Abstract
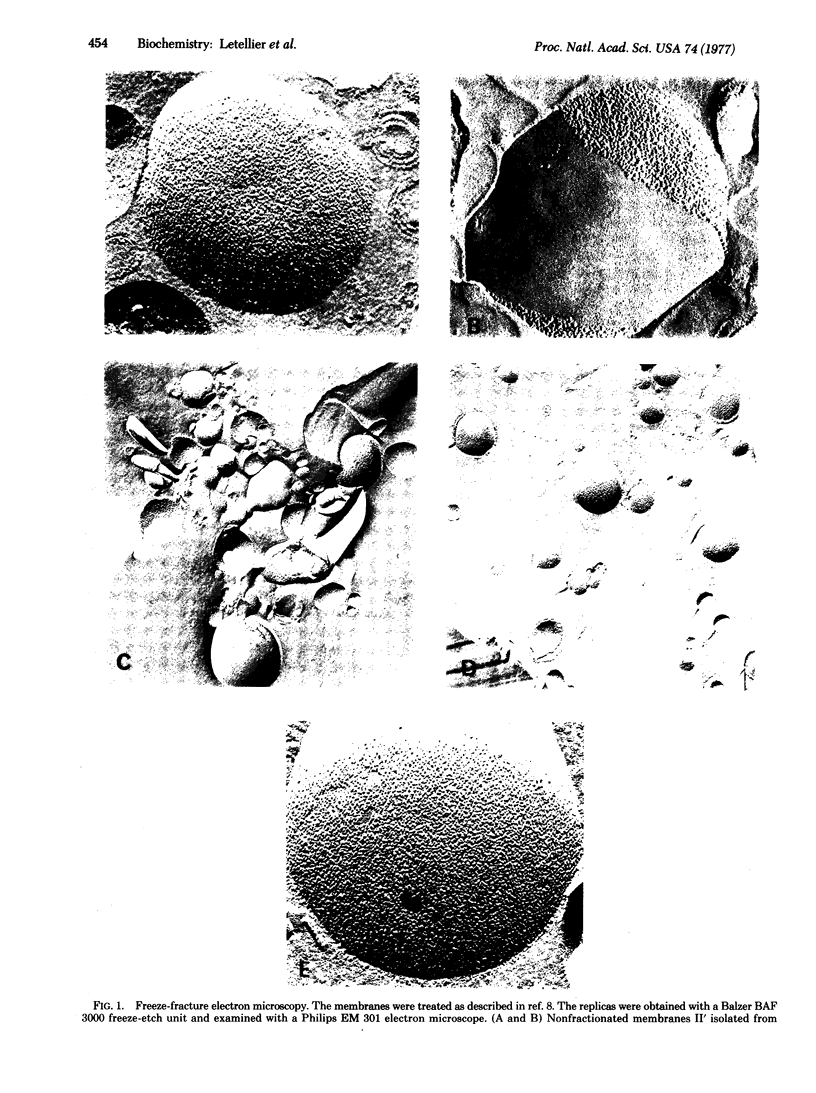
Lipid and protein segregations can be induced in E. coli cytoplasmic membranes by conformational transitions of their lipid hydrocarbon chains from a disordered to an ordered state. For E. coli strain K 1059 (an unsaturated fatty acid auxotroph) supplemented with linolenic acid, the segregation leads to large areas of membrane surfaces having distinctly different morphological characteristics (smooth compared with strongly particulated fracture faces, as visualized by freeze fracture electron microscopy). The different regions are physically separated by osmotic lysis of spheroplasts at temperatures below those of the order-disorder transition of the lipid hydrocarbon chains. The analysis of the different cytoplasmic membrane fractions provides a direct demonstration and allows a direct analysis of the segregation. As compared to the nonfractionated membranes, the membrane regions corresponding to the smooth fracture surfaces are poor in proteins, rich in lipids, and enriched in saturated fatty acids, while the membrane regions corresponding to the strongly particulated fracture surfaces are rich in proteins, poor in lipids, and enriched in unsaturated fatty acids. Quantitative information about the extent of these segregations is obtained from high-angle x-ray diffraction of the different membrane fractions and of the corresponding total lipid extracts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K. H., Staehelin L. A. Orientation of membrane vesicles from Escherichia coli as detected by freeze-cleave electron microscopy. J Bacteriol. 1974 Feb;117(2):888–899. doi: 10.1128/jb.117.2.888-899.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont Y., Gabriel A., Chabre M., Gulik-Krzywicki T., Schechter E. Use of a new detector for x-ray diffraction and kinetics of the ordering of the lipids in E. coli membranes and model systems. Nature. 1972 Aug 11;238(5363):331–333. doi: 10.1038/238331a0. [DOI] [PubMed] [Google Scholar]
- James R., Branton D. Lipid- and temperature-dependent structural changes in Acholeplasma laidlawii cell membranes. Biochim Biophys Acta. 1973 Oct 25;323(3):378–390. doi: 10.1016/0005-2736(73)90183-1. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga N. Ultrastructure of the cell envelope of Escherichia coli B after freeze-etching. J Bacteriol. 1970 Jan;101(1):297–303. doi: 10.1128/jb.101.1.297-303.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
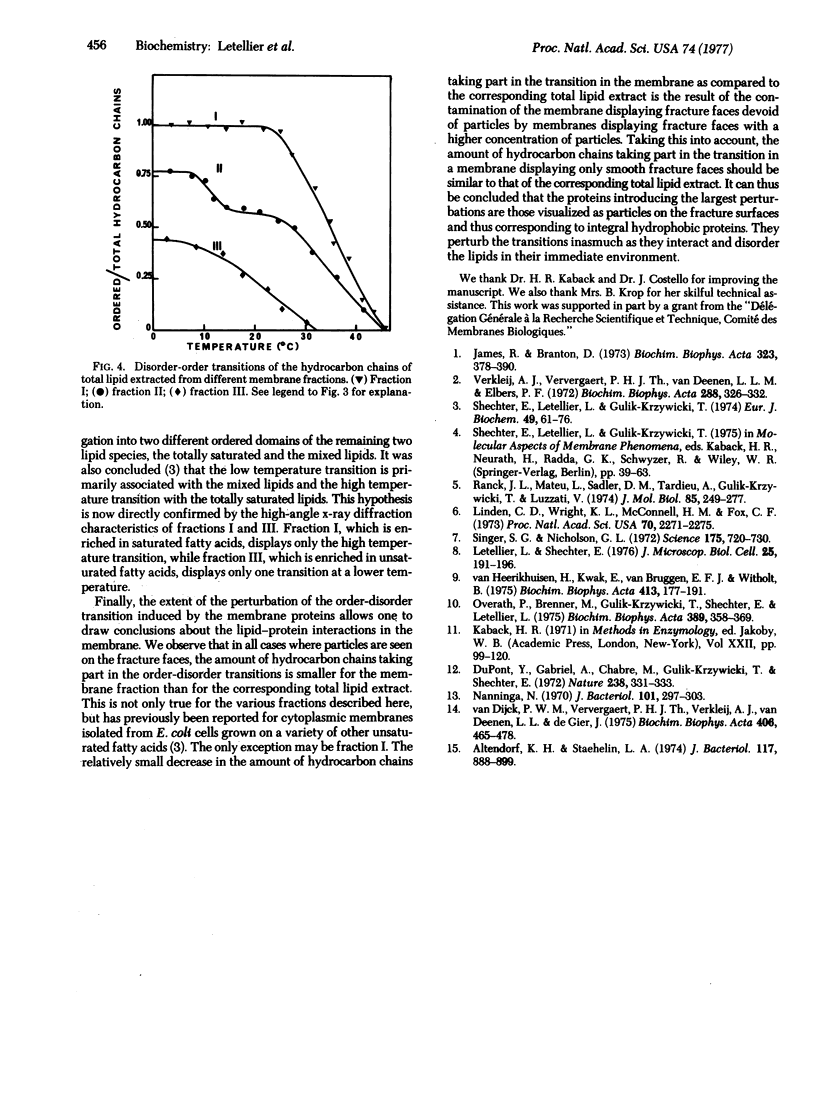
- Overath P., Brenner M., Gulik-Krzywicki T., Shechter E., Letellier L. Lipid phase transitions in cytoplasmic and outer membranes of Escherichia coli. Biochim Biophys Acta. 1975 May 6;389(2):358–369. doi: 10.1016/0005-2736(75)90328-4. [DOI] [PubMed] [Google Scholar]
- Ranck J. L., Mateu L., Sadler D. M., Tardieu A., Gulik-Krzywicki T., Luzzati V. Order-disorder conformational transitions of the hydrocarbon chains of lipids. J Mol Biol. 1974 May 15;85(2):249–277. doi: 10.1016/0022-2836(74)90363-5. [DOI] [PubMed] [Google Scholar]
- Shechter E., Letellier L., Gulik-Krzywicki G. Relations between structure and function in cytoplasmic membrane vesicles isolated from an Escherichia coli fatty-acid auxotroph. High-angle x-ray diffraction, freeze-etch electron microscopy and transport studies. Eur J Biochem. 1974 Nov 1;49(1):61–76. doi: 10.1111/j.1432-1033.1974.tb03811.x. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Van Dijck P. W., Ververgaert P. H., Verkleij A. J., Van Deenen L. L., De Gier J. Influence of Ca2+ and Mg2+ on the thermotropic behaviour and permeability properties of liposomes prepared from dimyristoyl phosphatidylglycerol and mixtures of dimyristoyl phosphatidylglycerol and dimyristoyl phosphatidylcholine. Biochim Biophys Acta. 1975 Nov 3;406(4):465–478. doi: 10.1016/0005-2736(75)90025-5. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Ververgaert P. H., van Deenen L. L., Elbers P. F. Phase transitions of phospholipid bilayers and membranes of Acholeplasma laidlawii B visualized by freeze fracturing electron microscopy. Biochim Biophys Acta. 1972 Nov 2;288(2):326–332. doi: 10.1016/0005-2736(72)90253-2. [DOI] [PubMed] [Google Scholar]
- van Heerikhuizen H., Kwak E., van Bruggen E. F., Witholt B. Characterization of a low density cytoplasmic membrane subfraction isolated from Escherichia coli. Biochim Biophys Acta. 1975 Dec 1;413(2):177–191. doi: 10.1016/0005-2736(75)90102-9. [DOI] [PubMed] [Google Scholar]







