Abstract
AIM: To conduct a network meta-analysis to evaluate the effectiveness of different chemotherapy regimens for patients with gastric cancer.
METHODS: PubMed (1966-2011.12), the Cochrane Library (2011 Issue 2) and EMBASE (1974-2011.12) were searched with the terms “gastric cancer” and “chemotherapy”, as well as the medical subject headings. References from relevant articles and conferences were also included. Patients who had previous gastric surgery, radiation before or after surgery or chemotherapy before surgery were excluded. In this study, only randomized controlled trials (RCTs) were considered, and the end-point was the overall mortality. Direct comparisons were performed using traditional meta-analysis whereas indirect comparisons were performed using network meta-analysis.
RESULTS: In total, 31 RCTs with 7120 patients were included. Five chemotherapy regimens, fluorouracil (FU) + BCNU, FU + methyl-CCNU (mCCNU), FU + cisplatin, FU + anthracyclines and FU + mitomycin c (MMC) + cytarabine (Ara-c), were found to be less beneficial in terms of overall mortality. In contrast, four chemotherapy regimens were effective for the patients after surgery, including FU + MMC + adriamycin (FMA), FU + MMC (FM), Tegafur and MMC, There was no significant difference in terms of overall mortality among these regimens. The evidence for the FM regimen and MMC regimen was poor. Additionally, the FMA regimen, which includes a variety of chemotherapy drugs and causes many side effects, was not better than the Tegafur regimen.
CONCLUSION: Although the four chemotherapy regimens were effective in patients with gastric cancer after surgery and the overall mortality revealed no significant difference among them in the network meta-analysis, thorough analysis of the results recommends Tegafur as the first-line adjuvant chemotherapy regimen for patients after complete resection.
Keywords: Gastric cancer, Chemotherapy, Randomized controlled trials, Indirect treatment comparison, Network meta-analysis
Core tip: Although adjuvant chemotherapy after complete resection of gastric cancer is therapeutically useful, which of the many regimens is most effective? To date, no regimen has been clearly recommended as the standard procedure post-operation; therefore, we performed a network meta-analysis, which is a useful tool to summarize the different clinical trials and to evaluate the effectiveness of different chemotherapy regimens for patients after complete resection of gastric cancer. Based on our findings, the Tegafur regimen, especially S-1, is the first therapy that should be recommend to the patients to reduce overall mortality.
INTRODUCTION
Gastric cancer (GC) remains the second leading cause of cancer-related deaths in the world and is the most common malignancy in Asia, South America and Eastern Europe. The overall outcome for patients with GC has not significantly improved over recent decades[1-4]. GC remains a considerable threat to public health around the world. Currently, complete resection still has the highest potential for curatively treating GC[5]. However, approximately 20%-60% of GC patients who have already had curative surgery develop recurrent diseases[6] and will need to undergo adjuvant chemotherapy.
No network meta-analysis has been conducted to compare the efficacy of different chemotherapy protocols for patients with GC. Network meta-analysis is a useful tool for summarizing different clinical trials[7], especially when many different regimens are effective for the same clinical condition. In this type of analysis, all binary comparisons are shown with labels indicating superiority, inferiority or no difference in a summary graph[8-12]. Some recent meta-analyses have indicated that adjuvant chemotherapy after complete resection produces a small survival benefit[13-18]. Several additional trials have also been conducted in this setting. However, they did not indicate which chemotherapy protocol had the best efficacy for treating patients who have undergone complete resection. There is no clearly recommended protocol for the standard treatment of patients with GC after complete resection, and a 5-fluorouracil (5-FU) and platinum-based regimen is usually administered. Surgeons need empirical evidence to determine the best treatment for GC patients. Therefore, it was deemed important to assess the benefits of various adjuvant chemotherapy regimens through a network meta-analysis based on data from all relevant randomized controlled trials (RCTs).
The purpose of this network meta-analysis was to evaluate the effectiveness of different chemotherapy regimens for patients with GC who had undergone surgery.
MATERIALS AND METHODS
Study selection
PubMed (1966.01-2011.12), the Cochrane Library (2011 Issue 12) and EMBASE (1974.01-2011.12) were searched with the terms “gastric cancer” and “chemotherapy”, as well as the medical subject headings. The relevant articles referenced in these publications were downloaded from the databases. The related article function was also used to widen the search results. All abstracts, comparative studies, non-randomized trials, and citations scanned were searched comprehensively. Additional searches were conducted by reviewing abstract booklets and review articles. Trials were included irrespective of the language in which they were reported.
Data extraction
Each article was critically reviewed by two researchers for eligibility in our network meta-analysis (Table 1). Only RCTs on palliative or adjuvant chemotherapy for treating GC patients who had undergone surgery were analyzed in this network meta-analysis. The two researchers extracted the data separately, which were then confirmed by a third researcher.
Table 1.
Characteristics of randomized trials included in the network meta-analysis
| Trial | Year | Postoperative chemotherapy regimens |
Sample size |
Overall mortality |
Follow-up (mo) | Jadad score | ||
| Chemotherapy group | Control group | Chemotherapy group | Control group | |||||
| Lawton et al[20] | 1981 | FU + BCNU | 13 | 12 | 11/13 | 10/12 | 60 | 2 |
| Stablein et al[21] | 1982 | FU + MCCNU | 71 | 71 | 29/71 | 40/71 | 48 | 3 |
| Higgins et al[22] | 1983 | FU + MCCNU | 156 | 156 | 121/156 | 117/156 | 36 | 3 |
| Nakajima et al[23] | 1984 | FM + Ara-c | 128 | 124 | 11/128 | 17/124 | 60 | 3 |
| Engstrom et al[24] | 1985 | FU + MCCNU | 91 | 89 | 57/91 | 51/89 | 24 | 3 |
| Schlag et al[25] | 1987 | FU + BCNU | 42 | 53 | 21/42 | 28/53 | 72 | 2 |
| Bonfanti et al[26] | 1988 | FU + MCCNU | 75 | 69 | 63/75 | 56/69 | 84 | 4 |
| Coombes et al[27] | 1990 | FMA | 131 | 148 | 101/133 | 123/148 | 68 | 3 |
| Estape et al[28] | 1991 | MMC | 33 | 37 | 16/33 | 31/37 | 120 | 2 |
| Krook et al[29] | 1991 | FA | 61 | 64 | 41/61 | 43/64 | 60 | 3 |
| Kim et al[30] | 1992 | MMC + FU | 77 | 94 | 54/77 | 71/94 | 60 | 2 |
| Grau et al[31] | 1993 | MMC | 68 | 66 | 40/68 | 49/66 | 105 | 2 |
| Hallissey et al[32] | 1994 | FMA | 138 | 145 | 101/138 | 110/145 | 60 | 3 |
| Macdonald et al[33] | 1995 | FMA | 93 | 100 | 59/93 | 68/100 | 114 | 2 |
| Lise et al[34] | 1995 | FMA | 155 | 159 | 88/155 | 99/159 | 78 | 3 |
| Tsavaris et al[35] | 1996 | FMA | 42 | 42 | 27/42 | 34/42 | 60 | 3 |
| Cirera et al[36] | 1999 | MMC + Tegafur | 76 | 76 | 33/ 76 | 44/72 | 37 | 3 |
| Nakajima et al[37] | 1999 | MMC + FU + UFT | 288 | 285 | 41/288 | 49/285 | 72 | 3 |
| Neri et al[38] | 2001 | Epirubicin + FU | 69 | 68 | 48/69 | 59/68 | 60 | 2 |
| Bajetta et al[39] | 2002 | FU + Adriamycin etoposide + cisplatin | 137 | 137 | 66/137 | 71/137 | 66 | 2 |
| Nashimoto et al[40] | 2003 | MMC + FU + Ara C | 128 | 124 | 11/128 | 23/124 | 69 | 2 |
| Popiela et al[41] | 2004 | FAM | 53 | 52 | 42/53 | 47/52 | 120 | 2 |
| Chipponi et al[42] | 2004 | Cisplatin + FU | 101 | 104 | 62/101 | 63/104 | 60 | 2 |
| Bouché et al[43] | 2005 | Cisplatin + FU | 127 | 133 | 68/127 | 77/133 | 97.8 | 3 |
| Nitti et al[44] | 2006 | FU + Adriamycin + methotrexate + LV | 103 | 103 | 54/103 | 49/103 | 60 | 3 |
| Nitti et al[44] | 2006 | FU + Epirubicin + methotrexate + LV | 91 | 100 | 63/91 | 64/100 | 60 | 3 |
| De Vita et al[45] | 2007 | FU + Epirubicin + LV + etoposide | 112 | 113 | 58/112 | 64/113 | 60 | 2 |
| Nakajima et al[46] | 2007 | Uracil-Tegafur | 95 | 95 | 18/95 | 30/95 | 60 | 4 |
| Di Costanzo et al[47] | 2008 | FU + Epirubicin + cisplatin + LV | 130 | 128 | 69/130 | 70/128 | 60 | 3 |
| Miyashiro et al[48] | 2011 | Cisplatin + FU | 132 | 132 | 50/132 | 52/132 | 60 | 4 |
| Sasako et al[49] | 2011 | S-1 | 529 | 530 | 149/529 | 206/530 | 60 | 4 |
FU: Fluorouracil; MCCNU: Methyl-CCNU; MMC: Mitomycin c; LV: Leucovorin; Ara-c: Cytarabine; CDHP: 5-Chloro-2,4-dihydropyrimidine; Oxo: Potassium oxonate; FM: FU + MMC; FMA: FU + MMC + adriamycin; S-1: Tegafur + CDHP + Oxo.
Inclusion criterion: Patients with GC after complete resection and age < 71 years.
Exclusion criteria: Patients who had previous gastric surgery, radiation before or after surgery, chemotherapy before surgery, a history of deep venous thrombosis or pulmonary embolism and severe cardiovascular, respiratory, hepatic or renal disease.
End point: Overall mortality was defined as the time from randomization to death from any cause, or to the last follow-up, which was used as the date of censoring.
Quality evaluation
The quality of the studies included was assessed using the Jadad score[19].
Statistical analysis
The traditional meta-analysis method was used for extracting the crude rates of our pre-specified clinical end-point for each treatment group when the trials reported suitable information. We summarized the available data on overall survival from the reported results in all trials, computing pooled odd ratios and their respective 95% confidence intervals (95%CI) by means of a fixed-effects model. All statistical analyses were performed using Review Manager (RevMan version 5.0), the Cochrane Collaboration’s software for preparing and maintaining Cochrane systematic reviews. We used the chi-square statistic to assess the heterogeneity between trials and the I2 statistic to assess the extent of inconsistency. Subgroup analysis was used to explore important clinical differences among trials that might be expected to affect the magnitude of the treatment effect.
Network meta-analysis was used after traditional meta-analysis. When efficient chemotherapy regimens were compared through network meta-analysis, the head-to-head comparisons (in this case, indirect comparisons) were handled and consequently assigned a statistical result in terms of superiority/inferiority or no difference along with the level of statistical significance. Statistical calculations and graph generation were carried out. The HR, with a 95%CI, for each indirect comparison was estimated according to the ITC software (Canadian Agency for Drugs and Technologies in Health, Indirect Treatment Comparison software, Ottawa, Ontario, Canada). This approach allows an indirect HR, with a 95%CI, to be estimated on the condition that both treatments included in the indirect comparison had been compared in actual trials against a common comparator.
Role of funding source
No sponsors were involved in the study design; during the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit the report for publication. All authors had access to the raw data. The corresponding author had full access to all of the data and the final responsibility to submit the report for publication.
RESULTS
Flow diagram of trial selection
In total, 31 RCTs, with a total of 7120 patients, were included (Figure 1) from the electronic databases. Figure 1 shows a flow chart of studies from the initial results of the publication searches to the final inclusion or exclusion. The RCTs that met the criteria for our analysis are described in Table 1. There were 12 RCTs that had a Jadad score of 2, 15 RCTs that had a Jadad score of 3 and 4 RCTs that had a Jadad score of 4.
Figure 1.
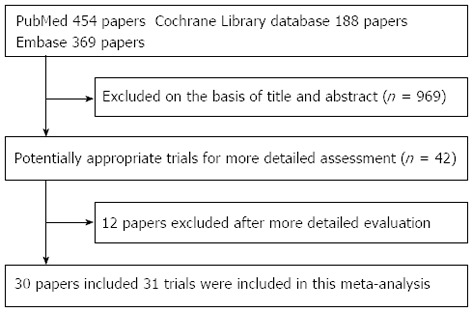
Flow diagram of trial selection.
Analysis of regimen groups
In terms of direct comparisons, this analysis divided the chemotherapy regimens into 9 subgroups, and 8 subgroups were assessed by the fixed effects models, while only 1 was assessed by the random effects models. In terms of overall mortality, at least 5 chemotherapy regimens were found to be of equal efficacy when compared to a blank control. The values of HR were as follows: 0.92 (95%CI: 0.43-1.96) for FU + BCNU regimen, 1.00 (95%CI: 0.76-1.32) for FU + methyl-CCNU (mCCNU) regimen, 0.93 (95%CI: 0.69-1.24) for FU + cisplatin regimen, 0.92 (95%CI: 0.74-1.14) for FU + anthracyclines regimen, and 0.67 (95%CI: 0.41-1.10) for FU + mitomycin c (MMC) + AraC regimen. In contrast, in terms of overall mortality, 4 chemotherapy regimens were found to be more effective than the blank control. The values of HR were as follows: 0.74 (95%CI: 0.58-0.94) for FAM regimen, 0.68 (95%CI: 0.49-0.94) for FM regimen, 0.60 (95%CI: 0.47-0.76) for Tegafur regimen, and 0.33 (95%CI: 0.13-0.86) for MMC regimen. These outcomes are described in Figures 2 and 3.
Figure 2.
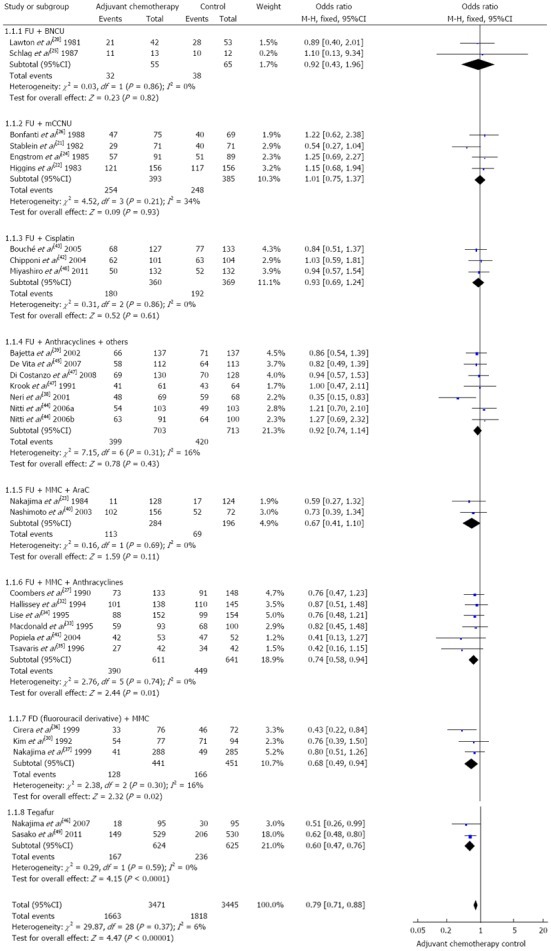
Eight subgroups in the fixed effects models.
Figure 3.
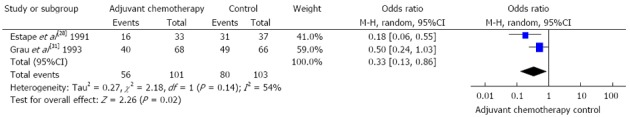
One subgroup in the random effects model. FU: Fluorouracil; mCCNU: Methyl-CCNU; MMC: Mitomycin c.
In terms of indirect comparisons, 4 chemotherapy regimens were found to be equal in terms of overall mortality. The values of HR were as follows: 1.09 (95%CI: 0.73-1.63) for 5-FU + adriamycin + MCC (FAM) regimen vs FM regimen; 1.23 (95%CI: 0.88-1.73) for 5-FU + MMC + adriamycin (FMA) regimen vs Tegafur regimen; 2.24 (95%CI: 0.85-5.95) for FMA regimen vs MMC regimen; 1.13 (95%CI: 0.76-1.70) for FM regimen vs Tegafur regimen; 2.06 (95%CI: 0.76-5.60) for FM regimen vs MMC regimen; and 1.82 (95%CI: 0.67-4.80) for Tegafur regimen vs MMC regimen. These outcomes are described in Figure 4.
Figure 4.
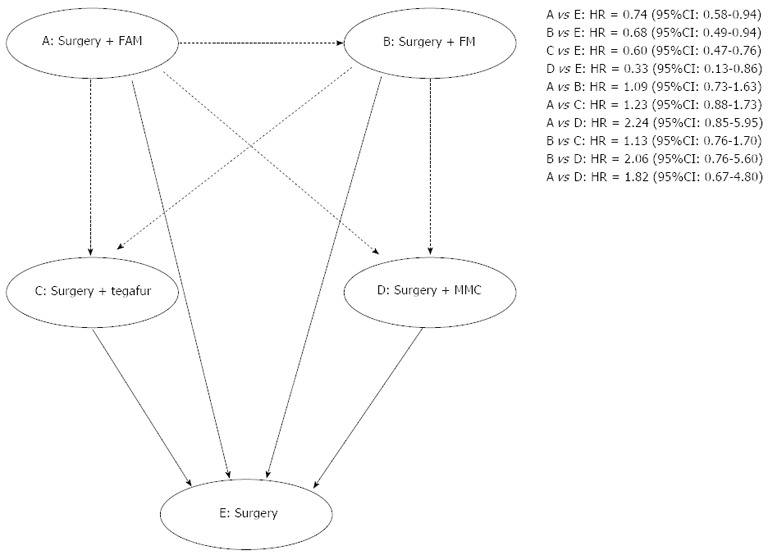
Network meta-analysis in terms of mortality. MMC: Mitomycin c; FAM: 5-fluorouracil, adriamycin, and mitomycin c.
DISCUSSION
In total, 31 RCTs, with a total of 7120 patients, were included in this analysis, and 12 RCTs had a Jadad score of 2, 15 RCTs had a Jadad score of 3, and 4 RCTs had a Jadad score of 4. This study divided these chemotherapy regimens into 9 subgroups. The result of this analysis indicated that 5 chemotherapy regimens had little benefit to the patients, including the FU + BCNU, FU + mCCNU, FU + cisplatin, FU + anthracyclines, and FU + MMC + AraC regimens. In contrast, 4 chemotherapy regimens were effective for patients after surgery, including the FMA, FM, Tegafur, and MMC regimens. In this study, Tegafur and the S-1 regimen were assigned to one regimen because S-1 was composed of Tegafur, CDHP and Oxo, as CDHP and Oxo reduced the side effects of Tegafur. As Tegafur is a fluorouracil derivative, the FM regimen was included in 3 RCTs. Additionally, anthracyclines, including adriamycin, epirubicin and doxorubicin, were part of the FMA regimen, which was included in 6 RCTs. Indirect comparisons were estimated according to the ITC software, and the results indicated that there was no difference among these four chemotherapy regimens in the terms of overall mortality.
Although this analysis indicated that MMC was effective for patients after surgery, the evidence for this result was poor because of the low quality of the 2 RCTs included. Specifically, one trial had a small sample size, and only 204 patients were contained in the subgroup analysis. Additionally, because there was also significant heterogeneity among the trials (P = 0.14, I2 = 54%), the analysis was carried out using the random effects models. The curative effect of MMC needs to be further validated. The evidence for the Tegafur regimen included 1249 patients, the RCTs were of high quality, and there was no significant heterogeneity among the trials (P = 0.59, I2 = 0%). Accordingly, the analysis was carried out using the fixed effects model, and we found strong evidence to confirm the efficacy of the Tegafur regimen. The joint application with 5-chloro-2,4-dihydropyrimidine (CDHP) and potassium oxonate (Oxo) reduced the side effects of Tegafur; therefore, the S-1 regimen (Tegafur + CDHP + Oxo) is recommended.
The combination of Tegafur and MMC in the FM regimen was similar to treatment with each component individually, as determined by indirect comparison, and further studies are needed to confirm which treatment is the primary effector. Additionally, if the side effects of Tegafur and MMC will reduce the overall efficacy, further studies are needed to identify an adjuvant that can reduce these side effects, as in the case of S-1. If the treatments have a mutual antagonist effect on each other, they should be used separately. As the evidence for the FM regimen is not very strong, larger sample sizes and multicenter RCTs are still needed. While the FMA regimen is available, surprisingly, it is not better than Tegafur or MMC. Traditional analysis indicated that the FU + anthracyclines regimen is not available, and thus, MMC may contribute to the efficacy of the FMA regimen to a great extent. Accordingly, based on these results, FMA is not recommended.
In summary, chemotherapy regimens, especially Tegafur, are available for GC. However, the efficacy of the FM regimen and MMC regimen needs to be further validated. The evidence for the Tegafur regimen is more credible, and S-1 may be the best current choice. Future studies should focus on identifying better adjuvants that can reduce the side effects of MMC as much as possible. Their combination could be a better regimen than S-1, and perhaps, the combination of MMC, Tegafur and adjuvant can achieve better outcomes than mono-chemotherapy alone. However, based on recent evidence, the Tegafur regimen, especially S-1, is most commonly recommended to patients after complete resection.
In conclusion, this analysis indicated that four chemotherapy regimens are effective for patients with GC after surgery, including the FMA regimen, FM regimen, Tegafur regimen and MMC regimen. However, the evidence for the FM regimen and MMC regimen was poor in terms of overall mortality. The FMA regimen, which includes many chemotherapy drugs and thus has many side effects, is not better than the Tegafur regimen. Based on this study, the Tegafur regimen is recommended as a better choice for doctors when dealing with GC patients after complete resection.
COMMENTS
Background
Gastric cancer is very common worldwide and, in most cases, will lead to serious health problems, even after complete resection. Currently, treatment with adjuvant and palliative chemotherapies are essential to prevent and treat recurrence disease. A standard chemotherapy regimen has not been established; therefore, the evaluation of which regimens may be better for gastric cancer patients is needed.
Research frontiers
This network meta-analysis was performed to evaluate the effectiveness of different chemotherapy regimens for patients with gastric cancer. The end point was overall mortality, which was defined as the time from randomization to death from any cause, or to the last follow-up.
Innovations and breakthroughs
The meta-analysis shows the following: four chemotherapy regimens [fluorouracil (FU) + mitomycin c + adriamycin, fluorouracil + mitomycin c (FM), tegafur and mitomycin c (MMC)] are effective for patients after surgery, whereas the other five regimens [fluorouracil + BCNU, FU + methyl-CCNU (mCCNU), FU + cisplatin, FU + anthracyclines and FU + mitomycin c + cytarabine] were found to be less beneficial.
Applications
From the analysis, Tegafur is recommended as the first-line adjuvant chemotherapy regimen for patients after complete resection. This recommendation is due to the high quality of the randomized controlled trials (RCTs), homogeneity among trials and fewer side effects.
Peer review
The current network meta-analysis evaluated the effectiveness of different chemotherapy regimens for gastric cancer patients after curative surgery, and we found that the outcomes and analysis were good. However, further RCTs are needed to study the FM regimen, MMC regimen and combination chemotherapy.
Footnotes
P- Reviewers: Hahm KB, Tiberio GAM, Zaniboni A, Zoli W S- Editor: Cui XM L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Akoh JA, Macintyre IM. Improving survival in gastric cancer: review of 5-year survival rates in English language publications from 1970. Br J Surg. 1992;79:293–299. doi: 10.1002/bjs.1800790404. [DOI] [PubMed] [Google Scholar]
- 2.Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer. 1998;1:125–133. doi: 10.1007/s101200050006. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura K, Ueyama T, Yao T, Xuan ZX, Ambe K, Adachi Y, Yakeishi Y, Matsukuma A, Enjoji M. Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer. 1992;70:1030–1037. doi: 10.1002/1097-0142(19920901)70:5<1030::aid-cncr2820700504>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Roder DM. The epidemiology of gastric cancer. Gastric Cancer. 2002;5 Suppl 1:5–11. doi: 10.1007/s10120-002-0203-6. [DOI] [PubMed] [Google Scholar]
- 5.Sasako M. Principles of surgical treatment for curable gastric cancer. J Clin Oncol. 2003;21:274s–275s. doi: 10.1200/JCO.2003.09.172. [DOI] [PubMed] [Google Scholar]
- 6.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadda V, Maratea D, Trippoli S, Messori A. Network meta-analysis. Results can be summarised in a simple figure. BMJ. 2011;342:d1555. doi: 10.1136/bmj.d1555. [DOI] [PubMed] [Google Scholar]
- 9.Fadda V, Maratea D, Trippoli S, Messori A. Treatments for macular degeneration: summarising evidence using network meta-analysis. Br J Ophthalmol. 2011;95:1476–1477. doi: 10.1136/bjophthalmol-2011-300316. [DOI] [PubMed] [Google Scholar]
- 10.Maratea D, Fadda V, Trippoli S, Messori A. Prevention of venous thromboembolism after major orthopedic surgery: indirect comparison of three new oral anticoagulants. J Thromb Haemost. 2011;9:1868–1870. doi: 10.1111/j.1538-7836.2011.04421.x. [DOI] [PubMed] [Google Scholar]
- 11.Messori A, Del Santo F, Maratea D. First-line treatments for hepatitis C. Aliment Pharmacol Ther. 2011;33:1383–1385. doi: 10.1111/j.1365-2036.2011.04672.x. [DOI] [PubMed] [Google Scholar]
- 12.Passaro D, Fadda V, Maratea D, Messori A. Anti-platelet treatments in acute coronary syndrome: simplified network meta-analysis. Int J Cardiol. 2011;150:364–367. doi: 10.1016/j.ijcard.2011.05.083. [DOI] [PubMed] [Google Scholar]
- 13.Janunger KG, Hafström L, Glimelius B. Chemotherapy in gastric cancer: a review and updated meta-analysis. Eur J Surg. 2002;168:597–608. doi: 10.1080/11024150201680005. [DOI] [PubMed] [Google Scholar]
- 14.Liu TS, Wang Y, Chen SY, Sun YH. An updated meta-analysis of adjuvant chemotherapy after curative resection for gastric cancer. Eur J Surg Oncol. 2008;34:1208–1216. doi: 10.1016/j.ejso.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, Cascinu S, Barni S, Labianca R, Torri V. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente) Ann Oncol. 2000;11:837–843. doi: 10.1023/a:1008377101672. [DOI] [PubMed] [Google Scholar]
- 16.Oba K, Morita S, Tsuburaya A, Kodera Y, Kobayashi M, Sakamoto J. Efficacy of adjuvant chemotherapy using oral fluorinated pyrimidines for curatively resected gastric cancer: a meta-analysis of centrally randomized controlled clinical trials in Japan. J Chemother. 2006;18:311–317. doi: 10.1179/joc.2006.18.3.311. [DOI] [PubMed] [Google Scholar]
- 17.Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 18.Zhao SL, Fang JY. The role of postoperative adjuvant chemotherapy following curative resection for gastric cancer: a meta-analysis. Cancer Invest. 2008;26:317–325. doi: 10.1080/07357900701834686. [DOI] [PubMed] [Google Scholar]
- 19.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 20.Lawton JO, Giles GR, Hall R, Bird GG, Matheson T. Chemotherapy following palliative resection of gastric cancer. Br J Surg. 1981;68:397–399. doi: 10.1002/bjs.1800680610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stablein DM, Douglass HO. Controlled trial of adjuvant chemotherapy following curative resection for gastric cancer[J] Cancer. 1982;49:1116–1122. doi: 10.1002/1097-0142(19820315)49:6<1116::aid-cncr2820490609>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Higgins GA, Amadeo JH, Smith DE, Humphrey EW, Keehn RJ. Efficacy of prolonged intermittent therapy with combined 5-FU and methyl-CCNU following resection for gastric carcinoma. A Veterans Administration Surgical Oncology, Group report. Cancer. 1983;52:1105–1112. doi: 10.1002/1097-0142(19830915)52:6<1105::aid-cncr2820520629>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima T, Takahashi T, Takagi K, Kuno K, Kajitani T. Comparison of 5-fluorouracil with ftorafur in adjuvant chemotherapies with combined inductive and maintenance therapies for gastric cancer. J Clin Oncol. 1984;2:1366–1371. doi: 10.1200/JCO.1984.2.12.1366. [DOI] [PubMed] [Google Scholar]
- 24.Engstrom PF, Lavin PT, Douglass HO, Brunner KW. Postoperative adjuvant 5-fluorouracil plus methyl-CCNU therapy for gastric cancer patients. Eastern Cooperative Oncology Group study (EST 3275) Cancer. 1985;55:1868–1873. doi: 10.1002/1097-0142(19850501)55:9<1868::aid-cncr2820550904>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 25.Schlag P. Adjuvant chemotherapy in gastric cancer. World J Surg. 1987;11:473–477. doi: 10.1007/BF01655812. [DOI] [PubMed] [Google Scholar]
- 26.Bonfanti G. Adjuvant treatments following curative resection for gastric cancer[J] Br J Surg. 1988;75:1100–1104. doi: 10.1002/bjs.1800751117. [DOI] [PubMed] [Google Scholar]
- 27.Coombes RC, Schein PS, Chilvers CE, Wils J, Beretta G, Bliss JM, Rutten A, Amadori D, Cortes-Funes H, Villar-Grimalt A. A randomized trial comparing adjuvant fluorouracil, doxorubicin, and mitomycin with no treatment in operable gastric cancer. International Collaborative Cancer Group. J Clin Oncol. 1990;8:1362–1369. doi: 10.1200/JCO.1990.8.8.1362. [DOI] [PubMed] [Google Scholar]
- 28.Estape J, Grau JJ, Lcobendas F, Curto J, Daniels M, Viñolas N, Pera C. Mitomycin C as an adjuvant treatment to resected gastric cancer. A 10-year follow-up. Ann Surg. 1991;213:219–221. doi: 10.1097/00000658-199103000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krook JE, O’Connell MJ, Wieand HS, Beart RW, Leigh JE, Kugler JW, Foley JF, Pfeifle DM, Twito DI. A prospective, randomized evaluation of intensive-course 5-fluorouracil plus doxorubicin as surgical adjuvant chemotherapy for resected gastric cancer. Cancer. 1991;67:2454–2458. doi: 10.1002/1097-0142(19910515)67:10<2454::aid-cncr2820671010>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Kim JP, Kwon OJ, Oh ST, Yang HK. Results of surgery on 6589 gastric cancer patients and immunochemosurgery as the best treatment of advanced gastric cancer. Ann Surg. 1992;216:269–278; discussion 278-279. doi: 10.1097/00000658-199209000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grau JJ, Estapé J, Alcobendas F, Pera C, Daniels M, Terés J. Positive results of adjuvant mitomycin-C in resected gastric cancer: a randomised trial on 134 patients. Eur J Cancer. 1993;29A:340–342. doi: 10.1016/0959-8049(93)90381-o. [DOI] [PubMed] [Google Scholar]
- 32.Hallissey MT, Dunn JA, Ward LC, Allum WH. The second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: five-year follow-up. Lancet. 1994;343:1309–1312. doi: 10.1016/s0140-6736(94)92464-3. [DOI] [PubMed] [Google Scholar]
- 33.Macdonald JS, Fleming TR, Peterson RF, Berenberg JL, McClure S, Chapman RA, Eyre HJ, Solanki D, Cruz AB, Gagliano R. Adjuvant chemotherapy with 5-FU, adriamycin, and mitomycin-C (FAM) versus surgery alone for patients with locally advanced gastric adenocarcinoma: A Southwest Oncology Group study. Ann Surg Oncol. 1995;2:488–494. doi: 10.1007/BF02307081. [DOI] [PubMed] [Google Scholar]
- 34.Lise M, Nitti D, Marchet A, Sahmoud T, Buyse M, Duez N, Fiorentino M, Dos Santos JG, Labianca R, Rougier P. Final results of a phase III clinical trial of adjuvant chemotherapy with the modified fluorouracil, doxorubicin, and mitomycin regimen in resectable gastric cancer. J Clin Oncol. 1995;13:2757–2763. doi: 10.1200/JCO.1995.13.11.2757. [DOI] [PubMed] [Google Scholar]
- 35.Tsavaris N, Tentas K, Kosmidis P, Mylonakis N, Sakelaropoulos N, Kosmas C, Lisaios B, Soumilas A, Mandrekas D, Tsetis A, et al. A randomized trial comparing adjuvant fluorouracil, epirubicin, and mitomycin with no treatment in operable gastric cancer. Chemotherapy. 1996;42:220–226. doi: 10.1159/000239446. [DOI] [PubMed] [Google Scholar]
- 36.Cirera L, Balil A, Batiste-Alentorn E, Tusquets I, Cardona T, Arcusa A, Jolis L, Saigí E, Guasch I, Badia A, et al. Randomized clinical trial of adjuvant mitomycin plus tegafur in patients with resected stage III gastric cancer. J Clin Oncol. 1999;17:3810–3815. doi: 10.1200/JCO.1999.17.12.3810. [DOI] [PubMed] [Google Scholar]
- 37.Nakajima T, Nashimoto A, Kitamura M, Kito T, Iwanaga T, Okabayashi K, Goto M. Adjuvant mitomycin and fluorouracil followed by oral uracil plus tegafur in serosa-negative gastric cancer: a randomised trial. Gastric Cancer Surgical Study Group. Lancet. 1999;354:273–277. doi: 10.1016/s0140-6736(99)01048-x. [DOI] [PubMed] [Google Scholar]
- 38.Neri B, Cini G, Andreoli F, Boffi B, Francesconi D, Mazzanti R, Medi F, Mercatelli A, Romano S, Siliani L, et al. Randomized trial of adjuvant chemotherapy versus control after curative resection for gastric cancer: 5-year follow-up. Br J Cancer. 2001;84:878–880. doi: 10.1054/bjoc.2000.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajetta E, Buzzoni R, Mariani L, Beretta E, Bozzetti F, Bordogna G, Aitini E, Fava S, Schieppati G, Pinotti G, et al. Adjuvant chemotherapy in gastric cancer: 5-year results of a randomised study by the Italian Trials in Medical Oncology (ITMO) Group. Ann Oncol. 2002;13:299–307. doi: 10.1093/annonc/mdf040. [DOI] [PubMed] [Google Scholar]
- 40.Nashimoto A, Nakajima T, Furukawa H, Kitamura M, Kinoshita T, Yamamura Y, Sasako M, Kunii Y, Motohashi H, Yamamoto S; Gastric Cancer Surgical Study Group, Japan Clinical Oncology Group. Randomized trial of adjuvant chemotherapy with mitomycin, Fluorouracil, and Cytosine arabinoside followed by oral Fluorouracil in serosa-negative gastric cancer: Japan Clinical Oncology Group 9206-1. J Clin Oncol. 2003;21:2282–2287. doi: 10.1200/JCO.2003.06.103. [DOI] [PubMed] [Google Scholar]
- 41.Popiela T, Kulig J, Czupryna A, Szczepanik AM, Zembala M. Efficiency of adjuvant immunochemotherapy following curative resection in patients with locally advanced gastric cancer. Gastric Cancer. 2004;7:240–245. doi: 10.1007/s10120-004-0299-y. [DOI] [PubMed] [Google Scholar]
- 42.Chipponi J, Huguier M, Pezet D, Basso N, Hay JM, Quandalle P, Jaeck D, Fagniez PL, Gainant A. Randomized trial of adjuvant chemotherapy after curative resection for gastric cancer. Am J Surg. 2004;187:440–445. doi: 10.1016/j.amjsurg.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Bouché O, Ychou M, Burtin P, Bedenne L, Ducreux M, Lebreton G, Baulieux J, Nordlinger B, Martin C, Seitz JF, Tigaud JM, Echinard E, Stremsdoerfer N, Milan C, Rougier P; Fédération Francophone de Cancérologie Digestive Group. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801) Ann Oncol. 2005;16:1488–1497. doi: 10.1093/annonc/mdi270. [DOI] [PubMed] [Google Scholar]
- 44.Nitti D, Wils J, Dos Santos JG, Fountzilas G, Conte PF, Sava C, Tres A, Coombes RC, Crivellari D, Marchet A, Sanchez E, Bliss JM, Homewood J, Couvreur ML, Hall E, Baron B, Woods E, Emson M, Van Cutsem E, Lise M; EORTC GI Group; ICCG. Randomized phase III trials of adjuvant FAMTX or FEMTX compared with surgery alone in resected gastric cancer. A combined analysis of the EORTC GI Group and the ICCG. Ann Oncol. 2006;17:262–269. doi: 10.1093/annonc/mdj077. [DOI] [PubMed] [Google Scholar]
- 45.De Vita F, Giuliani F, Orditura M, Maiello E, Galizia G, Di Martino N, Montemurro F, Cartenì G, Manzione L, Romito S, et al. Adjuvant chemotherapy with epirubicin, leucovorin, 5-fluorouracil and etoposide regimen in resected gastric cancer patients: a randomized phase III trial by the Gruppo Oncologico Italia Meridionale (GOIM 9602 Study) Ann Oncol. 2007;18:1354–1358. doi: 10.1093/annonc/mdm128. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima T, Kinoshita T, Nashimoto A, Sairenji M, Yamaguchi T, Sakamoto J, Fujiya T, Inada T, Sasako M, Ohashi Y; National Surgical Adjuvant Study of Gastric Cancer Group. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. Br J Surg. 2007;94:1468–1476. doi: 10.1002/bjs.5996. [DOI] [PubMed] [Google Scholar]
- 47.Di Costanzo F, Gasperoni S, Manzione L, Bisagni G, Labianca R, Bravi S, Cortesi E, Carlini P, Bracci R, Tomao S, Messerini L, Arcangeli A, Torri V, Bilancia D, Floriani I, Tonato M; Italian Oncology Group for Cancer Research, Dinota A, Strafiuso G, Corgna E, Porrozzi S, Boni C, Rondini E, Giunta A, Monzio Compagnoni B, Biagioni F, Cesari M, Fornarini G, Nelli F, Carboni M, Cognetti F, Enzo MR, Piga A, Romiti A, Olivetti A, Masoni L, De Stefanis M, Dalla Mola A, Camera S, Recchia F, De Filippis S, Scipioni L, Zironi S, Luppi G, Italia M, Banducci S, Pisani Leretti A, Massidda B, Ionta MT, Nicolosi A, Canaletti R, Biscottini B, Grigniani F, Di Costanzo F, Rovei R, Croce E, Carroccio R, Gilli G, Cavalli C, Olgiati A, Pandolfi U, Rossetti R, Natalini G, Foa P, Oldani S, Bruno L, Cascinu S, Catalano G, Catalano V, Lungarotti F, Farris A, Sarobba MG, Trignano M, Muscogiuri A, Francavilla F, Figoli F, Leoni M, Papiani G, Orselli G, Antimi M, Bellini V, Cabassi A, Contu A, Pazzola A, Frignano M, Lastraioli E, Saggese M, Bianchini D, Antonuzzo L, Mela M, Camisa R. Adjuvant chemotherapy in completely resected gastric cancer: a randomized phase III trial conducted by GOIRC. J Natl Cancer Inst. 2008;100:388–398. doi: 10.1093/jnci/djn054. [DOI] [PubMed] [Google Scholar]
- 48.Miyashiro I, Furukawa H, Sasako M, Yamamoto S, Nashimoto A, Nakajima T, Kinoshita T, Kobayashi O, Arai K; Gastric Cancer Surgical Study Group in the Japan Clinical Oncology Group. Randomized clinical trial of adjuvant chemotherapy with intraperitoneal and intravenous cisplatin followed by oral fluorouracil (UFT) in serosa-positive gastric cancer versus curative resection alone: final results of the Japan Clinical Oncology Group trial JCOG9206-2. Gastric Cancer. 2011;14:212–218. doi: 10.1007/s10120-011-0027-3. [DOI] [PubMed] [Google Scholar]
- 49.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]


