in symptom exacerbation among IBS patients. J Psychosom Res. 2008;64:119–128. doi: 10.1016/j.jpsychores.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 Suppl 8:28–36; discussion 37. [PubMed] [Google Scholar]
- 26.Lackner JM, Ma CX, Keefer L, Brenner DM, Gudleski GD, Satchidanand N, Firth R, Sitrin MD, Katz L, Krasner SS, et al. Type, rather than number, of mental and physical comorbidities increases the severity of symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:1147–1157. doi: 10.1016/j.cgh.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108:65–72. doi: 10.1016/s0002-9343(99)00299-5. [DOI] [PubMed] [Google Scholar]
- 30.Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005;39:S184–S193. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- 31.Kern MK, Shaker R. Cerebral cortical registration of subliminal visceral stimulation. Gastroenterology. 2002;122:290–298. doi: 10.1053/gast.2002.30989. [DOI] [PubMed] [Google Scholar]
- 32.El-Salhy M, Wendelbo I, Gundersen D. Serotonin and serotonin transporter in the rectum of patients with irritable bowel disease. Mol Med Rep. 2013;8:451–455. doi: 10.3892/mmr.2013.1525. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Ranjan P, Mittal B, Ghoshal UC. Serotonin transporter gene (SLC6A4) polymorphism in patients with irritable bowel syndrome and healthy controls. J Gastrointestin Liver Dis. 2012;21:31–38. [PubMed] [Google Scholar]
- 34.Kapeller J, Houghton LA, Mönnikes H, Walstab J, Möller D, Bönisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, et al. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967–2977. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]
- 35.Milne RJ, Heel RC. Ondansetron. Therapeutic use as an antiemetic. Drugs. 1991;41:574–595. doi: 10.2165/00003495-199141040-00006. [DOI] [PubMed] [Google Scholar]
- 36.Goldberg PA, Kamm MA, Setti-Carraro P, van der Sijp JR, Roth C. Modification of visceral sensitivity and pain in irritable bowel syndrome by 5-HT3 antagonism (ondansetron) Digestion. 1996;57:478–483. doi: 10.1159/000201377. [DOI] [PubMed] [Google Scholar]
- 37.Callahan MJ. Irritable bowel syndrome neuropharmacology. A review of approved and investigational compounds. J Clin Gastroenterol. 2002;35:S58–S67. doi: 10.1097/00004836-200207001-00011. [DOI] [PubMed] [Google Scholar]
- 38.Smart HL, Mayberry JF, Atkinson M. Alternative medicine consultations and remedies in patients with the irritable bowel syndrome. Gut. 1986;27:826–828. doi: 10.1136/gut.27.7.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang SH, Choi SW, Lee SJ, Chung WS, Lee HR, Chung KY, Lee ES, Moon HS, Kim SH, Sung JK, et al. The effects of lifestyle modification on symptoms and quality of life in patients with irritable bowel syndrome: a prospective observational study. Gut Liver. 2011;5:472–477. doi: 10.5009/gnl.2011.5.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris LR, Roberts L. Treatment for irritable bowel syndrome: patients’ attitudes and acceptability. BMC Complem Altern Med. 2008;8:65. doi: 10.1186/1472-6882-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langmead L, Chitnis M, Rampton DS. Use of complementary therapies by patients with IBD may indicate psychosocial distress. Inflamm Bowel Dis. 2002;8:174–179. doi: 10.1097/00054725-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Hussain Z, Quigley EM. Systematic review: Complementary and alternative medicine in the irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:465–471. doi: 10.1111/j.1365-2036.2006.02776.x. [DOI] [PubMed] [Google Scholar]
- 43.Yoon SL, Grundmann O, Koepp L, Farrell L. Management of irritable bowel syndrome (IBS) in adults: conventional and complementary/alternative approaches. Altern Med Rev. 2011;16:134–151. [PubMed] [Google Scholar]
- 44.Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, Kowalczykowski M, Miller FG, Kirsch I, Lembo AJ. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5:e15591. doi: 10.1371/journal.pone.0015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol. 2009;15:1548–1553. doi: 10.3748/wjg.15.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halpert A, Dalton CB, Diamant NE, Toner BB, Hu Y, Morris CB, Bangdiwala SI, Whitehead WE, Drossman DA. Clinical response to tricyclic antidepressants in functional bowel disorders is not related to dosage. Am J Gastroenterol. 2005;100:664–671. doi: 10.1111/j.1572-0241.2005.30375.x. [DOI] [PubMed] [Google Scholar]
- 47.Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58:367–378. doi: 10.1136/gut.2008.163162. [DOI] [PubMed] [Google Scholar]
- 48.Käll E, Lindström E, Martinez V. The serotonin reuptake inhibitor citalopram does not affect colonic sensitivity or compliance in rats. Eur J Pharmacol. 2007;570:203–211. doi: 10.1016/j.ejphar.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 49.Salari P, Abdollahi M. Systematic review of modulators of benzodiazepine receptors in irritable bowel syndrome: is there hope? World J Gastroenterol. 2011;17:4251–4257. doi: 10.3748/wjg.v17.i38.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camilleri M. Current and future pharmacological treatments for diarrhea-predominant irritable bowel syndrome. Expert Opin Pharmacother. 2013;14:1151–1160. doi: 10.1517/14656566.2013.794223. [DOI] [PubMed] [Google Scholar]
- 51.Heizer WD, Southern S, McGovern S. The role of diet in symptoms of irritable bowel syndrome in adults: a narrative review. J Am Diet Assoc. 2009;109:1204–1214. doi: 10.1016/j.jada.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Lea R, Whorwell PJ. The role of food intolerance in irritable bowel syndrome. Gastroenterol Clin North Am. 2005;34:247–255. doi: 10.1016/j.gtc.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Gholamrezaei A, Ardestani SK, Emami MH. Where does hypnotherapy stand in the management of irritable bowel syndrome? A systematic review. J Altern Complement Med. 2006;12:517–527. doi: 10.1089/acm.2006.12.517. [DOI] [PubMed] [Google Scholar]
- 54.Webb AN, Kukuruzovic RH, Catto-Smith AG, Sawyer SM. Hypnotherapy for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2007;(4):CD005110. doi: 10.1002/14651858.CD005110.pub2. [DOI] [PubMed] [Google Scholar]
- 55.Roberts L, Wilson S, Singh S, Roalfe A, Greenfield S. Gut-directed hypnotherapy for irritable bowel syndrome: piloting a primary care-based randomised controlled trial. Br J Gen Pract. 2006;56:115–121. [PMC free article] [PubMed] [Google Scholar]
- 56.Zijdenbos IL, de Wit NJ, van der Heijden GJ, Rubin G, Quartero AO. Psychological treatments for the management of irritable bowel syndrome. Cochrane Database Syst Rev. 2009;(1):CD006442. doi: 10.1002/14651858.CD006442.pub2. [DOI] [PubMed] [Google Scholar]
- 57.Hutton JM. Issues to consider in cognitive-behavioural therapy for irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2008;20:249–251. doi: 10.1097/MEG.0b013e3282f25185. [DOI] [PubMed] [Google Scholar]
- 58.Halpert A, Godena E. Irritable bowel syndrome patients’ perspectives on their relationships with healthcare providers. Scand J Gastroenterol. 2011;46:823–830. doi: 10.3109/00365521.2011.574729. [DOI] [PubMed] [Google Scholar]
- 59.Conboy LA, Macklin E, Kelley J, Kokkotou E, Lembo A, Kaptchuk T. Which patients improve: characteristics increasing sensitivity to a supportive patient-practitioner relationship. Soc Sci Med. 2010;70:479–484. doi: 10.1016/j.socscimed.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lang L. The Food and Drug Administration approves lubiprostone for irritable bowel syndrome with constipation. Gastroenterology. 2008;135:7. doi: 10.1053/j.gastro.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Sweetser S, Busciglio IA, Camilleri M, Bharucha AE, Szarka LA, Papathanasopoulos A, Burton DD, Eckert DJ, Zinsmeister AR. Effect of a chloride channel activator, lubiprostone, on colonic sensory and motor functions in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296:G295–G301. doi: 10.1152/ajpgi.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ford AC, Brandt LJ, Young C, Chey WD, Foxx-Orenstein AE, Moayyedi P. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2009;104:1831–1843; quiz 1844. doi: 10.1038/ajg.2009.223. [DOI] [PubMed] [Google Scholar]
- 63.Thompson CA. Novartis suspends tegaserod sales at FDA’s request. Am J Health Syst Pharm. 2007;64:1020. doi: 10.2146/news070044. [DOI] [PubMed] [Google Scholar]
- 64.Cremonini F, Nicandro JP, Atkinson V, Shringarpure R, Chuang E, Lembo A. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther. 2012;36:437–448. doi: 10.1111/j.1365-2036.2012.05208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bleser S. Alosetron for severe diarrhea-predominant irritable bowel syndrome: improving patient outcomes. Curr Med Res Opin. 2011;27:503–512. doi: 10.1185/03007995.2010.547933. [DOI] [PubMed] [Google Scholar]
- 66.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 67.Martínez-Vázquez MA, Vázquez-Elizondo G, González-González JA, Gutiérrez-Udave R, Maldonado-Garza HJ, Bosques-Padilla FJ. Effect of antispasmodic agents, alone or in combination, in the treatment of Irritable Bowel Syndrome: systematic review and meta-analysis. Rev Gastroenterol Mex. 2012;77:82–90. doi: 10.1016/j.rgmx.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460. doi: 10.1002/14651858.CD003460.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Darvish-Damavandi M, Nikfar S, Abdollahi M. A systematic review of efficacy and tolerability of mebeverine in irritable bowel syndrome. World J Gastroenterol. 2010;16:547–553. doi: 10.3748/wjg.v16.i5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanauer SB. The benefits of loperamide in the treatment of patients with IBS or IBD. Introduction. Rev Gastroenterol Disord. 2007;7 Suppl 3:S1–S2. [PubMed] [Google Scholar]
- 71.Palmer KR, Corbett CL, Holdsworth CD. Double-blind cross-over study comparing loperamide, codeine and diphenoxylate in the treatment of chronic diarrhea. Gastroenterology. 1980;79:1272–1275. [PubMed] [Google Scholar]
- 72.Thabane M, Marshall JK. Post-infectious irritable bowel syndrome. World J Gastroenterol. 2009;15:3591–3596. doi: 10.3748/wjg.15.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pimentel M, Morales W, Jee SR, Low K, Hwang L, Pokkunuri V, Mirocha J, Conklin J, Chang C. Antibiotic prophylaxis prevents the development of a post-infectious phenotype in a new rat model of post-infectious IBS. Dig Dis Sci. 2011;56:1962–1966. doi: 10.1007/s10620-010-1548-z. [DOI] [PubMed] [Google Scholar]
- 74.Basseri RJ, Weitsman S, Barlow GM, Pimentel M. Antibiotics for the treatment of irritable bowel syndrome. Gastroenterol Hepatol (N Y) 2011;7:455–493. [PMC free article] [PubMed] [Google Scholar]
- 75.Corinaldesi R, Stanghellini V, Cremon C, Gargano L, Cogliandro RF, De Giorgio R, Bartesaghi G, Canovi B, Barbara G. Effect of mesalazine on mucosal immune biomarkers in irritable bowel syndrome: a randomized controlled proof-of-concept study. Aliment Pharmacol Ther. 2009;30:245–252. doi: 10.1111/j.1365-2036.2009.04041.x. [DOI] [PubMed] [Google Scholar]
- 76.Huerta C, García Rodríguez LA, Wallander MA, Johansson S. Users of oral steroids are at a reduced risk of developing irritable bowel syndrome. Pharmacoepidemiol Drug Saf. 2003;12:583–588. doi: 10.1002/pds.836. [DOI] [PubMed] [Google Scholar]
- 77.Róka R, Ait-Belgnaoui A, Salvador-Cartier C, Garcia-Villar R, Fioramonti J, Eutamène H, Bueno L. Dexamethasone prevents visceral hyperalgesia but not colonic permeability increase induced by luminal protease-activated receptor-2 agonist in rats. Gut. 2007;56:1072–1078. doi: 10.1136/gut.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rezaie A, Nikfar S, Abdollahi M. The place of antibiotics in management of irritable bowel syndrome: a systematic review and meta-analysis. Arch Med Sci. 2010;6:49–55. doi: 10.5114/aoms.2010.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frissora CL, Cash BD. Review article: the role of antibiotics vs. conventional pharmacotherapy in treating symptoms of irritable bowel syndrome. Aliment Pharmacol Ther. 2007;25:1271–1281. doi: 10.1111/j.1365-2036.2007.03313.x. [DOI] [PubMed] [Google Scholar]
- 80.Saadi M, McCallum RW. Rifaximin in irritable bowel syndrome: rationale, evidence and clinical use. Ther Adv Chronic Dis. 2013;4:71–75. doi: 10.1177/2040622312472008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peralta S, Cottone C, Doveri T, Almasio PL, Craxi A. Small intestine bacterial overgrowth and irritable bowel syndrome-related symptoms: experience with Rifaximin. World J Gastroenterol. 2009;15:2628–2631. doi: 10.3748/wjg.15.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Halland M, Talley NJ. New treatments for IBS. Nat Rev Gastroenterol Hepatol. 2013;10:13–23. doi: 10.1038/nrgastro.2012.207. [DOI] [PubMed] [Google Scholar]
- 83.Shi J, Tong Y, Shen JG, Li HX. Effectiveness and safety of herbal medicines in the treatment of irritable bowel syndrome: a systematic review. World J Gastroenterol. 2008;14:454–462. doi: 10.3748/wjg.14.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu JP, Yang M, Liu YX, Wei M, Grimsgaard S. Herbal medicines for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2006;(1):CD004116. doi: 10.1002/14651858.CD004116.pub2. [DOI] [PubMed] [Google Scholar]
- 85.van Tilburg MA, Palsson OS, Levy RL, Feld AD, Turner MJ, Drossman DA, Whitehead WE. Complementary and alternative medicine use and cost in functional bowel disorders: a six month prospective study in a large HMO. BMC Complement Altern Med. 2008;8:46. doi: 10.1186/1472-6882-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fisher P, van Haselen R, Hardy K, Berkovitz S, McCarney R. Effectiveness gaps: a new concept for evaluating health service and research needs applied to complementary and alternative medicine. J Altern Complement Med. 2004;10:627–632. doi: 10.1089/acm.2004.10.627. [DOI] [PubMed] [Google Scholar]
- 87.Kong SC, Hurlstone DP, Pocock CY, Walkington LA, Farquharson NR, Bramble MG, McAlindon ME, Sanders DS. The Incidence of self-prescribed oral complementary and alternative medicine use by patients with gastrointestinal diseases. J Clin Gastroenterol. 2005;39:138–141. [PubMed] [Google Scholar]
- 88.Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280:1585–1589. doi: 10.1001/jama.280.18.1585. [DOI] [PubMed] [Google Scholar]
- 89.Drisko J, Bischoff B, Hall M, McCallum R. Treating irritable bowel syndrome with a food elimination diet followed by food challenge and probiotics. J Am Coll Nutr. 2006;25:514–522. doi: 10.1080/07315724.2006.10719567. [DOI] [PubMed] [Google Scholar]
- 90.Kim YJ, Ban DJ. Prevalence of irritable bowel syndrome, influence of lifestyle factors and bowel habits in Korean college students. Int J Nurs Stud. 2005;42:247–254. doi: 10.1016/j.ijnurstu.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 91.Levy RL, Linde JA, Feld KA, Crowell MD, Jeffery RW. The association of gastrointestinal symptoms with weight, diet, and exercise in weight-loss program participants. Clin Gastroenterol Hepatol. 2005;3:992–996. doi: 10.1016/s1542-3565(05)00696-8. [DOI] [PubMed] [Google Scholar]
- 92.Rao AS, Camilleri M, Eckert DJ, Busciglio I, Burton DD, Ryks M, Wong BS, Lamsam J, Singh R, Zinsmeister AR. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol. 2011;301:G919–G928. doi: 10.1152/ajpgi.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saberi-Firoozi M, Khademolhosseini F, Mehrabani D, Yousefi M, Salehi M, Heidary ST. Subjective lactose intolerance in apparently healthy adults in southern Iran: Is it related to irritable bowel syndrome? Indian J Med Sci. 2007;61:591–597. [PubMed] [Google Scholar]
- 94.Gupta D, Ghoshal UC, Misra A, Misra A, Choudhuri G, Singh K. Lactose intolerance in patients with irritable bowel syndrome from northern India: a case-control study. J Gastroenterol Hepatol. 2007;22:2261–2265. doi: 10.1111/j.1440-1746.2007.04986.x. [DOI] [PubMed] [Google Scholar]
- 95.Yang J, Deng Y, Chu H, Cong Y, Zhao J, Pohl D, Misselwitz B, Fried M, Dai N, Fox M. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:262–268.e1. doi: 10.1016/j.cgh.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 96.Bijkerk CJ, Muris JW, Knottnerus JA, Hoes AW, de Wit NJ. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19:245–251. doi: 10.1111/j.0269-2813.2004.01862.x. [DOI] [PubMed] [Google Scholar]
- 97.Ford AC, Talley NJ, Spiegel BM, Foxx-Orenstein AE, Schiller L, Quigley EM, Moayyedi P. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313. doi: 10.1136/bmj.a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Daley AJ, Grimmett C, Roberts L, Wilson S, Fatek M, Roalfe A, Singh S. The effects of exercise upon symptoms and quality of life in patients diagnosed with irritable bowel syndrome: a randomised controlled trial. Int J Sports Med. 2008;29:778–782. doi: 10.1055/s-2008-1038600. [DOI] [PubMed] [Google Scholar]
- 99.Villoria A, Serra J, Azpiroz F, Malagelada JR. Physical activity and intestinal gas clearance in patients with bloating. Am J Gastroenterol. 2006;101:2552–2557. doi: 10.1111/j.1572-0241.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- 100.Lustyk MK, Jarrett ME, Bennett JC, Heitkemper MM. Does a physically active lifestyle improve symptoms in women with irritable bowel syndrome? Gastroenterol Nurs. 2001;24:129–137. [PubMed] [Google Scholar]
- 101.Kuttner L, Chambers CT, Hardial J, Israel DM, Jacobson K, Evans K. A randomized trial of yoga for adolescents with irritable bowel syndrome. Pain Res Manag. 2006;11:217–223. doi: 10.1155/2006/731628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taneja I, Deepak KK, Poojary G, Acharya IN, Pandey RM, Sharma MP. Yogic versus conventional treatment in diarrhea-predominant irritable bowel syndrome: a randomized control study. Appl Psychophysiol Biofeedback. 2004;29:19–33. doi: 10.1023/b:apbi.0000017861.60439.95. [DOI] [PubMed] [Google Scholar]
- 103.Koch TR. Peppermint oil and irritable bowel syndrome. Am J Gastroenterol. 1998;93:2304–2305. doi: 10.1111/j.1572-0241.1998.02304.x. [DOI] [PubMed] [Google Scholar]
- 104.Liu JH, Chen GH, Yeh HZ, Huang CK, Poon SK. Enteric-coated peppermint-oil capsules in the treatment of irritable bowel syndrome: a prospective, randomized trial. J Gastroenterol. 1997;32:765–768. doi: 10.1007/BF02936952. [DOI] [PubMed] [Google Scholar]
- 105.Logan AC, Beaulne TM. The treatment of small intestinal bacterial overgrowth with enteric-coated peppermint oil: a case report. Altern Med Rev. 2002;7:410–417. [PubMed] [Google Scholar]
- 106.Rees WD, Evans BK, Rhodes J. Treating irritable bowel syndrome with peppermint oil. Br Med J. 1979;2:835–836. doi: 10.1136/bmj.2.6194.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sagduyu K. Peppermint oil for irritable bowel syndrome. Psychosomatics. 2002;43:508–509. doi: 10.1176/appi.psy.43.6.508. [DOI] [PubMed] [Google Scholar]
- 108.Cappello G, Spezzaferro M, Grossi L, Manzoli L, Marzio L. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: a prospective double blind placebo-controlled randomized trial. Dig Liver Dis. 2007;39:530–536. doi: 10.1016/j.dld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 109.Merat S, Khalili S, Mostajabi P, Ghorbani A, Ansari R, Malekzadeh R. The effect of enteric-coated, delayed-release peppermint oil on irritable bowel syndrome. Dig Dis Sci. 2010;55:1385–1390. doi: 10.1007/s10620-009-0854-9. [DOI] [PubMed] [Google Scholar]
- 110.Alam MS, Roy PK, Miah AR, Mollick SH, Khan MR, Mahmud MC, Khatun S. Efficacy of Peppermint oil in diarrhea predominant IBS - a double blind randomized placebo - controlled study. Mymensingh Med J. 2013;22:27–30. [PubMed] [Google Scholar]
- 111.Charrois TL, Hrudey J, Gardiner P, Vohra S. Peppermint oil. Pediatr Rev. 2006;27:e49–e51. doi: 10.1542/pir.27-7-e49. [DOI] [PubMed] [Google Scholar]
- 112.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytother Res. 2006;20:519–530. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- 113.Walker AF, Middleton RW, Petrowicz O. Artichoke leaf extract reduces symptoms of irritable bowel syndrome in a post-marketing surveillance study. Phytother Res. 2001;15:58–61. doi: 10.1002/1099-1573(200102)15:1<58::aid-ptr805>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 114.Bundy R, Walker AF, Middleton RW, Marakis G, Booth JC. Artichoke leaf extract reduces symptoms of irritable bowel syndrome and improves quality of life in otherwise healthy volunteers suffering from concomitant dyspepsia: a subset analysis. J Altern Complement Med. 2004;10:667–669. doi: 10.1089/acm.2004.10.667. [DOI] [PubMed] [Google Scholar]
- 115.Verspohl EJ, Ploch M, Windeck T, Klaes M, Schmidt T, Bauer K. Effect of two artichoke extracts (36_U and 36_EB) on rat ileum (with respect to bowel syndrome) and the peristaltic threshold. Phytomedicine. 2008;15:1002–1009. doi: 10.1016/j.phymed.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 116.Bundy R, Walker AF, Middleton RW, Booth J. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: a pilot study. J Altern Complement Med. 2004;10:1015–1018. doi: 10.1089/acm.2004.10.1015. [DOI] [PubMed] [Google Scholar]
- 117.Brinkhaus B, Hentschel C, Von Keudell C, Schindler G, Lindner M, Stützer H, Kohnen R, Willich SN, Lehmacher W, Hahn EG. Herbal medicine with curcuma and fumitory in the treatment of irritable bowel syndrome: a randomized, placebo-controlled, double-blind clinical trial. Scand J Gastroenterol. 2005;40:936–943. doi: 10.1080/00365520510023134. [DOI] [PubMed] [Google Scholar]
- 118.Rösch W, Vinson B, Sassin I. A randomised clinical trial comparing the efficacy of a herbal preparation STW 5 with the prokinetic drug cisapride in patients with dysmotility type of functional dyspepsia. Z Gastroenterol. 2002;40:401–408. doi: 10.1055/s-2002-32130. [DOI] [PubMed] [Google Scholar]
- 119.von Arnim U, Peitz U, Vinson B, Gundermann KJ, Malfertheiner P. STW 5, a phytopharmacon for patients with functional dyspepsia: results of a multicenter, placebo-controlled double-blind study. Am J Gastroenterol. 2007;102:1268–1275. doi: 10.1111/j.1572-0241.2006.01183.x. [DOI] [PubMed] [Google Scholar]
- 120.Madisch A, Holtmann G, Plein K, Hotz J. Treatment of irritable bowel syndrome with herbal preparations: results of a double-blind, randomized, placebo-controlled, multi-centre trial. Aliment Pharmacol Ther. 2004;19:271–279. doi: 10.1111/j.1365-2036.2004.01859.x. [DOI] [PubMed] [Google Scholar]
- 121.Simmen U, Kelber O, Okpanyi SN, Jaeggi R, Bueter B, Weiser D. Binding of STW 5 (Iberogast) and its components to intestinal 5-HT, muscarinic M3, and opioid receptors. Phytomedicine. 2006;13 Suppl 5:51–55. doi: 10.1016/j.phymed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 122.Ottillinger B, Storr M, Malfertheiner P, Allescher HD. STW 5 (Iberogast®)--a safe and effective standard in the treatment of functional gastrointestinal disorders. Wien Med Wochenschr. 2013;163:65–72. doi: 10.1007/s10354-012-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sallon S, Ben-Arye E, Davidson R, Shapiro H, Ginsberg G, Ligumsky M. A novel treatment for constipation-predominant irritable bowel syndrome using Padma Lax, a Tibetan herbal formula. Digestion. 2002;65:161–171. doi: 10.1159/000064936. [DOI] [PubMed] [Google Scholar]
- 124.Hofbauer S, Kainz V, Golser L, Klappacher M, Kiesslich T, Heidegger W, Krammer B, Hermann A, Weiger TM. Antiproliferative properties of Padma Lax and its components ginger and elecampane. Forsch Komplementmed. 2006;13 Suppl 1:18–22. doi: 10.1159/000091147. [DOI] [PubMed] [Google Scholar]
- 125.Gschossmann JM, Krayer M, Flogerzi B, Balsiger BM. Effects of the Tibetan herbal formula Padma Lax on visceral nociception and contractility of longitudinal smooth muscle in a rat model. Neurogastroenterol Motil. 2010;22:1036–1041, e269-e270. doi: 10.1111/j.1365-2982.2010.01512.x. [DOI] [PubMed] [Google Scholar]
- 126.Wang ZJ, Li HX, Wang JH, Zhang F. Effect of Shugan Jianpi Granule () on gut mucosal serotonin-positive cells in patients with irritable bowel syndrome of stagnated Gan-qi attacking Pi syndrome type. Chin J Integr Med. 2008;14:185–189. doi: 10.1007/s11655-008-9001-2. [DOI] [PubMed] [Google Scholar]
- 127.Pan F, Zhang T, Zhang YH, Xu JJ, Chen FM. Effect of Tongxie Yaofang Granule in treating diarrhea-predominate irritable bowel syndrome. Chin J Integr Med. 2009;15:216–219. doi: 10.1007/s11655-009-0216-7. [DOI] [PubMed] [Google Scholar]
- 128.Bian Z, Wu T, Liu L, Miao J, Wong H, Song L, Sung JJ. Effectiveness of the Chinese herbal formula TongXieYaoFang for irritable bowel syndrome: a systematic review. J Altern Complement Med. 2006;12:401–407. doi: 10.1089/acm.2006.12.401. [DOI] [PubMed] [Google Scholar]
- 129.Leung WK, Wu JC, Liang SM, Chan LS, Chan FK, Xie H, Fung SS, Hui AJ, Wong VW, Che CT, et al. Treatment of diarrhea-predominant irritable bowel syndrome with traditional Chinese herbal medicine: a randomized placebo-controlled trial. Am J Gastroenterol. 2006;101:1574–1580. doi: 10.1111/j.1572-0241.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 130.Medicine NCfCaA. What is complementary and alternative medicine? 2011. Available from: http://www.nccam.nih.gov/health/whatiscam.
- 131.Mehling WE, Wrubel J, Daubenmier JJ, Price CJ, Kerr CE, Silow T, Gopisetty V, Stewart AL. Body Awareness: a phenomenological inquiry into the common ground of mind-body therapies. Philos Ethics Humanit Med. 2011;6:6. doi: 10.1186/1747-5341-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eriksson EM, Möller IE, Söderberg RH, Eriksson HT, Kurlberg GK. Body awareness therapy: a new strategy for relief of symptoms in irritable bowel syndrome patients. World J Gastroenterol. 2007;13:3206–3214. doi: 10.3748/wjg.v13.i23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Johannesson E, Simrén M, Strid H, Bajor A, Sadik R. Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol. 2011;106:915–922. doi: 10.1038/ajg.2010.480. [DOI] [PubMed] [Google Scholar]
- 134.Tovey P. A single-blind trial of reflexology for irritable bowel syndrome. Br J Gen Pract. 2002;52:19–23. [PMC free article] [PubMed] [Google Scholar]
- 135.Cherniack EP. Use of complementary and alternative medicine to treat constipation in the elderly. Geriatr Gerontol Int. 2013;13:533–538. doi: 10.1111/ggi.12023. [DOI] [PubMed] [Google Scholar]
- 136.Lämås K, Graneheim UH, Jacobsson C. Experiences of abdominal massage for constipation. J Clin Nurs. 2012;21:757–765. doi: 10.1111/j.1365-2702.2011.03946.x. [DOI] [PubMed] [Google Scholar]
- 137.Harrington KL, Haskvitz EM. Managing a patient’s constipation with physical therapy. Phys Ther. 2006;86:1511–1519. doi: 10.2522/ptj.20050347. [DOI] [PubMed] [Google Scholar]
- 138.Zhou EH, Liu HR, Wu HG, Shi Y, Wang XM, Tan LY, Yao LQ, Zhong YS, Jiang Y, Zhang LL. Suspended moxibustion relieves chronic visceral hyperalgesia via serotonin pathway in the colon. Neurosci Lett. 2009;451:144–147. doi: 10.1016/j.neulet.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 139.Tian SL, Wang XY, Ding GH. Repeated electro-acupuncture attenuates chronic visceral hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat irritable bowel syndrome model. Life Sci. 2008;83:356–363. doi: 10.1016/j.lfs.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 140.Ma XP, Tan LY, Yang Y, Wu HG, Jiang B, Liu HR, Yang L. Effect of electro-acupuncture on substance P, its receptor and corticotropin-releasing hormone in rats with irritable bowel syndrome. World J Gastroenterol. 2009;15:5211–5217. doi: 10.3748/wjg.15.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vas J, Rivas-Ruiz F. Response to letter by Wu and Guo. Pain. 2013;154:2576. doi: 10.1016/j.pain.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 142.Kong J, Spaeth R, Cook A, Kirsch I, Claggett B, Vangel M, Gollub RL, Smoller JW, Kaptchuk TJ. Are all placebo effects equal? Placebo pills, sham acupuncture, cue conditioning and their association. PLoS One. 2013;8:e67485. doi: 10.1371/journal.pone.0067485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lembo AJ, Conboy L, Kelley JM, Schnyer RS, McManus CA, Quilty MT, Kerr CE, Drossman D, Jacobson EE, Davis RB. A treatment trial of acupuncture in IBS patients. Am J Gastroenterol. 2009;104:1489–1497. doi: 10.1038/ajg.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schneider A, Enck P, Streitberger K, Weiland C, Bagheri S, Witte S, Friederich HC, Herzog W, Zipfel S. Acupuncture treatment in irritable bowel syndrome. Gut. 2006;55:649–654. doi: 10.1136/gut.2005.074518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schneider A, Streitberger K, Joos S. Acupuncture treatment in gastrointestinal diseases: a systematic review. World J Gastroenterol. 2007;13:3417–3424. doi: 10.3748/wjg.v13.i25.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Anastasi JK, McMahon DJ, Kim GH. Symptom management for irritable bowel syndrome: a pilot randomized controlled trial of acupuncture/moxibustion. Gastroenterol Nurs. 2009;32:243–255. doi: 10.1097/SGA.0b013e3181b2c920. [DOI] [PubMed] [Google Scholar]
- 147.Lim B, Manheimer E, Lao L, Ziea E, Wisniewski J, Liu J, Berman B. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2006;(4):CD005111. doi: 10.1002/14651858.CD005111.pub2. [DOI] [PubMed] [Google Scholar]
- 148.Field T. Yoga clinical research review. Complement Ther Clin Pract. 2011;17:1–8. doi: 10.1016/j.ctcp.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 149.Brands MM, Purperhart H, Deckers-Kocken JM. A pilot study of yoga treatment in children with functional abdominal pain and irritable bowel syndrome. Complement Ther Med. 2011;19:109–114. doi: 10.1016/j.ctim.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 150.Cuellar NG. Hypnosis for pain management in the older adult. Pain Manag Nurs. 2005;6:105–111. doi: 10.1016/j.pmn.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 151.Hrezo RJ. Hypnosis: an alternative in pain management for nurse practitioners. Nurse Pract Forum. 1998;9:217–226. [PubMed] [Google Scholar]
- 152.Rainville P, Hofbauer RK, Paus T, Duncan GH, Bushnell MC, Price DD. Cerebral mechanisms of hypnotic induction and suggestion. J Cogn Neurosci. 1999;11:110–125. doi: 10.1162/089892999563175. [DOI] [PubMed] [Google Scholar]
- 153.Abrahamsen R, Baad-Hansen L, Zachariae R, Svensson P. Effect of hypnosis on pain and blink reflexes in patients with painful temporomandibular disorders. Clin J Pain. 2011;27:344–351. doi: 10.1097/AJP.0b013e3181ffbfcb. [DOI] [PubMed] [Google Scholar]
- 154.Lea R, Houghton LA, Calvert EL, Larder S, Gonsalkorale WM, Whelan V, Randles J, Cooper P, Cruickshanks P, Miller V, et al. Gut-focused hypnotherapy normalizes disordered rectal sensitivity in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:635–642. doi: 10.1046/j.1365-2036.2003.01486.x. [DOI] [PubMed] [Google Scholar]
- 155.Gonsalkorale WM, Miller V, Afzal A, Whorwell PJ. Long term benefits of hypnotherapy for irritable bowel syndrome. Gut. 2003;52:1623–1629. doi: 10.1136/gut.52.11.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gonsalkorale WM. Gut-directed hypnotherapy: the Manchester approach for treatment of irritable bowel syndrome. Int J Clin Exp Hypn. 2006;54:27–50. doi: 10.1080/00207140500323030. [DOI] [PubMed] [Google Scholar]
- 157.Gonsalkorale WM, Toner BB, Whorwell PJ. Cognitive change in patients undergoing hypnotherapy for irritable bowel syndrome. J Psychosom Res. 2004;56:271–278. doi: 10.1016/S0022-3999(03)00076-X. [DOI] [PubMed] [Google Scholar]
- 158.Palsson OS, Turner MJ, Johnson DA, Burnett CK, Whitehead WE. Hypnosis treatment for severe irritable bowel syndrome: investigation of mechanism and effects on symptoms. Dig Dis Sci. 2002;47:2605–2614. doi: 10.1023/a:1020545017390. [DOI] [PubMed] [Google Scholar]
- 159.Vlieger AM, Menko-Frankenhuis C, Wolfkamp SC, Tromp E, Benninga MA. Hypnotherapy for children with functional abdominal pain or irritable bowel syndrome: a randomized controlled trial. Gastroenterology. 2007;133:1430–1436. doi: 10.1053/j.gastro.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 160.Lindfors P, Ljótsson B, Bjornsson E, Abrahamsson H, Simrén M. Patient satisfaction after gut-directed hypnotherapy in irritable bowel syndrome. Neurogastroenterol Motil. 2013;25:169–e86. doi: 10.1111/nmo.12022. [DOI] [PubMed] [Google Scholar]
- 161.Lindfors P, Unge P, Arvidsson P, Nyhlin H, Björnsson E, Abrahamsson H, Simrén M. Effects of gut-directed hypnotherapy on IBS in different clinical settings-results from two randomized, controlled trials. Am J Gastroenterol. 2012;107:276–285. doi: 10.1038/ajg.2011.340. [DOI] [PubMed] [Google Scholar]
- 162.Moser G, Trägner S, Gajowniczek EE, Mikulits A, Michalski M, Kazemi-Shirazi L, Kulnigg-Dabsch S, Führer M, Ponocny-Seliger E, Dejaco C, et al. Long-term success of GUT-directed group hypnosis for patients with refractory irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol. 2013;108:602–609. doi: 10.1038/ajg.2013.19. [DOI] [PubMed] [Google Scholar]
- 163.Gerson CD, Gerson J, Gerson MJ. Group hypnotherapy for irritable bowel syndrome with long-term follow-up. Int J Clin Exp Hypn. 2013;61:38–54. doi: 10.1080/00207144.2012.700620. [DOI] [PubMed] [Google Scholar]
- 164.Lindfors P, Unge P, Nyhlin H, Ljótsson B, Björnsson ES, Abrahamsson H, Simrén M. Long-term effects of hypnotherapy in patients with refractory irritable bowel syndrome. Scand J Gastroenterol. 2012;47:414–420. doi: 10.3109/00365521.2012.658858. [DOI] [PubMed] [Google Scholar]
- 165.Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 166.Tsiros MD, Sinn N, Brennan L, Coates AM, Walkley JW, Petkov J, Howe PR, Buckley JD. Cognitive behavioral therapy improves diet and body composition in overweight and obese adolescents. Am J Clin Nutr. 2008;87:1134–1140. doi: 10.1093/ajcn/87.5.1134. [DOI] [PubMed] [Google Scholar]
- 167.Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain. 2007;127:276–286. doi: 10.1016/j.pain.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 168.Thase ME, Dubé S, Bowler K, Howland RH, Myers JE, Friedman E, Jarrett DB. Hypothalamic-pituitary-adrenocortical activity and response to cognitive behavior therapy in unmedicated, hospitalized depressed patients. Am J Psychiatry. 1996;153:886–891. doi: 10.1176/ajp.153.7.886. [DOI] [PubMed] [Google Scholar]
- 169.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5:355–362. [PMC free article] [PubMed] [Google Scholar]
- 170.Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, Emmott S, Proffitt V, Akman D, Frusciante K, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 171.Weinland SR, Morris CB, Dalton C, Hu Y, Whitehead WE, Toner BB, Diamant N, Leserman J, Bangdiwala SI, Drossman DA. Cognitive factors affect treatment response to medical and psychological treatments in functional bowel disorders. Am J Gastroenterol. 2010;105:1397–1406. doi: 10.1038/ajg.2009.748. [DOI] [PubMed] [Google Scholar]
- 172.Jones M, Koloski N, Boyce P, Talley NJ. Pathways connecting cognitive behavioral therapy and change in bowel symptoms of IBS. J Psychosom Res. 2011;70:278–285. doi: 10.1016/j.jpsychores.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 173.Ljótsson B, Hedman E, Andersson E, Hesser H, Lindfors P, Hursti T, Rydh S, Rück C, Lindefors N, Andersson G. Internet-delivered exposure-based treatment vs. stress management for irritable bowel syndrome: a randomized trial. Am J Gastroenterol. 2011;106:1481–1491. doi: 10.1038/ajg.2011.139. [DOI] [PubMed] [Google Scholar]
- 174.Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, Wessely S. Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial. Health Technol Assess. 2006;10:iii–iv, ix-x, 1-67. doi: 10.3310/hta10190. [DOI] [PubMed] [Google Scholar]
- 175.Mahvi-Shirazi M, Fathi-Ashtiani A, Rasoolzade-Tabatabaei S-K, Amini M. Irritable bowel syndrome treatment: cognitive behavioral therapy versus medical treatment. Arch Med Sci. 2008;8:123–129. doi: 10.5114/aoms.2012.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tkachuk GA, Graff LA, Martin GL, Bernstein CN. Randomized controlled trial of Cognitive-Behavioral Group Therapy for Irritable Bowel Syndrome in a Medical Setting. J Clin Psych Med Set. 2003;10:57–69. [Google Scholar]
- 177.Craske MG, Wolitzky-Taylor KB, Labus J, Wu S, Frese M, Mayer EA, Naliboff BD. A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations. Behav Res Ther. 2011;49:413–421. doi: 10.1016/j.brat.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lackner JM, Keefer L, Jaccard J, Firth R, Brenner D, Bratten J, Dunlap LJ, Ma C, Byroads M. The Irritable Bowel Syndrome Outcome Study (IBSOS): rationale and design of a randomized, placebo-controlled trial with 12 month follow up of self- versus clinician-administered CBT for moderate to severe irritable bowel syndrome. Contemp Clin Trials. 2012;33:1293–1310. doi: 10.1016/j.cct.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ljótsson B, Falk L, Vesterlund AW, Hedman E, Lindfors P, Rück C, Hursti T, Andréewitch S, Jansson L, Lindefors N, et al. Internet-delivered exposure and mindfulness based therapy for irritable bowel syndrome--a randomized controlled trial. Behav Res Ther. 2010;48:531–539. doi: 10.1016/j.brat.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 180.Gaylord SA, Palsson OS, Garland EL, Faurot KR, Coble RS, Mann JD, Frey W, Leniek K, Whitehead WE. Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. Am J Gastroenterol. 2011;106:1678–1688. doi: 10.1038/ajg.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ljótsson B, Hedman E, Lindfors P, Hursti T, Lindefors N, Andersson G, Rück C. Long-term follow-up of internet-delivered exposure and mindfulness based treatment for irritable bowel syndrome. Behav Res Ther. 2011;49:58–61. doi: 10.1016/j.brat.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 182.Tillisch K. Complementary and alternative medicine for functional gastrointestinal disorders. Gut. 2006;55:593–596. doi: 10.1136/gut.2005.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Birtwhistle RV. Irritable bowel syndrome: are complementary and alternative medicine treatments useful? Can Fam Physician. 2009;55:126–127, 128-129. [PMC free article] [PubMed] [Google Scholar]
- 184.Shen YH, Nahas R. Complementary and alternative medicine for treatment of irritable bowel syndrome. Can Fam Physician. 2009;55:143–148. [PMC free article] [PubMed] [Google Scholar]
- 185.Shen YH CAHCIM. AHC-Consortium of Academic Health Centers for Integrative Medicine 2013. Available from: http://www.imconsortium.org/


