Abstract
The hepatitis B virus (HBV) is considered to be a major public health problem worldwide, and a significant number of reports on nosocomial outbreaks of HBV infections have been reported. Prevention of indirect HBV transmission by contaminated objects is only possible through the use of infection-control principles, including the use of chemical biocides, which are proven to render the virus non-infectious. The virucidal activity of biocides against HBV cannot be predicted; therefore, validation of the virucidal action of disinfectants against HBV is essential. However, feasible HBV infectivity assays have not yet been established. Thus, surrogate models have been proposed for testing the efficacy of biocides against HBV. Most of these assays do not correlate with HBV infectivity. Currently, the most promising and feasible assay is the use of the taxonomically related duck hepatitis B virus (DHBV), which belongs to the same Hepadnaviridae virus family. This paper reviews the application of DHBV, which can be propagated in vitro in primary duck embryonic hepatocytes, for the testing of biocides and describes why this model can be used as reliable method to evaluate disinfectants for efficacy against HBV. The susceptibility levels of important biocides, which are often used as ingredients for commercially available disinfectants, are also described.
Keywords: Hepatitis B virus, Surrogate model, Duck hepatitis B virus, Disinfectants, Testing virucidal efficacy
Core tip: There is a need for disinfectants with proven virucidal activity against the hepatitis B virus (HBV). Feasible HBV infectivity assays are not available; therefore, the establishment of surrogate models for HBV infection is of high importance. This paper reviews the application of the most promising and feasible assay, the use of the duck hepatitis B virus, which can be propagated in vitro in primary duck embryonic hepatocytes. The paper also describes how and why this model can be used to evaluate the efficacy of disinfectants against HBV.
WHY EVALUATE BIOCIDES FOR THEIR EFFICACY AGAINST HEPATITIS B VIRUS?
Approximately 350 million people, 5% of the total population, are chronically infected with the hepatitis B virus (HBV)[1]. Thus, the hepatitis B is considered to be a major public health problem worldwide. Furthermore, nosocomial infections resulting from HBV, in patients during hospitalization and interventional procedures, as well as in health care workers, have been described[2,3]. Although infections attributed to transfusion of contaminated blood or blood products and transmission from infected health care workers have been reduced over the past decades by prophylactic measures, such as HBV screening of blood or vaccination of health care workers, there is still a significant number of reports about nosocomial outbreaks of HBV infections[4-7]. Common transmission pathways include the use of multi-dose vials[8], dental or biopsy equipment[9], dialysis units[10], contaminated finger-stick devices[11,12], contaminated acupuncture needles[13], reuse of syringes[14], endoscopes[15], or unsafe surgical and injection procedures[16-19]. Prevention of indirect HBV transmission by contaminated objects is only possible through the use of fundamental infection-control principles, including the use of chemical biocides[15,20], which are proven to destroy the viral infectivity. Thus, HBV must be inactivated as a result of the disinfection of instruments, surfaces, and biological materials. The use of biocides with proven microbiocidal activity against the pathogens most likely to contaminate a patients’ environment has been recommended by the United States Healthcare Infection Control Practices Advisory Committee as part of their guidelines to prevent the transmission of infectious agents in health care settings[21].
In general, HBV can be inactivated by chemical biocides, such as formaldehyde, glutaraldehyde or peracetic acid, which possess broad-spectrum virucidal activity according to the norm EN 14476:2007[22]. However, like all enveloped viruses[23], HBV is thought to be relatively sensitive to biocides. A German guideline for testing the virucidal activity of chemical disinfectants in the medical field characterizes disinfectants effective against enveloped viruses as biocides with limited virucidal activity. In contrast, disinfectants effective against non-enveloped and enveloped viruses are defined as biocides with virucidal activity[24,25]. Thus, broad-spectrum biocides, of which there are few, are not required for the inactivation of HBV like other blood-borne viruses. However, validation of the virucidal action of disinfectants against HBV is essential because the virucidal activity of biocides against HBV cannot be predicted[26]. In addition, human blood plasma protects the virus from inactivation. In the literature, HBV has been described as an enveloped virus that may be difficult to inactivate[27]. The virus possesses a relatively high thermal and dry resistance. At 25 °C and a relative air humidity of 42%, the HBV can be infectious for more than 1 wk[28]. Therefore, the virucidal efficacy of biocides against HBV must be validated using reliable and robust laboratory methods.
METHODS FOR TESTING THE EFFICACY OF BIOCIDES AGAINST HBV
The most common methods for the evaluation of virucidal activity of biocides are infectivity assays, which measure the infectivity of viruses in cell culture systems after the virus has been exposed to the biocide in suspension[24,25]. Recently, more practice-relevant methods have been developed, testing viral infectivity after exposure to viruses dried on non-porous surfaces[29]. These procedures mimic the conditions found in actual practice. A crucial component of these assays is that the tested viruses can easily be propagated in cell cultures and the infectivity can be determined reliably by the evaluation of virus-induced cytopathic changes or other methods detecting viral antigens, which are produced during the viral replication cycle. However, the in vitro propagation of non-cytopathogenic HBV is difficult, especially in obtaining human liver cells. Historically, the virucidal efficacy of biocides has been stringently determined in vivo through the use of chimpanzee infection assays, albeit with decreased sensitivity[30-34]. Currently, animal protection and economic reasons prohibit the use of higher primates for routine tests of commercial products[35]. For in vitro infectivity testing, the use of the hepatoma cell line HepG2[36], which has been described in the literature[27,37], has been debated[38]. In comparison, re-differentiated HepaRG cells[39] are well accepted and reproducible as an HBV infectivity system[38]. The specificity of this HBV infection model has been determined by both the neutralization capacity of HBV envelope protein-specific antibodies and the competition with an envelope-derived peptide. However, this infectivity system has not been applied in the past for testing the hepatitis B-virucidal activity of biocides. The reasons for this are the following: HepaRG cells are very expensive, can only be used in highly HBV-specialized laboratories and require highly concentrated HBV suspensions or human sera with a high viral load. Thus far, the most promising HBV infectivity assay seems to be the use of primary hepatocyte cultures derived from Tupaias, small-squirrel-like animals living in Southeast Asia[38,40]. However, the availability of Tupaia hepatocytes is limited, thus the model is too costly for routine use. Furthermore, purified virus must contain approximately 109 particles/mL to demonstrate an inactivation factor of at least 104[38]. The virus can be obtained from human serum by sedimentation in a density gradient[41].
Thus, surrogate models have often been reported for testing the efficacy of biocides against HBV. To measure the virucidal activity of disinfectants against HBV, Hilfenhaus et al[42] and Thraenhart et al[35] validated the integrity of viral DNA using a polymerase chain reaction (PCR) technique. Several groups[43,44] have also examined HBV inactivation by measuring the enzymatic activity of the viral DNA polymerase. In addition, the destruction of HBV antigenicity and the decrease in the immunochemical reactivity of different HBV antigens, such as HBsAg, HBcAg and HBeAg, was outlined to verify the virucidal efficacy of alcohol antiseptic, formaldehyde and peracetic acid-containing disinfectants[26,45,46]. Finally, the irreversible morphological alterations of HBV particles were determined to be an indicator of HBV inactivation by chemical biocides[43,47,48]. However, this test is subjective, and there is a qualitative but not a quantitative measurement. In conclusion, all of the abovementioned studies have shown that the results do not correlate with HBV infectivity.
DUCK HEPATITIS B VIRUS AS A SURROGATE VIRUS FOR HBV
The most promising and feasible assay for the evaluation of hepatitis B-virucidal efficacy of biocides is the use of the taxonomically related duck hepatitis B virus (DHBV) belonging to the same family-Hepadnaviridae-and within the genus Avihepadnavirus, while HBV is in the genus Orthohepadnavirus[49]. DHBV shares many physical properties with the closely related HBV but a sequence comparison of the two viruses indicated that there is a low nucleotide identity[49,50]. Furthermore, there are differences in the genome size (3.2 kb for Orthohepadnaviruses and 3.0 kb for Avihepadnaviriruses), and the host range of the viruses is restricted to mammals (Orthohepadnaviruses) or birds (Avihepadnaviruses). In addition, the Avihepadnaviriuses have larger core proteins and lack M surface protein[49]. In contrast to the Orthohepadnaviruses, some envelope proteins of the Avihepadnaviruses are not glycosylated but are phosphorylated. In addition, the proteins of the envelope are not connected by disulfide bridges and instead contain lysine side chains[51]. The model infection of DHBV in Pekin ducks has been used extensively for studying aspects of HBV infection in humans[50,52]. It has been concluded that DHBV and HBV differ primarily between the hosts they infect and the nature of the disease they produce. This has no bearing on the ability of disinfectants to abolish infectivity of the viruses[53]. Furthermore, the DHBV model has similar disinfectant inactivation kinetics to those observed in the limited studies of HBV transmission in chimpanzees[31,54]. Thus, DHBV infectivity tests have been used for testing the virucidal activity of chemical biocides against HBV in the United States and Australia[54-57] and have been proposed in Europe[58].
It is of great value that the DHBV is maintained in domestic duck flocks through vertical transmission from viremic ducks. The virus infects the developing liver in ovo and is not sufficiently recognized by the host immune system to produce hepatitis and liver disease or to eliminate the virus[49,59]. Thus, DHBV can be propagated in vivo in ducklings or in vitro in primary duck embryonic hepatocytes to assess viral infectivity[56,60-62]. Several authors have reported using in vivo DHBV assays[54,55,63-65]. To estimate DHBV infectivity, the diluted viral suspensions exposed to the biocides are injected intraperitoneally or intravenously into naïve ducklings not infected with DHBV. The ducklings are euthanized 2 wk later, and their livers are removed to be analyzed for DHBV DNA using PCR[66]. However, these in vivo tests conflict with ethical and legal aspects of animal protection. Therefore, the preferred method for testing the efficacy of disinfectants against DHBV is the in vitro assay. This protocol is in accordance with the recommendations of the United States Environmental Protection Agency[67].
Viral propagation of DHBV in duck embryonic hepatocytes is not trivial because DHBV is a non-cytopathogenic virus. This approach requires that additional tests, such as immunofluorescence[68], PCR[69] or Southern blotting[70], be used to verify the growth of the virus. Additionally, viral propagation requires a source of DHBV-free Pekin ducks, appropriate eggs for the preparation of embryonic hepatocytes, in vitro cultures of hepatocytes, and congenitally infected Pekin ducks as source of the virus. It is advantageous that experimental investigations on embryonated hen and duck eggs are, in general, regarded experimentally as in between in vivo and in vitro systems and do not conflict with ethical and legal aspects of animal protection[71].
IN VITRO DUCK HEPATITIS B VIRUS MODEL FOR TESTING VIRUCIDAL EFFICACY OF BIOCIDES
In Germany, an assay protocol for testing DHB-virucidal efficacy of biocides by DHBV infection of primary duck embryonic hepatocytes has been established and successfully evaluated for virucidal testing in several studies[68,72,73]. The primary duck embryonic hepatocytes were obtained from fertilized Pekin eggs and incubated for 21 d[68]; the liver tissue was harvested from several embryos[74]. A crucial step was to ensure the absence of DHBV in the source tissue using a qualitative PCR technique[68,75]. To digest the liver tissue, a solution comprised of trypsin, ethyl diamine tetraacetate solution, phosphate-buffered saline and glucose was effective. Digestion of the liver could be inhibited by the addition of fetal calf serum[69,76,77]. DHBV-negative cells were seeded in 24-well culture plates not containing collagen 1 such as CellBINDTM (Corning, Acton, United States)[74]. This step is necessary to ensure stable attachment of hepatocytes to the surface of culture vessels for successful DHBV propagation (Figure 1). The optimal growth medium can be modified according to previous reports[69,78,79]. This medium supports the maintenance of differentiating hepatocytes, which is important for the susceptibility of cells to the virus and for DHBV replication[60,80-82]. A suitable microenvironment can be achieved by coating the growth surface with Matrigel or other substrates containing extracellular matrix molecules[83,84]. Alternatively, the use of co-culture systems of hepatocytes with non-parenchymal liver cells has been described as a suitable method to maintain hepatocyte differentiation in vitro[85,86].
Figure 1.
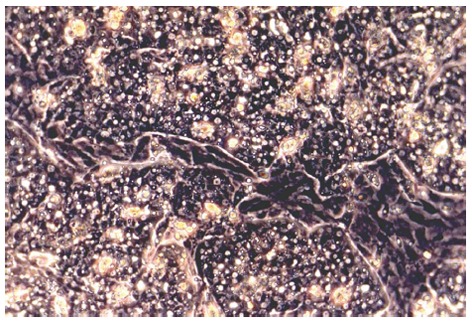
Primary duck embryonic hepatocytes grown in CeLLBINDTM plates at day 3 of cultivation. Monolayers of hepatocytes, which show typical polygonal morphology, are interrupted by areas of non-parenchymal cells (light microscopy, phase contrast, x 200).
The use of hepatocytes cryopreserved by the suspension method is also suitable when freshly isolated cells from the liver of duck embryos are not available due to seasonal differences[74]. Growth medium[74] was supplemented with 10% fetal calf serum and suitable cryoprotective agents, such as 10% DMSO or cryosafe-1. The freeze-thaw process does not significantly reduce the susceptibility of primary duck embryonic hepatocytes to DHBV infection, suggesting no loss of viral receptors on the cell surface.
As virus pool, DHBV-containing serum from congenitally infected ducks must be used[72,73]. Sera should contain between 106.0 and 108.0 tissue culture infective doses 50% of DHBV per mL, corresponding to 109.0 and 1011.0 DHBV genomic copies. To avoid reduction of viral titers, the uninterrupted storage of aliquots at -80 °C is strongly recommended. One should, however, be aware that the Pekin duck is an unreliable source of the test virus, which causes difficulties for standardization. Another disadvantage is that the titers of infectious virus are often too low to detect sufficient reduction of viral titers especially when cytotoxic biocides are tested[73]. On the other hand, the DHBV prepared from the DHBV DNA-transfected hepatoma cell line D2[87] is not suitable for testing virucidal efficacy of biocides either because this virus is more sensitive than the wild type DHBV naturally occurring in the serum of Pekin ducks[72].
Virucidal tests are recommended to be carried out in accordance with national guidelines for testing the virucidal efficacy of chemical disinfectants in human medical areas[25]. At the end of the chosen exposure time, the test compounds must be immediately removed from the mixture of virus and test formulation by rapid dilution of samples or the use of sephadex-based methods[88], particularly when cytotoxic residues must be removed. However, previous experience has shown that sephadex columns can withhold the infectious virus, thus leading to inaccurate results. It is recommended that the primary duck embryonic hepatocytes are infected at day 4 of cultivation. Due to the in vitro method of preparing of hepatocytes, the type I interferon system is stimulated, thereby inhibiting DHBV replication during the first 2-3 d after primary cell plating[89,90]. Thus, 4 d post-infection, a high number of DHBV-infected hepatocytes are infected[68,69]. On the other hand, DHBV-negative hepatocytes lose their susceptibility to DHBV infection after the day 5 of cultivation[60,80]. This results from the dedifferentiation of hepatocytes and/or the loss of the cellular receptor for virus attachment. The presence of DMSO in the cell culture medium is also critical for this process because DMSO not only allows maintenance of viral replication but also prolongs the susceptibility of cultured hepatocytes to DHBV infection[80]. Following viral infection, the cells should be incubated for at least 6 d to achieve infection rates of approximately 40% as shown by specific fluorescence, a surrogate marker of productive viral infection[68].
Indirect immunofluorescent antigen staining has been recommended for detection of DHBV surface(s) antigen in primary duck embryonic hepatocytes to verify DHBV infection[68,72]. To this end, a polyvalent rabbit anti-DHBs antiserum that is not commercially available must be used. As shown in Figure 2, the infected hepatocytes can be easily identified because they appear in clusters[79]. A 4-log10 reduction of infectivity (inactivation ≥ 99.99%) is regarded as evidence of sufficient virucidal activity[25]. As the guidelines of the United States Environmental Protection Agency[56] state, an in vitro assay requires a demonstration of at least 3-log10 reduction in viral titers beyond any disinfectant dilutions that exhibit cell culture cytotoxicity. Although fluorescent analysis has a subjective output and requires experience for the analysis of results, indirect immunofluorescence can be employed for routine testing and has been applied to detect a variety of human and animal viruses[91]. An advantage of this method is that the efficacy of biocides against DHBV infection can be rigorously evaluated because production of DHBV surface proteins in hepatocytes is a late step in the viral life cycle and correlates well with the production of mature virus particles[92]. In contrast, PCR-based methods identify the presence of viral DNA, but this may not necessarily correlate with the number of infectious virus particles[93].
Figure 2.
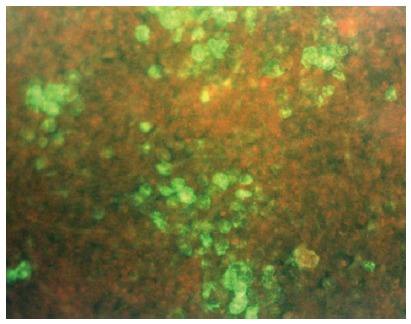
Detection of duck hepatitis B virus-specific surface antigen six days after inoculation of primary duck embryonic hepatocytes by indirect immunofluorescence. Polyvalent rabbit anti-DHBs (kindly provided by Dr. D. Glebe, Institute of Medical Virology, National Reference Centre for Hepatitis B and D, Justus Liebig University, Giessen, Germany) and goat anti-rabbit IgG Alexa Fluor® 488 (Life Technologies, Darmstadt, Germany) were used as antibodies (fluorescence microscopy, x 125).
EVALUATION OF BIOCIDES USING DUCK HBV
Several study groups in Australia, the United States, the United Kingdom and Germany have used the DHBV in vivo test system and the DHBV in vitro assay to evaluate the DHB-virucidal efficacy of chemical biocides or photochemical inactivation procedures. Table 1 gives an overview of the evaluated biocides and procedures of each study group. The majority of groups waived the in vivo test system. Since the year 2000, DHBV in vitro assays have been used almost exclusively. A recent study determined the DHB-virucidal activity of the following five different chemical biocides: ethanol, isopropanol, peracetic acid, glutaraldehyde and formaldehyde, which are often ingredients present in commercially available disinfectants[73]. Testing was carried out as modified quantitative suspension test[25] in the presence of a protein load of 10% fetal calf serum. Table 2 lists the minimal concentrations and contact times to reach virucidal efficacy. This means that ≥ 40% ethanol or isopropanol, ≥ 0.05% peracetic acid and ≥ 0.1% glutardiaaldehyde within ≥ 1 min significantly inactivate infectious DHBV corresponding to a 4-log10 reduction in viral titers. For a 0.7% formaldehyde solution, which resulted in high hepatocytotoxicity, a longer contact of ≥ 30 min is needed. These results show that the DHBV, as an enveloped virus, is considered to be relatively sensitive to inactivation by virucides. Limited, unpublished data with HBV and Tupaia hepatocytes corroborate these findings (personal communication: D. Glebe, Institute of Medical Virology, National Reference Centre for Hepatitis B and D, Justus Liebig University, Giessen, Germany). This is also in agreement with the susceptibility of levels of HBV detected by direct chimpanzee inoculation[30]. Thus, the results presented for DHBV are likely also valid for HBV. However, it must be considered for the interpretation of the in vitro data obtained by the quantitative suspension test that recommendations for the application of the agents in practice can be concluded only to a limited extent. Such favourable conditions as during the homogeneous suspension are seldom to be found in practice. Thus, results of the suspension test should not be regarded as practical application in every case but they allow conclusion of the efficacy of single disinfectants and, therefore, they also allow to compare the efficacy of different disinfectants[25]. For comparison, information on stability of HBV published by the World Health Organization[99] is summarized in Table 3. These biocides or measures, including concentrations, temperatures and contact times, are recommended for clinical practice to destroy infectious HBV. In contrast, Table 2 lists the minimal concentrations and contact times for the duck hepatitis B-virucidal activity of several biocides in the quantitative suspension test in which a protein load of 10% fetal calf serum was used. When selecting the most effective method for destroying infectious HBV, it should be taken into account that the amounts of serum HBV varies considerably among HBV-infected patients[100]. Thus, there can be differences in methods according to the level of viremia in patients.
Table 1.
Studies published in the literature to evaluate the efficacy of biocides against duck hepatitis B virus
| Year | Country | Ref. | Evaluated biocides or inactivation procedures |
| 1991 | Australia1 | Murray et al[54] | Glutaraldehyde; mix of glutaraldehyde, non-ionic alcohol derivate, quaternary compound and tri-ethyleneglycol surfactant |
| 1993 | United Kingdom1 | Tsiquaye et al[63] | Sodium hypochlorite; sodium dichloroisocyanurate |
| 1996 | Australia1 | Deva et al[94] | Glutaraldehyde |
| 1998 | United States2 | Eble et al[70] | Photochemical inactivation by 8-methoxypsoralen |
| 1999 | Australia1 | Chaufour et al[55] | Glutaraldehyde; ethylene oxide |
| 1999 | Australia1 | Vickery et al[64] | Hydrogen peroxide |
| 2000 | Australia1 | Vickery et al[95] | Glutaraldehyde |
| 2001 | United States2 | Wagner et al[96] | Photoinactivation by dimethylmethylene blue |
| 2002 | United States2 | Wang et al[69] | N-alkyl dimethyl benzyl ammonium chloride; alkyl dimethyl benzyl ammonium chloride |
| 2004 | United States2 | Moore et al[97] | Ethylene oxide |
| 2005 | Australia2 | Druce et al[65] | Ethylene oxide |
| 2006 | Germany2 | Sauerbrei et al[72] | Peracetic acid; povidone-iodine; formaldehyde |
| 2008 | United States2 | Roberts et al[98] | Ortho-phthalaldehyde |
| 2012 | Germany2 | Sauerbrei et al[73] | Ethanol; isopropanol; peracetic acid; glutaraldehyde; formaldehyde |
DHBV in vivo test system;
DHBV in vitro assay. DHBV: Duck hepatitis B virus.
Table 2.
Minimal concentrations and contact times for the duck hepatitis B virus-virucidal activity of ethanol, isopropanol, peracetic acid, glutaraldehyde and formaldehyde against duck hepatitis B virus in the presence of a protein load of 10% fetal calf serum
| Biocide | Concentration (%) | Contact time (min) |
| Ethanol | 40 | 1 |
| Isopropanol | 40 | 1 |
| Peracetic acid | 0.01 | 2 |
| 0.05 | 1 | |
| Glutaraldehyde | 0.05 | 2 |
| 0.1 | 0.5 | |
| Formaldehyde | 0.7 | 30 |
Results of quantitative suspension tests are shown[73].
Table 3.
Information on the stability of hepatitis B virus published by the World Health Organization[99]
| Biocide/measure | Concentration/temperature | Contact time | Remarks |
| Sodium hypochlorite | 0.25% | 3 min | Antigenicity of hepatitis B surface antigen is destroyed, infectivity is probably destroyed |
| Sodium hypochlorite | 5% | 10 min | Inactivation of virus |
| Glutaraldehyde | 2% (room temperature) | 5 min | Inactivation of virus |
| Glutaraldehyde | 2% (98 °C) | 2 min | Inactivation of virus |
| Formaldehyde | 5% | 2 min | Inactivation of virus |
| Isopropanol | 70% | 2 min | Inactivation of virus |
| Ethanol | 80% (11 °C) | 2 min | Inactivation of virus |
| Autoclaving | 121 °C | 20 min | Lost of infectivity |
| Heat sterilization | 160 °C | 1 h | Lost of infectivity |
Additionally, the study by Sauerbrei et al[73] has shown that biocides tested against DHBV are efficacious against the vaccinia virus strain Lister or the modified vaccinia Ankara strain[101], which are used in guidelines for the declaration of limited virucidal activity of biocides[25]. The testing of these viruses does not present any difficulties; therefore, it can be expected that in the absence of more direct tests, the results of DHBV, and even of the vaccinia virus or its modified Ankara strain, may be extrapolated to HBV. Therefore, the surrogate DHBV model can provide highly valuable data for the susceptibility of HBV to disinfectants.
Footnotes
P- Reviewers: Bock CT, Jin B, Lanini S, Shimizu Y, Lanini S S- Editor: Cui XM L- Editor: A E- Editor: Zhang DN
References
- 1.Hepatitis B vaccines. Wkly Epidemiol Rec. 2009;84:405–419. [PubMed] [Google Scholar]
- 2.Comstock RD, Mallonee S, Fox JL, Moolenaar RL, Vogt TM, Perz JF, Bell BP, Crutcher JM. A large nosocomial outbreak of hepatitis C and hepatitis B among patients receiving pain remediation treatments. Infect Control Hosp Epidemiol. 2004;25:576–583. doi: 10.1086/502442. [DOI] [PubMed] [Google Scholar]
- 3.Lanini S, Puro V, Lauria FN, Fusco FM, Nisii C, Ippolito G. Patient to patient transmission of hepatitis B virus: a systematic review of reports on outbreaks between 1992 and 2007. BMC Med. 2009;7:15. doi: 10.1186/1741-7015-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pourkarim MR, Verbeeck J, Rahman M, Amini-Bavil-Olyaee S, Forier AM, Lemey P, Maes P, Van Ranst M. Phylogenetic analysis of hepatitis B virus full-length genomes reveals evidence for a large nosocomial outbreak in Belgium. J Clin Virol. 2009;46:61–68. doi: 10.1016/j.jcv.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Burns K, Heslin J, Crowley B, Thornton L, Laoi BN, Kelly E, Ward E, Doody B, Hickey MM. Nosocomial outbreak of hepatitis B virus infection involving two hospitals in the Republic of Ireland. J Hosp Infect. 2011;78:279–283. doi: 10.1016/j.jhin.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Dencs A, Farkas A, Gyugos M, Kurcz A, Puskás E, Tresó B, Rusvai E, Barcsay E, Takács M. Phylogenetic analysis of a nosocomial transmission of hepatitis B virus at a paediatric haematology ward. Acta Microbiol Immunol Hung. 2011;58:23–29. doi: 10.1556/AMicr.58.2011.1.3. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Transmission of hepatitis B and C viruses in outpatient settings--New York, Oklahoma, and Nebraska, 2000-2002. MMWR Morb Mortal Wkly Rep. 2003;52:901–906. [PubMed] [Google Scholar]
- 8.Fisker N, Carlsen NL, Kolmos HJ, Tønning-Sørensen L, Høst A, Christensen PB. Identifying a hepatitis B outbreak by molecular surveillance: a case study. BMJ. 2006;332:343–345. doi: 10.1136/bmj.332.7537.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drescher J, Wagner D, Haverich A, Flik J, Stachan-Kunstyr R, Verhagen W, Wagenbreth I. Nosocomial hepatitis B virus infections in cardiac transplant recipients transmitted during transvenous endomyocardial biopsy. J Hosp Infect. 1994;26:81–92. doi: 10.1016/0195-6701(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 10.Carrilho FJ, Moraes CR, Pinho JR, Mello IM, Bertolini DA, Lemos MF, Moreira RC, Bassit LC, Cardoso RA, Ribeiro-dos-Santos G, et al. Hepatitis B virus infection in Haemodialysis Centres from Santa Catarina State, Southern Brazil. Predictive risk factors for infection and molecular epidemiology. BMC Public Health. 2004;4:13. doi: 10.1186/1471-2458-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bender TJ, Wise ME, Utah O, Moorman AC, Sharapov U, Drobeniuc J, Khudyakov Y, Fricchione M, White-Comstock MB, Thompson ND, et al. Outbreak of hepatitis B virus infections associated with assisted monitoring of blood glucose in an assisted living facility-Virginia, 2010. PLoS One. 2012;7:e50012. doi: 10.1371/journal.pone.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanini S, Garbuglia AR, Puro V, Solmone M, Martini L, Arcese W, Nanni Costa A, Borgia P, Piselli P, Capobionchi MR, et al. Hospital cluster of HBV infection: molecular evidence of patient-to-patient transmission through lancing device. PLoS One. 2012;7:e33122. doi: 10.1371/journal.pone.0033122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh B, Maguire H, Carrington D. Outbreak of hepatitis B in an acupuncture clinic. Commun Dis Public Health. 1999;2:137–140. [PubMed] [Google Scholar]
- 14.Thompson ND, Perz JF, Moorman AC, Holmberg SD. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998-2008. Ann Intern Med. 2009;150:33–39. doi: 10.7326/0003-4819-150-1-200901060-00007. [DOI] [PubMed] [Google Scholar]
- 15.Santos NC, Pinho JR, Lemos MF, Moreira RC, Lopes CM, Sacilotto MT, Tacla M, Pinheiro WS, Ramos LO. Risk of hepatitis B virus transmission by diagnostic hysteroscopy. Braz J Med Biol Res. 2004;37:683–689. doi: 10.1590/s0100-879x2004000500009. [DOI] [PubMed] [Google Scholar]
- 16.Redd JT, Baumbach J, Kohn W, Nainan O, Khristova M, Williams I. Patient-to-patient transmission of hepatitis B virus associated with oral surgery. J Infect Dis. 2007;195:1311–1314. doi: 10.1086/513435. [DOI] [PubMed] [Google Scholar]
- 17.Goldmann DA. Blood-borne pathogens and nosocomial infections. J Allergy Clin Immunol. 2002;110:S21–S26. doi: 10.1067/mai.2002.125337. [DOI] [PubMed] [Google Scholar]
- 18.Buster EH, van der Eijk AA, Schalm SW. Doctor to patient transmission of hepatitis B virus: implications of HBV DNA levels and potential new solutions. Antiviral Res. 2003;60:79–85. doi: 10.1016/j.antiviral.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Welch J, Webster M, Tilzey AJ, Noah ND, Banatvala JE. Hepatitis B infections after gynaecological surgery. Lancet. 1989;1:205–207. doi: 10.1016/s0140-6736(89)91213-0. [DOI] [PubMed] [Google Scholar]
- 20.Sattar SA, Tetro J, Springthorpe VS, Giulivi A. Preventing the spread of hepatitis B and C viruses: where are germicides relevant? Am J Infect Control. 2001;29:187–197. doi: 10.1067/mic.2001.114233. [DOI] [PubMed] [Google Scholar]
- 21.Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am J Infect Control. 2007;35:S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.British Standards Institute Staff. Chemical disinfectants and antiseptics. Virucidal quantitative suspension test for chemical disinfectants and antiseptics used in human medicine. Test method and requirements (phase 2, Step 1) London: B S I Standards; 2005. [Google Scholar]
- 23.Jülich WD, von Rheinbaben F, Steinmann J, Kramer A. On the virucidal efficacy of chemical and physical disinfectants or disinfection procedures. Hyg Med. 1993;18:303–326. [Google Scholar]
- 24.Robert Koch-Institut; Deutsche Gesellschaft zur Bekämpfung der Viruskrankheiten; Desinfektionsmittelkommission der Deutschen Gesellschaft für Hygiene und Mikrobiologie. [Evaluation and declaration of effectiveness of disinfectants against viruses. Position of the Virucide Study Group of the Robert Koch Institute (RKI) and the “Virus Disinfection” Professional Committee of the German Society for Control of “Virus Infections” and the Disinfectant Committee of the German Society of Public Health and Microbiology] Bundesgesundheitsblatt Gesundheitsforschung Gesundheits-schutz. 2004;47:62–66. doi: 10.1007/s00103-003-0754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blümel J, Glebe D, Neumann-Haefelin D, Rabenau HF, Rapp I, von Rheinbaben F, Ruf B, Sauerbrei A, Schwebke I, Steinmann J, et al. Guideline of “Deutsche Vereinigung zur Bekämpfung der Viruskrankheiten e.V.” (DVV; German Association for the Control of Virus Diseases) and Robert Koch-Institute (RKI; German Federal Health Authority) for testing the virucidal efficacy of chemical disinfectants in the human medical area. Hyg Med. 2009;34:293–299. [Google Scholar]
- 26.Jursch CA, Gerlich WH, Glebe D, Schaefer S, Marie O, Thraenhart O. Molecular approaches to validate disinfectants against human hepatitis B virus. Med Microbiol Immunol. 2002;190:189–197. doi: 10.1007/s00430-001-0103-0. [DOI] [PubMed] [Google Scholar]
- 27.Payan C, Cottin J, Lemarie C, Ramont C. Inactivation of hepatitis B virus in plasma by hospital in-use chemical disinfectants assessed by a modified HepG2 cell culture. J Hosp Infect. 2001;47:282–287. doi: 10.1053/jhin.2001.0945. [DOI] [PubMed] [Google Scholar]
- 28.Von Rheinbaben F, Wolf MH. Handbuch der viruswirksamen Desinfektion. Berlin: Springer; 2002. pp. 195–197. [Google Scholar]
- 29.Rabenau HF, Schwebke I, Steinmann J, Eggers M, Rapp I and the members of the expert committee for virus disinfection. Quantitative test for the evaluation of virucidal activity of chemical disinfectants on non-porous surfaces (For use in human medicine) Hyg Med. 2012;37:459–466. [Google Scholar]
- 30.Kobayashi H, Tsuzuki M, Koshimizu K, Toyama H, Yoshihara N, Shikata T, Abe K, Mizuno K, Otomo N, Oda T. Susceptibility of hepatitis B virus to disinfectants or heat. J Clin Microbiol. 1984;20:214–216. doi: 10.1128/jcm.20.2.214-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince DL, Prince HN, Thraenhart O, Muchmore E, Bonder E, Pugh J. Methodological approaches to disinfection of human hepatitis B virus. J Clin Microbiol. 1993;31:3296–3304. doi: 10.1128/jcm.31.12.3296-3304.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bond WW, Favero MS, Petersen NJ, Ebert JW. Inactivation of hepatitis B virus by intermediate-to-high-level disinfectant chemicals. J Clin Microbiol. 1983;18:535–538. doi: 10.1128/jcm.18.3.535-538.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prince AM, Stephan W, Brotman B. beta-propiolactone/ultraviolet irradiation: a review of its effectiveness for inactivation of viruses in blood derivatives. Rev Infect Dis. 1983;5:92–107. doi: 10.1093/clinids/5.1.92. [DOI] [PubMed] [Google Scholar]
- 34.Shikata T, Karasawa T, Abe K, Takahashi T, Mayumi M, Oda T. Incomplete inactivation of hepatitis B virus after heat treatment at 60 C for 10 hours. J Infect Dis. 1978;138:242–244. doi: 10.1093/infdis/138.2.242. [DOI] [PubMed] [Google Scholar]
- 35.Thraenhart O, Jursch C. Measures for disinfection and control of viral hepatitis. In: Block SS, editor. Disinfection, sterilisation, and preservation. 5th ed. Philadelphia: Lea & Febiger; 2001. pp. 585–615. [Google Scholar]
- 36.Bchini R, Capel F, Dauguet C, Dubanchet S, Petit MA. In vitro infection of human hepatoma (HepG2) cells with hepatitis B virus. J Virol. 1990;64:3025–3032. doi: 10.1128/jvi.64.6.3025-3032.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payan C, Pivert A, Kampf G, Ramont C, Cottin J, Lemarie C. Assessment of new chemical disinfectants for HBV virucidal activity in a cell culture model. J Hosp Infect. 2004;56 Suppl 2:S58–S63. doi: 10.1016/j.jhin.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 38.Gerlich WH, Glebe D. Methods for validation of hepatitis B virus inactivation. Dev Biol (Basel) 2004;118:113–122. [PubMed] [Google Scholar]
- 39.Glebe D, Aliakbari M, Krass P, Knoop EV, Valerius KP, Gerlich WH. Pre-s1 antigen-dependent infection of Tupaia hepatocyte cultures with human hepatitis B virus. J Virol. 2003;77:9511–9521. doi: 10.1128/JVI.77.17.9511-9521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, Guyomard C, Lucas J, Trepo C, Guguen-Guillouzo C. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci USA. 2002;99:15655–15660. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Köck J, Nassal M, MacNelly S, Baumert TF, Blum HE, von Weizsäcker F. Efficient infection of primary tupaia hepatocytes with purified human and woolly monkey hepatitis B virus. J Virol. 2001;75:5084–5089. doi: 10.1128/JVI.75.11.5084-5089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hilfenhaus J, Gröner A, Nowak T, Weimer T. Analysis of human plasma products: polymerase chain reaction does not discriminate between live and inactivated viruses. Transfusion. 1997;37:935–940. doi: 10.1046/j.1537-2995.1997.37997454021.x. [DOI] [PubMed] [Google Scholar]
- 43.Howard CR, Dixon J, Young P, van Eerd P, Schellekens H. Chemical inactivation of hepatitis B virus: the effect of disinfectants on virus-associated DNA polymerase activity, morphology and infectivity. J Virol Methods. 1983;7:135–148. doi: 10.1016/0166-0934(83)90003-4. [DOI] [PubMed] [Google Scholar]
- 44.Sherertz EF, Davis GL, Rice RW, Harris BA, Franzini DA. Transfer of hepatitis B virus by contaminated reusable needle electrodes after electrodesiccation in simulated use. J Am Acad Dermatol. 1986;15:1242–1246. doi: 10.1016/s0190-9622(86)70297-1. [DOI] [PubMed] [Google Scholar]
- 45.Frösner G, Jentsch G, Uthemann H. [Destroying of antigenicity and influencing the immunochemical reactivity of hepatitis B virus antigens (HBsAg, HBcAg and HBeAg) through disinfectants--a proposed method for testing (author’s transl)] Zentralbl Bakteriol Mikrobiol Hyg B. 1982;176:1–14. [PubMed] [Google Scholar]
- 46.Skelly J, Howard CR, Zuckerman AJ. Formaldehyde treatment of hepatitis B micelle vaccine. J Virol Methods. 1981;3:51–59. doi: 10.1016/0166-0934(81)90022-7. [DOI] [PubMed] [Google Scholar]
- 47.Thraenhart O, Kuwert EK, Scheiermann N, Dermietzel R, Paar D, Maruhn D, Alberti A, Richter HJ, Hotz J. Comparison of the morphological alteration and disintegration test (MADT) and the chimpanzee infectivity test for determination of hepatitis B virucidal activity of chemical disinfectants. Zentralbl Bakteriol Mikrobiol Hyg B. 1982;176:472–484. [PubMed] [Google Scholar]
- 48.Kuwert E, Thraenhart O, Dermietzel R, Scheiermann N. The morphological alteration and disintegration test (MADT) for quantitative and kinetic determination of hepato-virucidal effect of chemical disinfectants. Hyg Med. 1984;9:1–6. [Google Scholar]
- 49.Mason WS, Gerlich WH, Taylor JM, Kann M, Mizokami T, Loeb T, Sureau C, Magnius L, Norder H. Hepadnaviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier; 2012. pp. 445–455. [Google Scholar]
- 50.Schödel S, Weimer T, Fernholz D, Schneider R, Sprengel R, Wildner G, Will H. The biology of avian hepatitis B viruses. In: McLachlan A, editor. Molecular biology of the hepatitis B virus. Boca Raton, FL: CRC Press; 1991. pp. 53–80. [Google Scholar]
- 51.Modrow S, Falke D, Truyen U, Schätzl H. Molekulare Virologie. 3rd ed. Heidelberg: Spektrum; 2010. [Google Scholar]
- 52.Mason WS, Seal G, Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol. 1980;36:829–836. doi: 10.1128/jvi.36.3.829-836.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pugh JC, Ijaz MK, Suchmann DB. Use of surrogate models for testing efficacy of disinfectants against hepatitis B virus. Am J Infect Control. 1999;27:375–376. doi: 10.1016/s0196-6553(99)70064-7. [DOI] [PubMed] [Google Scholar]
- 54.Murray SM, Freiman JS, Vickery K, Lim D, Cossart YE, Whiteley RK. Duck hepatitis B virus: a model to assess efficacy of disinfectants against hepadnavirus infectivity. Epidemiol Infect. 1991;106:435–443. doi: 10.1017/s0950268800067480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaufour X, Deva AK, Vickery K, Zou J, Kumaradeva P, White GH, Cossart YE. Evaluation of disinfection and sterilization of reusable angioscopes with the duck hepatitis B model. J Vasc Surg. 1999;30:277–282. doi: 10.1016/s0741-5214(99)70138-2. [DOI] [PubMed] [Google Scholar]
- 56.United States Environmental Protection Agency. Protocol for testing the efficacy of disinfectants used to inactivate duck hepatitis B virus and to support corresponding label claims. 2000. Available from: http://www.epa.gov/oppad001/pdf_files/hbvprotocol.pdf.
- 57.Bishop N, Civitico G, Wang YY, Guo KJ, Birch C, Gust I, Locarnini S. Antiviral strategies in chronic hepatitis B virus infection: I. Establishment of an in vitro system using the duck hepatitis B virus model. J Med Virol. 1990;31:82–89. doi: 10.1002/jmv.1890310204. [DOI] [PubMed] [Google Scholar]
- 58.Steinmann J. Surrogate viruses for testing virucidal efficacy of chemical disinfectants. J Hosp Infect. 2004;56 Suppl 2:S49–S54. doi: 10.1016/j.jhin.2003.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jilbert AR, Miller DS, Scougall CA, Turnbull H, Burrell CJ. Kinetics of duck hepatitis B virus infection following low dose virus inoculation: one virus DNA genome is infectious in neonatal ducks. Virology. 1996;226:338–345. doi: 10.1006/viro.1996.0661. [DOI] [PubMed] [Google Scholar]
- 60.Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 61.Hantz O, Zoulim F. Duck hepatitis B virus primary hepatocyte culture model. Methods Mol Med. 2004;96:189–197. doi: 10.1385/1-59259-670-3:189. [DOI] [PubMed] [Google Scholar]
- 62.Marion PL, Cullen JM, Azcárraga RR, Van Davelaar MJ, Robinson WS. Experimental transmission of duck hepatitis B virus to Pekin ducks and to domestic geese. Hepatology. 1987;7:724–731. doi: 10.1002/hep.1840070418. [DOI] [PubMed] [Google Scholar]
- 63.Tsiquaye KN, Barnard J. Chemical disinfection of duck hepatitis B virus: a model for inactivation of infectivity of hepatitis B virus. J Antimicrob Chemother. 1993;32:313–323. doi: 10.1093/jac/32.2.313. [DOI] [PubMed] [Google Scholar]
- 64.Vickery K, Deva AK, Zou J, Kumaradeva P, Bissett L, Cossart YE. Inactivation of duck hepatitis B virus by a hydrogen peroxide gas plasma sterilization system: laboratory and ‘in use’ testing. J Hosp Infect. 1999;41:317–322. doi: 10.1053/jhin.1998.0516. [DOI] [PubMed] [Google Scholar]
- 65.Druce JD, Russell JS, Birch CJ, Vickery K, Harper RW, Smolich JJ. Cleaning and sterilization protocol for reused cardiac electrophysiology catheters inactivates hepatitis and coxsackie viruses. Infect Control Hosp Epidemiol. 2005;26:720–725. doi: 10.1086/502609. [DOI] [PubMed] [Google Scholar]
- 66.Freiman JS, Jilbert AR, Dixon RJ, Holmes M, Gowans EJ, Burrell CJ, Wills EJ, Cossart YE. Experimental duck hepatitis B virus infection: pathology and evolution of hepatic and extrahepatic infection. Hepatology. 1988;8:507–513. doi: 10.1002/hep.1840080313. [DOI] [PubMed] [Google Scholar]
- 67. Available from: http://www.epa.gov/oppad001/pdf_files/hbvprotresp.pdf.
- 68.Sauerbrei A, Schacke M, Schultz U, Egerer R, Merkle I, Glebe D, Gerlich W, Wutzler P. Alternative methods for validation of cell culture infection with duck hepatitis B virus. J Virol Methods. 2005;129:178–185. doi: 10.1016/j.jviromet.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 69.Wang CY, Giambrone JJ, Smith BF. Comparison of cell culture systems for duck hepatitis B virus using SyBr green quantitative PCR. J Virol Methods. 2002;106:175–184. doi: 10.1016/s0166-0934(02)00161-1. [DOI] [PubMed] [Google Scholar]
- 70.Eble BE, Corash L. Duck hepatitis B virus inactivation and 8-methoxypsoralen photoadduct formation in human platelet concentrates. Photochem Photobiol. 1998;67:700–713. [PubMed] [Google Scholar]
- 71.Bolls M, Ridell RJ, Worden AN. Animals and alternatives in toxicity testing. London: Academic Press; 1983. [Google Scholar]
- 72.Sauerbrei A, Schacke M, Glück B, Egerer R, Wutzler P. Validation of biocides against duck hepatitis B virus as a surrogate virus for human hepatitis B virus. J Hosp Infect. 2006;64:358–365. doi: 10.1016/j.jhin.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 73.Sauerbrei A, Schacke M, Glück B, Bust U, Rabenau HF, Wutzler P. Does limited virucidal activity of biocides include duck hepatitis B virucidal action? BMC Infect Dis. 2012;12:276. doi: 10.1186/1471-2334-12-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schacke M, Glück B, Wutzler P, Sauerbrei A. In vitro cultivation and cryopreservation of duck embryonic hepatocytes. J Virol Methods. 2009;157:25–31. doi: 10.1016/j.jviromet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 75.Protzer U, Nassal M, Chiang PW, Kirschfink M, Schaller H. Interferon gene transfer by a hepatitis B virus vector efficiently suppresses wild-type virus infection. Proc Natl Acad Sci USA. 1999;96:10818–10823. doi: 10.1073/pnas.96.19.10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pult I, Netter HJ, Bruns M, Prassolov A, Sirma H, Hohenberg H, Chang SF, Frölich K, Krone O, Kaleta EF, et al. Identification and analysis of a new hepadnavirus in white storks. Virology. 2001;289:114–128. doi: 10.1006/viro.2001.1115. [DOI] [PubMed] [Google Scholar]
- 77.Wang CY, Giambrone JJ, Smith BF. Development of viral disinfectant assays for duck hepatitis B virus using cell culture/PCR. J Virol Methods. 2002;106:39–50. doi: 10.1016/s0166-0934(02)00136-2. [DOI] [PubMed] [Google Scholar]
- 78.Prassolov A, Hohenberg H, Kalinina T, Schneider C, Cova L, Krone O, Frölich K, Will H, Sirma H. New hepatitis B virus of cranes that has an unexpected broad host range. J Virol. 2003;77:1964–1976. doi: 10.1128/JVI.77.3.1964-1976.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Funk A, Hohenberg H, Mhamdi M, Will H, Sirma H. Spread of hepatitis B viruses in vitro requires extracellular progeny and may be codetermined by polarized egress. J Virol. 2004;78:3977–3983. doi: 10.1128/JVI.78.8.3977-3983.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galle PR, Schlicht HJ, Kuhn C, Schaller H. Replication of duck hepatitis B virus in primary duck hepatocytes and its dependence on the state of differentiation of the host cell. Hepatology. 1989;10:459–465. doi: 10.1002/hep.1840100410. [DOI] [PubMed] [Google Scholar]
- 81.Pugh JC, Summers JW. Infection and uptake of duck hepatitis B virus by duck hepatocytes maintained in the presence of dimethyl sulfoxide. Virology. 1989;172:564–572. doi: 10.1016/0042-6822(89)90199-2. [DOI] [PubMed] [Google Scholar]
- 82.Schorr O, Borel C, Trepo C, Zoulim F, Hantz O. Effects of liver growth factors on hepadnavirus replication in chronically infected duck hepatocytes. J Hepatol. 2006;44:842–847. doi: 10.1016/j.jhep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 83.Bissell DM, Arenson DM, Maher JJ, Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987;79:801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takashi H, Katsumi M, Toshihiro A. Hepatocytes maintain their function on basement membrane formed by epithelial cells. Biochem Biophys Res Commun. 2007;359:151–156. doi: 10.1016/j.bbrc.2007.05.079. [DOI] [PubMed] [Google Scholar]
- 85.Morin O, Normand C. Long-term maintenance of hepatocyte functional activity in co-culture: requirements for sinusoidal endothelial cells and dexamethasone. J Cell Physiol. 1986;129:103–110. doi: 10.1002/jcp.1041290115. [DOI] [PubMed] [Google Scholar]
- 86.Utesch D, Oesch F. Dependency of the in vitro stabilization of differentiated functions in liver parenchymal cells on the type of cell line used for co-culture. In Vitro Cell Dev Biol. 1992;28A:193–198. doi: 10.1007/BF02631091. [DOI] [PubMed] [Google Scholar]
- 87.Condreay LD, Aldrich CE, Coates L, Mason WS, Wu TT. Efficient duck hepatitis B virus production by an avian liver tumor cell line. J Virol. 1990;64:3249–3258. doi: 10.1128/jvi.64.7.3249-3258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geller C, Fontanay S, Finance C, Duval RE. A new Sephadex-based method for removing microbicidal and cytotoxic residues when testing antiseptics against viruses: Experiments with a human coronavirus as a model. J Virol Methods. 2009;159:217–226. doi: 10.1016/j.jviromet.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lavine JE, Ganem D. Inhibition of duck hepatitis B virus replication by interferon-gamma. J Med Virol. 1993;40:59–64. doi: 10.1002/jmv.1890400112. [DOI] [PubMed] [Google Scholar]
- 90.Schultz U, Chisari FV. Recombinant duck interferon gamma inhibits duck hepatitis B virus replication in primary hepatocytes. J Virol. 1999;73:3162–3168. doi: 10.1128/jvi.73.4.3162-3168.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grandien M. Viral diagnosis by antigen detection techniques. Clin Diagn Virol. 1996;5:81–90. doi: 10.1016/0928-0197(96)00209-7. [DOI] [PubMed] [Google Scholar]
- 92.Gerlich W. Hepatitis B surface proteins. J Hepatol. 1991;13 Suppl 4:S90–S92. doi: 10.1016/0168-8278(91)90033-8. [DOI] [PubMed] [Google Scholar]
- 93.Sauerbrei A, Sehr K, Eichhorn U, Reimer K, Wutzler P. Inactivation of human adenovirus genome by different groups of disinfectants. J Hosp Infect. 2004;57:67–72. doi: 10.1016/j.jhin.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 94.Deva AK, Vickery K, Zou J, West RH, Harris JP, Cossart YE. Establishment of an in-use testing method for evaluating disinfection of surgical instruments using the duck hepatitis B model. J Hosp Infect. 1996;33:119–130. doi: 10.1016/s0195-6701(96)90096-1. [DOI] [PubMed] [Google Scholar]
- 95.Vickery K, Pajkos A, Cossart Y. Evaluation of the effectiveness of decontamination of dental syringes. Br Dent J. 2000;189:620–624. doi: 10.1038/sj.bdj.4800847. [DOI] [PubMed] [Google Scholar]
- 96.Wagner SJ, Skripchenko A, Pugh JC, Suchmann DB, Ijaz MK. Duck hepatitis B photoinactivation bydimethylmethylene blue in RBC suspensions. Transfusion. 2001;41:1154–1158. doi: 10.1046/j.1537-2995.2001.41091154.x. [DOI] [PubMed] [Google Scholar]
- 97.Moore TM, Gendler E, Gendler E. Viruses adsorbed on musculoskeletal allografts are inactivated by terminal ethylene oxide disinfection. J Orthop Res. 2004;22:1358–1361. doi: 10.1016/j.orthres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 98.Roberts CG, Chan-Myers HB, Favero MS. Virucidal activity of ortho-phthalaldehyde solutions against hepatitis B and C viruses. Am J Infect Control. 2008;36:223–226. doi: 10.1016/j.ajic.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 99.World Health Organization. Hepatitis B. 2002. Available from: http://www.who.int/csr/disease/hepatitis/HepatitisB_whocdscsrlyo2002_ 2.pdf.
- 100.Madan K, Batra Y, Jha JK, Kumar S, Kalra N, Paul SB, Singh R, Duttagupta S, Panda SK, Acharya SK. Clinical relevance of HBV DNA load in patients with chronic hepatitis B infection. Trop Gastroenterol. 2008;29:84–90. [PubMed] [Google Scholar]
- 101.Rabenau HF, Rapp I, Steinmann J. Can vaccinia virus be replaced by MVA virus for testing virucidal activity of chemical disinfectants? BMC Infect Dis. 2010;10:185. doi: 10.1186/1471-2334-10-185. [DOI] [PMC free article] [PubMed] [Google Scholar]


