Abstract
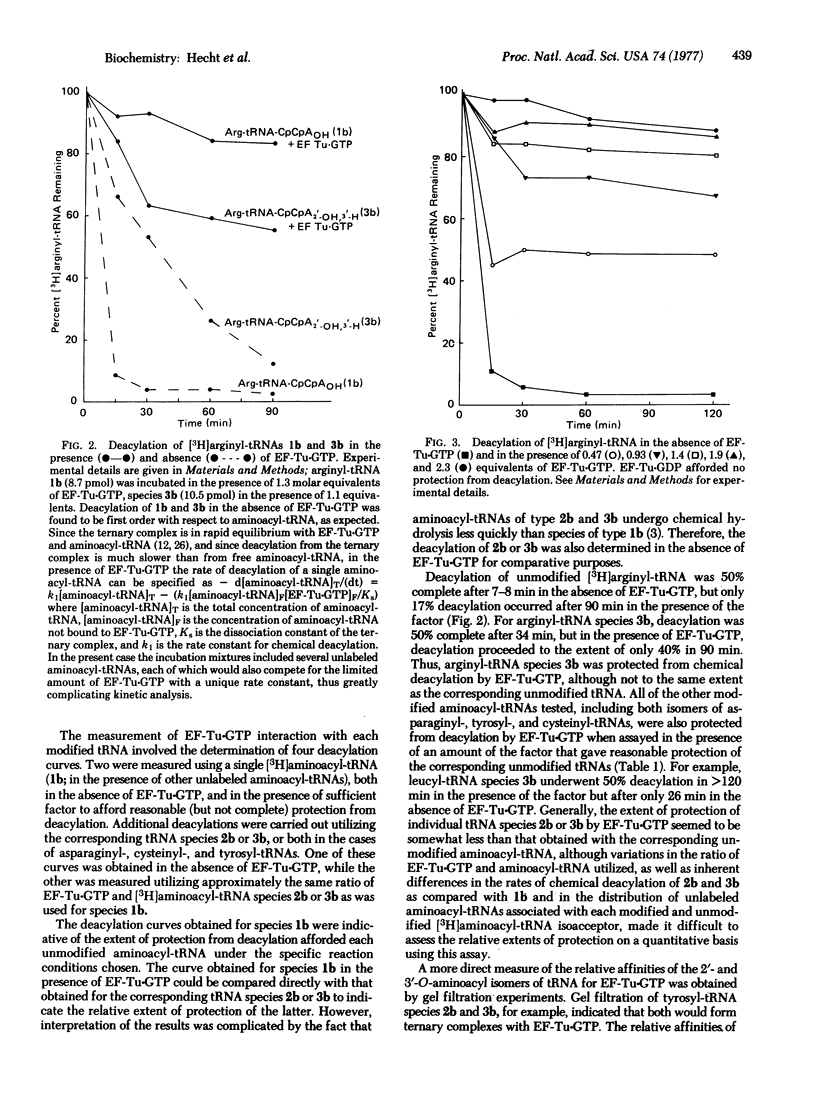
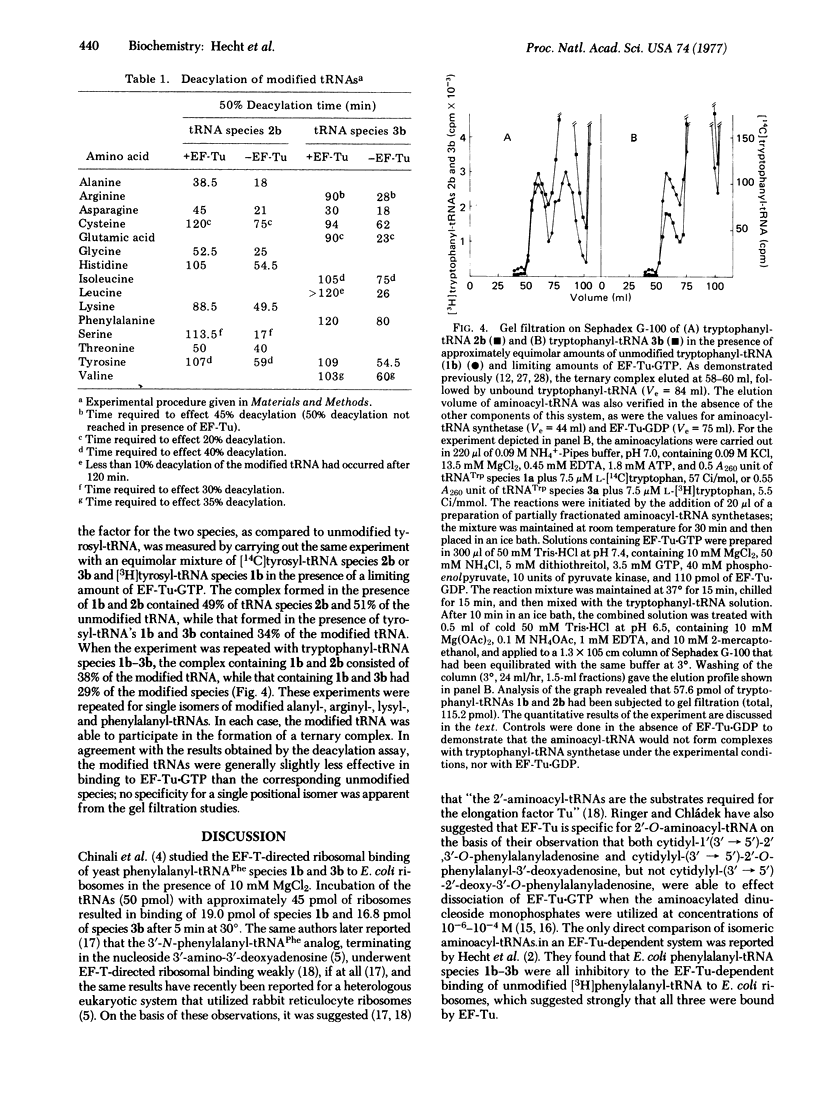
Recent suggestions that elongation factor Tu (EF-Tu) is specific for 2'-O-aminoacyl-tRNA, as compared with the 3'-isomer, prompted us to assay [3H]aminoacyl-tRNAs from Escherichia coli terminating in 2'- or 3'-deoxyadenosine for binding to EF-Tu to determine the possible positional specificity of the factor. Binding of modified aminaocyl-tRNAs to EF-Tu-GTP was measured both as a function of the ability of EF-Tu-GTP to diminish the rate of chemical deacylation of [3H]aminoacyl-tRNAs and by gel filtration of the individual ternary complexes. Fifteen different tRNA isoacceptors were tested by the deacylation procedure, including three (tRNAAsp, tRNACys, and tRNATyr) for which isomeric modified aminoacyl-tRNAs were available. All of the modified aminoacyl-tRNAs were protected fromdeacylation, although generally to a lesser extent than the corresponding unmodified species. Six modified tRNA isoacceptors (including tRNATrp and tRNATyr, for which both modified aminoacyl-tRNAs were accessible by enzymatic aminoacylation) were used in gel filtration experiments to permit direct measurement of the individual aminoacyl-tRNA-EF-Tu-GTP complexes. These experiments were also done in the presence of equimolar amounts of the corresponding unmodified [14C]aminoacyl-tRNAs, and the relative affinities for a limiting amount of EF-Tu-GTP were measured. The results were completely consistent with those obtained by the deacylation procedure and indicated that EF-Tu can bind to both positional isomers of aminoacyl-tRNA with no obvious preference for either.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Kawakita M., Kaziro Y. Studies on the polypeptide elongation factors from E. coli. IV. Crystalline Tu-GTP, Tu-Gpp(CH2)p, and phenylalanyl-tRNA-Tu-GTP complex. J Biochem. 1974 Aug;76(2):283–292. doi: 10.1093/oxfordjournals.jbchem.a130570. [DOI] [PubMed] [Google Scholar]
- Baksht E., de Groot N., Sprinzl M., Cramer F. Properties of tRNA species modified in the 3'-terminal ribose moiety in an eukaryotic ribosomal system. Biochemistry. 1976 Aug 10;15(16):3639–3646. doi: 10.1021/bi00661a035. [DOI] [PubMed] [Google Scholar]
- Beres L., Lucas-Lenard J. Studies on the fluorescence of the Y base of yeast phenylalanine transfer ribonucleic acid. Effect of pH, aminoacylation, and interaction with elongation factor Tu. Biochemistry. 1973 Sep 25;12(20):3998–4002. doi: 10.1021/bi00744a033. [DOI] [PubMed] [Google Scholar]
- Chinali G., Sprinzl M., Parmeggiani A., Cramer F. Participation in protein biosynthesis of transfer ribonucleic acids bearing altered 3'-terminal ribosyl residues. Biochemistry. 1974 Jul 16;13(15):3001–3010. doi: 10.1021/bi00712a001. [DOI] [PubMed] [Google Scholar]
- Fraser T. H., Rich A. Amino acids are not all initially attached to the same position on transfer RNA molecules. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3044–3048. doi: 10.1073/pnas.72.8.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser T. H., Rich A. Synthesis and aminoacylation of 3'-amino-3'-deoxy transfer RNA and its activity in ribosomal protein synthesis. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2671–2675. doi: 10.1073/pnas.70.9.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. A stepwise reaction yielding a complex between a supernatant fraction from E. coli, guanosine 5'-triphosphate, and aminoacyl-sRNA. Proc Natl Acad Sci U S A. 1968 Jan;59(1):179–183. doi: 10.1073/pnas.59.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S. M., Chinualt A. C. Position of aminoacylation of individual Escherichia coli and yeast tRNAs. Proc Natl Acad Sci U S A. 1976 Feb;73(2):405–409. doi: 10.1073/pnas.73.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S. M., Hawrelak S. D., Kozarich J. W., Schmidt F. J., Bock R. M. Chemical modifications of transfer RNA species. Transfer RNA's terminating in 2'- and 3'-O-methyladenosine. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1341–1347. doi: 10.1016/0006-291x(73)90648-7. [DOI] [PubMed] [Google Scholar]
- Hecht S. M., Kozarich J. W., Schmidt F. J. Isomeric phenylalanyl-tRNAs. Position of the aminoacyl moiety during protein biosynthesis. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4317–4321. doi: 10.1073/pnas.71.11.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Oashi Z., Harada F., Nishimura S., Oppenheimer N. J., Crain P. F., Liehr J. G., von Minden D. L., McCloskey J. A. Structure of the modified nucleoside Q isolated from Escherichia coli transfer ribonucleic acid. 7-(4,5-cis-Dihydroxy-1-cyclopenten-3-ylaminomethyl)-7-deazaguanosine. Biochemistry. 1975 Sep 23;14(19):4198–4208. doi: 10.1021/bi00690a008. [DOI] [PubMed] [Google Scholar]
- Klyde B. J., Bernfield M. R. Purification of chicken liver seryl transfer ribonucleic acid by complex formation with elongation factor EF-Tu:GTP. A general micromethod of aminoacyl transfer ribonucleic acid purification. Biochemistry. 1973 Sep 11;12(19):3752–3757. doi: 10.1021/bi00743a026. [DOI] [PubMed] [Google Scholar]
- Kozarich J. W., Chinault A. C., Hecht S. M. Ribonucleoside phosphates via phosphorimidazolidate intermediates. Synthesis of pseudoadenosine 5'-triphosphate. Biochemistry. 1973 Oct 23;12(22):4458–4463. doi: 10.1021/bi00746a024. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Gilham P. T., Söll D. An improved method for the purification of tRNA by chromatography on dihydroxyboryl substituted cellulose. Nucleic Acids Res. 1975 Jun;2(6):853–864. doi: 10.1093/nar/2.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Skoultchi A., Klein A., Lengyel P. Peptide chain elongation: discrimination against the initiator transfer RNA by microbial amino-acid polymerization factors. Nature. 1968 Dec 28;220(5174):1304–1307. doi: 10.1038/2201304a0. [DOI] [PubMed] [Google Scholar]
- Ringer D., Chládek S. Interaction of elongation factor Tu with 2'(3')-O-aminoacyloligonucleotides derived from the 3' terminus of aminoacyl-tRNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2950–2954. doi: 10.1073/pnas.72.8.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H., Sundari R. M. Structural requirements for recognition of Escherichia coli initiator and non-initiator transfer ribonucleic acids by bacterial T factor. J Biol Chem. 1974 Nov 25;249(22):7102–7110. [PubMed] [Google Scholar]
- Shorey R. L., Ravel J. M., Garner C. W., Shive W. Formation and properties of the aminoacyl transfer ribonucleic acid-guanosine triphosphate-protein complex. J Biol Chem. 1969 Sep 10;244(17):4555–4564. [PubMed] [Google Scholar]
- Sprinzl M., Cramer F. Accepting site for aminoacylation of tRNAphe from yeast. Nat New Biol. 1973 Sep 5;245(140):3–5. doi: 10.1038/newbio245003a0. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Cramer F. Site of aminoacylation of tRNAs from Escherichia coli with respect to the 2'- or 3'-hydroxyl group of the terminal adenosine. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3049–3053. doi: 10.1073/pnas.72.8.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach H., Miller D. L., Hachmann J. Studies on the role of factor Ts in polypeptide synthesis. Arch Biochem Biophys. 1970 Mar;137(1):262–269. doi: 10.1016/0003-9861(70)90433-9. [DOI] [PubMed] [Google Scholar]
- von der Haar F., Cramer F. Isoleucyl-tRNA synthetase from baker's yeast: the 3'-hydroxyl group of the 3'-terminal ribose is essential for preventing misacylation of tRNAIle-C-C-A with misactivated valine. FEBS Lett. 1975 Aug 15;56(2):215–217. doi: 10.1016/0014-5793(75)81094-5. [DOI] [PubMed] [Google Scholar]


