Abstract
Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) are effective weight loss surgeries that also improve glucose metabolism. Rapid, early rises of circulating insulin and glucagon-like peptide-1 (GLP-1) concentrations following food ingestion are characteristic of these procedures. The purpose of the current study was to test the hypothesis that postprandial hormone release is due to increased nutrient emptying from the stomach. Radioscintigraphy and chemical and radiolabeled tracers were used to examine gastric emptying in rat models of VSG and RYGB surgery. Intraduodenal nutrient infusions were used to assess intestinal GLP-1 secretion and nutrient sensitivity in VSG rats compared with shams. Five minutes after a nutrient gavage, the stomachs of RYGB and VSG rats were completely emptied, whereas only 6.1% of the nutrient mixture had emptied from sham animals. Gastric pressure was increased in VSG animals, and rats with this procedure did not inhibit gastric emptying normally in response to increasing caloric loads of dextrose or corn oil, and they did not respond to neural or endocrine effectors of gastric motility. Finally, direct infusion of liquid nutrients into the duodenum caused significantly greater GLP-1 release in VSG compared with shams, indicating that increases in GLP-1 secretion after VSG are the result of both greater gastric emptying rates and altered responses at the level of the intestine. These findings demonstrate greatly accelerated gastric emptying in rat models of RYGB and VSG. In VSG this is likely due to increased gastric pressure and reduced responses to inhibitory feedback from the intestine.
Keywords: glucagon-like peptide-1
the most effective treatment option currently available for obese and overweight patients seeking significant and lasting weight loss is bariatric surgery. Roux-en-Y gastric bypass (RYGB), currently the most widely utilized bariatric surgery, results in ∼30% excess weight loss and substantial improvements in metabolic comorbidities (5). RYGB involves a 90% reduction of stomach size and reconfiguration of the intestine whereby the pylorus, duodenum, and upper jejunum are completely excluded from nutrient exposure. Recently, the vertical sleeve gastrectomy (VSG) has gained popularity because of its simplicity and comparable efficacy with RYGB (7, 10, 18, 22, 33, 37). In VSG ∼80% of the stomach is removed along the greater curvature, and there is no physical manipulation of the pylorus or intestine.
Despite the dramatic anatomic differences, RYGB and VSG cause similar weight loss, improvements in glucose regulation, and increases in nutrient-dependent secretion of gut hormones (7, 33, 37). Both surgeries also produce long-term reductions in food intake and reduced meal size (8, 41, 51). Following meal ingestion, glucose levels rise rapidly in persons with RYBG or VSG, but clearance is rapid, and overall glucose tolerance improves in both diabetic and nondiabetic individuals (3, 37). This pattern of postprandial glucose excursion is due in great part to increased entry of meal glucose from the gut following RYGB (34). Increased nutrient delivery to the intestine has also been hypothesized to be the mechanism by which glucagon-like peptide-1 (GLP-1) release is enhanced after bariatric surgery. Most studies of humans with RYGB or VSG report increased rates of gastric emptying (2, 4, 27–30, 40); for VSG this was unanticipated, since the procedure preserves the pylorus (24). There is strong evidence from human studies that GLP-1 secretion is dependent primarily on rates of glucose appearance in the intestine (38, 39).
The rate of gastric emptying is controlled by mechanical factors in the stomach that are related primarily to volume and pressure but also inhibited though endocrine and neuronal inputs from the intestinal tract tied to nutrient absorption (6, 13). This complex regulatory system governs the appearance of carbohydrates and other nutrients into circulation and to critical organs such as the liver. In this study, indices of gastric emptying rate were optimized in surgically naïve rats and then assessed in sham- and bariatric-operated animals, using imaging and biochemical techniques. These models were used to explore the neuronal and endocrine regulation of gastric emptying after VSG along with the postingestive consequences of faster gastric emptying insulin and GLP-1 secretion.
METHODS
Animals.
Male Long-Evans rats were purchased from Harlan (Indianapolis, IN) and housed singly in the University of Cincinnati Laboratory Animals for Medical Science Facility at the Metabolic Diseases Institute under controlled conditions (12:12-h light-dark cycle, 50–60% humidity, 25°C). Animals had free access to standard rodent chow or high-fat diet and water unless noted. All procedures for animal use were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Surgery.
Animals received bariatric surgeries using standardized surgical procedures (7, 8). Before surgery, rats were given ad libitum access to water and a high (butter)-fat diet (4.54 kcal/g, 41% fat; Research Diets, New Brunswick, NJ) that was previously documented to produce significant weight gain and metabolic impairment (49). After 8 wk on a high-fat diet, fat mass was assessed using NMR (Echo MRI; Echo Medical Systems, Houston, TX) and used to assign surgical groups (RYGB, VSG, or sham) in a counterbalanced fashion. Surgeries and postoperative care were performed as described previously (7, 8).
Imaging/scintigraphy.
Rats were anesthetized with isoflurane and gavaged with either 2.5 ml of a liquid nutrient solution (Ensure Plus; Abbott, Columbus, OH) containing 37 MBq (1 mCi) of 99mTc Sulfur Colloid (99mTc SC) or a mixture of Ensure (1 ml) and barium sulfate (2.5 ml, 70% wt/vol) for X-ray visualization of GI anatomy. Immediately after the gavage, animals were placed in a supine position on the imaging tray and maintained under anesthesia. For the tracer studies, a radioisotopic phosphor screen was placed between the charge-coupled device camera and the imaging tray to allow for visualization of the isotope. A 230-s radioisotopic exposure of the stomach was taken every 5 min for 1 h (Carestream MS FX PRO, Woodbridge, CT). Prior to the 1-h radioistopic study, an X-ray (30-s exposure with no filtering) was taken for anatomic reference. The radioistopic data were processed by converting the images to photons·s−1·mm2. An auto region of interest (ROI) was drawn around the Ensure-99mTc mixture in the stomach using a threshold method where the lower limit is the minimum photon intensity in the 100% gastric content image (see Fig. 1A). For consistency, all images were displayed and evaluated with the same minimum photon intensity value. An autogenerated background (background nos. were within 99.6% of each other) was subtracted from each image. The change in net photon intensity over time was used as an index of gastric emptying rate. For the contrast studies, a radiographic phosphor screen was placed between the charge-coupled device camera and the imaging tray to obtain the X-ray images. A manual ROI was drawn around the stomach, visualized by the barium. Background-subtracted net X-ray intensity values were obtained from stomach ROIs for comparison evaluation between surgery rat groups (Fig. 2C).
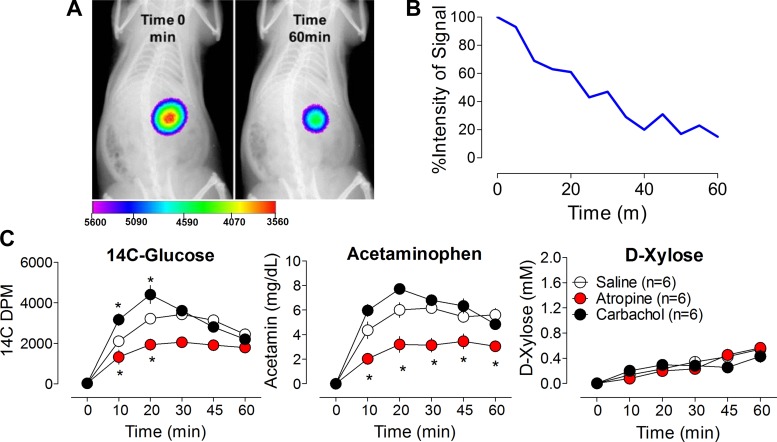
Fig. 1.
A: representative radioisotopic/X-ray overlay images showing gastric visualization of 99mTc Sulfur Colloid/Ensure mixture in rat stomach at time 0 and 60 min following oral gavage. B: changes in net photon intensity over time were used to index the rate of gastric emptying. C: plasma levels of 3 different markers for gastric emptying, [14C]glucose, acetaminophen (acetamin), and d-xylose, after intraperitoneal (ip) saline (○), atropine (red circles), or carbachol (●). Left: [14C]glucose levels were significantly higher after ip treatment with carbachol and significantly lower after ip atropine; *P < 0.05 vs. saline (Bonferroni posttests). Middle: atropine significantly reduced acetaminophen levels compared with saline treatment; *P < 0.05. Right: plasma levels of d-xylose were unaffected by ip treatment with atropine or carbachol compared with saline treatment.
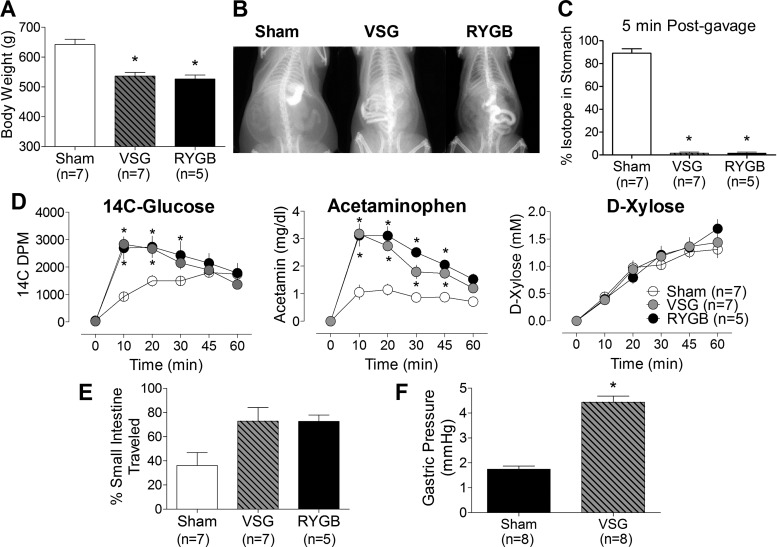
Fig. 2.
A: body weight in sham (open bar), vertical sleeve gastrectomy (VSG; black and gray hatched bar), and Roux-en-Y gastric bypass (RYGB; black bar) rats; *P < 0.05 vs. sham (Bonferroni posttests). B: images showing barium/Ensure contrast mixture 5 min after oral gavage in sham, RYGB, and VSG surgical groups. In the sham animal, most of the barium remains in the stomach, whereas in both the RYGB and VSG animals the barium and tracer are well into the intestine. C: the isotope (%initial intensity) remained in stomachs of sham rats but was completely emptied from the stomachs of VSG and RYGB rats; *P < 0.05. D: plasma appearance of 14C-labeled glucose, acetamin, and d-xylose over time in sham (○), VSG (gray circles), and RYGB (●). E: intestinal transit (expressed as %means ± SE of intestine travelled) in sham (open bar), VSG (black and gray hatched bar), and RYGB (black bar). F: postmortem gastric pressure in sham and VSG animals. *P < 0.05 vs. sham (unpaired t-test). DPM, disintegreations per minute.
Chemical tracer techniques for measuring gastric emptying.
Overnight-fasted, chow-fed Long-Evans rats with no prior surgical interventions were weighed and moved to a procedure room and allowed to acclimate for 2 h. Weight-matched groups had a baseline blood sample taken from a tail vein and were given an intraperitoneal (ip) injection of atropine, a muscarinic receptor antagonist known to inhibit gastric emptying, carbachol, an acetylcholine receptor agonist known to increase gastric emptying, or saline (n = 6/group). Animals were gavaged with a mixed volume (6 ml) of d-[14C]glucose (20 μCi), acetaminophen (100 mg/kg), and d-xylose (0.5 g/kg) and sampled at 10, 20, 30, 45, and 60 min after the gavage. A similar gavage procedure was used to study RYGB (n = 5), VSG (n = 7), or sham (n = 7), but with a 3-ml volume, and toluidine blue was added to the liquid gavage mixture for postmortem measurement of transit of chyme through the intestine. For this assessment the intestine was carefully removed and stretched to full length, and the length containing dye was compared with the total length of the small intestine. For RYGB rats, the biliopancreatic limb was included in total gut length to account for reflux into this region.
Pressure and volume effects.
Gastric pressure was measured in a separate group of animals 6 mo after VSG or sham surgery (n = 8/group). The pylorus and esophagus were ligated immediately after euthanization. Three milliliters of saline was infused into the stomach, and pressure was measured using a transducer (PX26–005GV; Omega Engineering, Stamford, CT). In a group of conscious rats with VSG or sham operations (n = 4–5/group), the effect of volume on gastric emptying was studied using gavage of 0.3, 1, or 3.0 ml of a viscous mixture of 2% methylcellulose and d-[14C]glucose (14.7 μci). The appearance of 14C in plasma between 0 and 60 min was used as an index of gastric emptying.
Neural influences on gastric emptying.
Four months after surgery, atropine (1 mg/kg ip) was given to overnight-fasted sham- or VSG-operated rats (n = 5/group) 15 min prior to orally delivered 50% dextrose (2.0 ml) mixed with an acetaminophen tracer (100 mg/kg) to study neural influences on gastric emptying. Plasma was sampled for the appearance of tracer at 0- and 10-min time points.
Effects of GLP-1 receptor activation on gastric emptying in VSG.
Six months after surgery, rats with VSG (n = 4) or sham (n = 5) were administered saline or the GLP-1 receptor agonist exendin-4 (50 μg/kg ip) 15 min prior to orally delivered 50% dextrose (2.0 ml) mixed with acetaminophen (100 mg/kg). All experiments were performed following an overnight fast, and each animal went through each condition via a counterbalance design. Blood samples were collected at 0 and 10 min. The role of endogenous GLP-1 secretion on gastric emptying after VSG was investigated in GLP-1R-knockout (n = 28) and wild-type littermate control mice (n = 14) 6 wk following VSG or a sham operation, as described previously (8). Mice were given an intraoral infusion of liquid diet (200 μl of Ensure Plus) mixed with acetaminophen (100 mg/kg) and blood sampled at 0 and 10 min. Mice in this experiment were previously part of another study (43).
Nutrient-induced inhibition of gastric emptying.
Five months after surgery, rats with VSG or sham procedures (n = 8–11/group) were gavaged with 2 ml of sucrose or corn oil at varying caloric densities (0.2, 1.0, and 3.0 kcal) following an overnight fast. The infusates included acetaminophen to estimate gastric emptying. Blood glucose and plasma insulin were also measured over 60 min.
Intestinal nutrient sensing and GLP-1 secretion.
At the time of VSG (n = 9) or sham (n = 12) surgery, an indwelling duodenal catheter was placed, tunneled subcutaneously, and exteriorized at the back of the neck. Beginning 1 mo postoperatively, rats were randomly allocated to receive 2.5 ml of 25% dextrose by oral gavage or through the tube directly into the duodenum (0.4 ml/min) following an overnight fast. The studies were repeated at 1-wk intervals such that each animal received both treatments. Blood was sampled at 0 and 15 min, and plasma was stored for assay of GLP-1.
Assays.
To measure plasma content of d-[14C]glucose, samples were deproteinized with BaOH and ZnSO4, and β-counting was performed with standard scintillant. Plasma d-xylose and acetaminophen were measured by calorimetric assay, as described previously (23, 36). Insulin was measured by ELISA (Crystal Chem, Downers Grove, IL), and GLP-1 was measured using MSD mesoscale assay (Gaithersburg, MD) according to the manufacturer's instructions.
Statistical analysis.
The primary data analysis was conducted with mixed-model ANOVAs, using treatment and surgery as the active factors. Bonferroni's post hoc test was performed for direct comparisons of individual groups. Two-tailed t-tests were used for comparisons of two groups. Statistical significance was set at P < 0.05 for all analyses. Data are presented as means ± SE.
RESULTS
Scintigraphy and chemical tracer techniques.
In rats given a mixed liquid meal containing 99mTc SC, there was a linear decrease in the stomach contents over time. This was anatomically verified in a second experiment in the same rats with a gavage of barium plus liquid meal. Figure 1, A and B, displays a representative image from the overlay of the 99mTc images.
In tests of surrogate measurements of gastric emptying in conscious rats, atropine and carbachol were given to decrease and increase gastric emptying rates, respectively. Plasma levels of d-[14C]glucose, acetaminophen, and d-xylose during these treatments are depicted in Fig. 1C. The pharmacological inhibition of gastric emptying by atropine was observed using both 14C and acetaminophen tracers relative to saline- or carbachol-treated animals. However, plasma levels of d-xylose were similar between the three treatment groups. These data indicate that 14C-labeled glucose and acetaminophen were better markers of gastric emptying rate, as both reflected the expected change in gastric emptying rate induced by carbachol and atropine compared with saline-treated animals.
Gastric emptying dynamics following bariatric surgery.
VSG and RYGB rats lost similar amounts of weight and were significantly lighter than sham-operated controls (Fig. 2A). There was significant passage of barium into the intestine of RYBG and VSG animals within the first 5 min following gavage, whereas little contrast passed from the stomach of sham animals (Fig. 2B). When this measurement was quantified with 99mTc SC, the liquid meal mixture was 100 ± 0% emptied from the pouch in RYGB rats and stomach in VSG animals in 5 min, whereas only 6.1 ± 6% was emptied from sham stomachs over this period (Fig. 2C). In fact, only 24 ± 15% of administered tracer mixture passed from the stomach of the control animals by 60 min after the gavage. d-[14C]glucose and acetaminophen also appeared much more rapidly in the plasma of RYGB and VSG rats compared with the sham animals, whereas plasma concentrations of d-xylose did not differ among groups. Faster gastric emptying rates were also associated with faster transit times. The passage of chyme labeled with toluidine blue traveled twice the distance down the GI tract in VSG (73 ± 11%) and RYGB (73 ± 5%) compared with sham animals (36 ± 11%) (Fig. 2E). Interestingly, dye was observed in the bypassed region of RYGB rats, suggesting some reflux through the jejunostomy. Despite this, the distance of the dye from the terminal ileum was similar between VSG (33 ± 13 cm) and RYGB (38 ± 7 cm) rats, implying that nutrients reached the same cell populations at a similar rate in these animals. Gastric pressure was significantly elevated after VSG (4.43 ± 0.25 mmHg) vs. sham (1.74 ± 0.13 mmHg) surgery (Fig. 2F), as expected. Beause of the open system produced by RYGB, gastric pressure could not be measured in these animals.
Regulation of gastric emptying by gavage volume.
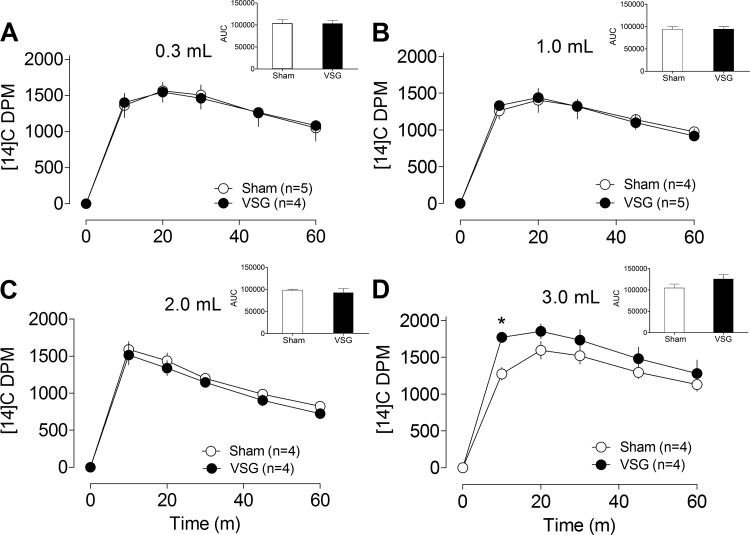
The effects of different gavage volumes on gastric emptying were studied using a noncaloric infusate (Fig. 3, A–D). The range of volumes tested was based on what animals were observed to eat voluntarily following VSG (8, 11). Rates of radiolabeled glucose appearance did not differ among sham and VSG animals gavaged with 0.3- to 2.0-ml volumes of the infusate. However, when administered at a volume of 3.0 ml, gastric emptying rates were significantly greater in VSG rats compared with sham animals (P < 0.05).
Fig. 3.
A–D: gastric emptying in sham (○) and VSG (●) rats orally gavaged with different volumes (0.3–3.0 ml) of a noncaloric methylcellulose solution mixed with 14C-labeled glucose. A–D, insets: area under the curve (AUC). *P < 0.05 compared with sham (Bonferroni posttests).
Neural and GLP-1R-mediated influences on gastric emptying.
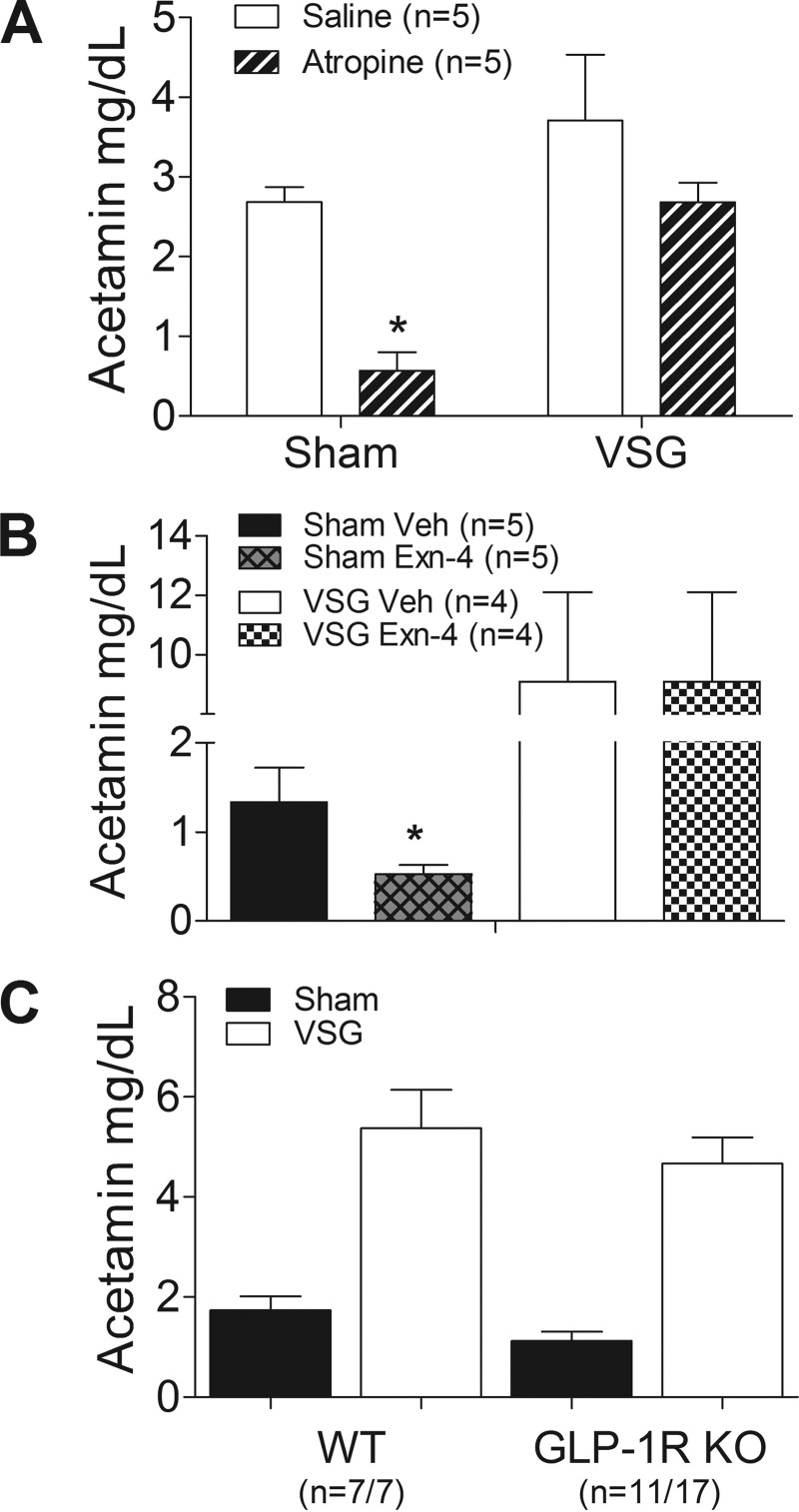
Innervation of gastric smooth muscle by efferent nerves inhibits motility through muscarinic receptor activation. As expected, gastric emptying was delayed in sham-operated rats given the muscarinic antagonist atropine compared with saline-treated rats (Fig. 4A). However, VSG rats were unresponsive to atropine, indicating that surgery abolished much of the neural regulation on gastric emptying in these animals.
Fig. 4.
A: gastric emptying in sham and VSG rats after ip saline or atropine injections. *P < 0.05 vs. saline (Bonferroni posttests). B: gastric emptying in Sham and VSG rats treated with either vehicle or exendin-4 (Exn-4). *P < 0.05 compared with saline. C: gastric emptying in wild-type (WT) or glucagon-like peptide-1 (GLP-1) receptor-deficient (GLP-1R KO) mice that underwent VSG or a sham operation. Note that gastric emptying was increased by VSG to a similar degree in both genotypes; P > 0.05 vs. WT.
Hormones released from the distal intestine, such as GLP-1, inhibit gastric emptying, a phenomenon referred to as the ileal brake. To test this effect in animals with VSG, the GLP-1R agonist exendin-4 was administered before a gavage of glucose and acetaminophen. In sham-operated rats, exendin-4 delayed gastric emptying compared with saline pretreatment, as expected (Fig. 4B). However, exendin-4 did not affect gastric emptying in VSG animals. To assess the role of endogenous GLP-1 on gastric motility after VSG, mice with targeted deletion of the GLP-1R were studied. Consistent with the pharmacological data, the absence of GLP-1R signaling did not alter gastric emptying in mice that received VSG compared with wild-type animals (Fig. 4C), implying that increased endogenous GLP-1 secretion after VSG has a minimal effect on gastric emptying.
Regulation of gastric emptying by nutrients.
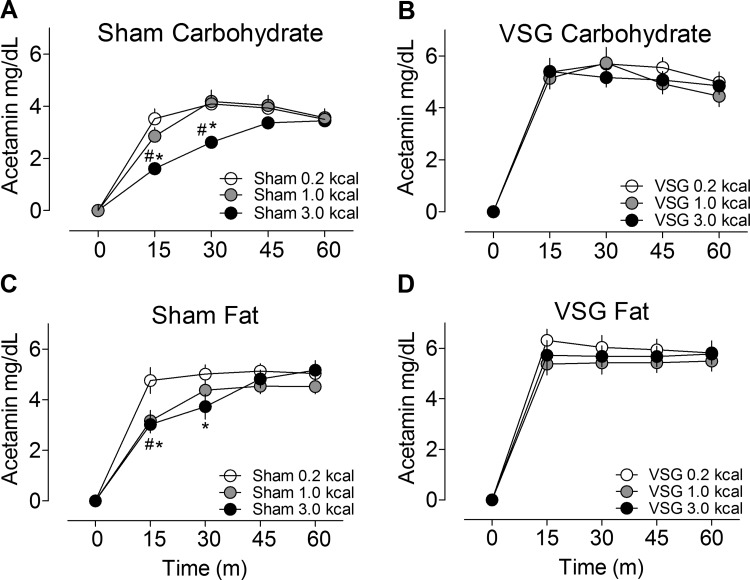
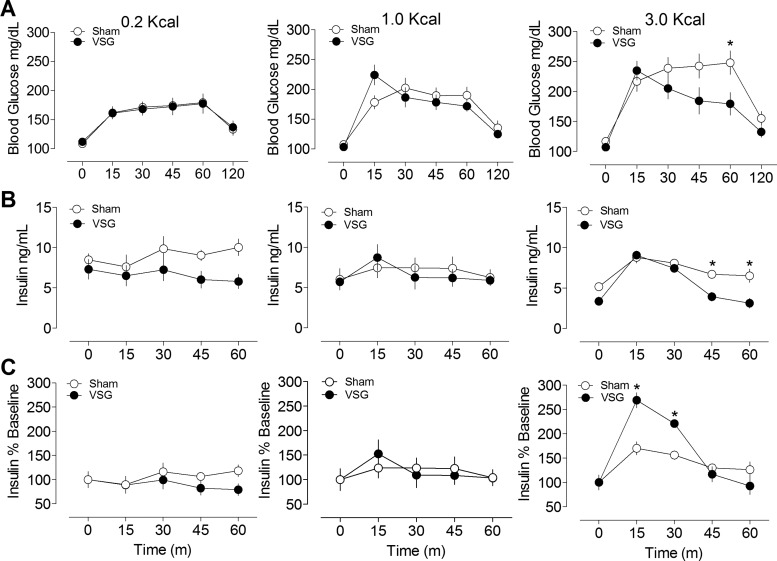
To investigate the role of nutrient density on gastric emptying, rats were given different amounts of carbohydrate or fat in a fixed volume. In sham-operated rats there was inhibition of gastric emptying with increasing density of both carbohydrate and fat calories (Fig. 5, A–D). However, in animals with VSG, gastric emptying was increased and did not differ among the caloric loads. In this set of experiments, VSG rats had greater prandial insulin secretion and lower glucose excursions than sham-operated rats at the highest carbohydrate caloric load (Fig. 6, A–F), suggesting that insulin secretion increases after these surgeries to compensate for faster emptying rates.
Fig. 5.
Gastric emptying in sham and VSG rats orally gavaged with different caloric densities of carbohydrate (A and B) or fat (C and D). #P < 0.05, 1.0 vs. 0.2 kcal; *P < 0.05, 3 vs. 0.2 kcal (Bonferroni posttests).
Fig. 6.
Blood glucose (A), plasma insulin levels (B), and %increase in insulin from baseline (C) in rats orally gavaged with different caloric densities of carbohydrate. Note the increase in early insulin secretion in VSG rats given the 3.0-kcal caloric load. *P < 0.05 compared with sham (Bonferroni posttests).
Intestinal nutrient sensing and GLP-1 secretion.
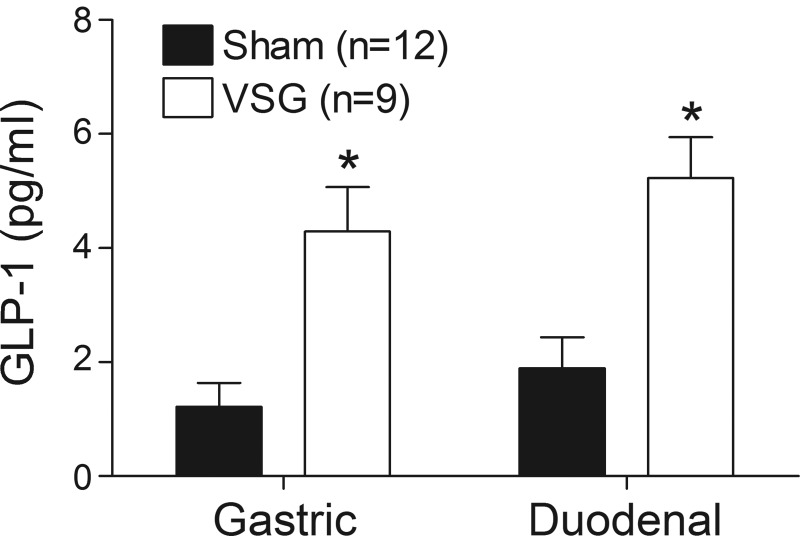
GLP-1 release is increased following bariatric surgery. To test the hypothesis that this response is determined by differences in the flux of nutrients into the intestine, equivalent concentrations of nutrients were infused directly into the duodenum of sham- and VSG-operated animals at identical rates. As expected, in rats given an oral nutrient gavage, plasma GLP-1 levels were significantly greater in VSG rats compared with controls (Fig. 7). Interestingly, the same increase was seen when nutrients were infused directly into the duodenum, indicating that intestinal factors contribute to increased GLP-1 secretion following surgery.
Fig. 7.
Plasma levels of GLP-1(7–36) in rats 10 min after receiving dextrose via oral gavage or following an intraduodenal infusion. *P < 0.05 vs. sham (Bonferroni posttests). Note that even when nutrients are infused directly into the intestine at an identical rate, GLP-1 secretion is greater in VSG rats relative to sham-operated controls.
DISCUSSION
The mechanisms underlying the success of bariatric surgery on weight loss and obesity-related comorbidities are still not clearly understood. We have developed rodent models of RYGB and VSG and in previous studies have demonstrated that these surgeries have comparable metabolic effects that are mediated through mechanisms beyond simple gastric restriction (7, 10, 11, 41, 42, 48). A central conundrum is the similar beneficial effects of RYGB and VSG, procedures that result in very different GI anatomy. In this study, we demonstrate that both VSG and RYGB also share rapid emptying of nutrients from the stomach into the intestine as a surgical effect. This was a consistent finding regardless of the technique used to assess gastric emptying rate. Moreover, we found that animals with VSG lose their response to many of the regulatory signals that normally control gastric emptying rate. It is also notable that VSG increased nutrient-induced GLP-1 secretion independent of the rate of delivery of nutrients into the intestine. We hypothesize that the chronic exposure of high-nutrient flux into the intestine enhances intestinal nutrient-sensing and ultimately the enhanced metabolic effects of the surgery.
Faster gastric emptying rates after VSG may result from the loss of receptive relaxation and the accommodation reflexes, a process whereby the stomach receives increasing volumes without concomitant increases in intragastric pressure (19). This reservoir function is specific to the proximal stomach, and removal of this region greatly increases intragastric pressure, resulting in accelerated gastric emptying (19). Therefore, it is possible that a similar process is responsible for the accelerated gastric emptying produced by VSG. Consistent with this hypothesis, patients that undergo subtotal gastrectomy to remove tumors or treat intractable peptic ulcers also display a pattern of accelerated glucose appearance and greater insulin secretion during meals (31, 35). Such patients are generally not obese, and changes in nutrient handling are regarded as an unwanted but tolerable side effect (31, 35). More recently, however, therapeutic potential for accelerated gastric emptying has been identified (28, 46). Therefore, in the present study we examined how changes in gastric emptying are potentially involved in the alleviation of metabolic derangements after bariatric surgery.
Although scintigraphy is a powerful method for assessing gastric emptying, it is a technique that may not be available to all investigators or for all experimental designs in rodent models. Therefore, we compared three different biochemical tracers as a means of indirect assessment of gastric emptying and the presentation of nutrients in plasma and with critical organs such as the liver and pancreas. Plasma levels of [14C]glucose and acetaminophen, but not d-xylose, followed the expected trends of decreased gastric emptying with atropine and increased gastric emptying with carbachol and bariatric surgery, whereas plasma d-xylose levels did not discriminate between pharmacological and surgical manipulations on gastric function. A key reason for this may be because there is gastric absorption of d-xylose in rats (45), which would tend to blur differences in gastric emptying under the conditions of the experiments presented here. In fact, in all models tested in these studies, the rate of d-xylose absorption was constant among groups, consistent with uptake starting at meal delivery and continuing through the course of observation. Hence, although d-xylose provides a surrogate for gastric emptying in human studies (36), it appears that acetaminophen, which is poorly absorbed in the stomach, is a superior nonradioactive indicator of gastric emptying in rats.
As expected, postmortem gastric pressure was elevated after VSG. To determine the extent to which increased gastric pressure contributed to faster emptying rates, we studied how various volumes of a viscous, noncaloric infusate affected gastric emptying in rats. To keep the conditions of the experiment as physiologically relevant as possible, volumes were chosen based on what rats consumed voluntarily (11, 43). Increased plasma levels of [14C]glucose with increased oral volumes in VSG rats are consistent with the notion that pressure effects contribute to faster gastric emptying. Our data are supported by multiple clinical studies that demonstrate that gastric pressure (50) and gastric emptying of liquids (2, 4), semisolids (29, 30), and solids (4, 27, 28, 40) are increased in patients with VSG compared with controls. What is surprising about these data is the extent to which these changes in nutrient handling overlap with RYGB.
Luminal perfusion of the intestine with acid, carbohydrate, lipid, protein, amino acids, or high-osmolality solutions decreases gastric motility and delays gastric emptying (6). In isolation the stomach does not respond to differences in caloric density or macronutrient composition. Rather, caloric composition in this context is sensed within the intestine (6, 16). This information is crucial for normal sequences of meal termination and is relayed to the stomach by intestinal peptide secretions that act as hormones as well as by the enteric and central nervous systems to control the rate of emptying (9). Roughly one-third of all calories empty the stomach prior to satiation (17, 47). In humans, accelerating gastric emptying pharmacologically reduces meal size (46). Hence, faster gastric emptying after VSG and RYGB provides a plausible physiological basis for reductions in food intake after these procedures.
Unlike sham-operated controls, VSG rats were unable to slow gastric emptying in response to increasing caloric densities of carbohydrate or fat. Pharmacological manipulation of muscarinic or GLP-1 receptors also failed to slow gastric emptying in VSG animals. Moreover, these effects were observed using a low-volume infusion that did not affect gastric emptying rate per se in VSG animals, implying that the removal of the gastric musculature and the neural networks contained within eliminates key factors that typically slow nutrient entry into intestine. There has been much speculation that rapid nutrient delivery directly into the midjejunum and beyond accounts for the tremendous increase in GLP-1 secretion after RYGB (12, 21, 25). Given the rapid gastric emptying that we observed in our study, the same could be hypothesized for VSG. In fact, we noted that both gastric emptying and plasma GLP-1 were greater in VSG animals given a gastric gavage of nutrients compared with shams. Interestingly, we found that increases in plasma GLP-1 also occurred when dextrose was infused directly into the duodenum of VSG animals, demonstrating that, in addition to faster gastric emptying, VSG results in intestinal effects that are independent of gastric function, possibly reflecting enhanced nutrient sensing at the level of the intestine. In support of this hypothesis, prandial levels of other hormones whose secretion is nutrient dependent are also increased after VSG, including PYY (14, 32, 33), cholecystokinin (32), gastric inhibitory polypeptide (44), and others (8, 14).
Limitations of our study include the focus on liquid nutrient and nonnutritive meals. Gastric digestion and emptying of solids are more complex and more prolonged. However, there are several advantages to studying gastric emptying in the liquid-phase. The labeling of solid meal components and lack of homogenous mixing of gastric contents can adversely affect measurements. Furthermore, the presence of a prolonged lag phase after solid meal ingestion can create situations in which the rate of gastric secretion may be greater than the rate of gastric emptying (15, 20). Secretion volumes are not trivial, especially given the sensitivity of VSG to volume-related increases in gastric emptying. Such confounds provide less than ideal conditions in which to study sensitive measurements of neural and endocrine influences on gastric emptying, as was the focus of our study. Moreover, others have already demonstrated that gastric emptying of solids is increased following VSG in humans (4, 28, 40) and in a rat (26) model of this procedure.
In summary, we report herein that animals with VSG and RYGB have similar and rapid gastric emptying compared with animals given a sham operation. Of the chemical techniques we used, acetaminophen may be the best indicator of gastric emptying in rodents since it can be done in conscious animals, is simple to perform, and requires minimal blood. Enhanced nutrient sensing and early satiety after VSG may be a physiological response to a perpetually faster gastric emptying rate. However, this hypothesis will need to be carefully evaluated. What is interesting is that despite the rapid nutrient entry into the system after VSG (and RYGB), overall glucose homeostasis is drastically and rapidly improved (1, 7, 8, 10, 18, 33). The sophistication of these outcomes challenges traditional explanations of VSG as a purely restrictive procedure.
GRANTS
This work was supported by grants from the National Institutes of Health (DK-082480 to D. A. Sandoval, DK-093848 to R. J. Seeley, DK-017844 to S. C. Woods, DK-57900 to D. A. D'Alessio, and IF32-HD-68103 to B. E. Grayson). A. P. Chambers has a Canadian Institutes of Health Research fellowship. D. A. Sandoval and R. J. Seeley are supported by a grant from Ethicon Endo-Surgery.
DISCLOSURES
A. P. Chambers, E. P. Smith, D. P. Begg, B. E. Grayson, S. Sisley, T. Greer, J. Sorrell, L. Lemmen, K. LaSance, and S. C. Woods have no conflicts of interest to declare. R. J. Seeley is a paid speaker for Ethicon Endosurgery, Novo Nordisk, and Merck. Dr. Seeley also provides research support for Ethicon Endosurgery, Novo Nordisk, Ablaris, Boehringer-Ingelheim, and Zealand, is a paid consultant for Ethicon Endosurgery, Novo Nordisk, Novartis, Angiochem, Takeda, Boehringer-Ingelheim, Eisai, Forest Pharmaceuticals, and Giuvadan, and receives equity from Zafgen. D. A. D'Alessio receives grant funding from Ethicon Endo-Surgery. Also, D. A. D'Alessio serves on a scientific advisory board at Ethicon Endo-Surgery, is a consultant for Givaudan, and receives grant funding from Ethicon Endo-Surgery, Boehringer-Ingelheim, and Novo Nordisk.
AUTHOR CONTRIBUTIONS
A.P.C., E.P.S., D.P.B., L.L., K.L., S.C.W., R.J.S., D.A.D., and D.A.S. contributed to the conception and design of the research; A.P.C., E.P.S., D.P.B., B.E.G., S.S., T.G., J.S., L.L., K.L., and D.A.S. performed the experiments; A.P.C., E.P.S., L.L., K.L., and D.A.S. analyzed the data; A.P.C., E.P.S., D.P.B., L.L., K.L., S.C.W., R.J.S., D.A.D., and D.A.S. interpreted the results of the experiments; A.P.C., L.L., K.L., and D.A.S. prepared the figures; A.P.C., D.A.D., and D.A.S. drafted the manuscript; A.P.C., E.P.S., D.P.B., B.E.G., S.S., T.G., J.S., L.L., K.L., S.C.W., R.J.S., D.A.D., and D.A.S. edited and revised the manuscript; A.P.C., E.P.S., D.P.B., B.E.G., S.S., T.G., J.S., L.L., K.L., S.C.W., R.J.S., D.A.D., and D.A.S. approved the final version of the manuscript.
REFERENCES
- 1.Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F, Basso N. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc 24: 1005–1010, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Baumann T, Kuesters S, Grueneberger J, Marjanovic G, Zimmermann L, Schaefer AO, Hopt UT, Langer M, Karcz WK. Time-resolved MRI after ingestion of liquids reveals motility changes after laparoscopic sleeve gastrectomy—preliminary results. Obes Surg 21: 95–101, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, Gastaldelli A, Chambers KT, Su X, Okunade A, Patterson BW, Klein S. Gastric bypass and banding equally improve insulin sensitivity and beta cell function. J Clin Invest 122: 4667–4674, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braghetto I, Davanzo C, Korn O, Csendes A, Valladares H, Herrera E, Gonzalez P, Papapietro K. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg 19: 1515–1521, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122: 248–256e5, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Calbet JA, MacLean DA. Role of caloric content on gastric emptying in humans. J Physiol 498: 553–559, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Pérez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141: 950–958, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschöp MH, Sandoval DA, Seeley RJ. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology 144: 50–52e5, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers AP, Sandoval DA, Seeley RJ. Integration of satiety signals by the central nervous system. Curr Biol 23: R379–R388, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers AP, Stefater MA, Wilson-Perez HE, Jessen L, Sisley S, Ryan KK, Gaitonde S, Sorrell JE, Toure M, Berger J, D'Alessio DA, Sandoval DA, Seeley RJ, Woods SC. Similar effects of roux-en-Y gastric bypass and vertical sleeve gastrectomy on glucose regulation in rats. Physiol Behav 105: 120–123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers AP, Wilson-Perez HE, McGrath S, Grayson BE, Ryan KK, D'Alessio DA, Woods SC, Sandoval DA, Seeley RJ. Effect of vertical sleeve gastrectomy on food selection and satiation in rats. Am J Physiol Endocrinol Metab 303: E1076–E1084, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab 89: 2608–2615, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Dent J, Sun WM, Anvari M. Modulation of pumping function of gastric body and antropyloric contractions. Dig Dis Sci 39: 28S–31S, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Dimitriadis E, Daskalakis M, Kampa M, Peppe A, Papadakis JA, Melissas J. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann Surg 257: 647–654, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Goetze O, Treier R, Fox M, Steingoetter A, Fried M, Boesiger P, Schwizer W. The effect of gastric secretion on gastric physiology and emptying in the fasted and fed state assessed by magnetic resonance imaging. Neurogastroenterol Motil 21: 725–742, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Hunt JN, Stubbs DF. The volume and energy content of meals as determinants of gastric emptying. J Physiol 245: 209–225, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan JM, Siemers WH, Smedh U, Schwartz GJ, Grill HJ. Gastric branch vagotomy and gastric emptying during and after intragastric infusion of glucose. Am J Physiol Regul Integr Comp Physiol 273: R1786–R1792, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 247: 401–407, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Kelly KA. Gastric emptying of liquids and solids: roles of proximal and distal stomach. Am J Physiol Gastrointest Liver Physiol 239: G71–G76, 1980 [DOI] [PubMed] [Google Scholar]
- 20.Kwiatek MA, Menne D, Steingoetter A, Goetze O, Forras-Kaufman Z, Kaufman E, Fruehauf H, Boesiger P, Fried M, Schwizer W, Fox MR. Effect of meal volume and calorie load on postprandial gastric function and emptying: studies under physiological conditions by combined fiber-optic pressure measurement and MRI. Am J Physiol Gastrointest Liver Physiol 297: G894–G901, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Laferrere B. Do we really know why diabetes remits after gastric bypass surgery? Endocrine 40: 162–167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakdawala MA, Bhasker A, Mulchandani D, Goel S, Jain S. Comparison between the results of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass in the Indian population: a retrospective 1 year study. Obes Surg 20: 1–6, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Maida A, Lovshin JA, Baggio LL, Drucker DJ. The glucagon-like peptide-1 receptor agonist oxyntomodulin enhances beta-cell function but does not inhibit gastric emptying in mice. Endocrinology 149: 5670–5678, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Marceau P, Biron S, St Georges R, Duclos M, Potvin M, Bourque RA. Biliopancreatic Diversion with Gastrectomy as Surgical Treatment of Morbid Obesity. Obes Surg 1: 381–387, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Mason EE. Ileal [correction of ilial] transposition and enteroglucagon/GLP-1 in obesity (and diabetic?) surgery. Obes Surg 9: 223–228, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Masuda T, Ohta M, Hirashita T, Kawano Y, Eguchi H, Yada K, Iwashita Y, Kitano S. A comparative study of gastric banding and sleeve gastrectomy in an obese diabetic rat model. Obes Surg 21: 1774–1780, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Melissas J, Daskalakis M, Koukouraki S, Askoxylakis I, Metaxari M, Dimitriadis E, Stathaki M, Papadakis JA. Sleeve gastrectomy-a “food limiting” operation. Obes Surg 18: 1251–1256, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Melissas J, Koukouraki S, Askoxylakis J, Stathaki M, Daskalakis M, Perisinakis K, Karkavitsas N. Sleeve gastrectomy: a restrictive procedure? Obes Surg 17: 57–62, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Melissas J, Leventi A, Klinaki I, Perisinakis K, Koukouraki S, de Bree E, Karkavitsas N. Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study. Ann Surg 258: 976–982, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Michalsky D, Dvorak P, Belacek J, Kasalicky M. Radical resection of the pyloric antrum and its effect on gastric emptying after sleeve gastrectomy. Obes Surg 23: 567–573, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Muir A. Postgastrectomy syndromes. Br J Surg 37: 165–178, 1949 [DOI] [PubMed] [Google Scholar]
- 32.Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, Kern B, von Fluee M, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg 22: 740–748, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterli R, Wolnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flue M, Beglinger C. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg 250: 234–241, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Rodieux F, Giusti V, D'Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 16: 298–305, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Roth DA, Meade RC. Hyperinsulinism-hypoglycemia in the postgastrectomy patient. Diabetes 14: 526–528, 1965 [DOI] [PubMed] [Google Scholar]
- 36.Salehi M, Vahl TP, D'Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab 93: 4909–4916, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366: 1567–1576, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schirra J, Katschinski M, Weidmann C, Schäfer T, Wank U, Arnold R, Göke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 97: 92–103, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seimon RV, Brennan IM, Russo A, Little TJ, Jones KL, Standfield S, Wishart JM, Horowitz M, Feinle-Bisset C. Gastric emptying, mouth-to-cecum transit, and glycemic, insulin, incretin, and energy intake responses to a mixed-nutrient liquid in lean, overweight, and obese males. Am J Physiol Endocrinol Metab 304: E294–E300, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Shah S, Shah P, Todkar J, Gagner M, Sonar S, Solav S. Prospective controlled study of effect of laparoscopic sleeve gastrectomy on small bowel transit time and gastric emptying half-time in morbidly obese patients with type 2 diabetes mellitus. Surg Obes Relat Dis 6: 152–157, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Stefater MA, Pérez-Tilve D, Chambers AP, Wilson-Pérez HE, Sandoval DA, Berger J, Toure M, Tschöp M, Woods SC, Seeley RJ. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 138: 2426–2436, 2436.e1–2436.e3, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefater MA, Sandoval DA, Chambers AP, Wilson-Pérez HE, Hofmann SM, Jandacek R, Tso P, Woods SC, Seeley RJ. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology 141: 939–949e1–4, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stefater MA, Wilson-Pérez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev 33: 595–622, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stemmer K, Bielohuby M, Grayson BE, Begg DP, Chambers AP, Neff C, Woods SC, Erben RG, Tschöp MH, Bidlingmaier M, Clemens TL, Seeley RJ. Roux-en-Y gastric bypass surgery but not vertical sleeve gastrectomy decreases bone mass in male rats. Endocrinology 22: 140–151, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stradley R, Farmer-Bailey P, Pasquini N, Rogers W, Sherding R. Gastric absorption of d-xylose in the rat: its influence on the d-xylose absorption test. J Lab Clin Med 107: 10–14, 1986 [PubMed] [Google Scholar]
- 46.Torra S, Ilzarbe L, Malagelada JR, Negre M, Mestre-Fusco A, Aguadé-Bruix S, Florensa E, Suñé P, Gras B, Hernandez JJ, Casamitjana R, Garcia MA, Ros FB, Delgado-Aros S. Meal size can be decreased in obese subjects through pharmacological acceleration of gastric emptying (The OBERYTH trial). Int J Obes (Lond) 35: 829–837, 2011 [DOI] [PubMed] [Google Scholar]
- 47.van der Velde P, Koslowsky I, Koopmans HS. Measurement of gastric emptying during and between meal intake in free-feeding Lewis rats. Am J Physiol Regul Integr Comp Physiol 276: R597–R605, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Wilson-Pérez HE, Chambers AP, Sandoval DA, Stefater MA, Woods SC, Benoit SC, Seeley RJ. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes (Lond) 37: 288–295, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr 133: 1081–1087, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Yehoshua RT, Eidelman LA, Stein M, Fichman S, Mazor A, Chen J, Bernstine H, Singer P, Dickman R, Beglaibter N, Shikora SA, Rosenthal RJ, Rubin M. Laparoscopic sleeve gastrectomy—volume and pressure assessment. Obes Surg 18: 1083–1088, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, Berthoud HR. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol 297: R1273–R1282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]