Abstract
It is estimated that 30% of the adult population in Japan is affected by nonalcoholic fatty liver disease (NAFLD). Fatty changes of the liver are generally diagnosed using imaging methods such as abdominal ultrasonography (US) and computed tomography (CT), but the sensitivity of these imaging techniques is low in cases of mild steatosis. Alanine aminotransferase levels may be normal in some of these patients, warranting the necessity to establish a set of parameters useful for detecting NAFLD, and the more severe form of the disease, nonalcoholic steatohepatitis (NASH). Although liver biopsy is currently the gold standard for diagnosing progressive NASH, it has many drawbacks, such as sampling error, cost, and risk of complications. Furthermore, it is not realistic to perform liver biopsies on all NAFLD patients. Diagnosis of NASH using various biomarkers, scoring systems and imaging methods, such as elastography, has recently been attempted. The NAFIC score, calculated from the levels of ferritin, fasting insulin, and type IV collagen 7S, is useful for the diagnosis of NASH, while the NAFLD fibrosis score and the FIB-4 index are useful for excluding NASH in cases of advanced fibrosis. This article reviews the limitations and merits of liver biopsy and noninvasive diagnostic tests in the diagnosis of NAFLD/NASH.
Keywords: Nonalcoholic fatty liver disease, Liver biopsy, Steatosis, Fibrosis, Nonalcoholic steatohepatitis
Core tip: Liver biopsies remain a gold standard, although the procedure has several limitations for the diagnosis of nonalcoholic steatohepatitis (NASH). The NAFIC score, calculated from the levels of ferritin, fasting insulin and type IV collagen 7S, is useful for diagnosing NASH, while the nonalcoholic fatty liver disease fibrosis score and the FIB-4 index are useful for excluding NASH in cases of advanced fibrosis.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent form of chronic liver disease in the world. According to a cooperative study group comprised of 10 institutions in Japan [Japan Study Group of NAFLD (JSG-NAFLD)], 29.7% of health checkup examinees (41.0% of men and 17.7% of women) had NAFLD[1], making it a major national disease of the 21st century. The long-term outcomes of NAFLD patients have been reported in several studies. Compared with matched control populations, NAFLD patients have an increased overall mortality, with the most common cause of death being cardiovascular disease (28% of total deaths). In addition, there is an increased risk of death from a variety of extrahepatic malignancies (25% of total deaths) and from liver disease (13% of total deaths), which is the third leading cause of death for these patients and the eleventh leading cause in the general population[2]. NAFLD can be classified as either nonalcoholic steatohepatitis (NASH) or simple steatosis. NASH carries a high risk of liver disease-related mortality such as deaths from hepatic cirrhosis and hepatocellular carcinoma. Simple steatosis, however, has a low risk of liver disease-related mortality. NASH can be differentiated from simple steatosis only by liver biopsy and is diagnosed when all of the following 3 criteria are met: (1) macrovesicular fatty change of hepatocytes; (2) inflammatory cell infiltration; and (3) ballooning degeneration of hepatocytes. However, liver biopsy is invasive, has drawbacks such as sampling error and cost and is not possible for all NAFLD patients. Thus, it is necessary to establish a method to efficiently detect progressive NASH in NAFLD patients to decrease liver disease-related mortality. This review summarizes the current limitations and problems of liver biopsy and noninvasive diagnostic methods for NAFLD/NASH in Japan and other countries and outlines future prospects for improved diagnostic practices.
NAFLD DIAGNOSIS
According to the latest guidelines established by the American Association for the Study of Liver Diseases (AASLD)[3], NAFLD is diagnosed when the following 4 criteria are met: (1) fatty change of the liver is observed by imaging or histologically; (2) no marked alcohol drinking habit is present (ethanol intake of < 210 g/wk for men and < 140 g/wk for women); (3) no presence of other factors inducing fatty change of the liver; and (4) no concomitant factors causing chronic liver disease are present. This section of the review focuses on diagnostic imaging methods and scoring systems for fatty change of the liver.
Usefulness and limitations of imaging methods in diagnosing fatty change of the liver
Simple, minimally invasive ultrasonography (US) is used for the imaging diagnosis of fatty liver in many cases. However, the sensitivity is low in mild cases with a fatty change of less than 20%-30%[4,5]. The dependency of the diagnosis on the subjective judgments of operators is also problematic[6]. Computed tomography (CT) is objective and capable of measuring the amount of visceral fat[6,7], but radiation exposure and cost are negative aspects of this methodology. Moreover, although fatty liver is diagnosed when the liver-to-spleen CT ratio (the L/S ratio) is below 0.9, the sensitivity is not high, and fatty liver cannot be ruled out even if the L/S ratio is 0.9 or higher[8]. Particularly, in cases of obesity and metabolic syndrome and in the absence of other factors inducing abnormal liver function, NAFLD/NASH should be considered even if fatty liver is not evident by imaging. It has been revealed that NAFLD/NASH is latently present in patients who are monitored for liver disorder of unknown causes. When liver biopsy was performed in 354 patients with abnormal liver function and in whom the disease could not be definitely diagnosed serologically, 64% had NAFLD[9]. In another study, liver biopsy was performed in 81 patients with chronic abnormal liver function of unknown cause, and simple steatosis and NASH were observed in 41 and 26 patients, respectively[10], suggesting the importance of performing liver biopsy. The severity of fatty change is not correlated with the advancement of fibrosis; rather, it decreases with the progression of fibrosis in NASH. Therefore, the grade of fatty change from imaging analysis should not be employed as an evaluation criterion for NAFLD severity. Magnetic resonance (MR) spectroscopy is reportedly the most accurate method for the quantification of fatty change[7,11-13], but currently, its use is limited to research.
The usefulness of US for the diagnosis of NAFLD is evaluated, to some extent, because of its simplicity. Recently, quantification of fatty change using US to supplement elastography has also occasionally been reported, and further development of this application is expected[14]. It is impossible to differentiate between NASH and simple steatosis using any imaging methods. At the same time, certain US and CT findings, such as irregularity of the liver surface, blunt margins of the liver, and splenomegaly, suggest the presence of chronic liver diseases, including NASH with advanced fibrosis, and can indicate the need for further attention. It has recently been reported that the differentiation between NASH and simple steatosis is possible using contrast-enhanced US[15].
Scoring systems for diagnosing fatty change of the liver
Because imaging has limited diagnostic value for NAFLD, as described above, the prediction of fatty change of the liver from general laboratory test values has been investigated. As shown in Table 1, various indices have been proposed, including the fatty liver index (FLI)[16], NAFLD liver fat score, hepatic steatosis index (HSI)[17], and Steato Test (ST)[18]. According to a report from Italy[19], FLI, calculated from the body mass index (BMI), waist circumference, and γ-glutamyl transferase (γGT) and triglyceride (TG) levels, is an independent risk factor for liver-related mortality. HSI, formulated based on data from approximately 10000 Korean patients, is a simple index calculated only from BMI, the aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio (AAR), sex, and the presence or absence of diabetes mellitus (DM)[17] Both the sensitivity and specificity of HSI are favorable compared to that observed in other scoring systems. A validation study involving Japanese patients is expected.
Table 1.
Indexes for the prediction of liver steatosis
| Index | Author(nation) | Paper (yr) | No. of subjects(fatty liver/ non-fatty liver) | Parameters | Cutoff values | Sens-itivity | Speci-ficity | AUROC | Diagnostic methods for hepatic steatosis |
| FLI | Bedogni(Italy) | BMC Gastroenterology (2006) | 228/268 | BMI, waist circumference, triglyceride, γGT | < 30 > 60 | 87.0%61.0% | 64.0%86.0% | 0.84 | US |
| NAFLD liver fat score1 | Kontronen(Finland) | Gastroenterology (2009) | 470 | MetS, type II diabetes, IRI, AST, AST/ALT ratio | -0.64 | 86.0% | 71.0% | 0.8720.863 | MRS |
| HSI | Lee (South Korea) | Dig Liver Dis (2010) | 5362/5362 (sex- and age- matched) | 8 × AST/ALT ratio + BMI + (+ 2 for females, + 2 for diabetes) | < 30 > 36 | 93.1% | 92.4% | 0.812 | US |
| ST | Poynaud(France) | Comp Hepatol(2005) | 744/140 | 12 parameters4 | 0.30 0.72 | 90% | 90.0% | 0.7920.8030.8630.723 | Biopsy |
| Park (South Korea) | Korean J Hepatol(2011) | 145/311 | ALT/AST > 1.5 (= 1 point)γGT > 50 IU/L (= 1 point)TG > 150 mg/dL (= 1 point)BMI 23-24.9 (= 2 points) ≥ 25 (= 3 points) | 3 | 71.7% | 75.9% | 0.797 | US | |
| Bajaj(India) | Indian J Med Res (2009) | 39/82 | IRI +1.6 × BMI + 1.9 × FPG | 1.6 | 84.6% | 76.0% | 0.76 | US |
PNPLA3 did not improve diagnostic accuracies;
Estimation group;
Validation group;
Alanine aminotransferase (ALT), α2-macroglobulin, apolipoprotein A-I, haptoglobin, total bilirubin, γ-glutamyl transferase (γGT), cholesterol, triglycerides, glucose, age, gender and body mass index (BMI). ROC: Receiver operating characteristics curve; US: Ultrasonography; MetS: Metabolic syndrome; MRS: Magnetic resonance spectroscopy, IRI: Immuno-reactive insulin; FPG: Fasting plasma glucose. FLI: Fatty liver index; HSI: Hepatic steatosis index; ST: Steato Test; AST: Aspartate aminotransferase; NAFLD: Nonalcoholic fatty liver disease; AUROC: Area under the receiver operating characteristic curve.
CURRENT STATUS AND PROBLEMS OF LIVER BIOPSY FOR DIAGNOSIS OF NASH: IS LIVER BIOPSY NECESSARY?
Pros and cons of liver biopsy for NAFLD
There is some controversy surrounding whether liver biopsy should be actively performed to make a definite diagnosis of NAFLD and its prognosis and to differentiate it from other diseases or if it should be avoided as much as possible[20]. Liver biopsy is essential to the definite diagnosis of NASH and is considered very useful in differentiating NASH from other diseases, making a prognosis, and judging the effects of therapeutic intervention. However, liver biopsy is inefficient in many non-advanced cases and has several drawbacks, such as sampling error and high cost, as described below. Furthermore, pathologists differ in their diagnosis and recognition of liver biopsy results, and there is no established treatment method for NASH even when it is diagnosed by liver biopsy. In the guidelines recently published by the AASLD, liver biopsy is suggested for complications of metabolic syndrome and a high serum ferritin level in patients with NASH, as well as in those suspected of having advanced fibrosis[3].
Limitations of liver biopsy
Sampling error: Only 1/50000 of the whole liver tissue is sampled during a liver biopsy, for which sampling error is of concern. To prevent sampling errors, it is essential to collect a sufficient amount of tissue; the use of a thick needle[21] and collection of 2 or more samples with a sufficient length are recommended. Making an accurate diagnosis of NASH is dependent on the length of the specimens[22], with a necessary length of 15-16 mm or longer to accurately evaluate fibrosis[23]. Ratziu et al[24] excised and compared two percutaneous liver biopsy samples from each of 51 NAFLD patients and observed that the consistency in fatty change was relatively high (78%), but the fibrosis stage was different between the two samples in 41% of the patients. In 35% of the cases with bridging fibrosis observed in one sample, only mild or no fibrosis was noted in the other sample. The inconsistency in ballooning degeneration of hepatocytes, an essential feature for the diagnosis of NASH, was 18%, suggesting that NASH may be overlooked when only one sample is collected. In other reports, the results differed by one or more stages between specimens biopsied from the left and right lobes in 30% of the patients[25], and the inflammatory findings were more inconsistent than those of fatty change and fibrosis between biopsied specimens from the left and right lobes[26]. The assessment criteria for the pathological diagnosis recently proposed by the AASLD specify that the right lobe should be biopsied first, and when the left lobe is biopsied before treatment, a sample should also be biopsied from the left lobe after treatment to judge the therapeutic effect[27].
Inter- and intra-observer variability: Inter- and intra-observer variability also presents a serious problem for the pathological diagnosis of NAFLD. Younossi et al[28] reported that the evaluations of fatty change (κ = 0.64) and fibrosis (κ = 0.60) were highly consistent among observers, but that the evaluation of inflammatory activity was inconsistent at a high rate (κ =0.33). It has also been shown that inter-observer variability remained even when training in histopathological observation was provided in an effort to reduce these inconsistencies[29]. In that study, the post-intervention κ value (0.39) was not significantly different from the pre-intervention κ value (0.27). Measures to solve this problem are needed.
Risk and complications: Regarding the complications of liver biopsy, the incidence of pain is reportedly 20%, but it increases to 84% when a mildly unpleasant feeling is included in the assessment. The incidence of serious complications and mortality has been reported to be 0.3%-0.57% and 0.01%, respectively[30-32]. To decrease complications, operators that are trained by an instructor with sufficient experience should perform biopsies, and operation with a US guide and the use of an aspiration-type biopsy needle are recommended[33,34].
Problems with pathological diagnosis: The pathological features of typical NASH, in addition to fat deposition in hepatocytes, include inflammatory cell (neutrophil and lymphocyte) infiltration in lobules, ballooning degeneration of hepatocytes, Mallory-Denk bodies, pericellular fibrosis, sinusoidal fibrosis, giant mitochondria, eosinophilic necrosis, and iron deposition. However, few NASH patients show all of these typical findings, and there are no integrated criteria to diagnose NASH based on them. Matteoni et al[35] classified NAFLD into 4 types: type 1, fat deposition alone; type 2, fat deposition and inflammatory cell infiltration in the parenchyma; type 3, fat deposition and ballooning degeneration of hepatocytes; and type 4, type 3 criteria plus Mallory-Denk bodies or fibrosis. The authors observed that the liver disease-related mortality during an approximately 8-year follow-up period was only 1.7% in the type 1-plus-type 2 group, but significantly increased to 11% in the type 3-plus-type 4 group. They proposed the definition of types 3 and 4 as NASH from a prognostic viewpoint (Table 2). Later, Rafiq et al[36] followed the course for a longer period and reported that liver disease-related mortality was only 2.7% in the first group but was 17.5% in the second group. Based on these findings, the following consensus has been reached in Japan: the gold standard for the diagnosis of NASH is liver biopsy, and NASH is diagnosed when all of the following 3 pathological findings are observed (i.e., those in types 3 and 4 of Matteoni’s classification): (1) macrovesicular fatty change of hepatocytes; (2) inflammatory cell infiltration; and (3) ballooning degeneration of hepatocytes. However, the differentiation between types 2 and 3 of Matteoni’s classification depends on the judgment of ballooning degeneration of hepatocytes, which is subjectively made by observers. Thus, the Nonalcoholic Steatohepatitis Clinical Research Network (NASH-CRN) proposed the classification of these two types by scoring the severities of fatty change (0-3 points), inflammation (0-3), and ballooning degeneration of hepatocytes (0-2) (0-8 points in total) by a system termed the NAS scoring system[37]. Cases with a score of 5 or higher or 2 or lower are regarded as NASH and non-NASH, respectively, and those with a score between these values are regarded as borderline cases. A NAS validation study was performed at NASH-CRN-affiliated institutions, and the utility of the system was reported in the United States. However, some researchers deem a NASH threshold of 5 points or higher as too insensitive, and they believe that it should be set at 4 or higher[38]. NAS is markedly reproducible, requires no special staining, is applicable for pediatric NASH, and is useful for assessing therapeutic effects in clinical studies. However, NAS is incapable of diagnosing NASH in patients with burned-out NASH, in whom fatty changes and inflammatory cell infiltration resolving in fibrosis has progressed; i.e., inflammatory findings have been improved by treatment and only fibrosis remains. Moreover, a divergence has been reported in pathological diagnosis using NAS between general and liver-specialized pathologists[39]. It has recently been reported in the United States that Matteoni’s classification scheme more faithfully reflects the diagnosis and prognosis of NASH than NAS[40]. In the future, NAS may be used as an index for judging therapeutic effects rather than as a diagnosis tool for NASH. Therefore, it is desirable to employ Matteoni’s classification when a diagnostician skilled in diagnosing NASH is present. Matteoni’s classification is useful for routine clinical practice, single-facility clinical studies, and investigation of long-term prognosis, such as carcinogenesis. However, NAS is useful for multicenter clinical studies involving several diagnosticians, many patients, and evaluation of the short-term therapeutic effects of drugs. According to a new definition of NASH proposed by Younossi et al[41], NASH is diagnosed for (1) any degree of steatosis along with centrilobular ballooning and/or Mallory-Denk bodies or (2) any degree of steatosis along with centrilobular pericellular/perisinusoidal fibrosis or bridging fibrosis. Younossi’s criteria almost perfectly agree with Matteoni’s classification, and these two definitions of NASH correlated significantly with the prediction of a higher liver-related mortality rate. Younossi’s criteria, which placed high importance on the presence of fibrosis, would enable the diagnosis of burned-out NASH in patients. Finally, Younossi’s criteria are now accepted by the NAFLD/NASH clinical practice guideline committee (under the chairmanship of Prof. Sumio Watanabe, Juntendo University) organized by the Japan Society of Gastroenterology. In Japan, the diagnosis of NASH will be based on the presence of hepatic steatosis plus ballooning, Mallory-Denk bodies, or fibrosis in the near future (Table 2).
Table 2.
Pathological criteria for the diagnosis of nonalcoholic steatohepatitis
| Criteria (yr) | Classifications | Definitions of NASH | Characteristics |
| Matteoni (1999) | Type 1: steatosis aloneType 2: steatosis with inflammationType 3: steatosis with hepatocyte balloningType 4: Type 3 plus MDB or fibrosis | Type 3 or 4 | Depend on the subjective judgments of observers(existence of hepatocyte balloning)Well correlation with liver-related mortalityInflammation is not included |
| NAS (2005) | Steatosis (0-3) Inflammation (0-3) Hepatocyte balloning (0-2) Total: 0 to 8 | Total scores: 5 to 8 | Numerical scoreLow sensitivity, NAS ≥ 4 may be better Fibrosis is not includedNo significant correlation with liver-related mortality Recommended use for assessing the therapeutic effect during clinical studies |
| Younossi (2011) | SteatosisHepatocyte balloningMDBFibrosis | Steatosis + Hepatocyte balloningor + MDBor + Fibrosis | Inflammation is not includedWell correlation with Matteoni’s classificationCan diagnose so-called burned-out NASHEssential validation study |
MDB: Mallory-Denk bodies; NAS: Nonalcoholic fatty liver disease activity score; NASH: Nonalcoholic steatohepatitis.
Miscellaneous: Liver biopsies may be performed at outpatient clinics to reduce costs overseas, but biopsy patients are hospitalized for several days in Japan. Performing liver biopsies for all NAFLD patients in Japan, estimated at 10 million, would be prohibitively expensive, and no cost-benefit analysis has been performed to date. In regard to the follow-up after liver biopsy, Toyoda et al[42] reported a very low follow-up rate in NAFLD patients compared with that in viral hepatitis patients, suggesting the need for more patient education.
NONINVASIVE DIAGNOSTIC METHODS FOR NASH
Several extensive reviews from Western countries have previously discussed noninvasive diagnostic methods for NASH or advanced fibrosis[43-45]. However, most of these papers described a simple enumeration of noninvasive tests. Thus, we here review biomarkers or scoring systems with critical appraisal to establish diagnostic algorithms that can be applicable even for Asian patients with NAFLD in clinical practice. Various parameters of oxidative stress, inflammation, apoptosis, and fibrosis have been reported to be useful for the noninvasive diagnosis of NASH[46]. Interest in cytokeratin in viral and nonviral hepatitis has been rapidly increasing during recent years, especially as proposed circulating biomarkers of hepatic necrosis and apoptosis[47]. Among those, circulating levels of cytokeratin-18 (CK18) fragments have been investigated extensively as novel biomarkers for the presence of steatohepatitis in patients with NAFLD. A recent meta-analysis, consisting of 10 studies with 838 patients, showed that CK18 fragments may be a useful biomarker for screening NASH[48]. Although these are very encouraging results, currently, this assay is not commercially available. Furthermore, as each study utilized a study-specific cut-off value, there is not an established congruent cut-off value for identifying steatohepatitis. According to the AASLD guidelines, CK18 is not recommended in routine clinical practice[3].
Differentiation between NASH and simple steatosis
Yilmaz et al[45] extensively reviewed biochemical diagnostic tests for differentiating simple steatosis from NASH. Here, we summarize scoring systems including multiple serum tests. The first evaluation of NASH was the HAIR scoring system, reported from Australia. This system comprises three scored components-hypertension (HTN), ALT level, and insulin resistance (IR)-that were established based on data from 105 weight loss surgery-treated obese patients[49]. Later, Palekar et al[50] of the Mayo Clinic investigated 80 NAFLD patients and reported the use of six criteria - age ≥ 50 years old, female sex, BMI ≥ 30 kg/m2, AST ≥ 45 IU/L, AAR ≥ 0.8, and hyaluronic acid ≥ 55 ng/mL - of which any three, when met, allowed the diagnosis of NASH with a sensitivity and specificity of 74% and 66%, respectively. The NashTest, developed in Europe, predicts the disease on the basis of 13 parameters[51]. A recently proposed equation (2.627 × ln [AST] + 2.13 for DM) comprises only 2 items, AST and the presence or absence of DM, attaching greater importance to simplicity[52]. Campos et al[53] proposed a NASH clinical scoring system composed of HTN, type 2 DM, AST ≥ 27 IU/L, ALT ≥ 27 IU/L, sleep apnea syndrome, and race (other than blacks). Nice’s French group recently reported the Nice model, in which CK18, ALT, and the presence or absence of metabolic syndrome is scored[54]. However, it is unclear whether these scoring systems are applicable for Japanese NAFLD patients because these reports from Western countries were based on severely obese patients treated with bariatric surgery, and no validation study has been adequately performed.
In Japan, Shimada et al[55] reported that early NASH and simple steatosis could be differentiated by a combination of 3 values: adiponectin (≤ 4.0 μg/mL), homeostasis model assessment of insulin resistance (HOMA-IR) (≥ 3.0), and type 4 collagen 7S (≥ 5.0 ng/mL). However, adiponectin cannot be measured at general practice sites. JSG-NAFLD proposed the NAFIC score, which comprises three items-ferritin, fasting insulin, and type 4 collagen 7S - for the screening of NASH. These three variables were extracted as factors independently contributing to NASH in an analysis of 177 NAFLD patients. The NAFIC system assigns one point for 200 (female) or 300 (male) ng/mL or higher ferritin, one point for 10 μU/mL or higher fasting insulin, and two points for 5.0 ng/mL or higher type 4 collagen 7S. The total of these points is regarded as the NAFIC score (Table 3), and the possibility of NASH is high when the NAFIC score is 2 or higher. The usefulness of this scoring system has been verified in a validation study involving 442 patients[56]. The three variables constituting the NAFIC score are parameters associated with the pathology of NASH, such as oxidative stress, IR and fibrosis. The relevance of the scoring parameters to NASH pathology and the fact that no complex calculation is required are advantageous. However, there are also problems to be addressed, such as the scoring of insulin-treated patients, usefulness for races other than Japanese, cost, and coverage by national health insurance. No established scoring system to screen for NASH is currently available, but the utility of the NAFIC score is expected to be investigated by a large-scale study in Japan.
Table 3.
Scoring systems for picking up nonalcoholic steatohepatitis or severe fibrosis in nonalcoholic fatty liver disease
| Index | NAFIC score | NAFLD fibrosis score | FIB4 index | |||
| Object | Predicting NASH | Excluding severe fibrosis (stage 3-4) | ||||
| Formula | Ferritin > 200 (female), 300 (male) ng/mL (= 1 point) Fasting insulin > 10 μU/mL (= 1 point) Type 4 collagen 7S > 5.0 ng/mL (= 2 points) Total: 0-4 points | -1.675 + 0.037 × age (yr) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glycemia /DM (yes = 1, no = 0) + 0.99 × AAR – 0.013 × PLT (× 109/L) - 0.66 × Alb (g/dL) | Age (yr) × AST (IU/L)/(PLT (109/L) × √ALT (IU/L) | |||
| Cut-off values | 1 | 2 | -1.455 | 0.676 | 1.30 | 2.67 |
| Sensitivity | 94%1 | 66%1 | 82%1 | 51%1 | 74% | 33% |
| 88%2 | 60%2 | 77%2 | 43%2 | |||
| Specificity | 48%1 | 91%1 | 77%1 | 98%1 | 71% | 98% |
| 43%2 | 87%2 | 71%2 | 96%2 | |||
| Positive predictive value | 31%1 | 90%1 | 56%1 | 90%1 | 43% | 80% |
| 66%2 | 85%2 | 52%2 | 82%2 | |||
| Negative predictive value | 86%1 | 67%1 | 93%1 | 85%1 | 90% | 83% |
| 75%2 | 64%2 | 88%2 | 80%2 | |||
Estimation group;
Validation group. AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; AAR: AST/ALT ratio; BMI: Body mass index; DM: Diabetes mellitus; PLT: Platelets; NASH: Nonalcoholic steatohepatitis; NAFLD: Nonalcoholic fatty liver disease.
Diagnosis of NASH with advanced fibrosis
Noninvasive diagnosis of liver fibrosis is one of the most rapidly evolving fields in recent years. A recent extensive review mentioned noninvasive diagnostic tests, including routine clinical parameters, fibrosis biomarkers, and imaging techniques in chronic hepatitis C, alcoholic liver disease, and NAFLD/NASH[44]. The stage of fibrosis has generally been diagnosed according to Brunt’s criteria[57] or Kleiner’s classification as proposed by NASH-CRN[37]. According to Brunt’s criteria, the severity of hepatic fibrosis is defined in terms of the following stages: Stage 1, zone 3 perisinusoidal fibrosis; Stage 2, zone 3 perisinusoidal fibrosis with portal fibrosis; Stage 3, zone 3 perisinusoidal fibrosis and portal fibrosis with bridging fibrosis; and Stage 4, cirrhosis[57]. Kleiner’s classification differs from Brunt’s criteria in that Stage 1 is subdivided into three substages: Substages 1a and 1b are zone 3 perisinusoidal and differ only by the character of collagen disposition (delicate or dense, respectively), and Substage 1c is portal or periportal (representing the pediatric pattern)[37]. Advanced fibrosis is classified as Stage 3 or 4.
A French group proposed the BAAT score (0-4 points) as a system to predict the grade of fibrosis, in which 1 point each is assigned to BMI ≥ 28 kg/m2, ALT 2 or more times greater than the normal upper limit, age ≥ 50 years old, and TG ≥ 1.7 mmol/L. For the differentiation of patients with Stage 2 or higher fibrosis, the negative predictive value (NPV) of a 0-1 point score was 100%. The same group developed the FibroTest, which is composed of bilirubin, γGT, γ globulin, haptoglobin, and α2-macroglobulin. From the United States, the Mayo Clinic proposed the NAFLD fibrosis score (NFS) [= -1.675 + 0.037 × age (year) + 0.094 × BMI (kg/m2) + 1.13 × IFG/DM (with = 1, without = 0) + 0.99 × AAR-0.013 × platelets (PLT) (× 109/L) - 0.66 ×Alb (g/dL)], calculated from readily measured routine parameters such as the age, PLT, albumin (Alb) level, AAR, fasting hyperglycemia (impaired fasting glucose, or IFG) or DM, and BMI (Table 3)[58]. NFS has been confirmed to be useful in predicting the progression of fibrosis regardless of whether the ALT level is normal or abnormal, even in bariatric surgery-treated obese patients. NFS is advantageous because it contains no items that require a special test and has been validated in many studies. The latest AASLD guidelines recommend the use of NFS for decision making for the application of liver biopsy[3]. Although NFS contains no items that require a special test, the calculation is complex, and the score is intermediate (NFS = -1.455 to 0.676) in approximately 25%-30% of patients [between low (NFS < -1.455) and high (NFS > 0.676) scores][2] for whom a liver biopsy is still unavoidable. The results of a validation study of NFS performed in China have been recently published[59], and the NPV of a low score was favorable and useful for the exclusion of advanced cases. However, the positive predictive value (PPV) of a high score was low, showing that the usefulness of NFS for detecting advanced cases in Asians remains questionable.
In the United States, Harrison et al[60] proposed a simple system, the BARD score, assigning one, two, and one point to BMI ≥ 28 kg/m2, AAR ≥ 0.8, and DM, respectively, and reported that the possibility of Stage 3 or 4 is very high when the total score is 2 or higher. The NPV was high, and the results were favorable in validation studies performed in Poland and Argentina. However, the usefulness of the BARD score for Japanese populations is questionable because the Japanese have lower BMIs than Western populations[61].
The FIB-4 index, calculated as: [age (year) × AST (IU/L)]/[PLT (109/L) × ALT (IU/L)], was proposed as a parameter of the progression of fibrosis in patients superinfected with human immunodeficiency virus/hepatitis C virus and was also investigated with regard to application for NAFLD (Table 3)[62]. Unlike other scoring systems, the FIB-4 index has the ability to identify Stage 3 or higher fibrosis. This index is advantageous because it is based on test values that are routinely measured in health checkups, the number of items is small, and the index is not influenced by the BMI. In a study performed by JSG-NAFLD involving Japanese subjects, the FIB-4 index was the most useful in differentiating patients with advanced fibrosis[63]. Furthermore, the usefulness for patients with normal ALT is comparable to that for patients with abnormal ALT[64]. Similar findings were confirmed in England: the FIB-4 index value was low in approximately 80% of the patients diagnosed with NAFLD during a health checkup, whereas a high value was noted in only approximately 1% of patients. As a parameter used alone, PLT is expected to be useful but carries the caveat that the counts are relatively high when fibrosis is severe. It has been shown that advanced fibrosis patients can be simply excluded using a combination of PLT and AAR (PAAR) (the possibility of Stage 3 or higher fibrosis is very low when the platelet count is 1950000 or greater with an AAR below 0.8)[65]. The AST to platelet ratio index (APRI) {[(AST level/upper limit of normal AST)/PLT (109/L)] × 100}, originally developed for hepatitis C patients, has also been suggested as a useful strategy for predicting significant fibrosis due to NASH. McPherson et al[66] compared five scoring systems, AAR, APRI, BARD, NFS, and the FIB-4 index, in a study involving 145 English NAFLD patients. Evaluation based on area under the receiver operating characteristic curve (AUROC) demonstrated that the FIB-4 index was the most favorable (0.86), followed by AAR (0.83), NFS (0.81), BARD (0.77), and APRI (0.67), and the PPVs of the FIB-4 index and of NFS were 75% and 79%, respectively. On the basis of these results, the authors recommended the FIB-4 index and NFS. The Nippon score was reported from a multicenter study performed with Japanese subjects in Nagasaki, Japan. The score was calculated by assigning one point each to the following characteristics: female sex, an age ≥ 60 years old, and the presence or absence of type 2 DM and hypertension (4 points in total). Although this system is very simple, it has not been confirmed to be superior to other scoring systems. Other scoring systems such as FibroMeter have been proposed as tests for the probability of advanced fibrosis, but additional studies are necessary for their validation. The above information suggest that, overall, the NFS and the FIB-4 indexes are the most recommendable scoring systems that are expected to be useful for Japanese patients because these systems have been relatively well validated in Japan and in other countries. However, both systems require further evaluation by performing prospective multicenter validation studies. The usefulness of elastography has attracted attention recently. FibroScan is very useful in predicting the progression of fibrosis in NAFLD patients[67], and is covered by national health insurance in Japan as of October 2011.
Scoring systems useful for predicting liver carcinogenesis and making a prognosis
There has been no study on the association of liver diseases with carcinogenesis, but Kawamura et al[68] reported that the annual liver carcinogenic rate in NAFLD patients was 0.043% and that APRI was useful in predicting liver carcinogenesis. It was recently reported that the scores derived from the fibrosis-predicting scoring systems NFS, APRI, and FIB-4 also serve as prognostic factors[69]; however, the prognostic value of these scores still requires verification in Japan.
Proposal of a diagnostic algorithm for NAFLD
There have been no established algorithms for the diagnosis of NAFLD/NASH. An algorithm for the management of NAFLD was suggested by Rafiq et al[70]. Liver biopsies should be considered if NAFLD patients show potential signs of cirrhosis, such as a hard edge of the liver, AST > ALT, and low albumin or platelets, or have abnormal ALT levels for more than 6 mo in spite of undergoing diet change and exercise therapy.
Based on the results of the multicenter study performed by JSG-NAFLD, it can be concluded that the FIB-4 index is useful for excluding advanced fibrosis patients[63], whereas the NAFIC score is useful for detecting NASH[56]. Thus, we would like to propose a diagnostic algorithm for NAFLD based on these data, as shown in Figure 1. First, the FIB-4 index is applied to every NAFLD patient. If the FIB-4 index is higher than 2.67, liver biopsy should be performed immediately. If the FIB-4 index is lower than 1.30, follow-up is recommended. If the FIB-4 index is between indeterminate ranges, an NAFIC score should be calculated. If the NAFIC score is above 2 points, liver biopsy should be considered. In our cooperative study with institutions performing health checkups, the FIB-4 score was high, intermediate, and low in approximately 1%, 19% and 80% of the NAFLD patients, respectively[65]. Accordingly, patients other than those with a low FIB-4 score, i.e., approximately 20% of the NAFLD patients, will be treated by hepatologists.
Figure 1.
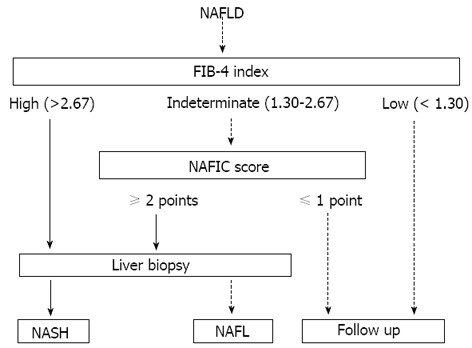
Proposed diagnostic algorithms combining non-invasive methods and liver biopsy. NAFLD: Nonalcoholic fatty liver disease; NASH: Nonalcoholic steatohepatitis; NAFL: Nonalcoholic fatty liver.
GENETIC PREDISPOSITION ASSOCIATED WITH THE PATHOGENESIS OF NAFLD/NASH
Genome-wide association studies (GWAS) offer a powerful technique for discovering novel associations between single-nucleotide polymorphisms (SNPs) and disease phenotypes. Romeo et al[71] first reported that a SNP in patatin-like phospholipase domain-containing protein 3 (PNPLA3) (rs738409 [G], encoding I148M), also termed adiponutrin, on chromosome 22 was strongly associated with increased hepatic fat levels, as well as with hepatic inflammation. This allele was most commonly observed in Hispanics, the group most susceptible to NAFLD among the 2111 subjects that comprised a mixed study population of Hispanics, African Americans, and European Americans. PNPLA3 is highly expressed in adipose tissue as well as in the liver, and the overexpression of PNPA3 promotes lipogenesis in mouse primary hepatocytes. In humans, hepatic PNPLA3 messenger RNA expression appears to be correlated with hepatic triglyceride content. Association studies[72-76], including one meta-analysis[76], confirm that the I148M polymorphism is also a strong modifier of NASH and progressive hepatic injury in various populations throughout the world. In addition to PNPLA3, other SNPs associated with NAFLD include neurocan, lysophospholipase-like 1, glucokinase regulatory protein, protein phosphatase 1 regulatory subunit 3b, and apolipoprotein C3[77-79]. However, it is unknown whether screening for these SNPs can facilitate the diagnosis of NASH or advanced fibrosis.
CONCLUSION
Currently, liver biopsy is essential for the diagnosis of NASH, but in the future, combining scoring systems and imaging methods may efficiently diagnose NAFLD/NASH. Whether these scoring systems reflect the long-term prognosis and carcinogenesis potential remains to be investigated. The development of an improved scoring system that will prove useful for efficiently detecting NASH and reducing liver disease-related deaths is expected in the future.
ACKNOWLEDGMENTS
The authors thank all of the members of Japan Study Group of NAFLD (JSG-NAFLD) for their assistance in preparation of this manuscript.
Footnotes
Supported by Scholarship Funds from MSD Co., Ltd. (to Sumida Y); Scholarship Funds from MSD Co., Ltd., and Dainippon Sumitomo Pharma Co., Ltd. (to Ioh Y)
P- Reviewers: Chamberlain S, Elena V, Yilmaz Y S- Editor: Zhai HH L- Editor: A E- Editor: Wu HL
References
- 1.Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, Chayama K, Saibara T. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586–595. doi: 10.1007/s00535-012-0533-z. [DOI] [PubMed] [Google Scholar]
- 2.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 4.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061–1067. doi: 10.1016/j.jhep.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 7.Roldan-Valadez E, Favila R, Martínez-López M, Uribe M, Méndez-Sánchez N. Imaging techniques for assessing hepatic fat content in nonalcoholic fatty liver disease. Ann Hepatol. 2008;7:212–220. [PubMed] [Google Scholar]
- 8.Iwasaki M, Takada Y, Hayashi M, Minamiguchi S, Haga H, Maetani Y, Fujii K, Kiuchi T, Tanaka K. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78:1501–1505. doi: 10.1097/01.tp.0000140499.23683.0d. [DOI] [PubMed] [Google Scholar]
- 9.Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001;35:195–199. doi: 10.1016/s0168-8278(01)00094-0. [DOI] [PubMed] [Google Scholar]
- 10.Daniel S, Ben-Menachem T, Vasudevan G, Ma CK, Blumenkehl M. Prospective evaluation of unexplained chronic liver transaminase abnormalities in asymptomatic and symptomatic patients. Am J Gastroenterol. 1999;94:3010–3014. doi: 10.1111/j.1572-0241.1999.01451.x. [DOI] [PubMed] [Google Scholar]
- 11.Cowin GJ, Jonsson JR, Bauer JD, Ash S, Ali A, Osland EJ, Purdie DM, Clouston AD, Powell EE, Galloway GJ. Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J Magn Reson Imaging. 2008;28:937–945. doi: 10.1002/jmri.21542. [DOI] [PubMed] [Google Scholar]
- 12.Machann J, Thamer C, Schnoedt B, Stefan N, Haring HU, Claussen CD, Fritsche A, Schick F. Hepatic lipid accumulation in healthy subjects: a comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn Reson Med. 2006;55:913–917. doi: 10.1002/mrm.20825. [DOI] [PubMed] [Google Scholar]
- 13.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 14.de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911–918. doi: 10.1111/j.1478-3231.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 15.Iijima H, Moriyasu F, Tsuchiya K, Suzuki S, Yoshida M, Shimizu M, Sasaki S, Nishiguchi S, Maeyama S. Decrease in accumulation of ultrasound contrast microbubbles in non-alcoholic steatohepatitis. Hepatol Res. 2007;37:722–730. doi: 10.1111/j.1872-034X.2007.00130.x. [DOI] [PubMed] [Google Scholar]
- 16.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, Yoon JH, Cho SH, Sung MW, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–508. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, Capron D, Abella A, Massard J, Ngo Y, et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10. doi: 10.1186/1476-5926-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calori G, Lattuada G, Ragogna F, Garancini MP, Crosignani P, Villa M, Bosi E, Ruotolo G, Piemonti L, Perseghin G. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology. 2011;54:145–152. doi: 10.1002/hep.24356. [DOI] [PubMed] [Google Scholar]
- 20.Laurin J. Motion - all patients with NASH need to have a liver biopsy: arguments against the motion. Can J Gastroenterol. 2002;16:722–726. doi: 10.1155/2002/614302. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein NS, Hastah F, Galan MV, Gordon SC. Fibrosis heterogeneity in nonalcoholic steatohepatitis and hepatitis C virus needle core biopsy specimens. Am J Clin Pathol. 2005;123:382–387. doi: 10.1309/EY72-F1EN-9XCB-1KXX. [DOI] [PubMed] [Google Scholar]
- 22.Vuppalanchi R, Unalp A, Van Natta ML, Cummings OW, Sandrasegaran KE, Hameed T, Tonascia J, Chalasani N. Effects of liver biopsy sample length and number of readings on sampling variability in nonalcoholic Fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:481–486. doi: 10.1016/j.cgh.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson SP, Bowers SP, Palekar NA, Ward JA, Pulcini JP, Harrison SA. Histopathologic variability between the right and left lobes of the liver in morbidly obese patients undergoing Roux-en-Y bypass. Clin Gastroenterol Hepatol. 2007;5:1329–1332. doi: 10.1016/j.cgh.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 25.Janiec DJ, Jacobson ER, Freeth A, Spaulding L, Blaszyk H. Histologic variation of grade and stage of non-alcoholic fatty liver disease in liver biopsies. Obes Surg. 2005;15:497–501. doi: 10.1381/0960892053723268. [DOI] [PubMed] [Google Scholar]
- 26.Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, Bass NM. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–880. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
- 27.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Younossi ZM, Gramlich T, Liu YC, Matteoni C, Petrelli M, Goldblum J, Rybicki L, McCullough AJ. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod Pathol. 1998;11:560–565. [PubMed] [Google Scholar]
- 29.Gawrieh S, Knoedler DM, Saeian K, Wallace JR, Komorowski RA. Effects of interventions on intra- and interobserver agreement on interpretation of nonalcoholic fatty liver disease histology. Ann Diagn Pathol. 2011;15:19–24. doi: 10.1016/j.anndiagpath.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 31.van der Poorten D, Kwok A, Lam T, Ridley L, Jones DB, Ngu MC, Lee AU. Twenty-year audit of percutaneous liver biopsy in a major Australian teaching hospital. Intern Med J. 2006;36:692–699. doi: 10.1111/j.1445-5994.2006.01216.x. [DOI] [PubMed] [Google Scholar]
- 32.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 33.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 34.Friedman LS. Controversies in liver biopsy: who, where, when, how, why? Curr Gastroenterol Rep. 2004;6:30–36. doi: 10.1007/s11894-004-0023-4. [DOI] [PubMed] [Google Scholar]
- 35.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 36.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 38.Hjelkrem M, Stauch C, Shaw J, Harrison SA. Validation of the non-alcoholic fatty liver disease activity score. Aliment Pharmacol Ther. 2011;34:214–218. doi: 10.1111/j.1365-2036.2011.04695.x. [DOI] [PubMed] [Google Scholar]
- 39.Juluri R, Vuppalanchi R, Olson J, Unalp A, Van Natta ML, Cummings OW, Tonascia J, Chalasani N. Generalizability of the nonalcoholic steatohepatitis Clinical Research Network histologic scoring system for nonalcoholic fatty liver disease. J Clin Gastroenterol. 2011;45:55–58. doi: 10.1097/MCG.0b013e3181dd1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, Goodman Z. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 42.Toyoda H, Kumada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Tada T. Markedly lower follow-up rate after liver biopsy in patients with non-alcoholic fatty liver diseases than those with viral hepatitis in Japan. BMC Res Notes. 2011;4:341. doi: 10.1186/1756-0500-4-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grandison GA, Angulo P. Can NASH be diagnosed, graded, and staged noninvasively? Clin Liver Dis. 2012;16:567–585. doi: 10.1016/j.cld.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martínez SM, Crespo G, Navasa M, Forns X. Noninvasive assessment of liver fibrosis. Hepatology. 2011;53:325–335. doi: 10.1002/hep.24013. [DOI] [PubMed] [Google Scholar]
- 45.Yilmaz Y, Ulukaya E. Toward a biochemical diagnosis of NASH: insights from pathophysiology for distinguishing simple steatosis from steatohepatitis. Curr Med Chem. 2011;18:725–732. doi: 10.2174/092986711794480122. [DOI] [PubMed] [Google Scholar]
- 46.Sumida Y, Eguchi Y, Ono M. Current status and agenda in the diagnosis of nonalcoholic steatohepatitis in Japan. World J Hepatol. 2010;2:374–383. doi: 10.4254/wjh.v2.i10.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yilmaz Y. Cytokeratins in hepatitis. Clin Chim Acta. 2011;412:2031–2036. doi: 10.1016/j.cca.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, Zhu Y, Zheng Q, Jiang J. Serum cytokeratin-18 in the diagnosis of non-alcoholic steatohepatitis: A meta-analysis. Hepatol Res. 2013:Jul 9; Epub ahead of print. doi: 10.1111/hepr.12197. [DOI] [PubMed] [Google Scholar]
- 49.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 50.Palekar NA, Naus R, Larson SP, Ward J, Harrison SA. Clinical model for distinguishing nonalcoholic steatohepatitis from simple steatosis in patients with nonalcoholic fatty liver disease. Liver Int. 2006;26:151–156. doi: 10.1111/j.1478-3231.2005.01209.x. [DOI] [PubMed] [Google Scholar]
- 51.Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F, Massard J, Bonyhay L, Tahiri M, Thabut D, et al. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34. doi: 10.1186/1471-230X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102:399–408. doi: 10.1111/j.1572-0241.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 53.Campos GM, Bambha K, Vittinghoff E, Rabl C, Posselt AM, Ciovica R, Tiwari U, Ferrel L, Pabst M, Bass NM, et al. A clinical scoring system for predicting nonalcoholic steatohepatitis in morbidly obese patients. Hepatology. 2008;47:1916–1923. doi: 10.1002/hep.22241. [DOI] [PubMed] [Google Scholar]
- 54.Anty R, Iannelli A, Patouraux S, Bonnafous S, Lavallard VJ, Senni-Buratti M, Amor IB, Staccini-Myx A, Saint-Paul MC, Berthier F, et al. A new composite model including metabolic syndrome, alanine aminotransferase and cytokeratin-18 for the diagnosis of non-alcoholic steatohepatitis in morbidly obese patients. Aliment Pharmacol Ther. 2010;32:1315–1322. doi: 10.1111/j.1365-2036.2010.04480.x. [DOI] [PubMed] [Google Scholar]
- 55.Shimada M, Kawahara H, Ozaki K, Fukura M, Yano H, Tsuchishima M, Tsutsumi M, Takase S. Usefulness of a combined evaluation of the serum adiponectin level, HOMA-IR, and serum type IV collagen 7S level to predict the early stage of nonalcoholic steatohepatitis. Am J Gastroenterol. 2007;102:1931–1938. doi: 10.1111/j.1572-0241.2007.01322.x. [DOI] [PubMed] [Google Scholar]
- 56.Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, Eguchi Y, Suzuki Y, Imai S, Kanemasa K, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:257–268. doi: 10.1007/s00535-010-0305-6. [DOI] [PubMed] [Google Scholar]
- 57.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 58.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 59.Wong VW, Wong GL, Chim AM, Tse AM, Tsang SW, Hui AY, Choi PC, Chan AW, So WY, Chan FK, et al. Validation of the NAFLD fibrosis score in a Chinese population with low prevalence of advanced fibrosis. Am J Gastroenterol. 2008;103:1682–1688. doi: 10.1111/j.1572-0241.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 60.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 61.Fujii H, Enomoto M, Fukushima W, Tamori A, Sakaguchi H, Kawada N. Applicability of BARD score to Japanese patients with NAFLD. Gut. 2009;58:1566–1567; author reply 1567. doi: 10.1136/gut.2009.182758. [DOI] [PubMed] [Google Scholar]
- 62.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. doi: 10.1186/1471-230X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoneda M, Fujii H, Sumida Y, Hyogo H, Itoh Y, Ono M, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, et al. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:1300–1306. doi: 10.1007/s00535-011-0436-4. [DOI] [PubMed] [Google Scholar]
- 65.Sumida Y, Ohno T, Sakai K, Kanemasa K, Imai S. Usefulness of combination of platelet count and AST/ALT ratio (PAAR index) for excluding advanced fibrosis in nonalcoholic fatty liver disease. Kanzo. 2011;52:383–386. Available from: https://www.jstage.jst.go.jp/article/kanzo/52/6/52_6_383/_pdf. [Google Scholar]
- 66.McPherson S, Anstee QM, Henderson E, Day CP, Burt AD. Are simple noninvasive scoring systems for fibrosis reliable in patients with NAFLD and normal ALT levels? Eur J Gastroenterol Hepatol. 2013;25:652–658. doi: 10.1097/MEG.0b013e32835d72cf. [DOI] [PubMed] [Google Scholar]
- 67.Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, Nozaki Y, Yonemitsu K, Higurashi T, Takahashi H, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD) Dig Liver Dis. 2008;40:371–378. doi: 10.1016/j.dld.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 68.Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, Kobayashi M, Saitoh S, Sezaki H, Akuta N, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–261. doi: 10.1038/ajg.2011.327. [DOI] [PubMed] [Google Scholar]
- 69.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rafiq N, Younossi ZM. Nonalcoholic fatty liver disease: a practical approach to evaluation and management. Clin Liver Dis. 2009;13:249–266. doi: 10.1016/j.cld.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 71.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hotta K, Yoneda M, Hyogo H, Ochi H, Mizusawa S, Ueno T, Chayama K, Nakajima A, Nakao K, Sekine A. Association of the rs738409 polymorphism in PNPLA3 with liver damage and the development of nonalcoholic fatty liver disease. BMC Med Genet. 2010;11:172. doi: 10.1186/1471-2350-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawaguchi T, Sumida Y, Umemura A, Matsuo K, Takahashi M, Takamura T, Yasui K, Saibara T, Hashimoto E, Kawanaka M, et al. Genetic polymorphisms of the human PNPLA3 gene are strongly associated with severity of non-alcoholic fatty liver disease in Japanese. PLoS One. 2012;7:e38322. doi: 10.1371/journal.pone.0038322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hernaez R, McLean J, Lazo M, Brancati FL, Hirschhorn JN, Borecki IB, Harris TB, Nguyen T, Kamel IR, Bonekamp S, et al. Association between variants in or near PNPLA3, GCKR, and PPP1R3B with ultrasound-defined steatosis based on data from the third national health and nutrition examination survey. Clin Gastroenterol Hepatol. 2013;11:1183–1190.e2. doi: 10.1016/j.cgh.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 77.Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, Gudnason V, Eiriksdottir G, Garcia ME, Launer LJ, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM, Dziura J, Lifton RP, Shulman GI. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362:1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gorden A, Yang R, Yerges-Armstrong LM, Ryan KA, Speliotes E, Borecki IB, Harris TB, Chu X, Wood GC, Still CD, et al. Genetic variation at NCAN locus is associated with inflammation and fibrosis in non-alcoholic fatty liver disease in morbid obesity. Hum Hered. 2013;75:34–43. doi: 10.1159/000346195. [DOI] [PMC free article] [PubMed] [Google Scholar]


