Abstract
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder characterized by unspecific symptoms. In clinical practice it is crucial to distinguish between non-inflammatory functional problems and inflammatory, malignant or infectious diseases of the GI tract. Differentiation between these involves the use of clinical, radiological, endoscopic, histological and serological techniques, which are invasive, expensive, time-consuming and/or hindered by inaccuracies arising from subjective components. A range of faecal markers now appears to have the potential to greatly assist in the differentiation of inflammatory bowel disease (IBD) and IBS. Faecal markers of neutrophil influx into the mucosa are reliable indicators of intestinal inflammation and their role has been mainly studied in discriminating IBD from non-IBD conditions (including IBS) rather than organic from non-organic diseases. Phagocyte-specific proteins of the S100 family (S100A12, calprotectin) are amongst the most promising faecal biomarkers of inflammation. Faecal leukocyte degranulation markers (lactoferrin, polymorphonuclear elastase and myeloperoxidase) have also been suggested as diagnostic tools for the differentiation of IBD and IBS. More recently, additional proteins, including granins, defensins and matrix-metalloproteases, have been discussed as differential diagnostic markers in IBD and IBS. In this review, some of the most promising faecal markers, which have the potential to differentiate IBD and IBS and to advance diagnostic practices, will be discussed.
Keywords: S100A12, Calprotectin, Lactoferrin, M2-pyruvate kinase, Polymorphonuclear elastase, Defensins, Granins, Irritable bowel syndrome
Core tip: Faecal markers of intestinal inflammation represent a practicable, non-invasive, inexpensive and objective diagnostic tool to differentiate organic [inflammatory bowel disease (IBD)] and functional [irritable bowel syndrome (IBS)] gastrointestinal diseases. Faecal markers have the potential to be incorporated into standard clinical practice for the routine assessment of IBS and IBD. Neutrophil-derived faecal biomarkers show a high diagnostic accuracy in the differentiation of IBD vs IBS. They can provide reassurance to the physicians that the clinical diagnosis of IBS is correct. Future progress in our knowledge about the biology of these proteins and the underlying pathogenesis of IBS will help translate IBD/IBS research into patient care.
IRRITABLE BOWEL SYNDROME
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal (GI) disorders, with a reported prevalence of approximately 10% to 15% worldwide[1]. The exact pathogenesis of IBS is only partially understood but seems to be multifactorial. There is evidence that heritability and genetics, environment and social learning, dietary or intestinal microbiota, low-grade inflammation and disturbances in the neuroendocrine system of the gut play a central role[2]. There is no medical therapy established to alter the natural history of IBS and most traditional therapies (e.g., bulking agents, antidiarrheals, antispasmodics) focus on improving individual symptoms. However, these symptom-based therapies have limited efficacy and as such novel and emerging therapies have been developed based upon the evolving understanding of the pathophysiology of IBS[3,4].
Though a variety of GI and extraintestinal symptoms and presentations are associated with IBS, it is primarily characterized by symptoms of abdominal pain or discomfort associated with an altered bowel function in the absence of any organic cause. Patients commonly report abnormal defecation ranging from diarrhoea to constipation, including a combination of the two, the degree of which can vary in both severity and duration[5,6]. Four subtypes of IBS were recognized: IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), mixed IBS (IBS-M), and unsubtyped IBS (IBS-U). IBS presents a challenge to gastroenterologists, with several groups having attempted to define a set of standardized symptom-based criteria for the diagnosis of IBS. Although no symptom-based criteria have ideal accuracy for diagnosing IBS[7], the third iteration of the Rome criteria (Rome III) and the Manning criteria are widely used by clinicians to diagnose IBS[8,9].
Since many GI disorders present with symptoms similar to IBS, it is important to exclude other causes. The diagnosis of IBS should be made using symptoms based on clinical criteria rather than excluding underlying organic disease by exhaustive investigation. Routine laboratory studies are normal in IBS and thus only a limited number of diagnostic studies are used to rule out other likely conditions. However, patients with alarm symptoms (e.g., fever, weight loss, blood in stools, nocturnal of progressive abdominal pain), laboratory abnormalities, abnormal physical findings, and/or a family history of inflammatory bowel disease (IBD) or colorectal cancer (CRC) require more extensive evaluation (e.g., imaging studies and/or colonoscopy)[2,3,10]. Otherwise, a limited number of diagnostic studies can rule out organic illness in the majority of patients and a sizeable number require no testing at all. However, whilst alarm symptoms (“red flags”) may have a relatively modest predictive value for identifying organic disease, their presence as exclusion criteria would result in many missed cases of IBS[11]. It is this large symptomatic overlap between functional and organic disease, in conjunction with the current lack of a biochemical, histopathological, or radiological diagnostic tests for IBS, which engenders the need for more definitive diagnostic tools[2].
FAECAL MARKERS OF INTESTINAL INFLAMMATION
A simple, reliable, reproducible and non-invasive test, with the ability to differentiate IBD from other GI condition, such as IBS, would be of substantial clinical utility. Serological markers (e.g., C-reactive protein, erythrocyte sedimentation rate) reflect the presence and intensity of a (systemic) inflammatory process and are not specific for intestinal inflammatory disease. Radiological and endoscopic techniques are invasive, time-consuming and/or expensive. Clinical disease (activity) scores are hindered by inaccuracies arising from subjective components. Faecal markers, however, offer a non-invasive approach to objectively measuring intestinal inflammation with the ability to differentiate organic and functional GI diseases. Stool markers are inexpensive, easily measured and therefore suitable for extensive use. Faecal markers include a heterogeneous group of substances that either leak from or are generated by the inflamed intestinal mucosa. The inflamed hyper-permeable gut mucosa is associated with increased protein cytokines and markers of neutrophil activation in faecal samples. Faecal markers of neutrophil influx into the mucosa are promising indicators of intestinal inflammation and their role has been mainly studied in discriminating IBD from non-IBD conditions (including IBS) rather than organic from non-organic diseases (Figure 1). Lactoferrin, polymorphonuclear (PMN) elastase and myeloperoxidase (MPO) are faecal markers of neutrophil degranulation. Of the proteins stored in neutrophilic granules, lactoferrin is the most accurate marker of intestinal inflammation. Importantly, lactoferrin, MPO and PMN elastase are not only expressed in neutrophils and show limited stability in stool samples at room temperature. Other faecal markers including alpha 1-antitrypsin, tumour necrosis factor alpha, lysozyme, and markers of eosinophil degranulation (e.g., eosinophil protein X, eosinophil cationic protein) have also been described as markers of intestinal inflammation but their clinical utility and/or diagnostic accuracy is inferior and data on their role in differentiating IBD from IBS are lacking or very limited[12-20]. The utility of other faecal markers [e.g., granins, defensins, matrix-metalloproteases (MMP)] in differentiating organic from functional disease has not been widely studied. More recently, neutrophil-derived S100 proteins have been identified as faecal markers for differentiating IBD and IBS. Proteins of the S100 family [S100A8/A9 (calprotectin), S100A12] are molecules released from the cytosol by activated or damaged cells under conditions of cell stress, followed by pro-inflammatory activation of pattern recognition receptors. S100 proteins are remarkably resistant to degradation by faecal bacteria, making them suitable markers for gut wall inflammation[14]. Faecal S100A12 and calprotectin are highly sensitive and specific markers of intestinal inflammation and exert a strong influence upon the pathogenesis of IBD[21]. In this review, some of the most promising faecal markers, which have the potential both to differentiate IBD and IBS and to advance diagnostic practices, will be discussed (Figure 1).
Figure 1.
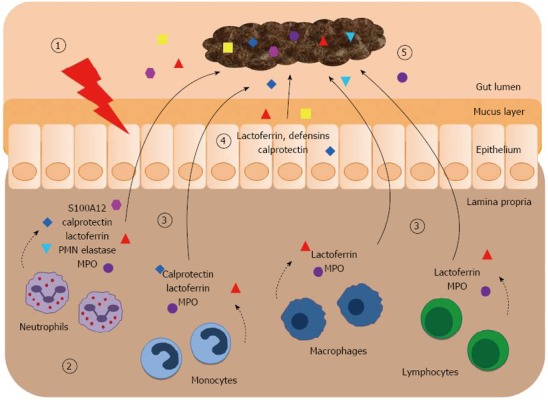
Faecal markers of intestinal inflammation. (1) Initially, unidentified triggers affect the epithelium and lead to an activation of the intestinal immune system; (2) The initiated immune response involves the influx of different innate immune cells (e.g., granulocytes, monocytes, macrophages) and cells of the adaptive immune system (e.g., T cells) into the affected mucosa. These cells actively secret inflammatory mediators or release granule proteins by cell degranulation. The contents of neutrophil granules [ lactoferrin, polymorphonuclear (PMN) elastase, myeloperoxidase (MPO)] have antimicrobial properties. The cytosol is the source of the damage associated molecular pattern proteins S100A8/A9 ( calprotectin) and S100A12 (); (3) During early stages of intestinal inflammation these released proteins spill over from the mucosa into the gut lumen; (4) Some of these factors (including defensins) are also released from the epithelium and the mucus layer; (5) In direct contact with the intestinal mucosa, the faecal stream contains the specific proteins of mucosal disease. The detection of these markers in faeces indicates the presence and degree of intestinal inflammation.
CALPROTECTIN
S100A8 [also known as calgranulin A and myeloid-related protein 8 (MRP8)] and S100A9 (calgranulin B, MRP14) are members of the S100 calcium-binding protein family. Both proteins are linked to the innate immune system and expressed in granulocytes, monocytes/macrophages and epithelial cells (Figure 1)[14]. The two proteins exist in multiple isoforms, the most abundant of which is the S100A8/S100A9 heterodimer (“calprotectin”)[22,23]. Calprotectin constitutes 60% of cytosolic protein in neutrophils and the influx of these neutrophils into the GI mucosa during inflammation is therefore proportional to the amount of measured faecal calprotectin[24,25]. Furthermore, calprotectin has not only shown resistance to degradation in faeces and stability at room temperature, but has also been reported to correlate well with 111Indium-labelled granulocyte scintigraphy[24,26]. It is these favourable characteristic prerequisites for the validity of a faecal biomarker that have witnessed the emergence of calprotectin as one of the most studied faecal biomarkers for intestinal inflammation[27].
Elevated faecal calprotectin levels have been reported in multiple organic GI diseases when compared with functional GI diseases (Table 1). In a large-scale study, Tibble et al[28] determined that at a cutoff value of 10 mg/L, faecal calprotectin had a sensitivity of 89% and a specificity of 79% for detecting organic disease, which performed better than the respective values for a positive Rome I criteria diagnosis (85% and 71% respectively). Following this, Costa et al[29] discussed the value of setting a cutoff point determined by the collective results of complete GI investigations on all patients with chronic abdominal pain and diarrhoea. For example, by using a cutoff of 60 μg/g they were able to produce their optimal diagnostic accuracy, with a sensitivity of 81% and a specificity of 88%[29]. In another study, for patients presenting with lower GI symptoms, D’Incà et al[30] reported a sensitivity, specificity and diagnostic accuracy of 78%, 83% and 80% respectively for diagnosing inflammatory disease, irrespective of diagnosis. Similar results have also been obtained in the paediatric population[31-33]. Carroccio et al[32] reported specificities which were in line with previous studies, but the sensitivities were far lower. This was attributed to a combination of a higher potential number of referrals for possible coeliac patients (due to their hospital being a tertiary centre for food intolerance), and the reported high frequency of negative calprotectin results for patients with coeliac disease. Furthermore, they highlighted the association between false-positive results for faecal calprotectin and both nonsteroidal anti-inflammatory drug use and liver cirrhosis, believed to be due to the mucosal abnormalities associated with each[34].
Table 1.
Studies investigating faecal markers in the differentiation of inflammatory bowel disease or healthy controls vs irritable bowel syndrome
| Study | Marker | Cutoff value | Se | Sp | PPV | NPV | Subjects (n) | CD (n) | UC (n) | IBS (n) | HC (n) | Other (n) | Other diagnosis (n) | Verification |
| Kaiser et al[35] 2007 | S100A12CP | > 0.8 mg/kg> 50 mg/kg | 86% 63% | 96% 86% | 98% 90% | 76% 51% | 195 | 32 | 27 | 24 | 24 | 88 | Bacterial (65) and viral (23) enteritis | Endoscopy/histology; immunohistochemistry |
| Sidler et al[42] 2008 | S100A12CP | > 10 mg/kg> 50 mg/kg | 97%100% | 97% 67% | 97% 75% | 97%100% | 61 | 30 | 1 | 14 | 0 | 16 | Reflux esophagitis (6), juvenile polyp (2); eosinophilic GI disorder (3), others (5) | Endoscopy/histology |
| Tibble et al[26] 2000 | CP | > 30 mg/L | 100% | 97% | - | - | 276 | 31 | 0 | 159 | 56 | 30 | Microscopic colitis (6), polyps (3), CRC (2), diverticulosis (19) | Radiology and/or colonoscopy |
| Tibble et al[28] 2002 | CP | > 10 mg/L | 89% | 79% | 76% | 89% | 602 | 102 | 87 | 339 | 0 | 74 | Coeliac disease (12), diarrhea (14), CRC (7), colitis (6), small bowel enteropathy (21), diverticulosis (14) | Radiology and/or colonoscopy |
| Carroccio et al[32] 2003 | CP | > 50 μg/g> 100 μg/g | 66% 46% | 84% 93% | 83% 90% | 68% 59% | 158 | 18 | 0 | 55 | 20 | 65 | Cow’s milk/food intolerance (22), coeliac disease (23), CRC/ polyps (3), diverticulosis (4), colitis (2), CD (9), giardiasis (2) | Endoscopy/histology (in selected patients only) |
| Fagerberg et al[33] 2006 | CP | > 50 μg/g | 95% | 93% | 95% | 93% | 36 | 10 | 7 | 5 | 0 | 14 | Indeterminate colitis/IBD (3), polyps (1), proctitis (1), food intolerance (4), others (5) | Endoscopy/histology |
| Sydora et al[47] 2012 | CP | > 150 μg/g(desk top device) | 56%-100% | 100% | - | - | 42 | 7 | 9 | 7 | 19 | 0 | - | Mayo clinic or harvey bradshaw index |
| Dolwani et al[91] 2004 | CP | > 60 μg/g | 100% | 79% | 60% | 100% | 138 | 25 | 0 | 24 | 26 | 63 | Symptoms of diarrhea and/or abdominal pain (63) | Barium follow through |
| Costa et al[29] 2003 | CP | > 50 μg/g | 83% | 82% | 90% | 71% | 239 | 49 | 82 | 48 | 34 | 26 | Intestinal neoplasms (26) | Colonoscopy and/or radiology |
| Canani et al[31] 2006 | CP | > 95 μg/g | 93% | 89% | 93% | 89% | 45 | 17 | 10 | 8 | 0 | 10 | Food allergy (5), infectious enterocolitis (4), familial Mediterranean fever (1) | Endoscopy, histology and radiology |
| Summerton et al[40] 2002 | CP | > 50 mg/kg | 82% | 73% | - | - | 134 | 4 | 10 | 7 | 28 | 85 | CRC (8), upper-GI lesions (44), diverticulosis (15), polyps (12), colon adenoma (6) | Upper or lower endoscopy |
| Dai et al[92] 2007 | LF | > 24 μg/g | 100% | 100% | - | - | 177 | 18 | 59 | 25 | 34 | 41 | Bacteria infectious bowel disease (41) | Colonoscopy |
| Walker et al[70] 2007 | LF | > 7.25 μg/mL | 84% | 97% | 99% | 55% | 170 | 79 | 62 | 7 | 22 | 0 | - | Endoscopy and/or radiology (in selected patients only) |
| Kane et al[68] 2003 | LF | > 4 μg/g | 86% | 100% | 100% | 87% | 271 | 104 | 80 | 31 | 56 | 0 | - | Clinical, radiographic, endoscopic, and histological criteria, as appropriate |
| Sidhu et al[71] 2010 | LF | > 7.25 µg/g | 67% | 96% | 87% | 87% | 465 | 104 | 126 | 137 | 98 | 0 | - | Colonoscopy (in selected patients only), questionnaires |
| Schoepfer et al[25] 2008 | CPLF | > 50 μg/mL> 7 μg/mL | 83% 87% | 100% 96% | 100% 98% | 74% 77% | 136 | 36 | 28 | 30 | 42 | 0 | - | Endoscopy/histology |
| D’Inca et al[30] 2007 | CPLF | > 50 mg/kg> 0.04 OD | 78% 80% | 83% 85% | 86% 87% | - | 144 | 31 | 46 | 20 | 0 | 47 | CRC (8), polyps (26), diverticulosis (11), CD (2) | Colonoscopy/histology |
| Otten et al[41] 2008 | CPCP FRTCP FRTLFLF FRT | > 50 mg/kg> 15 mg/kg> 60 mg/kg> 7.25 mg/mL> 128 ng/mL | 96%100% 61% 78% 78% | 87% 95% 98% 90% 99% | 65% 82% 88% 67% 95% | 99%100% 91% 94% 95% | 114 | 6 | 5 | 91 | 0 | 12 | Unspecified colitis/IBD (12) | Colonoscopy/sigmoidoscopy |
| Schröder et al[38] 2007 | CPLFPMNE | > 24 μg/g> 8.9 μg/g> 19 ng/g | 93% 82% 84% | 100%100% 87% | 100%100% 91% | 91% 80% 79% | 76 | 25 | 20 | 31 | 0 | 0 | - | Endoscopy/histology; clinical disease activity indices |
| Langhorst et al[36] 2008 | CPLFPMNE | > 48 μg/mL> 7.05 μg/mL> 0.062 μg/mL | 82% 87% 77% | 84% 77% 77% | - | - | 139 | 43 | 42 | 54 | 0 | 0 | - | Clinical disease activity indices; endoscopy |
| Langhorst et al[37] 2009 | HBD2 | MedianUC: 107 μg/gIBS: 76 μg/gHC: 30 μg/g | - | - | - | - | 100 | 0 | 30 | 46 | 24 | 0 | - | Endoscopy/histology; immunohistochemistry; faecal CP and LF |
| Ohman et al[87] 2012 | CgBSgII | < 0.48 nmol/g> 0.16 nmol/g | 78%80% | 69% 79% | - | - | 111 | 0 | 0 | 82 | 29 | 0 | - | Faecal CP; rectal sensitivity; colon transit time; questionnaires |
| Annaházi et al[86] 2013 | MMP-9 | > 0.245 ng/mL | 85% | 100% | - | - | 94 | 0 | 47 | 23 | 24 | 0 | - | Clinical and endoscopic Mayo score; faecal CP |
| Silberer et al[39] 2005 | CPLFPMNE | > 18.6 μg/g> 6.64 μg/g> 124 ng/g | 62%33%80% | 95% 95% 95% | - | - | 119 | 21 | 18 | 40 | 40 | 0 | - | Endoscopy/histology |
| Jeffery et al[82] 2009 | M2PKCP | > 4 U/mL> 50 μg/g | 67%93% | 88% 92% | 47%62% | 94%99% | 199 | 9 | 1 | 91 | 94 | 4 | Collagenous colitis (1), CRC (1), stricture (1), coeliac disease (1) | Colonoscopy (n = 87) or radiology (n = 4) |
| Chung-Faye et al[81] 2007 | M2PKCP | > 3.7 U/mL> 25 μg/g | 73%80% | 74% 74% | 89%87% | 57%65% | 131 | 31 | 50 | 43 | 0 | 7 | CRC (7) | Endoscopy/histology |
Se: Sensitivity; Sp: Specificity; PPV: Positive predictive value; NPV: Negative predictive value; CD: Crohn’s disease; UC: Ulcerative colitis; IBS: Irritable bowel syndrome; HC: Healthy control; CP: Calprotectin; LF: Lactoferrin; PMNE: Polymorphonuclear elastase; MMP: Matrix-metalloprotease; HBD: Human β-defensin; Cg: Chromogranin; Sg: Secretogranin; MPO: Myeloperoxidase; M2PK: M2-pyruvate kinase; IBD: Inflammatory bowel disease; GI: Gastrointestinal; CRC: Colorectal cancer; FRT: Faecal rapid test.
In efforts to highlight the potential of faecal calprotectin to distinguish between IBD and IBS specifically, a number of further studies have been performed[26,32,35-40] (Table 1). Langhorst et al[37] confirmed that faecal calprotectin was significantly raised (104 μg/g) in patients with active ulcerative colitis (UC) compared to faecal levels in patients with IBS (19 μg/g). In a slightly smaller prospective study, Schröder et al[38] reported that faecal calprotectin had a sensitivity of 93% and a specificity of 100% when differentiating IBD from IBS (cutoff 24.3 μg/g), though the diagnostic accuracy of calprotectin was not statistically significant superior to that of faecal lactoferrin or polymorphonuclear (PMN) elastase. The distinctly high diagnostic values found in this study were potentially due to a selection bias (their hospital represents a referral centre for IBD), which was supported by the exceptionally high number of patients suffering from IBD compared to IBS[38]. The comparative diagnostic accuracies between faecal calprotectin and other faecal markers have also been studied extensively[25,30,35,36,38,39,41,42]. Silberer et al[39] have reported a high and similar diagnostic accuracy of faecal calprotectin and PMN elastase for the differentiation of chronic IBD and IBS, which was superior to that of other leukocyte proteins in the faeces including lactoferrin and myeloperoxidase (MPO). In another such study, Schröder et al[38] reported that any combination of calprotectin, lactoferrin and PMN elastase did not improve their diagnostic accuracy in distinguishing between IBD and IBS, a result supported by other studies[36,38,41].
Correlation of faecal calprotectin with endoscopically and histologically assessed disease has always been the “gold standard” to ascertain its true prognostic value. Schoepfer et al[25] were able to demonstrate good correlation of faecal calprotectin with endoscopically assessed severity of disease in both Crohn's disease (CD) and UC. These findings were confirmed by a recent study reporting a significant correlation of faecal calprotectin levels and endoscopic disease activity in 126 included patients with IBD and 32 patients with IBS[43]. Following from this, the role of faecal calprotectin in being able to distinguish between IBD patients in remission with or without IBS symptoms has been investigated. Faecal calprotectin tends to be increased in subgroups of IBS-positive patients with IBD in remission, regardless of diagnosis[44,45]. Keohane et al[45] reported that both CD and UC patients with IBS-like symptoms had significantly higher faecal calprotectin levels than those without, most likely indicating the presence of ongoing subclinical inflammation rather than coexisting functional disease. Berrill et al[46] have reported that there is no statistical difference between the faecal calprotectin levels of patients with IBD in clinical remission with IBS-type symptoms compared with those without. While faecal calprotectin may be useful as a noninvasive marker to distinguish patients with IBD in need of intensified follow-up, the utility of faecal calprotectin as an aid to discriminate between inflammatory and functional symptoms in IBD patients remains uncertain.
There have also been some interesting results of other faecal calprotectin analysis techniques. Otten et al[41] reported that faecal rapid testing of calprotectin had an associated sensitivity and specificity of 100% and 95% respectively, at a cutoff value of 15 mg/kg. Interestingly, these results outperformed those of the standard enzyme-linked immunosorbent assay (ELISA) faecal calprotectin test (Table 1)[41]. Similarly, Sydora et al[47] found that “desk top” faecal analysis devices reported sensitivities of 56%-100% and specificities of 100% when differentiating between IBD and IBS (cutoff 150 µg/g). However, these data were generated from a very small cohort (Table 1), and though showing promise should nonetheless be treated with caution at present. In addition, it was recently reported that different faecal calprotectin ELISA kits show a between-assay variability[48].
Since many disorders present with symptoms similar to IBS, it is important to exclude other causes like IBD. Overall, calprotectin is the most widely studied faecal marker for the differentiation between IBD and IBS and a sensitive and specific marker of inflammatory activity in the gut (Table 2, Figure 2). Because of its high diagnostic accuracy in ruling out intestinal inflammation, many clinicians use faecal calprotectin as a noninvasive screen for IBD in their patients with IBS symptoms[49].
Table 2.
Overall diagnostic accuracy of faecal markers in the differentiation of inflammatory bowel disease vs irritable bowel syndrome in relation to the size of study cohorts
| Se | n | Sp | n | PPV | n | NPV | n | Ref. | |
| Calprotectin | 85% | 2984 | 85% | 2984 | 81% | 2274 | 82% | 2130 | [25,26,28-33,35,36,38-42,47,81,82,91] |
| S100A12 | 89% | 256 | 96% | 256 | 98% | 256 | 81% | 256 | [35,42] |
| Laktoferrin | 78% | 1811 | 94% | 1811 | 91% | 1376 | 82% | 1232 | [25,30,36,38,39,41,68,70,71,92] |
| M2-PK | 69% | 330 | 82% | 330 | 64% | 330 | 79% | 330 | [81,82] |
Se: Sensitivity; Sp: Specificity; PPV: Positive predictive value; NPV: Negative predictive value; n: Number of study subjects; M2PK: M2-pyruvate kinase.
Figure 2.
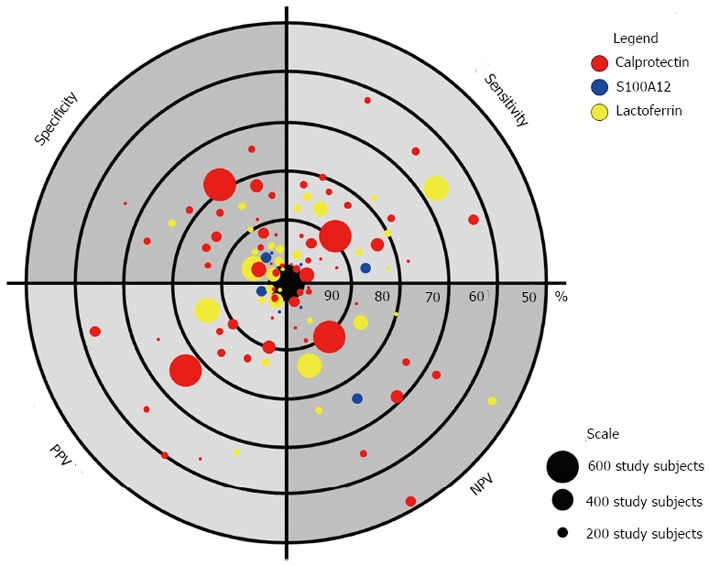
Diagnostic accuracy of faecal markers in the differentiation of organic gastrointestinal disease vs irritable bowel syndrome. The figure illustrates statistical measures of the diagnostic performance of different studies on the role of faecal markers in the diagnosis of irritable bowel syndrome. Sensitivities, specificities, positive predictive values (PPV), and negative predictive values (NPV) of different biomarker studies are represented with highest values close to the center of the “dartboard” (i.e., 100%). Each dot represents a biomarker study and different colors represent the type of the faecal marker (see legend). The size of each dot represents the number of included study subjects (see scale).
S100A12
S100A12, also known as calgranulin C or EN-RAGE (extra cellular newly identified receptor for advanced glycation end-products), is another member of the S100 calcium-binding protein family. In contrast to calprotectin, S100A12 is expressed almost exclusively by neutrophils (Figure 1) and does not form heterodimers with either S100A8, S100A9 or associate with the heterodimer S100A8/S100A9[50]. S100A12 was reported to function as a pro-inflammatory molecule, the binding of which to RAGE on endothelial cells, mononuclear phagocytes, and lymphocytes leads to upregulation of pro-inflammatory cytokines[51]. More recently, it was shown that S100A12 is a ligand of Toll-like receptor 4, amplifying monocyte activation and thus contributing to organ-specific as well as systemic inflammation[52]. Not surprisingly, S100A12 has been implicated in multiple inflammatory disorders[53-55]. S100A12 is strongly upregulated during chronic active IBD[21] and its release from intestinal mucosal specimens correlates to the intestinal inflammation status[56].
More recently, the value of S100A12 as a faecal biomarker of inflammatory conditions within the bowel has been investigated[42,57-62]. de Jong et al[62] showed that S100A12 was equally distributed in faeces, as well as being temperature stable for up to 7 d. Furthermore, in their study of 48 children, they reported that faecal S100A12 had a sensitivity of 96% and a specificity of 92% (cutoff 10 mg/kg) when distinguishing between healthy controls and the IBD group (mainly CD)[62]. In the wake of these findings we assessed the correlation between faecal S100A12 levels with endoscopic and histological findings in patients with IBD and IBS[35]. We demonstrated a sensitivity of 86% and a specificity of 96% (cutoff 0.8 mg/kg) when differentiating active IBD from IBS. Our study also showed a strong correlation between faecal S100A12 levels and endoscopically and histologically confirmed intestinal inflammation in both CD and UC. Our head-to-head comparison of faecal S100A12 and faecal calprotectin showed that faecal S100A12 was superior in distinguishing active IBD from IBS[35]. Similarly, in a prospective study of a paediatric population presenting with GI symptoms, Sidler et al[42] investigated the utility of faecal S100A12 compared to faecal calprotectin as a marker for intestinal inflammation. Children diagnosed with IBD (n = 31) had elevated faecal S100A12 (median 55.2 mg/kg) and faecal calprotectin (median 1265 mg/kg) levels when compared to 30 children without IBD (median S100A12 1.1 mg/kg; median calprotectin 30.5 mg/kg). The sensitivity and specificity of faecal S100A12 for the diagnosis of IBD (cutoff 10 mg/kg) were both 97%, whereas faecal calprotectin had a sensitivity of 100% and a specificity of only 67% (Table 1).
Though more recent studies into the role of S100A12 for diagnosis, prediction of outcomes and monitoring of disease responses for other GI diseases (including necrotizing enterocolitis and CRC) have been undertaken[58,59,63], further prospective studies into the role of S100A12 in distinguishing organic from functional disease are required to consolidate promising initial data (Table 2, Figure 2).
LACTOFERRIN
Lactoferrin is a multifunctional iron binding glycoprotein that is found in the secretions of most mucosal surfaces including tears, saliva, human breast milk, synovial fluid and serum[64]. Lactoferrin has been shown to exert bacteriocidal activity and is a major component of secondary granules released during the degranulation of polymorphonuclear neutrophils in response to inflammation[65,66]. In the intestinal lumen, the presence of inflammation triggers polymorphonuclear neutrophils to infiltrate the intestinal mucosa, causing a proportional increase of faecal lactoferrin levels (Figure 1)[67]. Lactoferrin demonstrates reasonable stability in faeces; it is unaffected by multiple freeze-thaw cycles, though it has been reported that after 48 h at room temperature, stool concentrations of lactoferrin declined slightly to 90% of their original levels[13,39,68].
Several studies have attempted to elucidate the utility of lactoferrin as a marker for intestinal inflammation, with variable outcomes[69]. Results were more variable when assessing the capabilities for lactoferrin as a distinguishing marker between IBS and IBD (Table 1). Compared to other proteins stored in neutrophilic granules such as PMN elastase, MPO, and human neutrophil lipocalin, Sugi et al[13] reported that lactoferrin was a superior faecal marker of neutrophil-derived intestinal inflammation. D’Incà et al[30] were able to quantify that, in colonoscopy referrals for lower GI symptoms, results of faecal lactoferrin assays yielded an overall sensitivity, specificity, positive predictive value (PPV), and diagnostic accuracy of 80%, 85%, 87% and 81% respectively in identifying intestinal inflammation. Similarly, Walker et al[70] reported that all of their included patients with IBS (n = 7) had normal levels of faecal lactoferrin (cutoff 7.25 μg/mL) and that the sensitivity, specificity, PPV and negative predictive value (NPV) for distinguishing individuals with IBD from those without IBD, were 84%, 97%, 99% and 55% respectively. Furthermore, in a recent meta-analysis, Gisbert et al[69] calculated the mean sensitivities and specificities of faecal lactoferrin in the diagnosis of IBD to be 80% and 82% respectively. Silberer et al[39] found that calprotectin and PMN elastase, but not lactoferrin, correlated with the severity of inflammation determined by ileocolonoscopy and were able to differentiate chronic IBD from IBS. When comparing receiver operating characteristic (ROC) curves calculated for healthy controls and patients with IBD, the areas under the curve (AUCs) for PMN elastase and calprotectin were 0.916 and 0.872 respectively, whilst that for lactoferrin was 0.693[39]. On the other hand, our recent review of studies on faecal markers of intestinal inflammation revealed that the diagnostic accuracy of faecal lactoferrin in the differentiation of IBD vs IBS had sensitivities and specificities between 56%-100% and 61%-100% respectively, with PPVs and NPVs of 59%-100% and 78%-99% respectively[14] (Table 1). In a more recent study, Sidhu et al[71] were further able to demonstrate that patients with inactive IBD had significantly higher median faecal lactoferrin levels than those with IBS. Of particular interest were the results of Otten et al[41] showing that new faecal rapid testing techniques for evaluating faecal lactoferrin in the primary care setting were at least comparable to the more standard ELISA tests when testing 114 patients referred for lower GI endoscopy for investigation of abdominal complaints (bloating, change in defecation frequency or consistency, or blood and mucus in the faeces) (Table 1).
Considering these positive results, the main disadvantages of faecal lactoferrin stem from its non-specificity to any particular organic disease and by the fact that it is not solely expressed by degranulated neutrophils. Lactoferrin is secreted endogenously by several mucosal epithelial cell types and can therefore act as a non-inflammatory induced source of faecal lactoferrin[72]. Furthermore, it has been reported that the use of non-steroidal anti-inflammatory drugs may increase the amount of lactoferrin detected in faeces, probably due to an associated induced enteropathy[32,73,74].
Similarly to S100 proteins, it should be emphasized that lactoferrin itself is not a marker of any specific organic disease, but rather of neutrophilic intestinal inflammation[75]. A negative faecal lactoferrin test, therefore, should only be seen as the absence of significant neutrophilic intestinal inflammation. It has consequently been proposed that faecal lactoferrin may have a role in excluding underlying inflammatory conditions thus removing the need for colonoscopy in patients presenting undifferentiated diarrhoea with no alarm symptoms[76]. In studies designed to compare IBD patients with healthy controls or IBS, direct comparison of calprotectin and lactoferrin revealed comparable levels of diagnostic accuracy (Tables 1 and 2)[25,30,36,38,39,41]. These conclusions support the notion that although lactoferrin may be of limited use in the direct classification or diagnosis of organic disease, it may yet have utility in IBD diagnosis.
M2-PYRUVATE KINASE
The glycolytic enzyme M2-pyruvate kinase (M2-PK) is a multifunctional protein, involved in several nonglycolytic pathways influencing cellular physiology including immunological responses, cellular growth and apoptosis[77]. The dimeric isoform of M2-PK (tumor M2-PK) is present in undifferentiated and proliferating tissues and M2-PK is upregulated in a range of GI malignancy[78]. The determination of M2-PK in stool samples was proposed as a new promising screening tool for CRC[79]. The usefulness of faecal M2-PK for the detection of intestinal inflammation was also studied in patients with IBD since these patients have increased cell turnover in the GI tract. The PK stool test requires a single, small and random faecal sample whilst the enzyme is stable for two days at room temperature[80]. Czub et al[80] have reported that faecal M2-PK could potentially be a useful marker for IBD activity with a better correlation for UC patients. Likewise, Turner et al[61] showed that faecal M2-PK reflects severity of paediatric UC by having very high faecal values. Furthermore, the authors demonstrated that faecal M2-PK has, in contrast to other faecal biomarkers (calprotectin, lactoferrin, S100A12), the best ability to predict steroid refractoriness in severe paediatric UC, but is still inferior to a clinical disease activity index[61]. Importantly, it has also been shown that faecal M2-PK is able to differentiate between patients with IBD or IBS (cutoff 3.7 U/mL) and that M2-PK and faecal calprotectin are highly significantly correlated[81]. In this study 67% of included patients (n = 88) had organic GI disease and faecal M2-PK had a sensitivity of 73%, specificity of 74%, PPV of 89%, and a NPV of 57% for IBD and CRC. These results were comparable to the diagnostic accuracy of faecal calprotectin (cutoff 25 μg/g) in the same patients with a sensitivity of 80%, specificity of 74%, PPV of 87%, and a NPV of 65% (Table 1). Jeffery et al[82] showed that, in a setting of a low prevalence or organic bowel disease, faecal M2-PK is able to differentiate organic disease from functional bowel disease (cutoff 4 U/mL) with a sensitivity of 67%, specificity of 88%, PPV of 47% and a NPV of 94%. In this study the incidence of functional bowel disorder was much higher (87% of included patients; n = 91) than in the aforementioned study (33% of included patients; n = 43) and the results showed that M2-PK does not perform as well as calprotectin (cutoff 50 µg/g; sensitivity 93%; specificity 92%, PPV 62%, NPV 99%) (Table 1)[82]. The authors concluded that use of calprotectin and M2-PK may be particularly advantageous as a rule-out test in clinical populations with a similar disease prevalence.
POLYMORPHONUCLEAR NEUTROPHIL ELASTASE
PMN elastase is a neutral serinproteinase, which is released from leucocyte granules as a mediator of inflammation by activation of neutrophils. Elastase is stable for four days in faeces at room temperature[39]. Silberer et al[39] showed that faecal PMN elastase levels in patients with IBS (n = 40) were in the range of healthy persons (n = 40). Faecal PMN elastase and calprotectin correlated with endoscopically classified severity of intestinal inflammation and yielded similar AUCs when ROC curves were calculated for healthy persons and patients with IBD (n = 39). The authors concluded that faecal PMN elastase and calprotectin are able to differentiate between chronic IBD and IBS. Similarly, Langhorst et al[36] showed that faecal PMN elastase, calprotectin and lactoferrin differentiate IBD and IBS. Patients with IBS (n = 54) demonstrated significantly lower levels of PMN elastase in stools when compared to patients with endoscopically active IBD (n = 60) and, interestingly, when compared with endoscopically inactive IBD (n = 25). The specificity and overall diagnostic accuracy of PMN elastase in patients with IBS were each 82% and slightly lower than for faecal lactoferrin (83%), faecal calprotectin (87%), and serum CRP (91%). Schröder et al[38] prospectively evaluated the diagnostic accuracy of faecal PMN elastase alone (cutoff 62 ng/g) and in combination with faecal calprotectin (cutoff 15 μg/g) and/or lactoferrin (cutoff 7.3 μg/g) to detect intestinal inflammation in patients with IBD (n = 45) and IBS (n = 31)[38]. The sensitivity, specificity, PPV, and NPV of faecal PMN elastase in distinguishing between IBD and IBS was 84%, 87%, 91% and 79%, respectively, and increased to 96%, 100%, 100% and 94%, respectively, when combined with faecal calprotectin ± lactoferrin. The odds ratio for having intestinal inflammation with an elevated faecal PMN elastase was 37 (95%CI: 12-116). However, the results of the study indicate an advantage of calprotectin over lactoferrin and PMN elastase in the detection of intestinal inflammation.
HUMAN β-DEFENSIN-2
Defensins belong to the class of protective antimicrobial peptides and play an important role in the host innate defense at the mucosal surface of the GI tract (Figure 1). Human β defensins (HBD) are expressed in the colon by epithelial cells and plasma cells. HBD-2 plays a crucial role in determining innate immune responses to bacteria in the gut. Cumulating evidence suggests a special role for HBD-2 as a marker for intestinal inflammation in IBD[83]. Interestingly, Langhorst et al[84] reported that elevated faecal levels of HBD-2 indicate an activation of innate immunity not only in IBD but also in IBS[37,84]. Faecal HBD-2 levels of patients with IBS (n = 46) were significantly elevated compared with health controls (n = 24) and similar to those in patients with active UC (n = 30), whereas faecal levels of calprotectin and lactoferrin did not differ between healthy controls and patients with IBS. These findings suggest a pro-inflammatory activation of the mucosal innate immune system in patients with IBS in the absence of endoscopic or histologic signs of inflammation. These results support the idea that IBS could be a (low-grade) inflammatory disorder though the functional significance remains to be established.
MYELOPEROXIDASE
MPO is another lysosomal protein that is released from granules of neutrophil granulocytes during inflammation (Figure 1). MPO produces oxygen radicals during the neutrophil’s respiratory burst, which are important in the killing of bacteria. MPO is stable for at least four days in feces at room temperature[39]. To date, MPO has shown to be of only limited utility as an inflammatory marker for IBD[85]. Thus, the use of MPO in the differentiation between IBS and IBD has not been widely studied (Table 1). In addition, Silberer et al[39] found that MPO separated healthy controls (n = 40) and patients with IBS (n = 40) from patients with chronic IBD (n = 39) less effectively than PMN elastase or calprotectin.
MATRIX-METALLOPROTEASE 9
MMPs are a family of zinc-dependent endopeptidases capable of degradation of extracellular matrix proteins. MMPs are secreted by various cell types including tumor cells and several immune cell types. MMP-9 is released from neutrophils and elevated in colonic biopsies, urine, and blood plasma of patients with UC[86]. Annaházi et al[86] compared faecal MMP-9 levels in patients with UC (n = 47) with those of patients with diarrhea predominant IBS-D (n = 23) and healthy controls (n = 24). Healthy controls and patients with IBS-D showed very low faecal MMP-9 levels compared with faecal levels of patients with UC. The sensitivity and specificity of faecal MMP-9 in distinguishing between UC and IBS-D was 85% and 100%, respectively (cutoff 0.245 ng/mL). Faecal MMP-9 levels correlated significantly with faecal calprotectin levels. The authors suggested that faecal MMP-9 could be a novel marker to help in the differential diagnosis of patients with diarrhea and abdominal pain. However, this is the first published study on the diagnostic role of faecal MMP-9 in IBD and IBS and further studies are needed to confirm these findings.
GRANINS
Granins are proteins expressed by cells of the enteric, endocrine, and immune system, and may broadly reflect activity of these systems. Chromogranins (Cg) and secretogranins (Sg) are precursors of several bioactive peptides and regulate a number of cellular functions. Öhman et al[87] assessed the association between faecal levels of Cg and Sg with IBS. The results showed that, compared to healthy controls (n = 29), IBS patients (n = 82) demonstrated higher levels of CgA, SgII, and SgIII, but lower levels of CgB. Thus, faecal levels of SgII, SgIII and CgB may be used to discriminate between IBS patients and healthy individuals. However, there was no disease control group included in this study, which therefore precludes the proper evaluation of faecal granins as diagnostic biomarkers. Faecal granins are however unlikely to be specific IBS markers since other diseases (e.g., coeliac disease) also manifest increased Cgs[88]. Furthermore, faecal calprotectin levels were not associated with the faecal concentrations of granins. Finally, the study design cannot differentiate whether the increased faecal levels of granins cause IBS or its symptoms, or merely reflect the phenotype of IBS. Elevation of faecal granins may serve as a marker for guiding medical treatment of IBS. However, the lack of specificity of faecal granins does not support the use of these proteins as positive biomarkers for IBS.
CONCLUSION
Extensive diagnostic tests in the evaluation of patients with typical symptoms of IBS and the absence of alarm features are not necessary[89]. A positive diagnostic strategy based on symptom-based criteria and simple blood tests is not inferior to a strategy of exclusion of organic disease with multiple unnecessary, expensive, and potentially harmful diagnostic tests and procedures[90]. Faecal surrogate markers of intestinal inflammation represent a practicable, inexpensive and objective diagnostic tool to differentiate organic and functional GI diseases. Neutrophil-derived faecal biomarkers show a high diagnostic accuracy in the differentiation of IBD vs IBS (Table 2) and could be useful in reducing unnecessary invasive investigations. Thus, these markers can provide reassurance to physicians that their clinical diagnosis of IBS is correct. Further studies are required to more comprehensively define and compare the role of these faecal proteins in the diagnosis and pathogenesis IBS. Nonetheless, faecal biomarkers have the potential to be incorporated into standard clinical practice for the routine assessment of IBS and IBD.
ACKNOWLEDGMENTS
The authors thank Dr. Trevelyan Menheniott for carefully reading the manuscript.
Footnotes
Supported by A research fellowship awarded to Däbritz J by the German Research Foundation, No. DFG DA1161/5-1
P- Reviewers: Koloski N, Montalto M, Wildt S S- Editor: Cui XM L- Editor: A E- Editor: Wu HL
References
- 1.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 2.El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18:5151–5163. doi: 10.3748/wjg.v18.i37.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford AC, Talley NJ. Irritable bowel syndrome. BMJ. 2012;345:e5836. doi: 10.1136/bmj.e5836. [DOI] [PubMed] [Google Scholar]
- 4.Chang JY, Talley NJ. An update on irritable bowel syndrome: from diagnosis to emerging therapies. Curr Opin Gastroenterol. 2011;27:72–78. doi: 10.1097/MOG.0b013e3283414065. [DOI] [PubMed] [Google Scholar]
- 5.Agréus L, Svärdsudd K, Nyrén O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109:671–680. doi: 10.1016/0016-5085(95)90373-9. [DOI] [PubMed] [Google Scholar]
- 6.Drossman DA, Morris CB, Hu Y, Toner BB, Diamant N, Leserman J, Shetzline M, Dalton C, Bangdiwala SI. A prospective assessment of bowel habit in irritable bowel syndrome in women: defining an alternator. Gastroenterology. 2005;128:580–589. doi: 10.1053/j.gastro.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1–35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 8.Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2:653–654. doi: 10.1136/bmj.2.6138.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 10.American Gastroenterology Association. American Gastroenterological Association medical position statement: irritable bowel syndrome. Gastroenterology. 2002;123:2105–2107. doi: 10.1053/gast.2002.37095b. [DOI] [PubMed] [Google Scholar]
- 11.Whitehead WE, Palsson OS, Feld AD, Levy RL, VON Korff M, Turner MJ, Drossman DA. Utility of red flag symptom exclusions in the diagnosis of irritable bowel syndrome. Aliment Pharmacol Ther. 2006;24:137–146. doi: 10.1111/j.1365-2036.2006.02956.x. [DOI] [PubMed] [Google Scholar]
- 12.Kristjánsson G, Venge P, Wanders A, Lööf L, Hällgren R. Clinical and subclinical intestinal inflammation assessed by the mucosal patch technique: studies of mucosal neutrophil and eosinophil activation in inflammatory bowel diseases and irritable bowel syndrome. Gut. 2004;53:1806–1812. doi: 10.1136/gut.2003.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugi K, Saitoh O, Hirata I, Katsu K. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil-derived proteins. Am J Gastroenterol. 1996;91:927–934. [PubMed] [Google Scholar]
- 14.Foell D, Wittkowski H, Roth J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut. 2009;58:859–868. doi: 10.1136/gut.2008.170019. [DOI] [PubMed] [Google Scholar]
- 15.Peterson CG, Eklund E, Taha Y, Raab Y, Carlson M. A new method for the quantification of neutrophil and eosinophil cationic proteins in feces: establishment of normal levels and clinical application in patients with inflammatory bowel disease. Am J Gastroenterol. 2002;97:1755–1762. doi: 10.1111/j.1572-0241.2002.05837.x. [DOI] [PubMed] [Google Scholar]
- 16.van der Sluys Veer A, Biemond I, Verspaget HW, Lamers CB. Faecal parameters in the assessment of activity in inflammatory bowel disease. Scand J Gastroenterol Suppl. 1999;230:106–110. doi: 10.1080/003655299750025624. [DOI] [PubMed] [Google Scholar]
- 17.de Silva DG, Mendis LN, Sheron N, Alexander GJ, Candy DC, Chart H, Rowe B. Concentrations of interleukin 6 and tumour necrosis factor in serum and stools of children with Shigella dysenteriae 1 infection. Gut. 1993;34:194–198. doi: 10.1136/gut.34.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen A, Hovdenak N, Karlsdottir A, Wentzel-Larsen T, Dahl O, Fagerhol MK. Faecal calprotectin and lactoferrin as markers of acute radiation proctitis: a pilot study of eight stool markers. Scand J Gastroenterol. 2004;39:1113–1118. doi: 10.1080/00365520410003614. [DOI] [PubMed] [Google Scholar]
- 19.Bischoff SC, Mayer J, Nguyen QT, Stolte M, Manns MP. Immunnohistological assessment of intestinal eosinophil activation in patients with eosinophilic gastroenteritis and inflammatory bowel disease. Am J Gastroenterol. 1999;94:3521–3529. doi: 10.1111/j.1572-0241.1999.01641.x. [DOI] [PubMed] [Google Scholar]
- 20.Saitoh O, Kojima K, Sugi K, Matsuse R, Uchida K, Tabata K, Nakagawa K, Kayazawa M, Hirata I, Katsu K. Fecal eosinophil granule-derived proteins reflect disease activity in inflammatory bowel disease. Am J Gastroenterol. 1999;94:3513–3520. doi: 10.1111/j.1572-0241.1999.01640.x. [DOI] [PubMed] [Google Scholar]
- 21.Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, Roth J. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52:847–853. doi: 10.1136/gut.52.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leukert N, Vogl T, Strupat K, Reichelt R, Sorg C, Roth J. Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. J Mol Biol. 2006;359:961–972. doi: 10.1016/j.jmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Pröpper C, Huang X, Roth J, Sorg C, Nacken W. Analysis of the MRP8-MRP14 protein-protein interaction by the two-hybrid system suggests a prominent role of the C-terminal domain of S100 proteins in dimer formation. J Biol Chem. 1999;274:183–188. doi: 10.1074/jbc.274.1.183. [DOI] [PubMed] [Google Scholar]
- 24.Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27:793–798. doi: 10.3109/00365529209011186. [DOI] [PubMed] [Google Scholar]
- 25.Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008;14:32–39. doi: 10.1002/ibd.20275. [DOI] [PubMed] [Google Scholar]
- 26.Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S, Foster R, Sherwood R, Fagerhol M, Bjarnason I. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47:506–513. doi: 10.1136/gut.47.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tibble JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450–460. doi: 10.1053/gast.2002.34755. [DOI] [PubMed] [Google Scholar]
- 29.Costa F, Mumolo MG, Bellini M, Romano MR, Ceccarelli L, Arpe P, Sterpi C, Marchi S, Maltinti G. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis. 2003;35:642–647. doi: 10.1016/s1590-8658(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 30.D’Incà R, Dal Pont E, Di Leo V, Ferronato A, Fries W, Vettorato MG, Martines D, Sturniolo GC. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007;22:429–437. doi: 10.1007/s00384-006-0159-9. [DOI] [PubMed] [Google Scholar]
- 31.Canani RB, de Horatio LT, Terrin G, Romano MT, Miele E, Staiano A, Rapacciuolo L, Polito G, Bisesti V, Manguso F, et al. Combined use of noninvasive tests is useful in the initial diagnostic approach to a child with suspected inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2006;42:9–15. doi: 10.1097/01.mpg.0000187818.76954.9a. [DOI] [PubMed] [Google Scholar]
- 32.Carroccio A, Iacono G, Cottone M, Di Prima L, Cartabellotta F, Cavataio F, Scalici C, Montalto G, Di Fede G, Rini G, et al. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Clin Chem. 2003;49:861–867. doi: 10.1373/49.6.861. [DOI] [PubMed] [Google Scholar]
- 33.Fagerberg UL, Lööf L, Myrdal U, Hansson LO, Finkel Y. Colorectal inflammation is well predicted by fecal calprotectin in children with gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2005;40:450–455. doi: 10.1097/01.mpg.0000154657.08994.94. [DOI] [PubMed] [Google Scholar]
- 34.Bini EJ, Lascarides CE, Micale PL, Weinshel EH. Mucosal abnormalities of the colon in patients with portal hypertension: an endoscopic study. Gastrointest Endosc. 2000;52:511–516. doi: 10.1067/mge.2000.108478. [DOI] [PubMed] [Google Scholar]
- 35.Kaiser T, Langhorst J, Wittkowski H, Becker K, Friedrich AW, Rueffer A, Dobos GJ, Roth J, Foell D. Faecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2007;56:1706–1713. doi: 10.1136/gut.2006.113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 37.Langhorst J, Junge A, Rueffer A, Wehkamp J, Foell D, Michalsen A, Musial F, Dobos GJ. Elevated human beta-defensin-2 levels indicate an activation of the innate immune system in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:404–410. doi: 10.1038/ajg.2008.86. [DOI] [PubMed] [Google Scholar]
- 38.Schröder O, Naumann M, Shastri Y, Povse N, Stein J. Prospective evaluation of faecal neutrophil-derived proteins in identifying intestinal inflammation: combination of parameters does not improve diagnostic accuracy of calprotectin. Aliment Pharmacol Ther. 2007;26:1035–1042. doi: 10.1111/j.1365-2036.2007.03457.x. [DOI] [PubMed] [Google Scholar]
- 39.Silberer H, Küppers B, Mickisch O, Baniewicz W, Drescher M, Traber L, Kempf A, Schmidt-Gayk H. Fecal leukocyte proteins in inflammatory bowel disease and irritable bowel syndrome. Clin Lab. 2005;51:117–126. [PubMed] [Google Scholar]
- 40.Summerton CB, Longlands MG, Wiener K, Shreeve DR. Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol. 2002;14:841–845. doi: 10.1097/00042737-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Otten CM, Kok L, Witteman BJ, Baumgarten R, Kampman E, Moons KG, de Wit NJ. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clin Chem Lab Med. 2008;46:1275–1280. doi: 10.1515/CCLM.2008.246. [DOI] [PubMed] [Google Scholar]
- 42.Sidler MA, Leach ST, Day AS. Fecal S100A12 and fecal calprotectin as noninvasive markers for inflammatory bowel disease in children. Inflamm Bowel Dis. 2008;14:359–366. doi: 10.1002/ibd.20336. [DOI] [PubMed] [Google Scholar]
- 43.D’Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, Geens P, Iwens D, Aerden I, Van Assche G, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. doi: 10.1002/ibd.22917. [DOI] [PubMed] [Google Scholar]
- 44.Jelsness-Jørgensen LP, Bernklev T, Moum B. Calprotectin Is a Useful Tool in Distinguishing Coexisting Irritable Bowel-Like Symptoms from That of Occult Inflammation among Inflammatory Bowel Disease Patients in Remission. Gastroenterol Res Pract. 2013;2013:620707. doi: 10.1155/2013/620707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keohane J, O’Mahony C, O’Mahony L, O’Mahony S, Quigley EM, Shanahan F. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? Am J Gastroenterol. 2010;105:1788, 1789–1794; quiz 1795. doi: 10.1038/ajg.2010.156. [DOI] [PubMed] [Google Scholar]
- 46.Berrill JW, Green JT, Hood K, Campbell AK. Symptoms of irritable bowel syndrome in patients with inflammatory bowel disease: examining the role of sub-clinical inflammation and the impact on clinical assessment of disease activity. Aliment Pharmacol Ther. 2013;38:44–51. doi: 10.1111/apt.12335. [DOI] [PubMed] [Google Scholar]
- 47.Sydora MJ, Sydora BC, Fedorak RN. Validation of a point-of-care desk top device to quantitate fecal calprotectin and distinguish inflammatory bowel disease from irritable bowel syndrome. J Crohns Colitis. 2012;6:207–214. doi: 10.1016/j.crohns.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Whitehead SJ, French J, Brookes MJ, Ford C, Gama R. Between-assay variability of faecal calprotectin enzyme-linked immunosorbent assay kits. Ann Clin Biochem. 2013;50:53–61. doi: 10.1258/acb.2012.011272. [DOI] [PubMed] [Google Scholar]
- 49.Furman DL, Cash BD. The role of diagnostic testing in irritable bowel syndrome. Gastroenterol Clin North Am. 2011;40:105–119. doi: 10.1016/j.gtc.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Vogl T, Pröpper C, Hartmann M, Strey A, Strupat K, van den Bos C, Sorg C, Roth J. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem. 1999;274:25291–25296. doi: 10.1074/jbc.274.36.25291. [DOI] [PubMed] [Google Scholar]
- 51.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 52.Foell D, Wittkowski H, Kessel C, Lüken A, Weinhage T, Varga G, Vogl T, Wirth T, Viemann D, Björk P, et al. Proinflammatory S100A12 can activate human monocytes via Toll-like receptor 4. Am J Respir Crit Care Med. 2013;187:1324–1334. doi: 10.1164/rccm.201209-1602OC. [DOI] [PubMed] [Google Scholar]
- 53.Hofmann MA, Drury S, Hudson BI, Gleason MR, Qu W, Lu Y, Lalla E, Chitnis S, Monteiro J, Stickland MH, et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun. 2002;3:123–135. doi: 10.1038/sj.gene.6363861. [DOI] [PubMed] [Google Scholar]
- 54.Foell D, Kane D, Bresnihan B, Vogl T, Nacken W, Sorg C, Fitzgerald O, Roth J. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology (Oxford) 2003;42:1383–1389. doi: 10.1093/rheumatology/keg385. [DOI] [PubMed] [Google Scholar]
- 55.Foell D, Seeliger S, Vogl T, Koch HG, Maschek H, Harms E, Sorg C, Roth J. Expression of S100A12 (EN-RAGE) in cystic fibrosis. Thorax. 2003;58:613–617. doi: 10.1136/thorax.58.7.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foell D, Wittkowski H, Ren Z, Turton J, Pang G, Daebritz J, Ehrchen J, Heidemann J, Borody T, Roth J, et al. Phagocyte-specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J Pathol. 2008;216:183–192. doi: 10.1002/path.2394. [DOI] [PubMed] [Google Scholar]
- 57.Däbritz J, Langhorst J, Lügering A, Heidemann J, Mohr M, Wittkowski H, Krummenerl T, Foell D. Improving relapse prediction in inflammatory bowel disease by neutrophil-derived S100A12. Inflamm Bowel Dis. 2013;19:1130–1138. doi: 10.1097/MIB.0b013e318280b1cd. [DOI] [PubMed] [Google Scholar]
- 58.Däbritz J, Jenke A, Wirth S, Foell D. Fecal phagocyte-specific S100A12 for diagnosing necrotizing enterocolitis. J Pediatr. 2012;161:1059–1064. doi: 10.1016/j.jpeds.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Däbritz J, Foell D, Wirth S, Jenke A. Fecal S100A12: identifying intestinal distress in very-low-birth-weight infants. J Pediatr Gastroenterol Nutr. 2013;57:204–210. doi: 10.1097/MPG.0b013e3182946eb2. [DOI] [PubMed] [Google Scholar]
- 60.Sipponen T, Haapamäki J, Savilahti E, Alfthan H, Hämäläinen E, Rautiainen H, Koskenpato J, Nuutinen H, Färkkilä M. Fecal calprotectin and S100A12 have low utility in prediction of small bowel Crohn’s disease detected by wireless capsule endoscopy. Scand J Gastroenterol. 2012;47:778–784. doi: 10.3109/00365521.2012.677953. [DOI] [PubMed] [Google Scholar]
- 61.Turner D, Leach ST, Mack D, Uusoue K, McLernon R, Hyams J, Leleiko N, Walters TD, Crandall W, Markowitz J, et al. Faecal calprotectin, lactoferrin, M2-pyruvate kinase and S100A12 in severe ulcerative colitis: a prospective multicentre comparison of predicting outcomes and monitoring response. Gut. 2010;59:1207–1212. doi: 10.1136/gut.2010.211755. [DOI] [PubMed] [Google Scholar]
- 62.de Jong NS, Leach ST, Day AS. Fecal S100A12: a novel noninvasive marker in children with Crohn’s disease. Inflamm Bowel Dis. 2006;12:566–572. doi: 10.1097/01.ibd.0000227626.72271.91. [DOI] [PubMed] [Google Scholar]
- 63.Karl J, Wild N, Tacke M, Andres H, Garczarek U, Rollinger W, Zolg W. Improved diagnosis of colorectal cancer using a combination of fecal occult blood and novel fecal protein markers. Clin Gastroenterol Hepatol. 2008;6:1122–1128. doi: 10.1016/j.cgh.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 64.Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999;37:281–286. doi: 10.1515/CCLM.1999.049. [DOI] [PubMed] [Google Scholar]
- 65.Baynes RD, Bezwoda WR. Lactoferrin and the inflammatory response. Adv Exp Med Biol. 1994;357:133–141. doi: 10.1007/978-1-4615-2548-6_13. [DOI] [PubMed] [Google Scholar]
- 66.Angriman I, Scarpa M, D’Incà R, Basso D, Ruffolo C, Polese L, Sturniolo GC, D’Amico DF, Plebani M. Enzymes in feces: useful markers of chronic inflammatory bowel disease. Clin Chim Acta. 2007;381:63–68. doi: 10.1016/j.cca.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 67.Guerrant RL, Araujo V, Soares E, Kotloff K, Lima AA, Cooper WH, Lee AG. Measurement of fecal lactoferrin as a marker of fecal leukocytes. J Clin Microbiol. 1992;30:1238–1242. doi: 10.1128/jcm.30.5.1238-1242.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kane SV, Sandborn WJ, Rufo PA, Zholudev A, Boone J, Lyerly D, Camilleri M, Hanauer SB. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol. 2003;98:1309–1314. doi: 10.1111/j.1572-0241.2003.07458.x. [DOI] [PubMed] [Google Scholar]
- 69.Gisbert JP, McNicholl AG, Gomollon F. Questions and answers on the role of fecal lactoferrin as a biological marker in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1746–1754. doi: 10.1002/ibd.20920. [DOI] [PubMed] [Google Scholar]
- 70.Walker TR, Land ML, Kartashov A, Saslowsky TM, Lyerly DM, Boone JH, Rufo PA. Fecal lactoferrin is a sensitive and specific marker of disease activity in children and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:414–422. doi: 10.1097/MPG.0b013e3180308d8e. [DOI] [PubMed] [Google Scholar]
- 71.Sidhu R, Wilson P, Wright A, Yau CW, D’Cruz FA, Foye L, Morley S, Lobo AJ, McAlindon ME, Sanders DS. Faecal lactoferrin--a novel test to differentiate between the irritable and inflamed bowel? Aliment Pharmacol Ther. 2010;31:1365–1370. doi: 10.1111/j.1365-2036.2010.04306.x. [DOI] [PubMed] [Google Scholar]
- 72.Levay PF, Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80:252–267. [PubMed] [Google Scholar]
- 73.Tibble JA, Sigthorsson G, Foster R, Scott D, Fagerhol MK, Roseth A, Bjarnason I. High prevalence of NSAID enteropathy as shown by a simple faecal test. Gut. 1999;45:362–366. doi: 10.1136/gut.45.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meling TR, Aabakken L, Røseth A, Osnes M. Faecal calprotectin shedding after short-term treatment with non-steroidal anti-inflammatory drugs. Scand J Gastroenterol. 1996;31:339–344. doi: 10.3109/00365529609006407. [DOI] [PubMed] [Google Scholar]
- 75.Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:524–534. doi: 10.1097/00054725-200606000-00013. [DOI] [PubMed] [Google Scholar]
- 76.Sutherland AD, Gearry RB, Frizelle FA. Review of fecal biomarkers in inflammatory bowel disease. Dis Colon Rectum. 2008;51:1283–1291. doi: 10.1007/s10350-008-9310-8. [DOI] [PubMed] [Google Scholar]
- 77.Gupta V, Bamezai RN. Human pyruvate kinase M2: a multifunctional protein. Protein Sci. 2010;19:2031–2044. doi: 10.1002/pro.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hathurusinghe HR, Goonetilleke KS, Siriwardena AK. Current status of tumor M2 pyruvate kinase (tumor M2-PK) as a biomarker of gastrointestinal malignancy. Ann Surg Oncol. 2007;14:2714–2720. doi: 10.1245/s10434-007-9481-x. [DOI] [PubMed] [Google Scholar]
- 79.Hardt PD, Mazurek S, Toepler M, Schlierbach P, Bretzel RG, Eigenbrodt E, Kloer HU. Faecal tumour M2 pyruvate kinase: a new, sensitive screening tool for colorectal cancer. Br J Cancer. 2004;91:980–984. doi: 10.1038/sj.bjc.6602033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Czub E, Herzig KH, Szaflarska-Popawska A, Kiehne K, Socha P, Woś H, Kamińska B, Błaszczyński M, Cichy W, Bała G, et al. Fecal pyruvate kinase: a potential new marker for intestinal inflammation in children with inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1147–1150. doi: 10.1080/00365520701320513. [DOI] [PubMed] [Google Scholar]
- 81.Chung-Faye G, Hayee B, Maestranzi S, Donaldson N, Forgacs I, Sherwood R. Fecal M2-pyruvate kinase (M2-PK): a novel marker of intestinal inflammation. Inflamm Bowel Dis. 2007;13:1374–1378. doi: 10.1002/ibd.20214. [DOI] [PubMed] [Google Scholar]
- 82.Jeffery J, Lewis SJ, Ayling RM. Fecal dimeric M2-pyruvate kinase (tumor M2-PK) in the differential diagnosis of functional and organic bowel disorders. Inflamm Bowel Dis. 2009;15:1630–1634. doi: 10.1002/ibd.20946. [DOI] [PubMed] [Google Scholar]
- 83.Langhorst J, Choi KE. The role of human defensins in gastrointestinal diseases. Expert Rev Clin Immunol. 2011;7:779–787. doi: 10.1586/eci.11.62. [DOI] [PubMed] [Google Scholar]
- 84.Langhorst J, Wieder A, Michalsen A, Musial F, Dobos GJ, Rueffer A. Activated innate immune system in irritable bowel syndrome? Gut. 2007;56:1325–1326. doi: 10.1136/gut.2007.125005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Judd TA, Day AS, Lemberg DA, Turner D, Leach ST. Update of fecal markers of inflammation in inflammatory bowel disease. J Gastroenterol Hepatol. 2011;26:1493–1499. doi: 10.1111/j.1440-1746.2011.06846.x. [DOI] [PubMed] [Google Scholar]
- 86.Annaházi A, Molnár T, Farkas K, Rosztóczy A, Izbéki F, Gecse K, Inczefi O, Nagy F, Földesi I, Szűcs M, et al. Fecal MMP-9: a new noninvasive differential diagnostic and activity marker in ulcerative colitis. Inflamm Bowel Dis. 2013;19:316–320. doi: 10.1002/ibd.22996. [DOI] [PubMed] [Google Scholar]
- 87.Ohman L, Stridsberg M, Isaksson S, Jerlstad P, Simrén M. Altered levels of fecal chromogranins and secretogranins in IBS: relevance for pathophysiology and symptoms? Am J Gastroenterol. 2012;107:440–447. doi: 10.1038/ajg.2011.458. [DOI] [PubMed] [Google Scholar]
- 88.Camilleri M. Editorial: fecal granins in IBS: cause or indicator of intestinal or colonic irritation? Am J Gastroenterol. 2012;107:448–450. doi: 10.1038/ajg.2011.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cremonini F, Cheng V, Lembo A. Diagnosing irritable bowel syndrome: no more million dollar work-up? Clin Gastroenterol Hepatol. 2013;11:963–964. doi: 10.1016/j.cgh.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 90.Begtrup LM, Engsbro AL, Kjeldsen J, Larsen PV, Schaffalitzky de Muckadell O, Bytzer P, Jarbøl DE. A positive diagnostic strategy is noninferior to a strategy of exclusion for patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:956–62.e1. doi: 10.1016/j.cgh.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 91.Dolwani S, Metzner M, Wassell JJ, Yong A, Hawthorne AB. Diagnostic accuracy of faecal calprotectin estimation in prediction of abnormal small bowel radiology. Aliment Pharmacol Ther. 2004;20:615–621. doi: 10.1111/j.1365-2036.2004.02128.x. [DOI] [PubMed] [Google Scholar]
- 92.Dai J, Liu WZ, Zhao YP, Hu YB, Ge ZZ. Relationship between fecal lactoferrin and inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1440–1444. doi: 10.1080/00365520701427094. [DOI] [PubMed] [Google Scholar]


