Abstract
Chronic hepatitis B virus (HBV) infection adversely influences the clinical outcomes of renal transplant recipients owing to increased hepatic complications. Management of HBV infection in kidney transplant recipients presents a challenge to clinicians, especially in endemic regions. Interferon precipitates renal allograft dysfunction. Treatment with lamivudine, the first oral nucleoside analogue available, resulted in effective viral suppression, reduced liver-related complications, and improved patient survival so that medium-term data showed comparable patient survival rates between hepatitis B surface antigen-positive and HBsAg-negative kidney transplant recipients in the era of effective antiviral therapies. Entecavir has replaced lamivudine as first-line therapy for treatment-naïve subjects in view of the propensity for drug resistance with the latter. Management of HBV infection in kidney transplant patients needs to take into consideration the nephrotoxicity of nucleoside/tide analogues such as adefovir and tenofovir. Prevention of HBV-related complications in kidney transplant recipients starts much earlier prior to transplantation, with vaccination of patients with chronic kidney disease and donor-recipient matching with regard to HBV status. In addition to anti-viral treatment, patients with chronic HBV infection must have regular surveillance for liver cancer and assessment for the development of cirrhosis.
Keywords: Hepatitis B, Kidney transplantation
Core tip: Treatment with oral nucleoside/tide analogues brought a new paradigm in the management of hepatitis B surface antigen-positive kidney transplant recipients, resulting in effective viral suppression, reduced hepatic complications, and improved patient survival, without compromising renal allograft outcome. Entecavir has replaced lamivudine as first-line therapy for treatment-naïve subjects given the propensity of lamivudine for selecting resistance. Due to the nephrotoxicity of adefovir and tenofovir, the optimal management of drug-resistant hepatitis B virus (HBV) remains to be defined. Other important measures to prevent HBV-related complications in renal transplant patients include early vaccination in non-immune subjects, donor-recipient matching of HBV status, and surveillance for liver cancer and cirrhosis.
INTRODUCTION
Hepatitis B virus (HBV) infection confers a significantly negative impact on the clinical outcomes of kidney allograft recipients. The inferior patient survival in hepatitis B surface antigen-positive (HBsAg+) renal transplant patients compared with the HBsAg-negative counterparts is attributed to increased hepatic complications such as chronic hepatitis and cirrhosis, fibrosing cholestatic hepatitis, and hepatocellular carcinoma[1-10]. Prevention and management of HBV infection in patients with renal failure is a major issue in endemic regions such as Asia, when the HBV carrier rate in the general population can exceed 10%. The reported prevalence of HBV infection among dialysis patients in the United States is often below 1.0%, whereas the prevalence rate is between 7.0% and 15% in the Asian-Pacific region[11-13]. While the incidence of HBV infection among dialysis patients has declined significantly over the past three decades because of widespread implementation of infection control measures, reduced need for transfusion and adherence to safe transfusion practices, and the introduction of HBV vaccination to neonates in many countries, over 350 million subjects worldwide carry HBV and thus it will remain a significant clinical issue for some time[14]. In this context, a considerable number of HBsAg-positive patients will undergo kidney transplantation[15].
PREVENTION OF DE NOVO HBV INFECTION IN RENAL TRANSPLANT RECIPIENTS
An effective immunization program in dialysis and chronic kidney disease patients is the cornerstone to prevent de novo HBV infection in renal transplant recipients. HBV vaccination should be given early in the course of chronic kidney disease owing to the relatively poor response in patients with significant renal impairment[16]. In the dialysis population, higher doses of vaccine are recommended, with post-vaccination and subsequent annual testing and booster administration if anti-HBs titer falls below 10 IU/L[17]. Intra-dermal injection may be considered in non-responders to enhance the vaccination efficacy[18]. The donor-recipient matching with regard to their HBV serological status significantly affects the risk of de novo HBV infection post-transplant. One must not transplant an HBsAg-positive allograft into a recipient who is negative for both HBsAg and anti-HBs, or de novo infection would occur and the course is often aggressive[19]. The risk of HBV transmission from HBsAg-negative anti-HBc positive donors to HBsAg-negative recipients is low, and the risk is even lower if the recipient is anti-HBs positive[20]. Accumulating experience suggests that it is safe to transplant an HBsAg-positive kidney to an HBsAg-negative recipient who has anti-HBs antibody under HBV immunoglobulin cover[21].
CLINICAL OUTCOMES OF HBSAG-POSITIVE RENAL TRANSPLANT RECIPIENTS IN THE PRE-ANTIVIRAL NUCLEOSIDE/TIDE ANALOGUE ERA
The clinical manifestations and diagnosis of HBV infection in kidney transplant recipients are generally similar to patients without renal disease, but due to the immunosuppressed state these individuals are more susceptible to progressive liver disease and severe life-threatening complications like fibrosing cholestatic hepatitis[10]. The significantly inferior survival of HBsAg-positive kidney transplant recipients in the “no-treatment” era was regarded unavoidable, and much of the mortality occurred relatively early, due to severe progressive liver disease[2-7,9]. In a meta-analysis of six observational studies, HBsAg-positivity was associated with a 2.49-fold risk of death after renal transplantation[22]. Liver-related complications were significantly increased in subjects with detectable serum HBV DNA or were HBeAg-positive[23]. The 10-and 20-year patient survival rates in HBsAg-positive kidney transplant recipients without anti-viral therapy were 85% and 71% respectively (vs 98% and 95% at 10 and 20 years in HBsAg-negative patients)[24].
Before the availability of oral nucleoside/tide analogues, chronic HBV infection was managed with interferon therapy. Interferons offer the advantage of sustained response with a finite duration of therapy in both HBeAg-positive and HBeAg-negative patients[25]. However, there has been data suggesting that the efficacy of interferon might be lower in endemic regions where most patients contract the infection during infancy, compared to non-endemic areas where the infection is contracted during adulthood[26,27]. Moreover, interferon should be avoided in kidney allograft recipients as it commonly precipitates allograft dysfunction and rejection[28,29], although one study suggested that interferon treatment might not be associated with acute rejection in HCV-positive kidney transplant recipients with low rejection risk[30]. With the advent of oral nucleoside/tide analogues which suppress HBV replication effectively, there was a dramatic change in the clinical course of HBsAg-positive kidney transplant recipients and a new paradigm of therapeutic management.
IMPACT OF NUCLEOSIDE/TIDE ANALOGUE THERAPY ON THE OUTCOMES OF HBSAG-POSITIVE RENAL TRANSPLANT RECIPIENTS
The current options of nucleoside/tide analogues include lamivudine, entecavir, telbivudine, adefovir and tenofovir (Table 1). The objective of treatment is to prevent HBV-related complications in these immunosuppressed individuals, and the indication to start treatment is based on the commencement of immunosuppressive therapy (the “prophylactic” approach) or the evidence of impending HBV reactivation (the “pre-emptive” approach). Due to a paucity of data, the optimal duration of antiviral treatmentin HBsAg-positive kidney transplant recipients remains undefined. Preliminary experience suggests that while most patients would require lifelong anti-viral suppression discontinuation may be cautiously attempted after stabilization, with success, in carefully selected low-risk patients[1].
Table 1.
The major clinical trials regarding the use of oral nucleoside/tides for HBsAg-positive kidney transplant recipients
| Oral Nucleoside/tides | Study design | n | Major treatment outcomes |
| Lamivudine | |||
| Rostaing et al[32] (1997) | Prospective | 6 | LAM as initial Rx → ALT normalization and HBV DNA undetectability in 4/6 patients |
| Chan et al[1] (2002) | Prospective | 11 | LAM as initial Rx → ALT normalization and HBV DNA undetectability in all patients; e-seroconversionrate (21.4%); markedly improved patient survival when compared to historical controls who had no anti-viral Rx (P < 0.001) |
| Fabrizi et al[33] (2004) | Meta-analysis | 184 | LAM as initial Rx → HBV-DNA undetectability [91% (95%CI: 86%-96%)], ALT normalization [81% (95%CI: 70%-92%)] and LAM-resistance [18% (95%CI: 10%-37%)] after 12 mo; e-seroconversion rate (0%-46%) in 4 trials |
| Thabut et al[34] (2004) | Prospective | 14 | LAM as initial Rx → HBV undetectability (57%) andALT normalization (57%) after 3 mo; LAM-resistance (57%) after median of 15 mo |
| Filik et al[31] (2006) | Prospective | 15 | LAM as initial Rx → HBV DNA undetectability (46.7%) after 2 yr |
| Yap et al[24] (2010) | Retrospective | 38 | LAM as initial Rx → LAM-resistance (64%) after 4 yr; improved long-term patient survival (83% vs 34% at 20-yr, P = 0.006) when compared to historical controls who had no anti-viral Rx |
| Adefovir | |||
| Fontaine et al[37] (2005) | Prospective | 11 | ADV as mono-therapy for LAM-resistant KTR → 5 log ↓ HBV DNA after 1 yr, only 1 patient had transient deterioration of allograft function |
| Kamar et al[40] (2009) | Prospective | 11 | ADV for LAM-resistant KTR → significant ↓ in HBV DNA (P = 0.01) and ALT normalization after 12 mo, ↑serum creatinine and proteinuria after 24 mo (P = 0.02) |
| Tse et al[43] (2010) | Retrospective | 4 | ADV for LAM-resistant KTR → 4 log ↓ HBV DNA and significant ↓ ALT levels (P = 0.029) after 18 mo, no significant change in allograft function |
| Lampertico et al[41] (2011) | Prospective | 11 | ADV as add-on Rx to LAM for LAM-resistant KTR → HBV undetectability (88%) after 3 yr; no significant changes in renal function and proteinuria |
| Lai et al[42] (2012) | Retrospective | 14 | ADV as mono- (n = 5) or add-on (n = 9) therapy in LAM-resistant KTR → HBV DNA undetectability [5 (35.7%) and 6 (42.8%) patients]after 12 and 24 mo with no virological breakthrough; ALT normalization in 13 patients (92.8%) after 1 yr; moderate to severe renal insufficiency (29%) |
| Entecavir | |||
| Kamar et al[48] (2008) | Prospective | 10 | ETV for ADV-resistant (n = 9) or LAM-resistant (n = 1) KTR → HBV DNA undetectability (50%) after 16.5 mo |
| Hu et al[47] (2012) | Prospective | 27 | ETV in KTR patients without LAM-resistance → HBV DNA undetectability (96% and 100%) after 12 and 24 mo, with no virological breakthrough |
| Tenofovir | |||
| Daudé et al[52] (2011) | Prospective | 3 | TFV as mono-therapy → HBV DNA undetectability (43%); no changes in allograft function |
ADV: Adefovir; ALT: Alanine transaminase; ETV: Entecavir; LAM: Lamivudine; KTR: Kidney transplant recipients; TFV: Tenofovir; HBV: Hepatitis B virus.
Lamivudine
Since lamivudine is the first amongst this class of drugs available for clinical use, it has yielded the majority of data on the management of HBsAg-positive renal transplant recipients. Lamivudine given as either prophylactic or pre-emptive treatment was proven superior to salvage therapy when liver dysfunction is evident[11,31]. Data from our group and other investigators have demonstrated that lamivudine was effective in suppressing HBV DNA and improving liver transaminase levels[1,22,32]. A meta-analysis which pooled data from 14 prospective clinical trials (a total of 184 patients) supported these observations[33]. With lamivudine as initial treatment, the mean rate of effective HBV DNA suppression, HBeAg clearance, alanine transaminase (ALT) normalization, and lamivudine-resistance was 91% (95%CI: 86%-96%), 27% (95%CI: 16%-39%), 81% (95%CI: 70%-92%), and 18% (95%CI: 10%-37%) respectively after a mean duration of 14 mo. The frequency of HBeAg seroconversion and lamivudine resistance correlated positively with treatment duration. Most importantly, treatment with lamivudine was associated with significantly improved patient survival[1,10,24]. With the use of lamivudine, the 10-year patient survival rate in HBsAg-positive renal transplant recipients was 81% and such results were nearly comparable to HBsAg-negative patients[24]. Although antiviral treatment has led to reduced mortality as a result of decreased hepatic complications (P = 0.036), liver-related deaths still accounted for 40% of mortalities in HBsAg-positive patients in the era of effective antiviral therapies, and 22.2% of all deaths that occurred in patients who had received antiviral treatment[24]. Prolonged treatment with lamivudine is associated with progressive increase in drug resistance and the cumulative probability of developing lamivudine-resistance was approximately 60% after 69 mo[24,33-35]. The emergence of lamivudine resistance can be associated with liver dysfunction, although one recent study showed that drug resistance did not have a significant negative impact on liver stiffness score, rate of HBeAg seroconversion rate, incidence of liver failure or hepatocellular carcinoma, or patient survival over 10-14 years of follow-up when rescue antiviral therapies are available[24].
Adefovir
Adefovir has similar activity against both wild-type and lamivudine-resistant HBV, this drug is nephrotoxic and the major clinical application of this antiviral agent is for the management of lamivudine-resistance[36]. Data regarding the management of lamivudine-resistance in kidney transplant recipients is relatively limited[37-39]. Prior to the availability of alternative nucleoside/tide analogues, it was the usual practice to continue lamivudine in patients who had developed lamivudine-resistance. Since the introduction of adefovir, there have been reports on its short-term efficacy either as mono- or add-on therapy in kidney transplant recipients[37-42]. The study by Fontaine et al[37] examined the use of adefovir as monotherapy in 11 post-kidney transplant patients with dosage adjustment according to renal function. Adefovir treatment led to a significant decline in serum HBV DNA with no virological breakthrough at one year and the drug was well-tolerated. Others have reported that adefovir as add-on therapy to lamivudine resulted in undetectable HBV DNA levels in 35.7%, 42.8% and 88.0% of lamivudine-resistant renal transplant recipients after 12, 24 and 36 mo[41,42]. There was no virological breakthrough and normalization of ALT was achieved in 92.8% of patients after 12 mo of treatment[42]. However, the virological response could be variable and relatively slow when compared with treatment-naïve subjects[43]. Nevertheless, rescue therapy with adefovir resulted in significantly better viral suppression and liver biochemistry compared with continuation of lamivudine (75% vs 14.3% had persistent normalization of ALT), and the clinical response was sustained for at least 24 mo[24]. Evidence of nephrotoxicity was observed in 30%-50% of renal allograft recipients despite dosage adjustment, and could necessitate treatment discontinuation[41,42]. In our experience, using adefovir in patients with serum creatinine below 150 μmol/L or creatinine clearances above 40 mL/min appeared safe, without evidence of worsening of renal allograft function during follow-up[24]. However, one must appreciate that the antiviral activity of adefovir at the currently approved dose is relatively weak, and efficacy could be further reduced with dose adjustment according to renal dysfunction.
Entecavir, tenofovir and telbivudine
Entecavir is effective in both treatment-naïve and lamivudine-resistant patients[44,45]. In immunosuppressed treatment-naïve post-renal transplant patients who required prolonged antiviral administration, entecavir is preferred due to its high resistance barrier and favorable safety profile[44,46]. A recent 2-year prospective study showed that the use of entecavir in treatment-naïve renal transplant recipients resulted in undetectable HBV DNA levels in 70%, 74%, 96% and 100% of patients after 12, 24, 52 and 104 wk respectively[47]. In this study, entecavir was associated with a more potent response than lamivudine and the tolerability profile was favorable. Experience regarding the use of entecavir in renal transplant recipients who had developed lamivudine- or adefovir-resistance had been examined in a small study with 10 solid organ transplant recipients (8 kidney allograft recipients)[48]. Treatment with entecavir resulted in an appreciable drop in HBV DNA levels and a 50% HBV undetectability in both HBeAg-positive and HBeAg-negative patients after 16.5 mo of therapy. Previously we had also reported the efficacy and tolerability of entecavir in lamivudine-resistant kidney allograft recipients, and showed that the virological response could be variable and relatively slower compared with treatment-naïve subjects[24,43]. Thus the response to entecavir in lamivudine-resistant subjects, and the subsequent emergence of entecavir-resistance, should be carefully monitored[49].
Tenofovir shows high efficacy in the treatment of treatment-naïve or lamivudine-resistant HBV infection[45,50]. There is little data in the renal transplant setting, and there is concern on its potential nephrotoxicity[51]. Daudé et al[52] reported the favorable short-term virological response and renal function stability in 7 solid organ transplant recipients (3 kidney allograft recipients) with a follow-up of 12 mo. Larger studies with longer follow-up duration are warranted to ascertain the long-term efficacy and effect on kidney allograft function. There is currently no data on the use of telbivudine in renal transplant recipients but it would be worthwhile to explore the use of this agent in treatment-naïve kidney allograft recipients given its relatively low resistance rate, lack of nephrotoxicity, and the relatively lower cost compared with other nucleoside/tide analogues[53,54].
CONCLUSION
The outcome and management of HBsAg-positive kidney transplant recipients have changed dramatically over the past few decades (Figure 1). Prior to the advent of effective and safe therapy, HBV infection had such a severe negative impact on patient survival that some centres regarded HBsAg sero-positivity as a contraindication against kidney transplantation. In the era of effective nucleoside/tide analogue therapy the 8-10 year survival rate of HBsAg-positive kidney transplant recipients is approaching that of HBsAg-negative subjects. The access to optimal therapy is limited by the cost of drugs in some places, unfortunately often in endemic regions when the treatment is needed most. The management of patients with drug resistant HBV infection remains a challenge, as is the nephrotoxic impact of some effective anti-viral agents. Apart from the treatment of HBV infection with anti-viral agents, the importance of regular surveillance for liver complications cannot be over-emphasized. In this regard, the data clearly shows that early detection of liver tumour with ultrasound and alpha-fetoprotein level measurement markedly increases the resection rate and patient survival[55-57].
Figure 1.
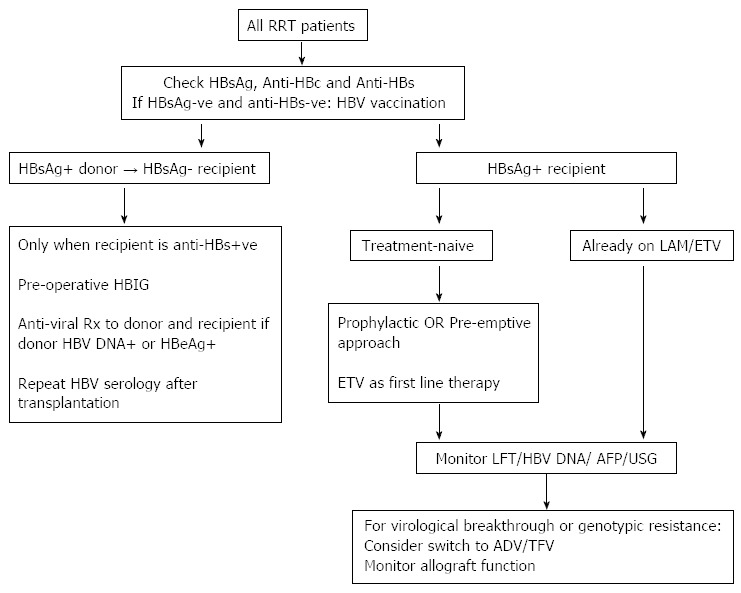
Management algorithm of hepatitis B virus infection in renal transplant recipients. ADV: Adefovir; AFP: Alpha-fetoprotein; ETV: Entecavir; HBIG: Hepatitis B hyperimmune globulin; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; LAM: Lamivudine; LFT: Liver function test; RRT: Renal replacement therapy; TFV: Tenofovir; USG: Ultrasonography.
Footnotes
Supported by Wai Hung Charitable Foundation, Endowment Fund
P- Reviewers: Dongiovanni P, Sanai FM S- Editor: Cui XM L- Editor: A E- Editor: Zhang DN
References
- 1.Chan TM, Fang GX, Tang CS, Cheng IK, Lai KN, Ho SK. Preemptive lamivudine therapy based on HBV DNA level in HBsAg-positive kidney allograft recipients. Hepatology. 2002;36:1246–1252. doi: 10.1053/jhep.2002.36156. [DOI] [PubMed] [Google Scholar]
- 2.Pirson Y, Alexandre GP, Ypersele C. Long-term effect of hbs antigenemia on patient survival after renal transplantation. N Engl J Med. 1977;296:194–196. doi: 10.1056/NEJM197701272960404. [DOI] [PubMed] [Google Scholar]
- 3.Parfrey PS, Forbes RD, Hutchinson TA, Beaudoin JG, Dauphinee WD, Hollomby DJ, Guttmann RD. The clinical and pathological course of hepatitis B liver disease in renal transplant recipients. Transplantation. 1984;37:461–466. doi: 10.1097/00007890-198405000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Harnett JD, Zeldis JB, Parfrey PS, Kennedy M, Sircar R, Steinmann TI, Guttmann RD. Hepatitis B disease in dialysis and transplant patients. Further epidemiologic and serologic studies. Transplantation. 1987;44:369–376. doi: 10.1097/00007890-198709000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Rao KV, Kasiske BL, Anderson WR. Variability in the morphological spectrum and clinical outcome of chronic liver disease in hepatitis B-positive and B-negative renal transplant recipients. Transplantation. 1991;51:391–396. [PubMed] [Google Scholar]
- 6.Bang BK, Yang CW, Yoon SA, Kim YS, Chang YS, Yoon YS, Koh YB. Prevalence and clinical course of hepatitis B and hepatitis C liver disease in ciclosporin-treated renal allograft recipients. Nephron. 1995;70:397–401. doi: 10.1159/000188635. [DOI] [PubMed] [Google Scholar]
- 7.Parfrey PS, Forbes RD, Hutchinson TA, Kenick S, Farge D, Dauphinee WD, Seely JF, Guttmann RD. The impact of renal transplantation on the course of hepatitis B liver disease. Transplantation. 1985;39:610–615. doi: 10.1097/00007890-198506000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Fornairon S, Pol S, Legendre C, Carnot F, Mamzer-Bruneel MF, Brechot C, Kreis H. The long-term virologic and pathologic impact of renal transplantation on chronic hepatitis B virus infection. Transplantation. 1996;62:297–299. doi: 10.1097/00007890-199607270-00025. [DOI] [PubMed] [Google Scholar]
- 9.Mathurin P, Mouquet C, Poynard T, Sylla C, Benalia H, Fretz C, Thibault V, Cadranel JF, Bernard B, Opolon P, et al. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29:257–263. doi: 10.1002/hep.510290123. [DOI] [PubMed] [Google Scholar]
- 10.Chan TM, Wu PC, Li FK, Lai CL, Cheng IK, Lai KN. Treatment of fibrosing cholestatic hepatitis with lamivudine. Gastroenterology. 1998;115:177–181. doi: 10.1016/s0016-5085(98)70380-4. [DOI] [PubMed] [Google Scholar]
- 11.Oguchi H, Miyasaka M, Tokunaga S, Hora K, Ichikawa S, Ochi T, Yamada K, Nagasawa M, Kanno Y, Aizawa T. Hepatitis virus infection (HBV and HCV) in eleven Japanese hemodialysis units. Clin Nephrol. 1992;38:36–43. [PubMed] [Google Scholar]
- 12.Su Y, Yan R, Duan Z, Norris JL, Wang L, Jiang Y, Xing W, Chen Y, Xiao Y, Li L, et al. Prevalence and risk factors of hepatitis C and B virus infections in hemodialysis patients and their spouses: a multicenter study in Beijing, China. J Med Virol. 2013;85:425–432. doi: 10.1002/jmv.23486. [DOI] [PubMed] [Google Scholar]
- 13.Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18:52–61. doi: 10.1111/j.1525-139X.2005.18108.x. [DOI] [PubMed] [Google Scholar]
- 14.Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis. 2010;14:1–21, vii. doi: 10.1016/j.cld.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Han DJ, Kim TH, Park SK, Lee SK, Kim SB, Yang WS, Park JS, Jung JG, Yu ES, Kim SC. Results on preemptive or prophylactic treatment of lamivudine in HBsAg (+) renal allograft recipients: comparison with salvage treatment after hepatic dysfunction with HBV recurrence. Transplantation. 2001;71:387–394. doi: 10.1097/00007890-200102150-00008. [DOI] [PubMed] [Google Scholar]
- 16.DaRoza G, Loewen A, Djurdjev O, Love J, Kempston C, Burnett S, Kiaii M, Taylor PA, Levin A. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis. 2003;42:1184–1192. doi: 10.1053/j.ajkd.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Rangel MC, Coronado VG, Euler GL, Strikas RA. Vaccine recommendations for patients on chronic dialysis. The Advisory Committee on Immunization Practices and the American Academy of Pediatrics. Semin Dial. 2000;13:101–107. doi: 10.1046/j.1525-139x.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 18.Choy BY, Peiris JS, Chan TM, Lo SK, Lui SL, Lai KN. Immunogenicity of intradermal hepatitis B vaccination in renal transplant recipients. Am J Transplant. 2002;2:965–969. doi: 10.1034/j.1600-6143.2002.21014.x. [DOI] [PubMed] [Google Scholar]
- 19.Wolf JL, Perkins HA, Schreeder MT, Vincenti F. The transplanted kidney as a source of hepatitis B infection. Ann Intern Med. 1979;91:412–413. doi: 10.7326/0003-4819-91-3-412. [DOI] [PubMed] [Google Scholar]
- 20.Wachs ME, Amend WJ, Ascher NL, Bretan PN, Emond J, Lake JR, Melzer JS, Roberts JP, Tomlanovich SJ, Vincenti F. The risk of transmission of hepatitis B from HBsAg(-), HBcAb(+), HBIgM(-) organ donors. Transplantation. 1995;59:230–234. [PubMed] [Google Scholar]
- 21.Jiang H, Wu J, Zhang X, Wu D, Huang H, He Q, Wang R, Wang Y, Zhang J, Chen J. Kidney transplantation from hepatitis B surface antigen positive donors into hepatitis B surface antibody positive recipients: a prospective nonrandomized controlled study from a single center. Am J Transplant. 2009;9:1853–1858. doi: 10.1111/j.1600-6143.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- 22.Fabrizi F, Martin P, Dixit V, Kanwal F, Dulai G. HBsAg seropositive status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant. 2005;5:2913–2921. doi: 10.1111/j.1600-6143.2005.01113.x. [DOI] [PubMed] [Google Scholar]
- 23.Fairley CK, Mijch A, Gust ID, Nichilson S, Dimitrakakis M, Lucas CR. The increased risk of fatal liver disease in renal transplant patients who are hepatitis Be antigen and/or HBV DNA positive. Transplantation. 1991;52:497–500. doi: 10.1097/00007890-199109000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Yap DY, Tang CS, Yung S, Choy BY, Yuen MF, Chan TM. Long-term outcome of renal transplant recipients with chronic hepatitis B infection-impact of antiviral treatments. Transplantation. 2010;90:325–330. doi: 10.1097/TP.0b013e3181e5b811. [DOI] [PubMed] [Google Scholar]
- 25.Sonneveld MJ, Janssen HL. Pros and Cons of Peginterferon Versus Nucleos(t)ide Analogues for Treatment of Chronic Hepatitis B. Curr Hepat Rep. 2010;9:91–98. doi: 10.1007/s11901-010-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuen MF, Hui CK, Cheng CC, Wu CH, Lai YP, Lai CL. Long-term follow-up of interferon alfa treatment in Chinese patients with chronic hepatitis B infection: The effect on hepatitis B e antigen seroconversion and the development of cirrhosis-related complications. Hepatology. 2001;34:139–145. doi: 10.1053/jhep.2001.25273. [DOI] [PubMed] [Google Scholar]
- 27.Marcellin P, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Jin R, Gurel S, Lu ZM, Wu J, et al. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136:2169–2179.e1-4. doi: 10.1053/j.gastro.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Rostaing L, Modesto A, Baron E, Cisterne JM, Chabannier MH, Durand D. Acute renal failure in kidney transplant patients treated with interferon alpha 2b for chronic hepatitis C. Nephron. 1996;74:512–516. doi: 10.1159/000189444. [DOI] [PubMed] [Google Scholar]
- 29.Durlik M, Gaciong Z, Rowińska D, Rancewicz Z, Lewandowska D, Kozłowska B, Wyzgał J, Soluch L, Walewska-Zielecka B, Rowiński W, et al. Long-term results of treatment of chronic hepatitis B, C and D with interferon-alpha in renal allograft recipients. Transpl Int. 1998;11 Suppl 1:S135–S139. doi: 10.1007/s001470050445. [DOI] [PubMed] [Google Scholar]
- 30.Sanai FM, Mousa D, Al-Mdani A, Al-Shoail G, Al-Ashgar H, Al Meshari K, Al-Qahtani A, Saadeh M, Bzeizi KI, Aleid H. Safety and efficacy of peginterferon-α2a plus ribavirin treatment in renal transplant recipients with chronic hepatitis C. J Hepatol. 2013;58:1096–1103. doi: 10.1016/j.jhep.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Filik L, Karakayali H, Moray G, Dalgiç A, Emiroğlu R, Ozdemir N, Colak T, Gür G, Yilmaz U, Haberal M. Lamivudine therapy in kidney allograft recipients who are seropositive for hepatitis B surface antigen. Transplant Proc. 2006;38:496–498. doi: 10.1016/j.transproceed.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 32.Rostaing L, Henry S, Cisterne JM, Duffaut M, Icart J, Durand D. Efficacy and safety of lamivudine on replication of recurrent hepatitis B after cadaveric renal transplantation. Transplantation. 1997;64:1624–1627. doi: 10.1097/00007890-199712150-00025. [DOI] [PubMed] [Google Scholar]
- 33.Fabrizi F, Dulai G, Dixit V, Bunnapradist S, Martin P. Lamivudine for the treatment of hepatitis B virus-related liver disease after renal transplantation: meta-analysis of clinical trials. Transplantation. 2004;77:859–864. doi: 10.1097/01.tp.0000116448.97841.6d. [DOI] [PubMed] [Google Scholar]
- 34.Thabut D, Thibault V, Bernard-Chabert B, Mouquet C, Di Martino V, Le Calvez S, Opolon P, Benhamou Y, Bitker MO, Poynard T. Long-term therapy with lamivudine in renal transplant recipients with chronic hepatitis B. Eur J Gastroenterol Hepatol. 2004;16:1367–1373. doi: 10.1097/00042737-200412000-00022. [DOI] [PubMed] [Google Scholar]
- 35.Chan TM, Tse KC, Tang CS, Lai KN, Ho SK. Prospective study on lamivudine-resistant hepatitis B in renal allograft recipients. Am J Transplant. 2004;4:1103–1109. doi: 10.1111/j.1600-6143.2004.00467.x. [DOI] [PubMed] [Google Scholar]
- 36.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–1751. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Fontaine H, Vallet-Pichard A, Chaix ML, Currie G, Serpaggi J, Verkarre V, Varaut A, Morales E, Nalpas B, Brosgart C, et al. Efficacy and safety of adefovir dipivoxil in kidney recipients, hemodialysis patients, and patients with renal insufficiency. Transplantation. 2005;80:1086–1092. doi: 10.1097/01.tp.0000178305.39231.a2. [DOI] [PubMed] [Google Scholar]
- 38.Garcia A, Mazuecos A, González P, Diaz F, Garcia T, Ceballos M, Rivero M. Treatment with adefovir dipivoxil in a renal transplant patient with renal insufficiency and lamivudine-resistant hepatitis B infection. Transplant Proc. 2005;37:1462–1463. doi: 10.1016/j.transproceed.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 39.de Silva HJ, Dassanayake AS, Manamperi A, de Silva AP. Treatment of lamivudine-resistant hepatitis B infection in post-renal transplant patients with adefovir dipivoxil: preliminary results. Transplant Proc. 2006;38:3118–3120. doi: 10.1016/j.transproceed.2006.08.186. [DOI] [PubMed] [Google Scholar]
- 40.Kamar N, Huart A, Tack I, Alric L, Izopet J, Rostaing L. Renal side effects of adefovir in hepatitis B virus-(HBV) positive kidney allograft recipients. Clin Nephrol. 2009;71:36–42. doi: 10.5414/cnp71036. [DOI] [PubMed] [Google Scholar]
- 41.Lampertico P, Viganò M, Facchetti F, Invernizzi F, Aroldi A, Lunghi G, Messa PG, Colombo M. Long-term add-on therapy with adefovir in lamivudine-resistant kidney graft recipients with chronic hepatitis B. Nephrol Dial Transplant. 2011;26:2037–2041. doi: 10.1093/ndt/gfr174. [DOI] [PubMed] [Google Scholar]
- 42.Lai HW, Chang CC, Chen TH, Tsai MC, Chen TY, Lin CC. Safety and efficacy of adefovir therapy for lamivudine-resistant hepatitis B virus infection in renal transplant recipients. J Formos Med Assoc. 2012;111:439–444. doi: 10.1016/j.jfma.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Tse KC, Yap DY, Tang CS, Yung S, Chan TM. Response to adefovir or entecavir in renal allograft recipients with hepatitic flare due to lamivudine-resistant hepatitis B. Clin Transplant. 2010;24:207–212. doi: 10.1111/j.1399-0012.2009.01090.x. [DOI] [PubMed] [Google Scholar]
- 44.Yuen MF, Seto WK, Fung J, Wong DK, Yuen JC, Lai CL. Three years of continuous entecavir therapy in treatment-naïve chronic hepatitis B patients: VIRAL suppression, viral resistance, and clinical safety. Am J Gastroenterol. 2011;106:1264–1271. doi: 10.1038/ajg.2011.45. [DOI] [PubMed] [Google Scholar]
- 45.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 46.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 47.Hu TH, Tsai MC, Chien YS, Chen YT, Chen TC, Lin MT, Chang KC, Chiu KW. A novel experience of antiviral therapy for chronic hepatitis B in renal transplant recipients. Antivir Ther. 2012;17:745–753. doi: 10.3851/IMP2097. [DOI] [PubMed] [Google Scholar]
- 48.Kamar N, Milioto O, Alric L, El Kahwaji L, Cointault O, Lavayssière L, Sauné K, Izopet J, Rostaing L. Entecavir therapy for adefovir-resistant hepatitis B virus infection in kidney and liver allograft recipients. Transplantation. 2008;86:611–614. doi: 10.1097/TP.0b013e3181806c8c. [DOI] [PubMed] [Google Scholar]
- 49.Yim HJ, Seo YS, Yoon EL, Kim CW, Lee CD, Park SH, Lee MS, Park CK, Chae HB, Kim MY, et al. Adding adefovir vs. switching to entecavir for lamivudine-resistant chronic hepatitis B (ACE study): a 2-year follow-up randomized controlled trial. Liver Int. 2013;33:244–254. doi: 10.1111/liv.12036. [DOI] [PubMed] [Google Scholar]
- 50.Jenh AM, Thio CL, Pham PA. Tenofovir for the treatment of hepatitis B virus. Pharmacotherapy. 2009;29:1212–1227. doi: 10.1592/phco.29.10.1212. [DOI] [PubMed] [Google Scholar]
- 51.Krummel T, Parvez-Braun L, Frantzen L, Lalanne H, Marcellin L, Hannedouche T, Moulin B. Tenofovir-induced acute renal failure in an HIV patient with normal renal function. Nephrol Dial Transplant. 2005;20:473–474. doi: 10.1093/ndt/gfh640. [DOI] [PubMed] [Google Scholar]
- 52.Daudé M, Rostaing L, Sauné K, Lavayssière L, Basse G, Esposito L, Guitard J, Izopet J, Alric L, Kamar N. Tenofovir therapy in hepatitis B virus-positive solid-organ transplant recipients. Transplantation. 2011;91:916–920. doi: 10.1097/TP.0b013e3182100f59. [DOI] [PubMed] [Google Scholar]
- 53.Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, Heathcote EJ, Rasenack J, Bzowej N, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576–2588. doi: 10.1056/NEJMoa066422. [DOI] [PubMed] [Google Scholar]
- 54.Gane EJ, Wang Y, Liaw YF, Hou J, Thongsawat S, Wan M, Moon YM, Jia J, Chao YC, Niu J, et al. Efficacy and safety of prolonged 3-year telbivudine treatment in patients with chronic hepatitis B. Liver Int. 2011;31:676–684. doi: 10.1111/j.1478-3231.2011.02490.x. [DOI] [PubMed] [Google Scholar]
- 55.Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–1014. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 56.Zoli M, Magalotti D, Bianchi G, Gueli C, Marchesini G, Pisi E. Efficacy of a surveillance program for early detection of hepatocellular carcinoma. Cancer. 1996;78:977–985. doi: 10.1002/(SICI)1097-0142(19960901)78:5<977::AID-CNCR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 57.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]


