Abstract
Background
The genus Orbivirus includes a number of important pathogenic viruses, including Bluetongue virus (BTV), African horse sickness virus (AHSV), and Epizootic hemorrhagic disease virus (EHDV). In this study we describe the isolation and characterization of an Orbivirus strain isolated from Anopheles maculatus mosquitoes collected in Tibet, China.
Methods and Results
Initial viral screening identified a viral strain (XZ0906) that caused significant cytopathic effect (CPE) in BHK-21 cells, including rounding, cell rupture, and floating. Although CPE was not observed in insect cells (C6/36), these cells supported viral replication. Polyacrylamide gel analysis revealed a genome consisting of 10 segments of double-stranded RNA (dsRNA), with a distribution pattern of 3-3-3-1. 454 high throughput sequencing of culture supernatant was used for viral identification. Complete genome sequencing was performed by Sanger sequencing in combination with 5′-RACE and 3′-RACE. Sequence analysis demonstrated that all 5′- and 3′- untranslated regions (UTRs) for each of the 10 genome segments contained a series of six highly conserved nucleotides. In addition, homology analysis and phylogenetic analysis based on amino acid sequence was completed, and all results show that virus XZ0906 was not a member of any known species or serotype of Orbivirus, indicating it to be a new species within the genus Orbivirus.
Conclusions
The isolated Orbivirus strain was designated Tibet Orbivirus, TIBOV to denote the location from which it was isolated. TIBOV is a novel orbivirus species which is isolated from Anopheles maculatus mosquitoes collected in Tibet, China.
Introduction
There are currently 22 confirmed species of the genus Orbivirus in the family Reoviridae [1]. This genus includes a number of important pathogenic viruses, including Bluetongue virus (BTV), African horse sickness virus (AHSV), and Epizootic hemorrhagic disease virus (EHDV) [1], [2], which are spread primarily through insect vectors, such as Culicoides midges, ticks, mosquitoes, and phlebotomine flies [1], [3]–[6].
Orbiviruses contain a multi-segmented, double-stranded RNA genome, consisting of 10 segments (Seg1–Seg10) of various length, which are identified according to their molecular weight [7]. Partial nucleotide sequences for each of the gene segments for many of the Orbiviruses have been published, along with complete genome sequences of some species [3], [5], [8]–[10], allowing for detailed classification and phylogenetic analysis of Orbiviruses.
This study describes a viral strain (XZ0906) isolated from Anopheles maculatus specimens collected in Tibet, China. All the results of initial viral screening showed a difference between XZ0906 and Yunnan Orbivirus (YUOV), an orbivirus also isolated from China. After whole genome sequencing, amino acid homology and molecular phylogenetic analysis, XZ0906, which is designated as Tibet Orbivirus (TIBOV), is identified as a novel species of the genus Orbivirus.
Materials and Methods
1. Cell culture
Aedes albopictus C6/36 cells and BHK-21 (Baby hamster kidney) cells (ATCC) were used in this study [11], and both cell lines were kept in our laboratory. C6/36 cells were maintained in medium with 45% RMPI 1640 and 45% DMEM (Invitrogen) supplemented with 10% inactive fetal bovine serum (FBS, Invitrogen) and 100 U/mL penicillin and streptomycin. Cells were propagated and maintained at 28°C [11]–[13]. BHK-21 cells were grown in minimal essential medium with Eagle's balanced salt solution supplemented with 10% FBS (Invitrogen), 2 mM glutamine, 0.12% NaHCO3, and 100 U/mL penicillin and streptomycin. BHK-21 cells were propagated and maintained at 37°C under a 5% CO2 atmosphere [11]–[13].
2. Viral isolation
Mosquito samples were collected in Medog County (altitude 1000 m) in the Nyingchi area of Tibet, China during the summer of 2009, and transported to the laboratory in liquid nitrogen containers, following morphological classification and species identification on-site. All specimens were homogenized and centrifuged at 12000×g for 30 min at 4°C. To isolate the virus, 150 µL of supernatant was then added to monolayers of both C6/36 and BHK-21 cells, and cultured at 28 and 37°C, respectively, in a 5% CO2 incubator. Cells were monitored at 24-h intervals to identify cytopathic effects (CPE) associated with infection [11]–[13].
3. dsRNA-polyacrylamide gel electrophoresis
Viral RNA was isolated as described previously, and analyzed by polyacrylamide gel electrophoresis [13].
4. Preparation of viral DNA and RNA and 454 sequencing
Viral DNA was extracted from 200-µL aliquots of virus-infected BHK-21 cell culture supernatants using a QIAamp DNA Blood Mini Kit (Qiagen). Viral RNA was extracted from 140-µL aliquots of virus-infected BHK-21 cell culture supernatant using a QIAamp Viral RNA Mini Kit (Qiagen) according to the manufacturer's instructions. cDNA was made with a Ready-To-Go kit (GE Healthcare) using random hexanucleotide primers. Samples were then amplified as described previously [14], [15]. Amplification products were pooled, adaptor-ligated, and sequenced at the Washington University Genome Sequencing Center on the 454 GS-FLX platform (454 Life Sciences, Branford, CT).
Because the nucleic acids used for sequencing contained a mixture of host cell DNA and viral RNA, sequencing reads were filtered using the custom informatics pipeline VirusHunter [16] to identify viral sequences. Sequences identified as most similar to viruses in the genus Orbivirus, as well as those that had no significant hit to any sequence in the GenBank database, were assembled with Newbler (454 Life Sciences) using the default parameters. Sequences were trimmed to remove primer sequences prior to data analysis and assembly.
5. Complete genome sequencing including 5′- and 3′-untranslated regions
Reverse-transcription polymerase chain reaction (RT-PCR) was performed to fill in gaps between viral gene sequences obtained with 454 sequencing using contig-specific primers. Total viral RNA was extracted as described in Step 4, cDNA was generated by reverse transcription, and used as a template for complete genome amplification. Next, a set of specific primers was designed to amplify each segment of the viral genome and the amplification products were sequenced using the Sanger method (Table 1). 5′-RACE and 3′-RACE systems (Rapid Amplification of cDNA Ends), Version 2.0 (Invitrogen) were used to amplify the 5′- and 3′-UTRs from each of the 10 segments, respectively. 5′-RACE was performed according to the manufacturer's instructions. For 3′-RACE, a PolyA tail was first added to RNA using a PolyA polymerase. 3′-UTR sequences were then generated by RT-PCR using sequence-specific and oligo-dT-adapter primers. Sequence assembly was performed resulting in a complete viral genome.
Table 1. Primers used in this study.
| Primer | Sequence (5′-3′) | Position | Orientation |
| 6-1-1F | GTAAAATCACAATGGTCG | 1–18 | Sense |
| 6-1-1R | TAGCAGCAACTCCCCAAG | 826–843 | Antisense |
| 6-1-2F | TGGAGGAAGAGGGCGTGAG | 679–697 | Sense |
| 6-1-2R | TAGAACCCTTTGTTTGGT | 1531–1548 | Antisense |
| 6-1-3F | AGTCAAGAAAAGGTTTGG | 1385–1402 | Sense |
| 6-1-3R | CTGAGCGTAAAATAGCGT | 2310–2327 | Antisense |
| 6-1-4F | ATTTAGCCATGATAGACACG | 2152–2171 | Sense |
| 6-1-4R | GAGACAATCGCCCTGGTG | 3064–3081 | Antisense |
| 6-1-5F | ATGCGACCCATACATAAA | 2874–2891 | Sense |
| 6-1-5R | CTCGTCCTCCGTCACAAC | 3786–3803 | Antisense |
| 6-1-6F | CTGAAATAATGGATGCGGTTGA | 3019–3040 | Sense |
| 6-1-6R | GTAAGTGTATCACGGGCGCGCTAAT | 3926–3950 | Antisense |
| 6-2-1F | GTAAAAACTGACGATGGACGAATTC | 1–25 | Sense |
| 6-2-1R | CGCATCCGCTCTTGAAAT | 940–957 | Antisense |
| 6-2-2F | ATTTGAGAAGTGGGAGTT | 760–777 | Sense |
| 6-2-2R | TTCATGTACGGTGGTAAG | 1549–1566 | Antisense |
| 6-2-3F | TTATAGATGGTGATTTGCTT | 1428–1447 | Sense |
| 6-2-3R | CATCCTTACTTCTGACGC | 2270–2287 | Antisense |
| 6-2-4F | GGGCATACGGCGGAGAAT | 2021–2038 | Sense |
| 6-2-4R | GTAAGTTTAAACTGTGTGGTGATCG | 2864–2888 | Antisense |
| 6-3-1F | GTAAAATTTCCGTGGCGATGGCTGA | 1–25 | Sense |
| 6-3-1R | ACCGCAGGGTTTATAGGT | 824–841 | Antisense |
| 6-3-2F | GCTCGGACCCACTTTACC | 637–654 | Sense |
| 6-3-2R | TGCTGCCACAAGCATCAG | 1515–1532 | Antisense |
| 6-3-3F | TATAATGGATGGGCTGTC | 1356–1373 | Sense |
| 6-3-3R | GTAGTCTGGCAATCTCGT | 2248–2265 | Antisense |
| 6-3-4F | TATTGGAGCGTGAAGCAT | 2056–2073 | Sense |
| 6-3-4R | GTAAGTGTATTCCCGTTGCAGTCGG | 2745–2769 | Antisense |
| 6-4-1F | GTAAAAACATGCCGGAGCCACATGC | 1–25 | Sense |
| 6-4-1R | TAGGCGATCCTCAGCAAA | 855–872 | Antisense |
| 6-4-2F | CGACAGACCAAAAGATAT | 734–751 | Sense |
| 6-4-2R | TCAACACGTAATCCAATA | 1565–1582 | Antisense |
| 6-4-3F | TGCAGCGCCTAAAACGAT | 986–1003 | Sense |
| 6-4-3R | GTAAGTGTAACATGCCTTCCAGATC | 1954–1978 | Antisense |
| 6-5-1F | GTAAAAAAGTTCTTCGTCGACTGCC | 1–25 | Sense |
| 6-5-1R | ACCAGCGTCATCGGCATC | 955–972 | Antisense |
| 6-5-2F | CACCGACAGAAGCAAGGC | 789–806 | Sense |
| 6-5-2R | GTAAGTGTAAGTTCGATAGAGCGAA | 1751–1775 | Antisense |
| 6-6-1F | GTAAAAAAGATCGCCTTACGTGCAG | 1–25 | Sense |
| 6-6-1R | GCTTATCCCCGCAACCAA | 915–932 | Antisense |
| 6-6-2F | AAGGGATGCAAGAGGAGG | 655–672 | Sense |
| 6-6-2R | GTAAGTTTAAGATCTAATTACGCTG | 1612–1636 | Antisense |
| 6-7-1F | GTAAAAATTTGGTGAAGATGGACGC | 1–25 | Sense |
| 6-7-1R | TCGCTGCTCGCAAACCGT | 853–870 | Antisense |
| 6-7-2F | GTGGTTGCCTGGAATGGA | 681–698 | Sense |
| 6-7-2R | GTAAGTGTAATTTGGGAAAACGTAT | 1141–1165 | Antisense |
| 6-8-1F | GTAAAAAATTCCTAGCAACCATGGA | 1–25 | Sense |
| 6-8-1R | CCACCTTTGACCACCTTA | 866–883 | Antisense |
| 6-8-2F | GGTAACCGAGATTCGCTCAA | 524–543 | Sense |
| 6-8-2R | GTAAGTTTAAATTCCCTCCCCTATA | 1118–1142 | Antisense |
| 6-9-1F | GTAAAAAATTGCTTATGTCAGCTGC | 1–25 | Sense |
| 6-9-1R | TGAGCACTACCCACCCTC | 565–582 | Antisense |
| 6-9-2F | AAGAAGATTCGGTGGTGG | 286–303 | Sense |
| 6-9-2R | GTAAGTTTTAAATTGCTACGGTCAG | 1076–1100 | Antisense |
| 6-10-1F | GTAAAAAAGAATGTGGTTGTCATGC | 1–25 | Sense |
| 6-10-1R | CGATTTGGCCCGTTAGCA | 587–604 | Antisense |
| 6-10-2F | GATGACGGATGGAATGGC | 159–176 | Sense |
| 6-10-2R | GTAAGTTGGGTGAATGCGGTGAACT | 808–832 | Antisense |
6. Molecular detection of viral genes in cell culture
Viral replication was detected in infected C6/36 and BHK21 cells using RT-PCR for specific regions for TIBOV segment 1 and segment 2. Total RNA was extracted from cell culture supernatants as described in Step 4. cDNA was then generated by reverse transcription, and used as a template for RT-PCR. Gene amplification was performed using primers 6-1-5R and 6-1-5F (primers for Seg1), 6-2-2R and 6-2-2F (primers for Seg2), etc.; detailed sequence information for all primer sequences is shown in Table 1. PCR was performed under the following conditions: one cycle of denaturation at 95°C for 5 min, 35 cycles of 95°C for 1 min (denaturation), 52°C for 1 min (annealing), and 72°C for 1 min (extension), followed by a final extension at 72°C for 10 min. Amplification products were analyzed by gel electrophoresis on a 1% agarose gel.
7. Nucleotide and amino acid sequence analysis
Sequences were identified by BLAST analysis (http://www.ncbi.nlm.nih.gov/BLAST/). Multiple sequence alignments were performed using the Clustal X2 software. Phylogenetic analysis of amino acid sequences for each Orbivirus gene segment were performed using the MEGA 5.04 software package (www.megasoftware.net). Amino acid sequences were analyzed using PredictProtein (http://www.predictprotein.org/). The background information for all virus strains used in this study is shown in Table 2.
Table 2. Information of all virus strains used in this study.
| Genus | Species | Abbreviation | Strain/Serotype | GenBank accession no. | |
| VP1(RdRp) | T 2 | ||||
| Genus Orbivirus | African horsesickness virus | AHSV-1 | HS29-62/serotype1 | FJ183364 | FJ183365 |
| African horsesickness virus | AHSV-2 | HS 02-07/serotype2 | FJ196584 | FJ196585 | |
| African horsesickness virus | AHSV-4 | HS32-62/serotype4 | JQ796724 | JQ796725 | |
| African horsesickness virus | AHSV-9 | E41-02(Or)/serotype9 | U94887 | DQ868776 | |
| Bluetongue virus | BTV-1 | SZ97-1/serotype1 | JN848759 | JN848760 | |
| Bluetongue virus | BTV-1A | Australia | NA | P20608 | |
| Bluetongue virus | BTV-2 | BTV-2IT(L)/serotype2 | JN255862 | JN255863 | |
| Bluetongue virus | BTV-4 | BTV-4IT(L)/serotype4 | JN255882 | JN255883 | |
| Bluetongue virus | BTV-6 | USA2006-01/serotype6 | GQ506536 | GQ506537 | |
| Bluetongue virus | BTV-9 | BTV-9IT(L)/serotype9 | JN255902 | JN255903 | |
| Bluetongue virus | BTV-12 | BTV12-PT2003/serotype12 | GU390658 | GU390659 | |
| Bluetongue virus | BTV-13 | USA | NA | Q65750 | |
| Bluetongue virus | BTV-1S | South Africa | NA | P56582 | |
| Bluetongue virus | BTV-17 | USA | NA | P03539 | |
| Changuinola virus | CGLV | BeAr478620 | HQ397615 | NA | |
| Corriparta virus | CORV | CSIRO1740 | HQ397617 | NA | |
| Corriparta virus | CORV | MRM1 | NA | AAM96695 | |
| Epizootic hemorrhagic disease virus | EHDV-1 | New Jersey/serotype1 | NC_013396 | NC_013397 | |
| Epizootic hemorrhagic disease virus | EHDV-2 | Ibaraki/serotype2 | AM745077 | AM745078 | |
| Epizootic hemorrhagic disease virus | EDHV-2 | Alberta/serotype2 | AM744997 | AM744999 | |
| Epizootic hemorrhagic disease virus | EHDV-6 | 318/serotype6 | AM745067 | AM745068 | |
| Epizootic hemorrhagic disease virus | EHDV-7 | CSIRO 775/serotype7 | AM745047 | AM745048 | |
| Equine encephalosis virus | EEV | HS103-06 | FJ183384 | FJ183385 | |
| Eubenangee virus | EUBV | AUS1963/01 | JQ070376 | JQ070377 | |
| Great Island virus | GIV | CanAr-42 | ADM88592 | ADM88593 | |
| Broadhaven virus | BRDV | BRDV | NA | P35934 | |
| Kemerovo virus | KEMV | EgAn 1169-61 | ADM88609 | ADM88610 | |
| Lipovnik virus | LIPV | CzArLip-91 | ADM88603 | ADM88604 | |
| Tribec virus | TRBV | TRBV | ADM88606 | ADM88607 | |
| Itupiranga virus | ITUV | BeAr312086 | HQ397639 | NA | |
| Matucare virus | MATV | MARU21343 | HQ397640 | NA | |
| Orungo virus | ORUV | IBH11306-84 | HQ397641 | NA | |
| Palyam virus | PALV | Chuzan | BAA76549 | BAA34936 | |
| St Croix River virus | SCRV | SCRV | AAG34363 | AAG34364 | |
| Umatilla virus | UMAV | USA1969/01 | AEE98368 | AEE98369 | |
| Stretch Lagoon | SLOV | K49460 | ACH91290 | ACH91291 | |
| Wallal virus | WALV | Ch12048 | NA | AAM96693 | |
| Warrego virus | WARV | V5080 | ABM92924 | ABM92926 | |
| Warrego virus | WARV | Ch9935 | AAM96690 | AAM96692 | |
| Wongorr virus | WGRV | CSIRO51 | HQ397668 | NA | |
| Wongorr virus | WGRV | mrm13443 | NA | U56992 | |
| Wongorr virus | WGRV | Paroo-River | NA | U56993 | |
| Wongorr virus | WGRV | V199 | NA | U56991 | |
| Yunnan orbivirus | YUOV | YOV-77-2 | YP443925 | YP443926 | |
| Middle point orbivirus | MPOV | DPP4440 | ABU95014 | ABU95015 | |
| Genus Phytoreovirus | Rice dwarf virus | RDV-A | A | BAA14222 | NA |
| Rice dwarf virus | RDV-Ch | Chinese | AAB18743 | NA | |
| Rice dwarf virus | RDV-H | H | BAA01074 | NA | |
| Genus Rotavirus | Rotavirus A (Bovine rotavirus A) | BoRV-A/UK | UK WT BRV4A | CAA39085 | NA |
| Rotavirus A (Bovine rotavirus A) | SiRV-A/SA11 | Simian | AAC58684 | NA | |
| Rotavirus C (Porcine rotavirus C) | PoRV-C/Co | Co | AAB00801 | NA | |
| Genus Seadornavirus | Banna virus | BAV | BAV-Ch | AAF77631 | NA |
| Kadipiro virus | KDV | JKT-7075 | AAF78848 | NA | |
| Liao ning virus | LNV | LNSV-NE9731 | AAQ83562 | NA | |
| Genus Cardoreovirus | Eriocheir sinensis reovirus | ESRV | 905 | AAT11887 | NA |
| Genus Mimoreovirus | Micromonas pusilla reovirus | MPRV | MPRV | AAZ94041 | NA |
| Genus Aquareovirus | Aquareovirus A (Chum salmon reovirus) | CSRV | CSRV | AAL31497 | NA |
| Aquareovirus A(Striped bass reovirus) | SBRV | SBRV | AAM93410 | NA | |
| Aquareovirus C(Grass carp reovirus) | GCRV | GCRV | AAG10436 | NA | |
| Aquareovirus C (Golden shiner reovirus) | GSRV | GSRV | AAM92745 | NA | |
| Aquareovirus G(Golden ide reovirus) | GIRV | GIRV | AAM93415 | NA | |
| Genus Cypovirus | Dendrlymus punctatus cytoplas-mic polyhedrosis virus-1 | DsCPV-1 | DsCPV-1 | AAN46860 | NA |
| Lymantria dispar cytoplasmic polyhedrosis virus-14 | LdCPV-14 | LdCPV-14 | AAK73087 | NA | |
| Genus Coltivirus | Colorado tick fever virus | CTFV | Florio | AAK00595 | NA |
| Eyach virus | EYAV | Fr578 | AAM18342 | NA | |
| GenusDinovernavirus | Aedes pseudoscutellaris reovirus | APRV | APRV | AAZ94068 | NA |
| Genus Fijivirus | Nilaparvata lugens reovirus | NLRV-Iz | Izumo | BAA08542 | NA |
| Genus Mycoreovirus | Mycoreovirus 1(Cryphonectria parasitica reovirus) | CpMYRV-1 | 9B21 | AAP45577 | NA |
| Mycoreovirus 3 (Rosellinia anti-rot virus) | RnMYRV-3 | RArV | BAC98431 | NA | |
| Genus Orthoreovirus | Mammalian orthoreovirus 1 | MRV-1 | Lang | AAA47234 | NA |
| Mammalian orthoreovirus 2 | MRV-2 | Jones | AAA47245 | NA | |
| Mammalian orthoreovirus 3 | MRV-3 | Dearing | AAA47255 | NA | |
| Mammalian orthoreovirus 4 | MRV-4 | Ndelle | AAL36027 | NA | |
| Genus Oryzavirus | Rice ragged stunt virus | RRSV-Th | Thai | AAC36456 | NA |
Note: NA, Not available.
Results
1. Isolation of viral strains
A. maculatus mosquitoes collected from Tibet, China were homogenized, and the supernatant added to monolayers of C6/36 and BHK-21 cells. Severe CPE was observed in BHK-21 cells three days after inoculation with mosquito lysate XZ0906, characterized by cell rounding, lysis, and floating cells (Figure 1). However, no obvious pathological changes were seen in C6/36 cells cultured with the same mosquito lysate for five days, or after three consecutive passages. Despite the lack of CPE in C6/36 cells, Orbivirus Seg1 and Seg2 could be detected by RT-PCR in the supernatant of third-generation C6/36 cultures (Figure 2), indicating that virus XZ0906 could replicate in C6/36 cells.
Figure 1. CPE of virus XZ0906 on BHK-21 cells after three days of infection.
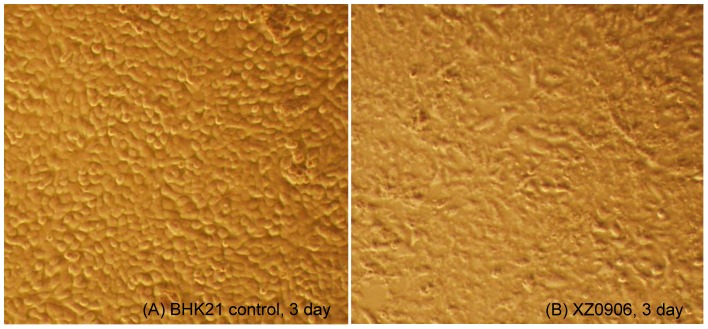
BHK-21 cells were grown to 80% confluence and inoculated with supernatant harvested from mosquito specimen XZ0906. (A) control BHK21; (B) CPE caused by XZ0906, including rounding, cell rupture.
Figure 2. PCR Identification of virus XZ0906 in the culture supernatant of BHK-21 and C6/36 cells.
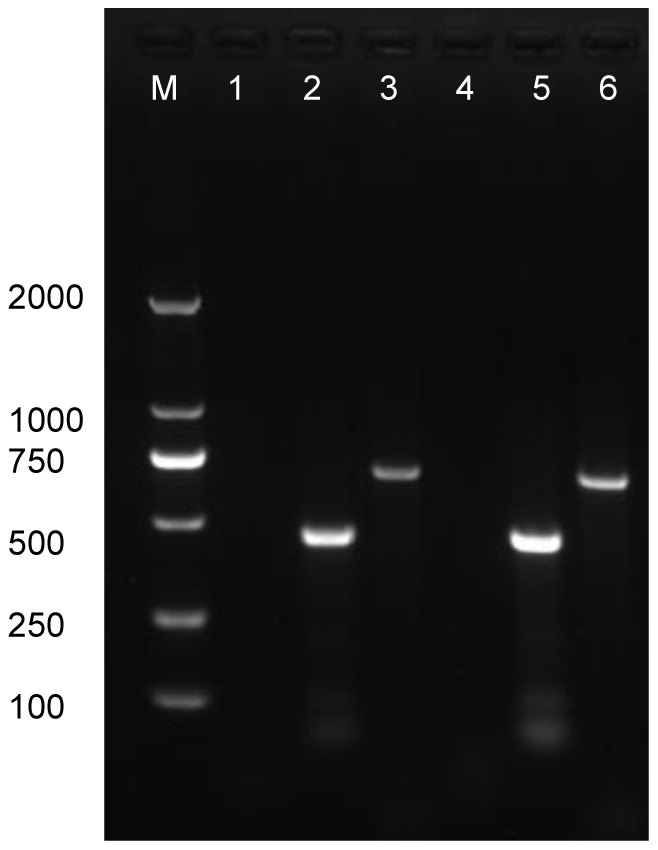
M, Marker DL2000; 1. BHK-21 cell control; 2. BHK-21 cells inoculated with virus XZ0906, the target is an amplicon of 480bp from Segment 1 of XZ0906; 3. BHK-21 cells inoculated with XZ0906, the target is an amplicon of 740bp from Segment 2 of XZ0906; 4. C6/36 cell control; 5. C6/36 cells inoculated with virus XZ0906, the target is an amplicon of 480bp from Segment 1 of XZ0906; 6. C6/36 cells inoculated with virus XZ0906, the target is an amplicon of 740bp from Segment 2 of XZ0906.
2. Identification of a segmented dsRNA genome
Viral RNA was harvested from the culture supernatant of infected BHK-21 cells, and analyzed by polyacrylamide gel electrophoresis (PAGE), revealing a genome consisting of 10 dsRNA segments, whose migration pattern was 3-3-3-1 (Figure 3). Within this pattern Seg2 migrated to the same region as Seg3; Seg5 and Seg6 were also difficult to distinguish, indicating that these segments had similar molecular weights. Segments 7, 8, and 9 were also similar in terms of molecular weights, but were easily distinguished from Seg10.
Figure 3. Electrophoretic migration patterns of the dsRNA of virus XZ0906 as determined by polyacrylamide gel electrophoresis.

The standard discontinuous polyacrylamide slab gel electrophoresis was used here with a 3.5% acrylamide concentration gel and 10% acrylamide separation gel. After staining with silver nitrate,the genome of XZ0906 was visualized separated into 10 distinct bands.
3. Preliminary identification of virus XZ0906 using 454 sequencing
Following random PCR amplification, samples were pooled (with barcodes) along with other samples, and sequenced using the Roche/454 FLX Titanium platform, producing a total of 24,929 reads. Sequence data were analyzed using the customized data analysis pipeline VirusHunter [16], identifying 85 unique reads which exhibited 28.1–84.9% sequence identity to viruses in the genus Orbivirus.
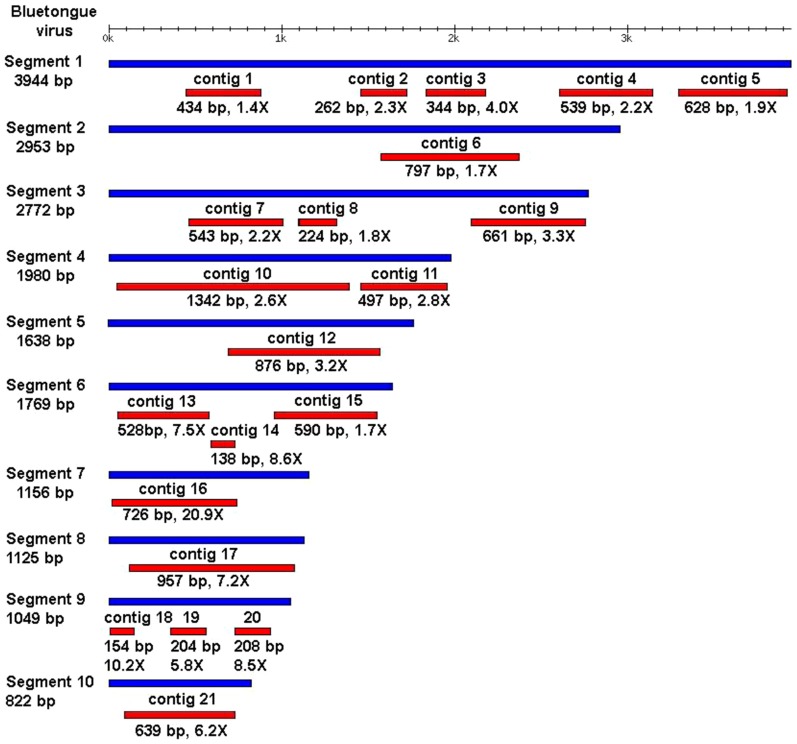
All individual reads with detectable similarity to Orbivirus, as well as those sharing no detectable sequence similarity with any sequence in the GenBank database, were used as inputs and assembled into contigs using the Newbler assembler. Twenty-one contigs were assembled, of 138–1342 bp in length, with the greatest similarity to BTV at a coverage depth of 1.4–20.9-fold (Figure 4). Almost-complete RNA sequences were obtained for segments 7, 8, 10. Segments 1, 3, 4, 6 and 9 were represented by two to five contigs; a single contig was identified for segments 2 and 5.
Figure 4. Contigs assembled from 454 sequencing reads compared with BTV.
Blue bars represent RNA segments from the BTV reference genome; red bars represent assembled viral contigs. Contig lengths and coverage are shown below each of the respective contigs.
4. Sequencing and analysis of virus XZ0906 and other Orbiviruses
RT-PCR amplification was used to close the gaps between contigs for each of the 10 segments. Primer walking, together with 5′- and 3′-RACE, were used to sequence the 5′- and 3′-ends of each segment. Finally, Sanger sequencing was employed to confirm sequences using primers newly designed for each of the 10 RNA segments (Table 1); complete sequences for this virus XZ0906 have been deposited in GenBank under accession number(genome segments KF746187 to KF746196).
Sequence analysis identified a stretch of six highly conserved nucleotides present at the ends of the 5′- and 3′-UTRs (5′-GUAAAA and ACUUAC-3′, respectively) for each of 10 gene segments (Table 3).
Table 3. Lengths of the coding and untranslated regions of each of the 10 genomic segments of virus XZ0906.
| Segment | Length (bp) | Protein (aa) | 5′ UTR | 3′ UTR | ||
| Length (bp) | Terminal sequence | Length (bp) | Terminal sequence | |||
| S1 | 3950 | 1304 | 11 | 5′-GUAAAAUC-- | 24 | --ACACUUAC-3′ |
| S2 | 2888 | 946 | 13 | 5′-GUAAAAAC-- | 34 | --AAACUUAC-3′ |
| S3 | 2769 | 899 | 17 | 5′-GUAAAAUU-- | 52 | --ACACUUAC-3′ |
| S4 | 1978 | 643 | 8 | 5′-GUAAAAAC-- | 38 | --ACACUUAC-3′ |
| S5 | 1775 | 554 | 31 | 5′-GUAAAAAA-- | 79 | --ACACUUAC-3′ |
| S6 | 1636 | 526 | 26 | 5′-GUAAAAAA-- | 29 | --AAACUUAC-3′ |
| S7 | 1165 | 349 | 17 | 5′-GUAAAAAU-- | 98 | --ACACUUAC-3′ |
| S8 | 1142 | 359 | 20 | 5′-GUAAAAAA-- | 42 | --AAACUUAC-3′ |
| S9 | 1100 | 346 | 14 | 5′-GUAAAAAA-- | 45 | --AAACUUAC-3′ |
| S10 | 832 | 234 | 21 | 5′-GUAAAAAA-- | 106 | --CAACUUAC-3′ |
Significant differences were observed in both the nucleotide and amino acid sequences of virus XZ0906 relative to other members of the genus Orbivirus (Table 4). The VP1 protein (RNA-dependent RNA polymerase, RdRp), encoded by Seg1, shared 35.3% (SCRV)-72.9% (EHDV-6) identity at the amino acid level to the six selected Orbiviruses. Protein T2, encoded by Seg3 of XZ0906, shared 22.9% (SCRV) to 75.9% (BTV-6) identity (Table 4).
Table 4. Comparison of each segment between virus XZ0906 and other Orbiviruses in nucleotide numbers and amino acid identities.
| Segment | AHSV-4 | BTV-6 | EHDV-6 | PALV | SCRV | YUOV | ||||||
| nt | aa(%) | nt | aa(%) | nt | aa(%) | nt | aa(%) | nt | aa(%) | nt | aa(%) | |
| S1 | 3965 | 1305(59.8) | 3944 | 1302(71.9) | 3942 | 1302(72.9) | 3930 | 1295(59.2) | 4089 | 1345(35.3) | 3993 | 1315(47.8) |
| S2 | 3229 | 1060(9.9) | 2922 | 955(28.8) | 2971 | 972(24.6) | 3055 | 1002(15.6) | 2747 | 890(16.7) | 2900 | 940(16.3) |
| S3 | 2792 | 905(58.5) | 2772 | 901(75.9) | 2768 | 899(75.8) | 2774 | 904(58.0) | 2024 | 654(13.1) | 2688 | 873(8.8) |
| S4 | 1978 | 642(50.5) | 1981 | 644(65.5) | 1983 | 644(64.4) | 1967 | 640(48.7) | 2017 | 643(34.2) | 1993 | 645(40.7) |
| S5 | 1748 | 548(27.6) | 1769 | 552(38.5) | 1803 | 551(41.6) | 1764 | 545(25.3) | 1664 | 517(8.8) | 1957 | 574(20.1) |
| S6 | 1566 | 505(43.6) | 1637 | 526(58.4) | 1641 | 527(61.4) | 1610 | 521(43.3) | 1657 | 517(8.6) | 1683 | 535(31.6) |
| S7 | 1167 | 349(56.7) | 1157 | 349(69.1) | 1162 | 349(69.3) | 1151 | 348(54.1) | 1463 | 462(8.8) | 1504 | 435(17.2) |
| S8 | 1166 | 365(36.3) | 1125 | 354(47.3) | 1186 | 373(44.5) | 1059 | 333(40.3) | 1256 | 379(9.9) | 1191 | 355(16.4) |
| S9 | 1160 | 366(32.9) | 1046 | 328(52.4) | 1140 | 359(46.5) | 877 | 272(43.3) | 764 | 232(35.3) | 1082 | 338(39.8) |
| S10 | 756 | 217(30.7) | 822 | 229(53.9) | 810 | 228(51.0) | 728 | 211(28.0) | 764 | 224(17.4) | 825 | 253(14.9) |
Note: As the T2 protein of Orbiviruses had important functions in virus protein/RNA structure and assembly, amino acid homology analysis for the T2 protein of TIBOV (T2 = VP3) compared to the T2 proteins of the above mentioned orbiviruses is presented:
AHSV-4(T2 = VP3):58.5%; BTV-6(T2 = VP3):75.9%; EHDV-6(T2 = VP3):75.8%; PALV(T2 = VP3):58.0%;
SCRV(T2 = VP2):22.9%; YUOV(T2 = VP2):37.6%.
5. Phylogenetic analysis and classification of virus XZ0906
5.1. Phylogenetic analysis of virus XZ0906 based on VP1 amino acid sequences
To better understand the taxonomic classification of virus XZ0906, the amino acid sequences of 37 VP1 proteins (Table 2) covering 14 genera within the family Reoviridae were obtained from GenBank, and used to construct a phylogenetic tree. These 37 virus strains (including different species and different serotype of one species) readily clustered into 14 evolutionary branches, with virus XZ0906 clustering within the genus Orbivirus branch (Figure 5(A)). To further establish the taxonomic classification of virus XZ0906, VP1 amino acid sequences from 28 known Orbivirus strains were used to construct a phylogenetic tree specific to this genus (Table 2). This analysis shows that virus XZ0906 forms a unique phylogenetic branch independent of any known Orbivirus species (Figure 5(B)).
Figure 5. Phylogenetic analysis of VP1 amino acid sequences from (A) Reoviridae and (B) Orbivirus strains.
(C) Phylogenetic analysis of T2 amino acid sequences from 29 Orbivirus strains. These analysis employed a neighbor-joining method (using the P-distance algorithm) using the MEGA version 5.04 software package (www.megasoftware.net). Bootstrap probabilities for each node were calculated using 1000 replicates. Scale bars indicate the number of amino acids substitutions per site. In Figure 5(C), as many of the available sequences are incomplete, analysis is based on partial sequences (residues 356-567 relative to the BTV-1A sequence). Abbreviations and serotypes (or strain name) are shown in the radial tree image of Figure 5. GenBank accession numbers and further details of the sequences can be found in Table 2.
5.2. Phylogenetic analysis based on the T2 protein amino acid sequence
The amino acid sequence of the T2 protein is an important marker used to classify species within the genus Orbivirus. T2 amino acid sequences from 29 known Orbivirus strains, along with the equivalent region from virus XZ0906, were selected to construct a phylogenetic tree. This analysis showed that virus XZ0906 is independent of any known Orbivirus species (Figure 5(C)). From these results, we determined virus XZ0906 to represent a novel species within genus Orbivirus. This novel species was given the name Tibet Orbivirus, TIBOV to reflect the location from which it was isolated.
Discussion
According to the 9th meeting report of the International Committee on the Taxonomy of Viruses (ICTV), the Reoviridae family consists of 15 genera: Orbivirus, Rotavirus, Seadornavirus, Phytoreovirus, Cardoreovirus, Mimoreovirus, Aquareovirus, Coltivirus, Cypovirus, Dinovernavirus, Fijivirus, Idnoreovirus, Mycoreovirus Orthoreovirus, and Oryzavirus [1]. All Reoviridae genomes consist of multi-segmented dsRNA; for example, the genome of Seadornavirus, Rotavirus, and Orbivirus contain 12, 11, and 10 dsRNA segments, respectively [2], [10], [17], [18]. Here we describe a novel orbivirus species isolated from mosquitoes collected in Tibet. This virus has many features characteristic of orbiviruses.
UTRs were detected at both the 5′ and 3′-ends of all 10 TIBOV gene segments. The lengths of these UTRs were highly variable; however, all 3′-UTRs contained a stretch of six highly conserved nucleotides at the end, which is a defining molecular characteristic used in the identification of Orbiviruses [8]. For BTV, AHSV, PALV, and Equine encephalosis virus (EEV), this stretch of six conserved nucleotides is readily detected in the 3′-UTRs of each gene segment [1], [4]; however, no such sequences are found at their corresponding 5′-ends. Among the 10 gene segments in Yunnan virus (YUOV), a recently identified Orbivirus isolated from mosquitoes in Yunnan, China, nine (Seg2–Seg10) contained a conserved seven-nucleotide sequence at the 5′-UTR end, but only three conserved nucleotide sequences at the 3′-end [4]. Among the 10 gene segments of Tibet Orbivirus, TIBOV, six conserved nucleotide sequences were detected in both end of the 5′- and 3′-UTRs (5′-GUAAAA and ACUUAC-3′, respectively); these sequences were distinct from those in any other Orbivirus species.
The Orbivirus RNA-dependent RNA polymerases (RdRp), which is encoded by the Seg1 gene (VP1), is an important marker for species identification [4], [8]. The VP1 protein sequence similarities of TIBOV to those of other Orbivirus species were 35.3–72.9% (Table 4), indicating that TIBOV constituted a novel member of the genus Orbiviruses. In addition, the T2 protein of Orbivirus is used to classify serotypes within the genus, with a threshold >91% identity at the amino acid level [4], [19], [20]. Such as Middle Point orbivirus (MPOV), which is isolated from Australian bovine serum specimens in 1998, exhibited up to 99% identity with YUOV, indicating that MPOV and YUOV were different serotypes of the same virus species [21]. The T2 protein from TIBOV shared 22.9–75.9% amino acid identity with those from six representative Orbiviruses, including YUOV (37.6%), well below the 91% threshold (Table 4). Together with phylogenetic analysis of both VP1(Figure 5(A),5(B)) and T2 (Figure 5(C)) amino acid sequences, we can draw a conclusion that TIBOV represented a new species within the genus Orbivirus, rather than a serotype of a previously described Orbivirus.
As previously described, YUOV is a new species of Orbivirus isolated from Culex tritaeniorhynchus specimens collected in Yunnan, China [4]. The genome of this virus consists of 10 dsRNA segments, and exhibits a 3-4-2-1 migration pattern when resolved in an agarose gel. Preliminary analysis of this virus showed clear CPE in Aedes albopictus cells, but no CPE or viral replication in mammalian cells (BHK and Vero cells) [4]. TIBOV isolated from mosquito specimens collected in Tibet in this study represents the second Orbivirus isolated from mosquito specimens in China. Substantial differences between YUOV and TIBOV were evident, including replication and toxicity to insect and mammalian cells, migration profiles, protein sequences, and the presence of conserved nucleotide sequences in the 5′-UTR and 3′-UTRs. Together, these results demonstrate that TIBOV is distinct from YUOV, and highlights the level of genetic diversity within Orbiviruses in China.
Orbiviruses can be transmitted by ticks or other hematophagous insect-vectors, including Culicoides, mosquitoes, and sand flies [1], [9]. The phylogenetic analyses (Figure 5(C)) indicated that TIBOV, isolated from A. maculatus, clustered with Orbiviruses which are transmitted primarily by Culicoides [1], [4], [9], [10], such as BTV, EHDV, and AHSV. TIBOV is more distantly related to Orbiviruses which are isolated from mosquito specimens, such as YUOV [3], Peruvian horse sickness virus (PHSV) [9], Umatilla virus (UMAV) [10], and Stretch Lagoon Orbivirus (SLOV) [9], [10]. Further study is necessary to determine if TIBOV is transmitted exclusively through A. maculatus, or can be spread by other blood-sucking insects.
TIBOV was isolated from A. maculatus specimens collected at a pigsty in rural Tibet. It is currently unknown whether TIBOV can infect either humans or animals. In order to determine whether this virus poses a risk to public health, serological studies to define potential human and animal exposures to TIBOV are needed.
Funding Statement
This work is supported by grants from National Natural Science Foundation of China (81290342), The Ministry of Science and Technology, China (2011CB504702) and Development Grant of State Key Laboratory for Infectious Disease Prevention and Control (2008SKLID105). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Attoui H, Mertens PPC, Becnel J, Belaganahalli S, Bergoin M, et al. (2011) Ninth Report of the International Committee on Taxonomy of Viruses. In: Andrew MQ King, Michael J Adams, Eric B Carstens, Elliot J Lefkowitz, editors. Family: Reoviridae. London: Elsevier/Academic Press. pp.541–603.
- 2.Mertens PPC (1999) In Encyclopedia of Virology, 2nd edn. In AGranoff&R.G., editors. Orbiviruses and coltiviruses-general features. Webster. London: Academic Press. pp. 1043–1061.
- 3. Attoui H, Jaafar FM, Belhouchet M, Aldrovandi N, Tao S, et al. (2005) Yunnan Orbivirus, a new Orbivirus species isolated from Culex tritaeniorhynchus mosquitoes in China. J Gen Virol 86: 3409–3417. [DOI] [PubMed] [Google Scholar]
- 4. Moss SR, Jones LD, Nuttall PA (1992) Comparison of the major structural core proteins of tick-borne and Culicoides-borne Orbiviruses . J Gen Virol 73: 2585–2590. [DOI] [PubMed] [Google Scholar]
- 5. Attoui H, Stirling JM, Munderloh UG, Billior F, Brookes SM, et al. (2001) Complete sequence characterization of the genome of the St Croix River virus, a new Orbivirus isolated from cells of Ixodes scapularis . J Gen Virol 82: 795–804. [DOI] [PubMed] [Google Scholar]
- 6. Belaganahalli MN, Maan S, Maan NS, Nomikou K, Pritchard I, et al. (2012) Full Genome Sequencing and Genetic Characterization of Eubenangee Viruses Identify Pata Virus as a Distinct Species within the Genus Orbivirus . PLoS One 7(3): e31911 10.1371/journal.pone.0031911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy P (2007) Fields Virology, 5th Edition. In Knipe DM, Howley PM, editors. Orbiviruses: Virion Structure. Wolters Kluwer-Lippincott Williams & Wilkins: Philadelphia. pp. 1976.
- 8. Mertens PPC, Diprose J, Maan S, Singh KP, Attoui H, et al. (2004) Bluetongue virus replication, molecular and structural biology. Vet Ital 40(4): 426–437. [PubMed] [Google Scholar]
- 9. Belhouchet M, Jaafar FM, Tesh R, Grimes J, Maan S, et al. (2010) Complete sequence of Great Island virus and comparison with the T2 and outer-capsid proteins of Kemerovo, Lipovnik and Tribec viruses (genus Orbivirus, family Reoviridae). J Gen Virol 91: 2985–2993. [DOI] [PubMed] [Google Scholar]
- 10. Belaganahalli MN, Maan S, Maan NS, Tesh R, Attoui H, et al. (2011) Umatilla Virus Genome Sequencing and Phylogenetic Analysis: Identification of Stretch Lagoon Orbivirus as a New Member of the Umatilla virus Species. PLoS One 6(8): e23605 10.1371/journal.pone.0023605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li MH, Fu SH, Chen WX, Wang HY, Guo YH, et al. (2011) Genotype V Japanese Encephalitis Virus Is Emerging. PLoS Negl Trop Dis 5(7): e1231 10.1371/journal.pntd.0001231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li YX, Li MH, Fu SH, Chen WX, Liu QY, et al. (2011) Japanese encephalitis, Tibet, China. Emerg Infect Dis 17(5): 934–936 10.3201/eid1705.101417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang J, Zhang H, Sun X, Fu S, Wang H, et al. (2011) Distribution of mosquitoes and mosquito-borne arboviruses in Yunnan Province near the China-Myanmar-Laos border. Am J Trop Med Hyg 85 (5): 738–746 10.4269/ajtmh.2011.10-0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang D, Coscoy L, Zylberberg M, Avila PC, Boushey HA, et al. (2002) Microarray-based detection and genotyping of viral pathogens. Proc Natl Acad Sci U S A 99(24): 15687–15692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang D, Urisman A, Liu YT, Springer M, Ksiazek TG, et al. (2003) Viral discovery and sequence recovery using DNA microarrays. PLoS Biol 1(2): E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao G, Krishnamurthy S, Cai Z, Popov VL, Travassos da Rosa AP, et al. (2013) Identification of Novel Viruses Using VirusHunter—An Automated Data Analysis Pipeline. PLoS One 8(10): e78470 10.1371/journal.pone.0078470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Attoui H, Billoir F, Biagini P, de Micco P, de Lamballerie X (2000) Complete sequence determination and genetic analysis of Banna virus and Kadipiro virus: proposal for assignment to a new genus (Seadornavirus) within the family Reoviridae . J Gen Virol 81(Pt 6): 1507–1515. [DOI] [PubMed] [Google Scholar]
- 18. Matthijnssens J, Ciarlet M, McDonald SM, Attoui H, Bányai K, et al. (2011) Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch Virol 156(8): 1397–1413 10.1007/s00705-011-1006-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grimes JM, Burroughs JN, Gouet P, Diprose JM, Malby R, et al. (1998) The atomic structure of the bluetongue virus core. Nature 395(6701): 470–478. [DOI] [PubMed] [Google Scholar]
- 20. Gouet P, Diprose JM, Grimes JM, Malby R, Burroughs JN, et al. (1999) The highly ordered double-stranded RNA genome of bluetongue virus revealed by crystallography. Cell 97(4): 481–490. [DOI] [PubMed] [Google Scholar]
- 21. Cowled C, Melville L, Weir R, Walsh S, Hyatt A, et al. (2007) Genetic and epidemiological characterization of Middle Point orbivirus, a novel virus isolated from sentinel cattle in northern Australia. J Gen Virol 88(Pt 12): 3413–3422. [DOI] [PubMed] [Google Scholar]