X chromosome cellular mosaicism for IRAK1 expression in mice increases circulating B cell numbers and preconditions for improved sepsis outcome.
Keywords: X-chromosome, gender, skewing, inflammation, sex chromosomes, B cells
Abstract
ChrX cellular mosaicism for X-linked genetic polymorphisms in females versus the single ChrX representation in males denotes a genetic difference, which may contribute to gender bias in the inflammatory response. This hypothesis was tested in female F1 offspring of consomic mice (BL6J-ChrXA/J/NaJ) that were homokaryotic or mosaic for the active BL6 and AJ ChrXs or for IRAK1 deficiency linked to the BL6 ChrX. Sepsis was initiated by CLP. IRAK1-deficient and IRAK1-mosaic mice showed similar protection from sepsis-induced mortality and reduced IL-6 and IL-10 release compared with WT. BM cellularity and blood B cell counts were increased in naive IRAK1-mosaic mice compared with WT-mosaic or IRAK1-deficient animals. Sepsis-induced BM cell depletion was greater in IRAK1-mosaic mice compared with WT-mosaic or IRAK1-deficient subjects, whereas splenic B and T cell depletion was less in IRAK1-mosaic and IRAK1-deficient than WT-mosaic mice. Skewing toward AJ or BL6-ChrX-expressing cells was assessed by testing allele-specific expression of strain-variant Xkrx and BTK genes. In naive IRAK1-mosaic mice, BM and blood cells with the active BL6-ChrX, were greater than cells expressing the AJ-ChrX (cell ratio 2.5 in IRAK1-mosaic; 1.5 in WT-mosaic mice). Sepsis decreased cell ratios more in IRAK1-mosaic than in WT-mosaic mice. The study reveals functional variability in cellular mosaicism for IRAK1 expression and natural X-linked polymorphisms during sepsis. Mosaicism for IRAK1 expression is accompanied by skewing toward deficient immune cell populations, producing a phenotype that is preconditioned for improved sepsis outcome similar to that observed in IRAK1 deficiency.
Introduction
Female ChrX mosaicism represents a unique condition, because as a result of random ChrX inactivation, the respective expression of maternal or paternal ChrX in individual cells is expected to manifest different functional phenotypes driven by polymorphic differences between the parental ChrXs [1–3]. In contrast to females, males carry and express only one ChrX, and the X-linked cellular phenotype in males is governed exclusively by maternal polymorphisms [1]. Multiple physiological, pathological, and disease processes are gender-biased, resulting in improved or worsened clinical outcomes in females and males. Females have a better general-health status and longer lifespan; furthermore, although still controversial, multiple studies showed that females compared with males present improved outcome following trauma, shock, and sepsis [4–14]. In contrast, autoimmune diseases are more frequent in females than males [15, 16].
A large number of studies tested the role of sex hormones on different cell types and functions, as well as gender-biased disease outcomes, and there is ample evidence indicating the important roles of estrogens, testosterones, and their derivatives, modulating a variety of cell functions and pathology [5–13]. In the clinical context, estrogens are often beneficial, whereas testosterones worsen outcome following trauma or infections. However, it is also becoming evident that sex hormones are not the only players causing gender differences in the inflammatory response. For example, gender differences in the inflammatory response or susceptibility to infection can also manifest between prepubertal girls and boys or postmenopausal females and elderly males [17–20]. These observations suggest a role of additional contributory factors, which are independent of sex hormones, in modulating the host response.
An important fact is that X-linked risk alleles always affect more males than females at the population level, which is the result of the lack of X-linked heterozygosity in males and the associated population frequency distributions of X-linked polymorphisms [1, 21]. It is also well-known that in severe X-linked genetic defects, mostly males are affected, and “heterozygous” (i.e., mosaic) female carriers are protected from the disease, or their symptoms are reduced greatly [2, 3]. However, the mechanism of protection in carrier females or the immune-modulatory effects of cellular mosaicism for common X-linked polymorphisms and risk alleles during the innate immune response have not been investigated thoroughly.
Previously, we tested some aspects of this question in proof-of-concept studies using an X-linked NOX2-deficient model. Animals mosaic for NOX2 expression carried two phagocyte populations: one-half of the phagocytes had a functioning NOX2 complex, whereas the other one-half was defective [22, 23]. We found that phagocyte mosaicism for normal and defective oxidative burst resulted in a unique, inflammatory phenotype and decreased mortality following sepsis or endotoxemia [22, 23].
The use of in-bred mouse strains, deficient or mosaic for a particular X-linked immune-competent gene, is an excellent tool for testing the effects of cellular mosaicism of a particular defect on the immune response. However, it is also evident that defective immune functions could be modulated further by other common X-linked polymorphisms present in humans or outbred animal populations. Furthermore, in any given population, the abundance of X-linked polymorphic variants and haplotype and linkage variations among the ChrXs is expected to manifest multiple phenotypic differences in mosaic cell subpopulations among female subjects. The impact of common X-linked polymorphisms in the context of immune cell mosaicism and immunomodulation has not been investigated.
Thus, with the use of a mouse model, the current study aimed to elucidate whether X-linked polymorphic mosaicism presents an increased functional variability and how this relates to sepsis outcome. We used a consomic mouse strain (BL6J-ChrXA/J/NaJ), in which the ChrX of the BL6 strain is substituted with the ChrX from the AJ strain. A slate of natural polymorphic differences is known between the AJ and BL6 ChrXs, which allowed us to test whether X-linked polymorphic differences between the BL6 and AJ variants in the simultaneous presence or absence of IRAK1 deficiency alter the response and outcome following septic peritonitis. ChrX activity and immune-cell skewing during the septic response were assessed by allele-specific quantitative real-time expression assays for the AJ and BL6 exonic SNP variants of Xkrx (rs29271257) and BTK (rs13484006). The X-linked IRAK1 was selected as the gene of immunomodulation, as it was shown that IRAK1 deficiency improves outcome following burn and endotoxemia [24–27]. We also demonstrated recently that IRAK1 deficiency dampened inflammation and decreased sepsis-associated mortality in hemizygous males [28]. Furthermore, previous human studies indicated that a variant IRAK1 haplotype with increased kinase activity worsened the clinical outcome in septic patients [29, 30]. We used the CLP model, which mimics peritonitis-induced sepsis and is accepted as a clinically relevant experimental model of polymicrobial septicemia [31–33]. To control for the confounding effects of male sex hormones, all experiments were performed on female animals.
We found that mice with cellular mosaicism for the expression of WT AJ and IRAK1-deficient BL6 ChrXs show WBC skewing toward the IRAK1-deficient cell subpopulations. This is consistent with a similar survival advantage of IRAK1-mosaic and homozygous IRAK1-deficient mice compared with WT subjects.
MATERIALS AND METHODS
Reagents
Endotoxin-free, cell culture-grade buffers, media, and reagents were used in the experiments. Flourochrome-conjugated antibodies, assay diluents, lysing, and permeabilizing flow cytometry solutions and kits were purchased from BD Biosciences and BD PharMingen (San Diego, CA, USA). All other reagents and chemicals of the highest grade available were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Animals and CLP
The initial IRAK1-deficient breeders on BL6 background were obtained from James Thomas (The University of Texas Southwestern Medical Center, Dallas, TX, USA). ChrX consomic (BL6J-ChrXA/J/NaJ) and WT (BL6J) animals were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Colonies of homozygous IRAK1-deficient, BL6J WT, and consomic WT (BL6J-ChrXA/J/NaJ) mice were maintained at our animal facility. In parallel breeding colonies, “WT-mosaic” animals were generated by crossing consomic BL6J-ChrXA/J/NaJ and BL6J mice. Offspring from this crossing produces mice with mosaic expression for the WT AJ and WT BL6 ChrXs, whereas all of the autosomal chromosomes are BL6. IRAK1-mosaic mice were generated by crossing consomic BL6J-ChrXA/J/NaJ and IRAK1-deficient animals. These mice have mosaic expression for the WT AJ and the IRAK1-deficient BL6 ChrXs, whereas all of the autosomal chromosomes are BL6. To control the confounding effects of meiotic recombination, only F1 of WT-mosaic or IRAK1-mosaic female offspring was used in the experiments. Animals used in the study were 12–16 weeks old.
Polymicrobial septic peritonitis was induced using the CLP model, as described earlier [23, 31]. Briefly, animals were anesthetized by an i.p. injection of Nembutal (5 mg/100 g body weight). A midline abdominal incision was made. The cecum was exposed, ligated, and punctured through opposing walls at two sites with a 20-gauge hypodermic needle. Animals were resuscitated by the s.c. injection of isotonic, pyrogen-free saline solution (0.04 ml/g body weight), immediately postoperatively and also at 18 h post-CLP. When animals were followed for longer than 20 h, they received daily saline resuscitations at the same dose. In pilot experiments, we compared naive controls and sham operations (opening the abdomen, moving the intestine, but no ligation or puncture) and found no remarkable increase in inflammatory markers [34]; therefore, we used nontreated, naive animals as controls in the study. Experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the New Jersey Medical School.
Genotyping
Animals enrolled in experiments were retested and genotype- and karyotype-confirmed before final analyses. The IRAK1 KO mutant mouse was generated by replacing a 7-kb gene with a neo-selection cassette. The deleted region included 3.5 kb of the upstream regulatory region and all of exons from 1–8 and a portion of Exon 9 [35]. Total genomic DNA was isolated from tail clippings using the REDExtract-N-Amp tissue PCR kit (Sigma-Aldrich). DNA was subjected to PCR amplification using forward primers complementary to the phosphoglycerine kinase-neo insert or WT sequences, respectively, and a common downstream primer. Forward primers, WT: 5′-GCAAGCCAGAGCAGTACTGTG-3′; IRAK1 KO (NEO): 5′-GCCTTCTATCGCCTTCTTGACG-3′; common reverse primer: 5′-GCCTCTGTAAGAGATCAGGTAG-3′. PCR reaction was carried out in the presence of 2 mM MgCl2 with the following cycling: 94°C for 2 min, followed by 35 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 2 min, 30 s, with the final elongation of 72°C for 7 min. PCR amplicons were resolved on 0.8% agarose gels.
Mosaic karyotype for the BL6 and AJ X-chromosomes was confirmed by testing for the Xkrk gene variant. DNA from the tail was extracted and amplified using the REDExtract-N-Amp tissue PCR kit (Sigma-Aldrich). Amplicon of 240 bp was amplified using common reverse primer: 5′-CTTCGGAGTCAAAGTGTTACTGAA-3′; control forward primer: 5′-CTTGTGTTAACCCAGACCCATC-3′; AJ forward primer: 5′-TGAGTTCTCAACCCTTTCCC-3′, and BL6J forward primer: 5′-TGAGTTCTCAACCCTTTCCG-3′. The temperatures cycling were: 94°C, 2 min, followed by 30 cycles (of 94°C, 30 s; 53°C, 30 s; and 72°C, 30 s) and then 72°C, 5 min. The amplified product was resolved on 3% agarose gel.
Allele-specific mRNA expression assay
Allele-specific gene expression for Xkrx (rs13484006; C/G, a conserved and constitutively expressed membrane protein) and BTK (rs29271257; A/G, synonymous mutation) was monitored by a real-time quantitative RT-PCR method using an Applied Biosystems 7500 Real-Time PCR system. RNA was extracted from 10 million cells from BM or spleen or 30 mg tissue from lung and liver using the Qiagen RNeasy Mini Kit. RNA for WBC was extracted after lysis by hypotonic ammonium chloride-Tris. Total RNA (500 ng/reaction for BM, spleen, lung, and liver or 100 ng for WBC or sorted WBC) was transcribed to cDNA by a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems).
Allele-specific real-time assays were carried out using primers matched or mismatched at the 3′ end with the variant mutation. The sequence-specific FAM Dye/MGB probes were designed for the nonvariant region in between the forward and reverse primers. The following allele-specific primer pairs and sequence-specific amplification probes were used:
Xkrx reverse primer: 5′-CTTATCTGATTTCCATTGGGGTC-3′
Xkrx AJ forward primer: 5′-TCTGAGTTCTCAACCCTTTCCC-3′
Xkrx BL6 forward primer: 5′-CTGAGTTCTCAACCCTTTCCG-3′
Xkrx probe: FAM 5′-TGAAGAGTGAGCGCAGGGGGTG-3′ MGB
BTK reverse primer: 5′-GCACCAATCTCCACAACCG-3′
BTK AJ forward primer: 5′-GCTCGCCACCACGGTAA-3′
BTK C57 forward primer: 5′-GCTCGCCACCACGGTAG-3′
BTK probe: FAM 5′-CTCCTCGCCCTTTCGCAATTGTAAG-3′ MGB
Each reaction was performed in duplicates. The ubiquitous, eukaryotic 18S rRNA (FAM Dye/MGB probe; Applied Biosystem) was used to normalize the data across samples. Before starting the animal experiments, probe specificity and the quantitative range for the allelic SNP variants of Xkrx and BTK were tested thoroughly in vitro. With the use of the listed allele-specific primers and probes, amplification occurred only when the primer matched the expected variant for Xkrx, as well as BTK. Serial dilutions of the initial specimen indicated that the assay can quantify allelic ratio changes under induced conditions as well. The comparison of Xkrx and BTK expression levels among tissues showed a ten- to 50-fold-greater expression level in immune-competent tissues (blood, BM, spleen) than parenchymal organs (lung, liver).
Blood, splenocyte, and BM cell isolation and incubations
Blood was collected into heparinized tubes via cardiac puncture from fully anesthetized animals. Following the exsanguination, femurs and spleen were collected from the same animals and BM and spleen cells isolated as described in details previously [23].
Flow cytometry
BM, blood, and spleen flow cytometry analyses and gating strategy have been described in detail previously [36]. The number of PMNs and lymphocyte subsets in blood and spleen was determined by the number of total cell counts and the percent distribution of CD3+CD4+, CD3+CD8+ T cells; CD19+ B cells; and CD11b+ myeloid cells using antibodies against CD markers conjugated with FITC, APC, PerCP, or PE (BD Biosciences) in three- or four-color incubations. BM cell composition was determined by the cell distribution of CD45+CD19+CD11b− (B cells) and CD11b+CD45+CD19− (myeloid cells). FACS acquisitions were performed in a centralized flow cytometry facility. At least 30,000 events were collected for each analysis. Blood differentials and cell counts in BM and spleen were determined using a computerized cell counter (HemaTrue Veterinary Hematology Analyzer; Heska, Loveland, CO, USA).
Flow cytometry cell sorting
Whole blood was centrifuged at 1200 rpm for 5 min. Buffy coat was removed and lysed with hypotonic ammonium chloride for 3 min at 37°C. Cells were then washed with RPMI containing glutamine and 2% FBS, twice by centrifugation, at 1200 rpm for 5 min. The pellet was resuspended in 0.45 ml RPMI buffer. Cells were labeled with surface markers CD3-FITC, CD11B-PerCP, and CD19-APC for 15 min. Cell suspensions were loaded to a cell sorter (FACSAria II; BD Biosciences) and cells separated by gating, according to positive staining for surface markers, as well as forward- and side-scatter properties. Each cell type (5×104) was collected and washed with PBS containing 1% FBS by centrifugation at 1200 rpm for 5 min and then processed for mRNA isolation and expression analyses.
ELISAs
ELISA kits for cytokine assays were purchased from BD Biosciences. Plasma from freshly drawn heparinized blood was stored at −85°C until analysis. ELISAs were performed according to the manufacturer's protocol. All of the compared samples from different genotypes were run simultaneously in duplicates on one plate. Values were determined from a calibration curve run parallel with the samples.
Statistical analysis
Statistical calculations were performed using JMP software (SAS Institute, Cary, NC, USA). Results were analyzed using ANOVA, followed by t-test for pair-wise comparisons or Tukey-Kramer's test for multiple comparisons. We used the long-rank test to assess survival differences among groups. Different study components were performed on six to eight different animals from each of the in vivo treatment groups, unless indicated otherwise. Significant difference was concluded at P < 0.05, unless noted otherwise.
RESULTS
Sepsis-induced mortality and cytokine release in IRAK1 mosaicism
The comparison of sepsis-induced mortality among female WT and IRAK1-deficient and -mosaic mice on the BL6 background indicated that homozygous IRAK1-deficient and -mosaic mice were similarly protected from CLP-induced mortality compared with WT animals (Fig. 1A). Next, with the use of consomic (BL6J-ChrXA/J/NaJ) and BL6 mice, as well as their F1 crosses, we tested whether female mosaicism for the expression of WT AJ and BL6 ChrXs results in any differences in sepsis-induced survival compared with females who express the BL6 or AJ ChrX in all cells (homokaryotypes). We found that mortality was similar in WT consomic animals when homokaryotic or mosaic for the AJ or BL6 ChrXs (Fig. 1B). These observations indicated that IRAK1 deficiency and mosaicism similarly improve sepsis survival, whereas polymorphic differences between the WT AJ and WT BL6 ChrXs per se have no impact on sepsis outcome. To test whether survival advantage was associated with differences in bacterial load, we compared septicemia among these genotypes, 20 h following CLP (Fig. 1C). We found no statistical differences in bacteremia among the groups.
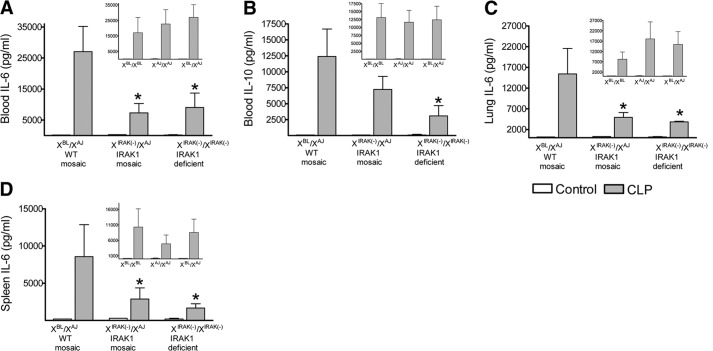
Figure 1. IRAK1 deficiency or mosaicism for IRAK1 expression similarly protects against sepsis-induced mortality.
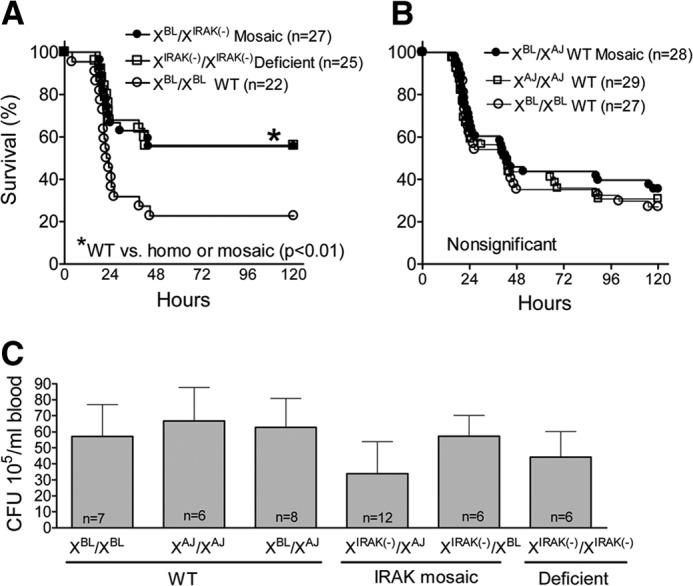
Animals with different X-chromosome karyotypes were produced, as described in Materials and Methods. Animals were subjected to CLP and monitored for 5 days. (A) Observations comparing WT (XBL/XBL), IRAK1-deficient [XIRAK(−)/XIRAK(−)], and IRAK1-mosaic [XBL/XIRAK(−)] mice on the BL6 background. (B) Observations on WT mice using BL6J-ChrXA/J/NaJ (XAJ/XAJ), BL6 (XBL/XBL), and their F1 crosses (XBL/XAJ). (C) Bacterial CFUs, 20 h following CLP. *Statistically significant difference between IRAK1-deficient or IRAK1-mosaic compared with WT.
The similar bacteremia in these animals suggested that the survival advantages are likely to be associated with alterations in the inflammatory response rather than differences in bacterial load. Therefore, WT-mosaic and IRAK1-mosaic animals were generated, and WT- and IRAK1-mosaic animals, both carrying the same natural X-linked polymorphisms, were tested for the inflammatory response and subsequently for allele-specific mRNA expression following sepsis. Figure 2 indicates that the sepsis-induced increase in IL-6 content in blood, lung, and spleen was reduced similarly in IRAK1-mosaic and IRAK1-deficient mice compared with WT-mosaic animals. Sepsis-induced increase in blood IL-10 concentration was also decreased in IRAK1-deficient mice compared with WT-mosaic, whereas IRAK1-mosaic mice displayed an intermediate IL-10 level. As shown in insets in Fig. 2, sepsis-induced blood, spleen, and lung IL-6 and IL-10 responses were similar in animals that were homokaryotic or mosaic for the WT BL6 or AJ ChrXs. Furthermore, the testing of WT- and IRAK1-mosaic mice on the BL6 background also indicated blunted CLP-induced IL-6 and IL-10 responses in IRAK1-mosaic mice compared with WT (data not shown). These observations together demonstrated that mosaicism for IRAK1 expression partially decreases sepsis-induced cytokine release, similarly to that observed in IRAK1 deficiency.
Figure 2. IRAK1 mosaicism blunts cytokine release following sepsis.
Animals were subjected to CLP, and 20 h later, blood (A and B) was collected and IL-6 and IL-10 concentrations determined. IL-6 was also measured in lung (C) and spleen (D). For comparison, responses from IRAK1-deficient homokaryotype mice are also shown. Insets show responses of WT mice with the indicated karyotype. Mean ± sem; n = 6–8. *Statistically significant difference compared with WT.
Sepsis-induced cell-composition changes in IRAK1 mosaicism
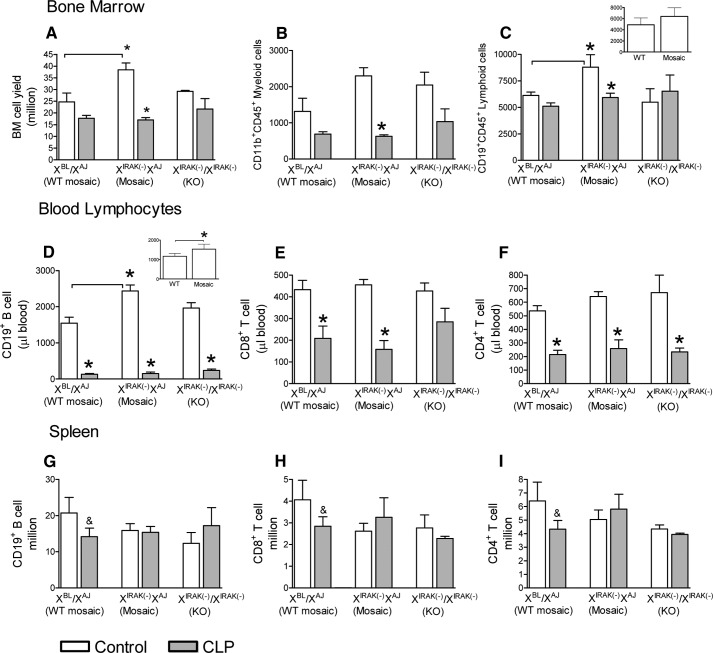
Next, we tested cell-composition alterations in BM, blood, and spleen following sepsis. Under control (unchallenged) conditions, BM cellularity was increased significantly in mosaic animals compared with WT (Fig. 3A), which was mostly accounted for by the increase in BM lymphocyte content (Fig. 3C). Compared with controls, the sepsis-induced decrease in BM myeloid and lymphoid cell content relative to controls was statistically significant in mosaic animals, whereas changes in WT-mosaic and IRAK1-deficient animals did not reach significant levels (Fig. 3A–C).
Figure 3. IRAK1 mosaicism displays unique cell-composition pattern under baseline and septic conditions.
Animals were subjected to CLP, and 20 h later, BM cell yield (A) and myeloid (B) and lymphoid cell counts (C) were determined and compared with values from unmanipulated control mice. B cell (D) and helper or cytotoxic T cell counts (E and F). Splenic lymphocyte composition was also determined and compared between septic and control mice (G–I). For comparison, responses from IRAK1-deficient homokaryotype mice are also shown. (C and D) Insets depict B cell-count values from naive WT- and IRAK1-mosaic mice on the BL6 background. *Statistically significant difference compared with control within a genotype or as indicated by connecting lines, P < 0.05; &Trend at P < 0.1. Mean ± sem; n = 6–8 animals in each group.
Consistent with increased baseline BM lymphocyte content, circulating B cells were also elevated in IRAK1-mosaic mice compared with WT-mosaic under control conditions (Fig. 3D). The increased blood-lymphocyte count in mosaic animals compared with WT was also present when tested in BL6 animals, but the increase did not reach significant levels in BM (insets to Fig. 3C and D). Sepsis markedly decreased circulating B as well as CD4+ and CD8+ T cells compared with controls, and the only exception is a blunted drop in CD8+ T cells in IRAK1-deficient mice (Fig. 3E and F). Sepsis-induced changes in the number of circulating neutrophils did not reach statistically significant levels (data not shown). A tendency for sepsis-induced depletion in splenic B and CD8+ and CD4+ T cells was present in WT-mosaic mice (P<0.1 vs. controls), whereas sepsis caused no depletion in splenic B or T cells in IRAK1-mosaic or IRAK1-deficient mice (Fig. 3G–I).
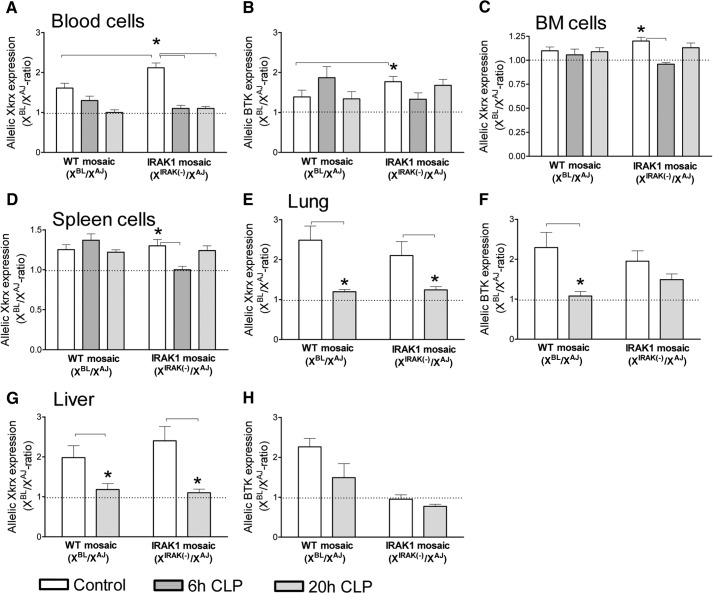
Sepsis-induced changes in allele-specific gene expression
As described in Materials and Methods, SNP variants of Xkrx and BTK were used as probes in assessing cellular skewing or changes in ChrX expression during sepsis. We compared allelic expression ratios in WBC, BM cells, and splenocytes under control and septic conditions, 6 h and 20 h following CLP (Fig. 4A–D). In nonseptic control mice, the BL6/AJ ratio for Xkrx expression in WBC was elevated in IRAK1-mosaic compared with WT-mosaic mice (Fig. 4A). Compared with controls, 6 h and 20 h following CLP, the BL6/AJ ratio for Xkrx expression was decreased in WBC from IRAK1-mosaic mice but not in WT-mosaic animals (Fig. 4A). WBC BL6/AJ ratio for BTK expression in control animals was also increased in IRAK1-mosaic mice compared with WT-mosaic; however, sepsis did not result in significant changes in BTK expression ratios (Fig. 4B). Testing allelic expression ratios in BM (Fig. 4C) and spleen (Fig. 4D) indicated a statistically significant decrease in the BL6/AJ ratio for Xkrx expression at 6 h after initiating sepsis in IRAK1-mosaic mice. In WT-mosaic animals, sepsis also decreased the mean value of BL6/AJ ratios; however, the differences did not reach statistically significant levels. WBC BL6/AJ ratio for BTK expression in control animals was increased in IRAK1-mosaic mice compared with WT-mosaic; however, sepsis did not result in significant changes in BTK expression ratios (Fig. 4B). The value of allelic BTK expression ratios was approximately one in BM and spleen from WT-mosaic and IRAK1-mosaic mice under control as well as septic conditions (data not shown).
Figure 4. Allelic Xkrx and BTK expression ratios in WT-mosaic and IRAK1-mosaic animals under baseline and septic conditions.
Circulating WBC (A and B), BM cells (C), and splenocytes (D) from control animals (n=8) and mice septic for 6 h (n=8) or 20 h (n=8–12) were analyzed for allelic mRNA expression ratios of Xkrx (A, C, and D) and BTK (B). Lung and liver (n=6) were also analyzed, 20 h after CLP (E–H). Mean ± sem. *Statistically significant difference, as indicated by connecting lines. Dotted line indicates 1/1-ratio.
As lung and liver are sites of cell infiltration during inflammation, we also determined allelic expression ratios in these organs (Fig. 4E–H). Compared with controls, sepsis resulted in a decrease in the BL6/AJ ratio for Xkrx expression in lung and liver in WT- and IRAK1-mosaic mice (Fig. 4E and G). Sepsis decreased the allelic BL6/AJ ratio for BTK expression in lung from WT-mosaic mice, whereas the sepsis-induced decrease was blunted in IRAK1-mosaic mice in lung as well as liver (Fig. 4F and H).
Allelic expression changes in individual subjects
As baseline cell ratios expressing different parental ChrXs vary among individuals that may confound sepsis-induced effects, we performed a follow-up study on allelic Xkrx expression in a self-control design (Fig. 5). In this set, WBCs from the same subject were tested for allelic expression ratios, 2 weeks before CLP and then again 20 h post-CLP. Untreated control mice were also tested twice, 2 weeks apart. This approach allowed testing sepsis-induced changes within an individual, independent of the initial value of the allelic expression ratio. Figure 5 indicates that sepsis resulted in a marked decrease in the BL6/AJ ratio for Xkrx expression in each IRAK1-mosaic subject (P<0.001). In WT-mosaic mice, the sepsis-induced decrease in the BL6/AJ Xkrx expression ratio did not reach a statistically significant level (P=0.125). However looking at individual responses, there was a clear tendency of a decreased BL6/AJ allelic expression ratio following sepsis. Allelic Xkrx expression ratios were similar in unchallenged control animals from WT- or IRAK1-mosaic mice when tested 2 weeks apart (Fig. 5).
Figure 5. Sepsis-induced changes in WBC allelic Xkrx expression ratios as determined before and after sepsis within the same subject.
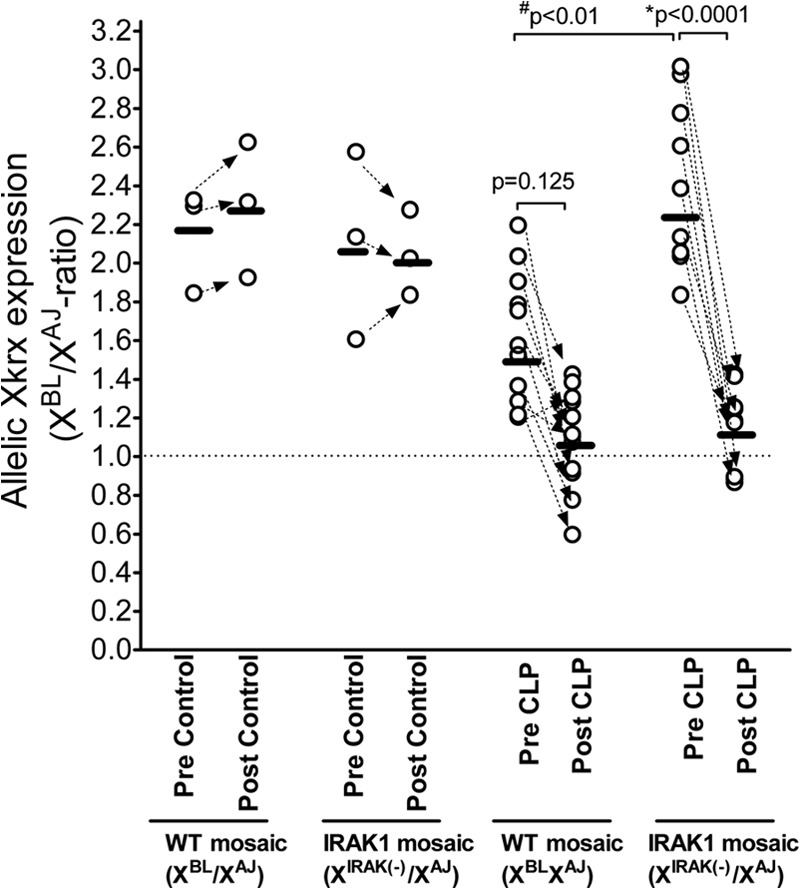
First, blood samples from naïve, unmanipulated animals were collected and Xkrx allelic expression ratios determined (depicted as “Pre Control” or “Pre CLP”). Two weeks later, some animals were left unmanipulated (“Post Control”) or subjected to sepsis for 20 h (“Post CLP”). Blood was sampled again and analyzed for allelic Xkrx expression ratios. Corresponding values in individual animals are connected by arrows. P values obtained after testing for statistical differences among groups are also shown.
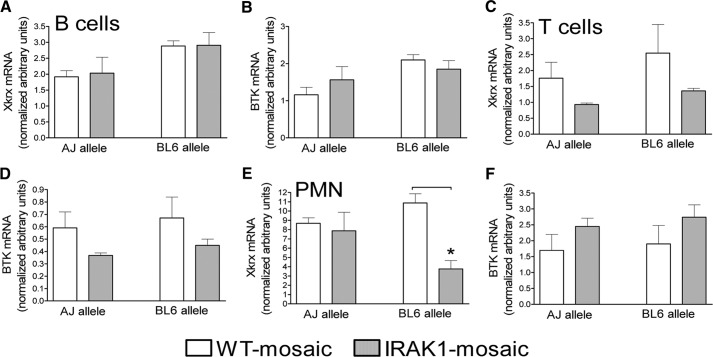
Allelic expression levels in neutrophils and lymphocytes
Lastly, we determined allelic Xkrx and BTK expression levels in neutrophils and B and T lymphocytes. Figure 6A indicates that the Xkrx expression level for the AJ and BL6 alleles was several-fold [4–8] greater in neutrophils than B and T cells when normalized to 18S RNA in WT-mosaic animals (please note different scale in y axes in Fig. 6A, C, and E). In contrast, 18S-normalized BTK expression was similar in neutrophils and B and T cells (one to two range; Fig. 6B, D, and F). IRAK1 mosaicism resulted in a marked and statistically significant decrease in the BL6 allele expression in neutrophils compared with cells from WT-mosaic mice (Fig. 6E). However other allelic Xkrx and BTK expression differences between cells from IRAK1- and WT-mosaic animals did not reach statistically significant levels (Fig. 6).
Figure 6. Allelic Xkrx and BTK expression levels in myeloid and lymphoid cells isolated from blood and BM.
Blood neutrophils (PMN) and B and T lymphocytes (B cells and T cells, respectively) were separated by flow cytometry cell sorting and mRNA extracted as described in Materials and Methods. Allelic Xkrx and BTK expression levels, normalized for 18S RNA in B cells (A and B), T cells (C and D), and neutrophils (E and F), were determined. Mean ± sem; n = 4. *Statistically significant difference, as indicated by connecting line.
DISCUSSION
This study reveals for the first time that mosaicism for IRAK1 expression provides the same protection against sepsis-induced mortality as observed in homozygous IRAK1 deficiency. The protection against sepsis-associated mortality is accompanied by similarly down-regulated IL-6 and IL-10 release in IRAK1-mosaic and -deficient subjects. Based on the observations from the tested X-linked allele-specific gene-expression rates, the most likely mechanism of protection is an increased representation of IRAK1-deficient cells in the BM and blood of healthy IRAK1-mosaic subjects, which renders IRAK1-mosaic animals toward a “more deficient” phenotype at the initial phase of the septic insult. The current observations on IRAK1-deficient females are in agreement with our previous studies using hemizygous IRAK1-deficient male animals, which also indicated protection against sepsis in IRAK1 deficiency compared with WT [28].
The fact that bacteremia in IRAK1-mosaic or -deficient females was not different from WT animals—similar to earlier findings, comparing WT and hemizygous IRAK1-deficient males [28]—indicates that a down-regulated, systemic inflammatory response rather than a decreased bacterial load is associated with survival advantage in IRAK1 deficiency and mosaicism. The partially decreased cytokine responses, including the anti-inflammatory IL-10, are most probably the results of blunted IRAK1-mediated cell activation [37, 38]. These studies together reveal that dampening inflammation by fully or partially depleted IRAK1 activity is advantageous during the septic response.
In agreement with these observations, independent studies on inherently polymorphic human cohorts also demonstrated that subjects with a high-activity IRAK1-variant haplotype manifest an adverse clinical course, including increased incidence of septic shock and associated mortality compared with patients with the WT haplotype [29, 30]. Females who were heterozygous for the IRAK1-variant and WT haplotypes showed no adverse clinical consequences in these cohorts [29, 30]. Our previous animal studies on IRAK1-deficient hemizygous males [28], as well as our current observations on females, are in agreement with the human observations by showing benefit in IRAK1-depleted conditions and also demonstrating protection against sepsis-associated mortality in mosaic females.
The conclusion that IRAK1-mosaic mice are predisposed toward more representation of deficient mosaic subpopulations in healthy subjects is supported by increased cellularity of BM and elevated circulating B cells in IRAK1-mosaic mice, together with elevated expression levels of Xkrx and in part, BTK, from the ChrX that carries the IRAK1 defect. Importantly, following a septic insult, the allelic Xkrx expression BL/AJ ratio decreases in blood and BM from IRAK1-mosaic subjects, which indicate increased peripheral recruitment and BM release of the IRAK1-deficient WBC subpopulation relative to WT cells in mosaic animals. These observations together indicate that under healthy as well as septic conditions, the response of mosaic animals is skewed toward the deficient phenotype, which is consistent with the observed, similar protection in mosaic and deficient subjects.
Our findings also reveals that IRAK1 mosaicism is not simply an intermediate between WT and IRAK1 deficiency, but it presents its own phenotype. One example supporting this conclusion is the increased BM cellularity in mosaic animals relative to WT or deficient cells, which suggests that a cross-talk between WT and deficient subpopulations may boost BM cell production and release, which is absent in WT- or homozygous-deficient mice. Another example is the overlapping mortality advantage in deficient and mosaic animals instead of a presumed intermediate course of mosaic mice in between WT and deficient mortality rates. In this context, we propose that a blunted activation of the IRAK1-deficient subpopulation of cells within mosaic subjects slows the amplification cascade of the inflammatory response. This could be achieved through decreased responsiveness and reduced mediator release by the deficient subpopulation, resulting in blunted intercellular signaling between deficient and WT cells, whereby preventing vicious amplification cycles of cell activation during the septic course.
One of the interesting findings of the study was that IRAK1 mosaicism mostly impacted on sepsis-induced B cell responses. This is reflected in elevated B cell content in BM and blood, which was accompanied by greater B cell number changes following sepsis in these tissue compartments relative to WT or deficient animals. In accordance with observations on IRAK1-deficient males [28], the sepsis-induced B cell decrease in spleen was also blunted in female IRAK1-deficient as well as IRAK1-mosaic mice. These observations are noteworthy in view of recent studies demonstrating an important role of B cells during the initial phase of sepsis, indicating that depleting B cells in mice worsens sepsis outcome [39]. Additionally, lymphocyte apoptosis during late sepsis is deleterious, as this has been well established in mice and humans [40, 41]. Based on these facts, the baseline increase in BM and circulating B cell numbers in IRAK1-mosaic mice may have also contributed to preconditioning for improved sepsis outcome in these animals. Whereas TLR-mediated signaling is an important component of B cell activation [39–44], the fine molecular as well as intercellular signaling mechanism, resulting in the unique mosaic phenotype in IRAK1 mosaicism, remains to be elucidated.
The AJ and BL6J mouse strains show differences in pathophysiological responses to endotoxemia, virus infections, and susceptibilities to other disease conditions [45–50]. Previous studies BL6 mice (commercial nonconsomic strains) showed that castrated males carrying the AJ ChrX display a markedly improved outcome following CLP-induced sepsis compared with males carrying the BL6 ChrX [12, 49, 51, 52]. These male animals were generated by bidirectional breeding, where an AJ male was paired with a BL6 female or vice versa, which produced males carrying the AJ or BL6 ChrX, whereas they were heterokaryotic for the autosomal chromosomes. These studies suggested that differences between the AJ and BL6 ChrX in the absence of testosterones may be important in determining CLP-induced sepsis outcome. In our current study, however, first using female consomic mice, in which only the AJ and BL6 ChrX was different, and the autosomal chromosomes were identical, we did not observe any differences in sepsis-induced mortality among mice homokaryotic or mosaic for the WT AJ or BL6 ChrXs (Fig. 1B). Additionally, we observed that sepsis-induced mortality was similar among consomic males carrying the WT AJ or WT BL6 ChrXs under castrated or normal conditions (unpublished observations). Thus, the studies published previously [12, 49, 51, 52], together with our current observations, indicate that innate X-linked polymorphism between the AJ and BL6 strains in the absence of autosomal polymorphisms is not responsible for determining sepsis mortality in the CLP model used.
However, despite the fact that consomic animals, which were homokaryotic or mosaic for the WT BL6 and AJ ChrXs, showed no differences in CLP-induced mortality, it cannot be ruled out that innate X-linked polymorphic differences between the AJ and BL6 variants may modulate or skew cell activation and repair processes without impacting on sepsis mortality. This latter notion is supported by observations from the “self-controlled” experiments (Fig. 5), which indicated a borderline significant but consistent drop in variant Xkrx BL/AJ ratios in WT-mosaic animals in response to sepsis. Importantly, however, it was also evident that the simultaneous presence of mosaicism for IRAK1 expression greatly enhanced this skewed response (Fig. 5). The observations on hepatic and pulmonary allelic expression ratios are more difficult to interpret as a result of the low baseline expression of Xkrx and BTK in these organs. Thus, expression ratios in control animals reflect conditions in the parenchyma combined with the relatively small number of resident immune cells. In contrast, values under septic conditions are most likely the reflections of newly recruited immune cells combined with parenchymal responses. Nevertheless, the sepsis-induced decrease in BL6/AJ-variant expression ratios in these organs is most likely the reflection of skewed sepsis-induced cell recruitment toward WBCs, in which the AJ ChrX is active.
Lastly, it is important to emphasize that despite the reliability and accuracy of the allele-specific, gene-expression assay used, the interpretation of the results has some limitations. This is because cell-composition changes in organs during the septic response are the reflections of sometimes opposing cellular events, including de novo cell recruitment, apoptosis, necrosis, local cell activation, and proliferation, which may impact different cell types at different degrees. Additionally, in mosaic animals, cellular gene-expression levels are expected to be influenced by the simultaneous presence or absence of IRAK1 expression within a cell, as we observed on sorted neutrophils and B and T cells, which could also influence subsequent cell responses to sepsis. Furthermore, similar to IRAK1, BTK is also part of the TLR/MyD88 signaling complex with critical roles in TLR-mediated B cell activation [40, 53, 54] but also participating in mediating phagocyte responses [55]. Thus, activated BTK signaling and associated changes in BTK expression, combined with dynamic cell trafficking impacting mosaic subsets at different degrees, could mask some of the mosaic cell-ratio changes when assessed by allelic BTK expression during sepsis. This is a likely explanation of why allelic BTK responses were not as marked as observed for Xkrk. Nevertheless, despite these limitations, our study demonstrates clearly that increased cellular variability driven by mosaic expression of IRAK1 and other naturally occurring X-linked polymorphisms presents a unique phenotype and modulates the inflammatory response toward improved clinical outcome during polymicrobial sepsis.
In summary, our studies reveal that mosaicism for IRAK1 expression manifests the same protection against sepsis-induced mortality as observed in homozygous female or hemizygous male [28] subjects. The mechanism of protection in mosaic subjects is, at least in part, a result of an increased representation of IRAK1-deficient cells in the BM and blood, which preconditions the host for an improved septic response similar to that observed in deficient subjects. The observations support the hypothesis [1] that female ChrX mosaicism represents increased functional and cellular variability, resulting in phenotypes that are more responsive to the dynamically changing pathophysiology during inflammation compared with male phenotypes, where the inflammatory response is modulated by a lone ChrX. The observations are in agreement with human studies, which indicated worsened sepsis outcome of high activity IRAK1 variant haplotype in hemizygous males and homozygous females and found no adverse effects in heterozygous females during septic conditions.
ACKNOWLEDGMENTS
This study was supported by U.S. National Institutes of Health-National Institute of General Medical Sciences Grant R01GM084932 and a University of Medicine and Dentistry of New Jersey Foundation grant.
Footnotes
- AJ
- A/J mouse strain
- APC
- allophycocyanin
- BL6
- C57BL/6j mouse strain
- BM
- bone marrow
- BTK
- Bruton's kinase
- ChrX
- X-Chromosome
- CLP
- cecal ligation and puncture
- IRAK1
- IL-1R-associated kinase 1
- KO
- knockout
- MGB
- minor groove binder
- neo
- neomycin
- NOX2
- NADPH oxidase 2
- PMN
- polymorphonuclear neutrophil
- SNP
- single-nucleotide polymorphism
- WBC
- white blood cell
- Xkrx
- X-kell blood group precursor-related
AUTHORSHIP
R.C. and Z.S. designed the experiments, performed final analyses, and formulated the manuscript. R.C., S.F., Z.H.N., and B.C. performed experiments and analytical procedures. J.A.T. provided the IRAK1-deficient mice and consulted the principal investigator. R.D. assisted in allelic probe designs.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1. Spolarics Z. (2007) The X-files of inflammation: cellular mosaicism of X-linked polymorphic genes and the female advantage in the host response to injury and infection. Shock 27, 597–604 [DOI] [PubMed] [Google Scholar]
- 2. Libert C., Dejager L., Pinheiro I. (2010) The X chromosome in immune functions: when a chromosome makes the difference. Nat. Rev. Immunol. 10, 594–604 [DOI] [PubMed] [Google Scholar]
- 3. Migeon B. R. (2006) The role of X inactivation and cellular mosaicism in women's health and sex-specific diseases. JAMA 295, 1428–1433 [DOI] [PubMed] [Google Scholar]
- 4. Sperry J. L., Vodovotz Y., Ferrell R. E., Namas R., Chai Y. M., Feng Q. M., Jia W. P., Forsythe R. M., Peitzman A. B., Billiar T. R. (2012) Racial disparities and sex-based outcomes differences after severe injury. J. Am. Coll. Surg. 214, 973–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu P. Y., Death A. K., Handelsman D. J. (2003) Androgens and cardiovascular disease. Endocr. Rev. 24, 313–340 [DOI] [PubMed] [Google Scholar]
- 6. Chen C. C., Parker C. R., Jr., (2004) Adrenal androgens and the immune system. Semin. Reprod. Med. 22, 369–377 [DOI] [PubMed] [Google Scholar]
- 7. Angele M. K., Schwacha M. G., Ayala A., Chaudry I. H. (2000) Effect of gender and sex hormones on immune responses following shock. Shock 14, 81–90 [DOI] [PubMed] [Google Scholar]
- 8. Kovacs E. J., Messingham K. A., Gregory M. S. (2002) Estrogen regulation of immune responses after injury. Mol. Cell. Endocrinol. 193, 129–135 [DOI] [PubMed] [Google Scholar]
- 9. Choudhry M. A., Bland K. I., Chaudry I. H. (2006) Gender and susceptibility to sepsis following trauma. Endocr. Metab. Immune Disord. Drug Targets 6, 127–135 [DOI] [PubMed] [Google Scholar]
- 10. George R. L., McGwin G., Jr., Windham S. T., Melton S. M., Metzger J., Chaudry I. H., Rue L. W., III (2003) Age-related gender differential in outcome after blunt or penetrating trauma. Shock 19, 28–32 [DOI] [PubMed] [Google Scholar]
- 11. Gannon C. J., Pasquale M., Tracy J. K., McCarter R. J., Napolitano L. M. (2004) Male gender is associated with increased risk for postinjury pneumonia. Shock 21, 410–414 [DOI] [PubMed] [Google Scholar]
- 12. De Maio A., Torres M. B., Reeves R. H. (2005) Genetic determinants influencing the response to injury, inflammation, and sepsis. Shock 23, 11–17 [DOI] [PubMed] [Google Scholar]
- 13. Deitch E. A., Livingston D. H., Lavery R. F., Monaghan S. F., Bongu A., Machiedo G. W. (2007) Hormonally active women tolerate shock-trauma better than do men: a prospective study of over 4000 trauma patients. Ann. Surg. 246, 447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sperry J. L., Friese R. S., Frankel H. L., West M. A., Cuschieri J., Moore E. E., Harbrecht B. G., Peitzman A. B., Billiar T. R., Maier R. V., et al. (2008) Male gender is associated with excessive IL-6 expression following severe injury. J. Trauma 64, 572–578 [DOI] [PubMed] [Google Scholar]
- 15. Selmi C., Brunetta E., Raimondo M. G., Meroni P. L. (2012) The X chromosome and the sex ratio of autoimmunity. Autoimmun. Rev. 11, A531–A537 [DOI] [PubMed] [Google Scholar]
- 16. Invernizzi P., Pasini S., Selmi C., Gershwin M. E., Podda M. (2009) Female predominance and X chromosome defects in autoimmune diseases. J. Autoimmun. 33, 12–16 [DOI] [PubMed] [Google Scholar]
- 17. Barrow R. E., Herndon D. N. (1990) Incidence of mortality in boys and girls after severe thermal burns. Surg. Gynecol. Obstet. 170, 295–298 [PubMed] [Google Scholar]
- 18. Wells J. C. (2000) Natural selection and sex differences in morbidity and mortality in early life. J. Theor. Biol. 202, 65–76 [DOI] [PubMed] [Google Scholar]
- 19. Adrie C., Azoulay E., Francais A., Clec'h C., Darques L., Schwebel C., Nakache D., Jamali S., Goldgran-Toledano D., Garrouste-Orgeas M., et al. (2007) Influence of gender on the outcome of severe sepsis: a reappraisal. Chest 132, 1786–1793 [DOI] [PubMed] [Google Scholar]
- 20. Crimmins E. M., Hayward M. D., Saito Y. (1996) Differentials in active life expectancy in the older population of the United States. J. Gerontol. B Psychol. Sci. Soc. Sci. 51, S111–S120 [DOI] [PubMed] [Google Scholar]
- 21. Schaffner S. F. (2004) The X chromosome in population genetics. Nat. Rev. Genet. 5, 43–51 [DOI] [PubMed] [Google Scholar]
- 22. Chandra R., Federici S., Hasko G., Deitch E. A., Spolarics Z. (2010) Female X-chromosome mosaicism for gp91phox expression diversifies leukocyte responses during endotoxemia. Crit. Care Med. 38, 2003–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chandra R., Federici S., Nemeth Z. H., Horvath B., Pacher P., Hasko G., Deitch E. A., Spolarics Z. (2011) Female X-chromosome mosaicism for NOX2 deficiency presents unique inflammatory phenotype and improves outcome in polymicrobial sepsis. J. Immunol. 186, 6465–6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swantek J. L., Tsen M. F., Cobb M. H., Thomas J. A. (2000) IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J. Immunol. 164, 4301–4306 [DOI] [PubMed] [Google Scholar]
- 25. Thomas J. A., Tsen M. F., White D. J., Horton J. W. (2002) IRAK contributes to burn-triggered myocardial contractile dysfunction. Am. J. Physiol. Heart Circ. Physiol. 283, H829–H836 [DOI] [PubMed] [Google Scholar]
- 26. Thomas J. A., Haudek S. B., Koroglu T., Tsen M. F., Bryant D. D., White D. J., Kusewitt D. F., Horton J. W., Giroir B. P. (2003) IRAK1 deletion disrupts cardiac Toll/IL-1 signaling and protects against contractile dysfunction. Am. J. Physiol. Heart Circ. Physiol. 285, H597–H606 [DOI] [PubMed] [Google Scholar]
- 27. Verdrengh M., Thomas J. A., Hultgren O. H. (2004) IL-1 receptor-associated kinase 1 mediates protection against Staphylococcus aureus infection. Microbes Infect. 6, 1268–1272 [DOI] [PubMed] [Google Scholar]
- 28. Chandra R., Federici S., Bishwas T., Nemeth Z. H., Deitch E. A., Thomas J. A., Spolarics Z. (2013) IRAK1-dependent signaling mediates mortality in polymicrobial sepsis. Inflammation [E-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 29. Arcaroli J., Silva E., Maloney J. P., He Q., Svetkauskaite D., Murphy J. R., Abraham E. (2006) Variant IRAK-1 haplotype is associated with increased nuclear factor-κB activation and worse outcomes in sepsis. Am. J. Respir. Crit. Care Med. 173, 1335–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Toubiana J., Courtine E., Pene F., Viallon V., Asfar P., Daubin C., Rousseau C., Chenot C., Ouaaz F., Grimaldi D., et al. (2010) IRAK1 functional genetic variant affects severity of septic shock. Crit. Care Med. 38, 2287–2294 [DOI] [PubMed] [Google Scholar]
- 31. Baker C. C., Chaudry I. H., Gaines H. O., Baue A. E. (1983) Evaluation of factors affecting mortality-rate after sepsis in a murine cecal ligation and puncture model. Surgery 94, 331–335 [PubMed] [Google Scholar]
- 32. Dejager L., Pinheiro I., Dejonckheere E., Libert C. (2011) Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 19, 198–208 [DOI] [PubMed] [Google Scholar]
- 33. Remick D. G., Newcomb D. E., Bolgos G. L., Call D. R. (2000) Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock 13, 110–116 [DOI] [PubMed] [Google Scholar]
- 34. Nemeth Z. H., Csoka B., Wilmanski J., Xu D., Lu Q., Ledent C., Deitch E. A., Pacher P., Spolarics Z., Hasko G. (2006) Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J. Immunol. 176, 5616–5626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas J. A., Allen J. L., Tsen M., Dubnicoff T., Danao J., Liao X. C., Cao Z., Wasserman S. A. (1999) Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J. Immunol. 163, 978–984 [PubMed] [Google Scholar]
- 36. Chandra R., Villanueva E., Feketova E., Machiedo G. W., Hasko G., Deitch E. A., Spolarics Z. (2008) Endotoxemia down-regulates bone marrow lymphopoiesis but stimulates myelopoiesis: the effect of G6PD deficiency. J. Leukoc. Biol. 83, 1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maitra U., Deng H., Glaros T., Baker B., Capelluto D. G., Li Z., Li L. (2012) Molecular mechanisms responsible for the selective and low-grade induction of proinflammatory mediators in murine macrophages by lipopolysaccharide. J. Immunol. 189, 1014–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang Y., Li T., Sane D. C., Li L. (2004) IRAK1 serves as a novel regulator essential for lipopolysaccharide-induced interleukin-10 gene expression. J. Biol. Chem. 279, 51697–51703 [DOI] [PubMed] [Google Scholar]
- 39. Kelly-Scumpia K. M., Scumpia P. O., Weinstein J. S., Delano M. J., Cuenca A. G., Nacionales D. C., Wynn J. L., Lee P. Y., Kumagai Y., Efron P. A., et al. (2011) B cells enhance early innate immune responses during bacterial sepsis. J. Exp. Med. 208, 1673–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peck-Palmer O. M., Unsinger J., Chang K. C., Davis C. G., McDunn J. E., Hotchkiss R. S. (2008) Deletion of MyD88 markedly attenuates sepsis-induced T and B lymphocyte apoptosis but worsens survival. J. Leukoc. Biol. 83, 1009–1018 [DOI] [PubMed] [Google Scholar]
- 41. Hotchkiss R. S., Chang K. C., Swanson P. E., Tinsley K. W., Hui J. J., Klender P., Xanthoudakis S., Roy S., Black C., Grimm E., et al. (2000) Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat. Immunol. 1, 496–501 [DOI] [PubMed] [Google Scholar]
- 42. Suzuki N., Saito T. (2006) IRAK-4—a shared NF-κB activator in innate and acquired immunity. Trends Immunol. 27, 566–572 [DOI] [PubMed] [Google Scholar]
- 43. Gan L., Li L. (2006) Regulations and roles of the interleukin-1 receptor associated kinases (IRAKs) in innate and adaptive immunity. Immunol. Res. 35, 295–302 [DOI] [PubMed] [Google Scholar]
- 44. Browne E. P. (2012) Regulation of B-cell responses by Toll-like receptors. Immunology 136, 370–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Forkert P. G. (2010) Mechanisms of lung tumorigenesis by ethyl carbamate and vinyl carbamate. Drug Metab. Rev. 42, 355–378 [DOI] [PubMed] [Google Scholar]
- 46. Hall D., Poussin C., Velagapudi V. R., Empsen C., Joffraud M., Beckmann J. S., Geerts A. E., Ravussin Y., Ibberson M., Oresic M., et al. (2010) Peroxisomal and microsomal lipid pathways associated with resistance to hepatic steatosis and reduced pro-inflammatory state. J. Biol. Chem. 285, 31011–31023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dominguez-Punaro M. L., Segura M., Radzioch D., Rivest S., Gottschalk M. (2008) Comparison of the susceptibilities of C57BL/6 and A/J mouse strains to Streptococcus suis serotype 2 infection. Infect. Immun. 76, 3901–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Malley J., Matesic L. E., Zink M. C., Strandberg J. D., Mooney M. L., De Maio A., Reeves R. H. (1998) Comparison of acute endotoxin-induced lesions in A/J and C57BL/6J mice. J. Hered. 89, 525–530 [DOI] [PubMed] [Google Scholar]
- 49. Fulton W. B., Reeves R. H., Takeya M., De Maio A. (2006) A quantitative trait loci analysis to map genes involved in lipopolysaccharide-induced inflammatory response: identification of macrophage scavenger receptor 1 as a candidate gene. J. Immunol. 176, 3767–3773 [DOI] [PubMed] [Google Scholar]
- 50. Leisewitz A. L., Rockett K., Kwiatkowski D. (2008) BCG-malaria co-infection has paradoxical effects on C57BL/6 and A/J mouse strains. Parasite Immunol. 30, 1–12 [DOI] [PubMed] [Google Scholar]
- 51. Trentzsch H., Stewart D., De Maio A. (2003) Genetic background conditions the effect of sex steroids on the inflammatory response during endotoxic shock. Crit. Care Med. 31, 232–236 [DOI] [PubMed] [Google Scholar]
- 52. Torres M. B., Trentzsch H., Stewart D., Mooney M. L., Fuentes J. M., Saad D. F., Reeves R. H., De Maio A. (2005) Protection from lethal endotoxic shock after testosterone depletion is linked to chromosome X. Shock 24, 318–323 [DOI] [PubMed] [Google Scholar]
- 53. Dye J. R., Palvanov A., Guo B., Rothstein T. L. (2007) B cell receptor cross-talk: exposure to lipopolysaccharide induces an alternate pathway for B cell receptor-induced ERK phosphorylation and NF-κ B activation. J. Immunol. 179, 229–235 [DOI] [PubMed] [Google Scholar]
- 54. Ruprecht C. R., Lanzavecchia A. (2006) Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur. J. Immunol. 36, 810–816 [DOI] [PubMed] [Google Scholar]
- 55. Fiedler K., Sindrilaru A., Terszowski G., Kokai E., Feyerabend T. B., Bullinger L., Rodewald H. R., Brunner C. (2011) Neutrophil development and function critically depend on Bruton tyrosine kinase in a mouse model of X-linked agammaglobulinemia. Blood 117, 1329–1339 [DOI] [PubMed] [Google Scholar]