Abstract
Previously, we reported that low-dose leptin infusions into the fourth ventricle produced a small but significant increase in body fat. These data contrast with reports that injections of higher doses of leptin into the fourth ventricle inhibit food intake and weight gain. In this study, we tested whether exogenous leptin in the fourth ventricle opposed or contributed to weight loss caused by third ventricle leptin infusion by blocking diffusion of CSF from the third to the fourth ventricle. Male Sprague-Dawley rats received third ventricle infusions of PBS or 0.3 μg leptin/24 h from miniosmotic pumps. After 4 days, rats received a 3-μl cerebral aqueduct injection of saline or of thermogelling nanoparticles (hydrogel) that solidified at body temperature. Third ventricle leptin infusion inhibited food intake and caused weight loss. Blocking the aqueduct exaggerated the effect of leptin on food intake and weight loss but had no effect on the weight of PBS-infused rats. Leptin reduced both body fat and lean body mass but did not change energy expenditure. Blocking the aqueduct decreased expenditure of rats infused with PBS or leptin. Infusion of leptin into the third ventricle increased phosphorylated STAT3 in the VMHDM of the hypothalamus and the medial NTS in the hindbrain. Blocking the aqueduct did not change hypothalamic p-STAT3 but decreased p-STAT3 in the medial NTS. These results support previous observations that low-level activation of hindbrain leptin receptors has the potential to blunt the catabolic effects of leptin in the third ventricle.
Keywords: food intake, body composition, calorimetry, signal transducer and activator of transcription 3
leptin, a cytokine that is released predominantly from white adipose tissue, is hypothesized to function as a negative feedback signal in the regulation of energy balance (36). It is well established that central or peripheral administration of leptin to normal-weight animals inhibits food intake and causes weight loss (13). There are multiple isoforms of the leptin receptor (34), but the isoform with a long intracellular domain (Lepr) has been associated with the impact of leptin on energy balance (5). Activation of Lepr results in phosphorylation, dimerization, and translocation of the transcription factor signal transducer and activator of transcription 3 (STAT3) to the nucleus (1), and this has been reported to be critical for leptin's effects on energy balance (3). Lepr is expressed at low levels in most peripheral tissues and in multiple areas of the brain. There are higher levels of expression in hypothalamic nuclei and in the nucleus tractus solitarius (NTS) in the hindbrain (4, 31).
A large number of studies examining the effects of leptin on energy balance have focused on the role of Lepr located in the hypothalamus, but there is increasing evidence that hindbrain Lepr also has the ability to modify food intake, body weight, and body temperature (12). These receptors are located in sites that integrate signals related to long-term energy balance with vagal afferent gastrointestinal signals of satiety (24) and peripheral signals of energy status (29). It appears that leptin enhances the inhibitory effect of some of these signals on food intake. In direct contrast to these studies, data from chronic decerebrate rats, in which a surgical transection is made to neurally isolate the caudal brainstem from the forebrain, indicate that loss of neural efferent information related to leptin activation of the forebrain not only prevents peripheral leptin from inducing weight loss but increases adiposity by suppressing energy expenditure (18). These data suggest that selective activation of hindbrain Lepr produces a state of positive energy balance. The observations from the study with chronic decerebrate rats were subsequently confirmed when low doses of leptin infused into the fourth ventricle produced a small but significant increase in body fat and when fourth ventricle infusion of a leptin receptor antagonist caused a significant reduction in body fat (16). Infusion of the antagonist did not modify the weight loss caused by peripheral leptin infusions, and therefore, the catabolic effect of blocking Lepr in the hindbrain was present only in baseline, nonstimulated conditions (16).
When leptin is injected or infused into the third ventricle, it has the potential to diffuse through the ventricular system and activate hindbrain Lepr in addition to those in the hypothalamus that are the intended target of leptin administration. The objective of this study was to test whether inadvertent activation of hindbrain Lepr in rats receiving third ventricle infusions of leptin contributed to or blunted the catabolic action of leptin. This was achieved by blocking the aqueduct of the rats to prevent flow of cerebrospinal fluid (CSF) from the third to the fourth ventricle using a thermogelling nanoparticle suspension (hydrogel) that was liquid at room temperature but solid at body temperature (22, 25).
METHODS
Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were housed with lights on for 12 h each day from 0700. Initially, they were individually housed in wire mesh cages. The animals had free access to chow (LabDiet 5012; PMI Nutrition International) and water throughout the study. Each rat had a Nylabone (Nylabone Products) in their cage for enrichment. All animal procedures were approved by the Institutional Animal Care Use Committee of Georgia Regents University. After 4–7 days of adaptation to the environment, each rat was fitted with a third ventricle infusion cannula and an injection guide cannula in the aqueduct. Coordinates for cannula placement were on the midline of a level skull and −2.8 mm anteroposterior, −9.0 mm ventral to the bregma for the third ventricle and −8.0 mm anterioposterior, and 0.0 mm lateral and −5.0 ventral to the bregma for the aqueduct (28). Five to seven days after surgery, the rats weighed ∼310 g and were individually housed in an indirect calorimeter (TSE LabMaster, Metabolic Research Platform; TSE Systems International), with cage temperature maintained at 20–21°C. Oxygen consumption, carbon dioxide production, and activity were measured on each cage once every 39 min. Each morning, the calorimeter was stopped at the end of a cycle of measurement between 7:15 and 7:30 AM. Food hoppers and water bottles were refilled and the rats weighed. Cage bedding was changed every 2nd day. The calorimeter was restarted at 8 AM.
After 1 day of acclimation, baseline energy expenditure, respiratory exchange ratio (RER), activity, and food intake were measured for 3 days. On the 4th day the rats were divided into two weight-matched groups, and a Model 1002 Alzet pump (Durect) was attached to the third ventricle infusion cannula to deliver PBS or 0.3 μg leptin/24 h (rat recombinant leptin; R & D Systems) in a volume of 0.25 μl/h. On the 4th day of infusion, rats in each treatment group were subdivided into two groups that received a 3-μl injection of either saline or hydrogel (lot no. 256-3) into the aqueduct over a period of 3 min, using an infusion pump (PHD 2000 infusion pump; Harvard Apparatus). This resulted in four treatment groups: PBS-saline, PBS-hydrogel, leptin-saline, and leptin-hydrogel. Hydrogel is a suspension of thermogelling nanoparticles that is liquid at room temperature but solid at body temperature (22). It was provided as a generous gift by the late Dr. Zhibing Hu, Department of Physics, University of North Texas, Denton, TX. The suspension was autoclaved and held on ice before injection. The tubing connecting the Hamilton syringe to the injector was also surrounded by ice during the injection.
On the 7th day of infusion, food was removed from the cages at 7 AM, and rats were decapitated between 10 AM and 12 PM. Trunk blood was collected for measurement of serum leptin (rat leptin RIA kit; Millipore), insulin (rat insulin RIA kit; Millipore), and glucose (glucose assay kit; Sigma Chemical). Inguinal, epididymal, retroperitoneal, and mesenteric white fat depots and intrascapular brown fat (IBAT) were dissected and weighed. The liver was dissected and weighed. IBAT and a sample of inguinal fat were snap-frozen for determination of uncoupling protein 1 (UCP1) protein content by Western blot (rabbit polyclonal to UCP1, ab23841; Abcam), as described previously (17). Tissue blocks of the hypothalamus or brainstem were collected, and phosphorylated STAT3 (p-STAT3), suppressor of cytokine signaling 3 (SOCS3), p-ERK1, and p-ERK2 were measured by Western blot, as described previously (6). All primary antibodies were obtained from Cell Signaling Technology. All other tissues were added back to the carcass, which was analyzed for composition, as described previously (15).
The study was completed using cohorts of rats because there were only 12 calorimetry cages. A total of 40 rats entered the study, one PBS-saline rat was excluded based on a failure to eat >5 g of food during the 72 h following the aqueduct injection. Data from four additional rats were excluded because food intake during the last 72 h of the study was greater than two standard deviations from the mean for their experimental group (1 PBS-saline, two leptin-saline, one leptin-hydrogel).
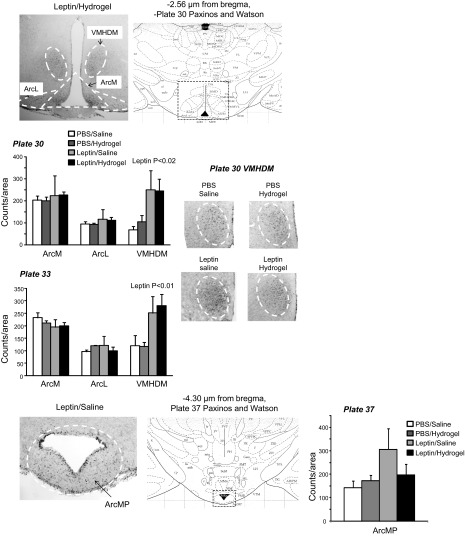
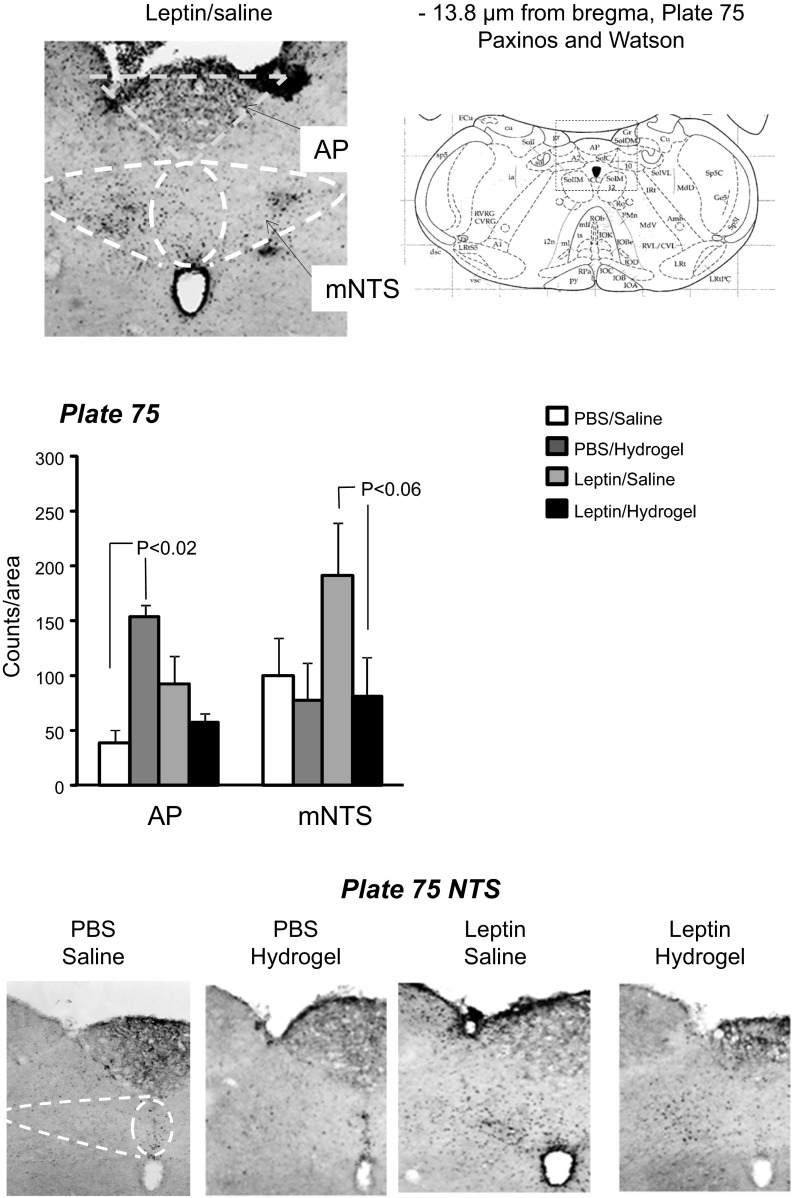
A second set of 16 rats (4 rats/group) was used to detect p-STAT3 in the hypothalamus and hindbrain by immunohistochemistry. The rats were cannulated and treated as described above, except that they were housed in hanging wire mesh cages for the duration of the experiment. There were four treatment groups: PBS-saline, PBS-hydrogel, leptin-saline, and leptin-hydrogel. On the last day of the study, the rats were anesthetized with ketamine-xylazine (90 mg/kg, 10 mg/kg). CSF was collected by puncturing the cistern magna with a 23G vein butterfly set, and leptin concentration was measured by ELISA (Leptin Quantikine ELISA kit; R & D Systems). The rats were transcardially perfused with 150 ml of ice-cold heparinized saline, followed by 250 ml of 4% paraformaldehyde and then 100 ml of heparinized saline. The brain was removed and held in 4% paraformaldehyde at 4°C overnight before being transferred to 25% sucrose and 1% sodium azide solution and held at 4°C until sectioned. Thirty-micrometer sections were made through hypothalamic and hindbrain tissue, and every fourth section was used for p-STAT3 immunostaining of free-floating sections, as described previously (14). The specificity of the antibody was confirmed by running three sets of sections without primary antibody. None of these sections stained positive for p-STAT3 (data not shown). Images were taken using an Olympus BX51 microscope. The p-STAT3 was quantified by manual counting in the following areas: dorsomedial ventromedial hypothalamic nucleus (VMHDM), medial arcuate nucleus (ArcM), and lateral arcuate nucleus (ArcL) at −2.56 and −3.30 μm from the bregma [plates 30 and 33 of the Paxinos and Watson (28) rat brain atlas], medial posteriorarcuate nucleus (ArcMP) at −4.30 μm from the bregma [plate 37 of Paxinos and Watson (28) rat brain atlas], area postrema (AP), and medial NTS at −13.80 μm from the bregma [plate 75 of the Paxinos and Watson (28) rat brain atlas]. The NTS adjacent to the AP was the only area of the hindbrain that showed p-STAT3. Others have reported additional Lepr expression at a more rostral site in rats injected intraperitoneally (ip) with 4 mg leptin/kg (23) and in mice injected with 5 mg leptin/kg ip. (8). The difference in p-STAT3 distribution may be due to the method of leptin administration (acute peripheral injection vs. chronic central infusion) or the amount of leptin delivered.
Hydrogel placement.
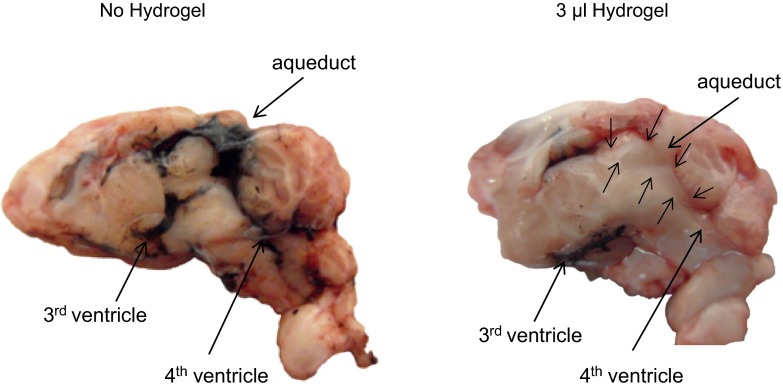
The volume of hydrogel needed to effectively block the aqueduct was tested in rats fitted with aqueduct guide cannulae. One week after surgery, the rats were anesthetized with ketamine-xylazine and injected with 2–8 μl of hydrogel. Thirty minutes later, 3 μl of india ink was injected into the third ventricle, using the coordinates described above for placement of third ventricle infusion cannula. Ten minutes after the ink injection, the rat was decapitated, the brain was removed under a stream of warm air to ensure that the hydrogel remained solid, and the brain was cut along the midline to determine the location of the ink. Three microliters of hydrogel was the minimal volume needed to prevent leakage of ink from the third ventricle into the cerebral aqueduct and the fourth ventricle, as shown in Fig. 1.
Fig. 1.
Cross-sections along the midline of the brains from rats that received an aqueduct injection of 3 μl of saline (left) or hydrogel (right). Thirty minutes later, 3 μl of india ink was injected into the 3rd ventricle, and the rats were euthanized after an additional 10 min. Small arrows indicate the location of the hydrogel.
Data analysis.
Statistically significant differences between treatment groups were determined using Statistica software version 9.0 (StatSoft, Tulsa, OK). Differences were considered significant at P < 0.05. Daily measurements of food intake, body weight, energy expenditure, RER, and activity were compared by repeated-measures analysis of variance. Single end point measurements and measurements at a specific time point were compared by two-way ANOVA, with third ventricle infusion and aqueduct injection as the dependent variables. Repeated-measures analysis was used to compare the same animals at different time points during the experiment. Post hoc differences were determined using Duncan's multiple range test.
RESULTS
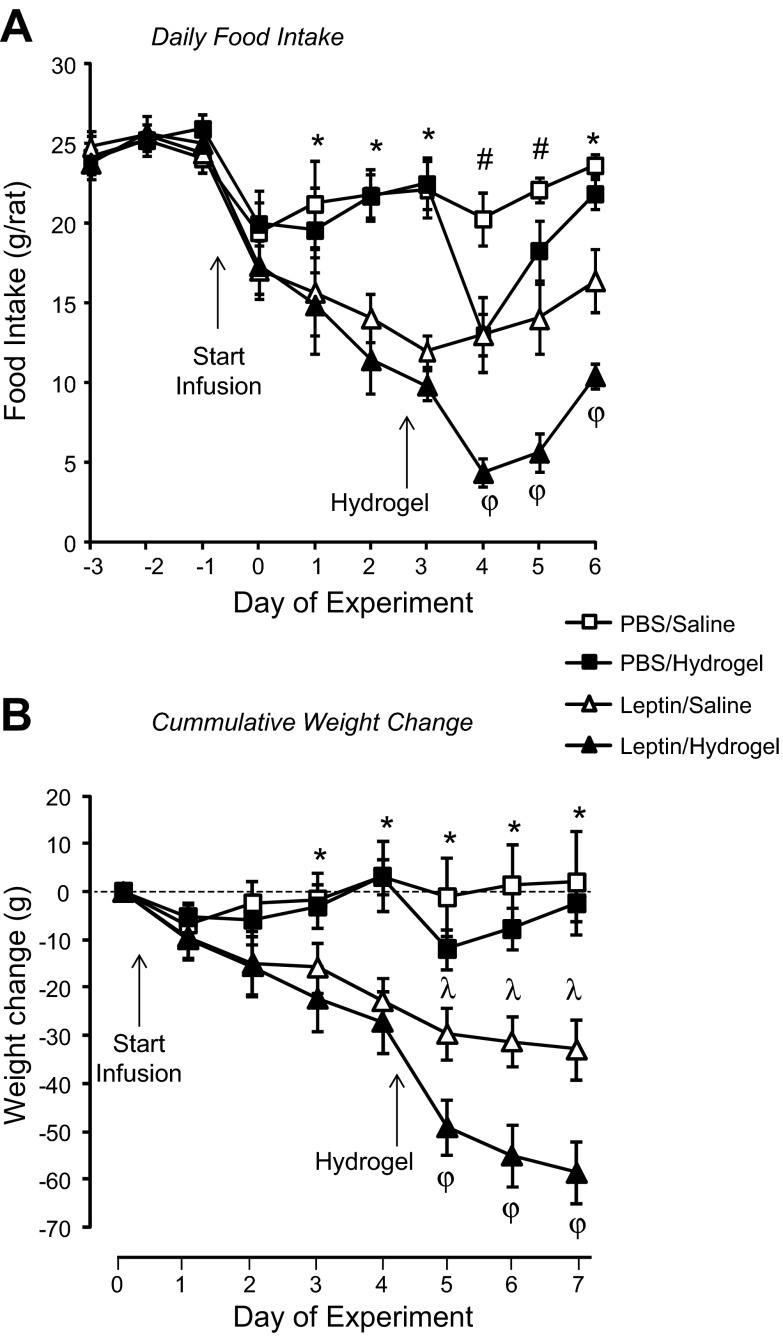
There were no differences in food intake of the different groups of rats during the baseline period (Fig. 2). There was no effect of PBS infusion on food intake during the first 3 days of third ventricle infusion, but injection of hydrogel into the aqueduct caused a transient 48-h inhibition of food intake in PBS infused rats (Fig. 2). Third ventricle infusion of 0.3 μg leptin/24 h caused a progressive decline in food intake of all of the rats during the first 3 days of infusion (Fig. 2). During the last 3 days of the experiment, the intake of leptin-saline rats showed a trend (P < 0.06) to increase compared with the first 3 days of leptin infusion (Fig. 2). Leptin-hydrogel rats ate less than leptin-saline rats on each of the last 3 days of the study [P (F): leptin P < 0.0001 (52), hydrogel P < 0.01 (7), day P < 0.0001 (7), leptin × day P < 0.001 (4), hydrogel × day P < 0.001 (4); Fig. 2].
Fig. 2.
Daily food intake (A) and weight change (B) of rats receiving 3rd ventricle infusions of PBS or 0.3 μg leptin/24 h. Data are means ± SE for groups of 7–9 rats. *Significant difference (P < 0.05) between PBS- and leptin-infused rats; #PBS-saline rats are different from all other groups; λleptin-saline rats are different from all other groups; φleptin-hydrogel rats are different from all other groups.
Rats receiving third ventricle infusions of PBS neither gained nor lost weight (Fig. 2). By contrast, there was continuous and significant weight loss in all rats that received leptin infusions (Fig. 2). Injection of hydrogel caused weight loss in all rats compared with their respective controls. For the PBS-infused animals, a 10-g weight loss on the day after hydrogel injection was recovered by the end of the experiment. Hydrogel exaggerated the rate of weight loss in leptin-infused rats such that weight loss in leptin-hydrogel rats was significantly greater than that of leptin-saline rats on all 3 days following hydrogel injection (Fig. 2) [P (F): leptin P < 0.001 (19), hydrogel not significant (NS), day P < 0.0001 (22), leptin × day P < 0.0001 (26), hydrogel × day P < 0.0001 (6)].
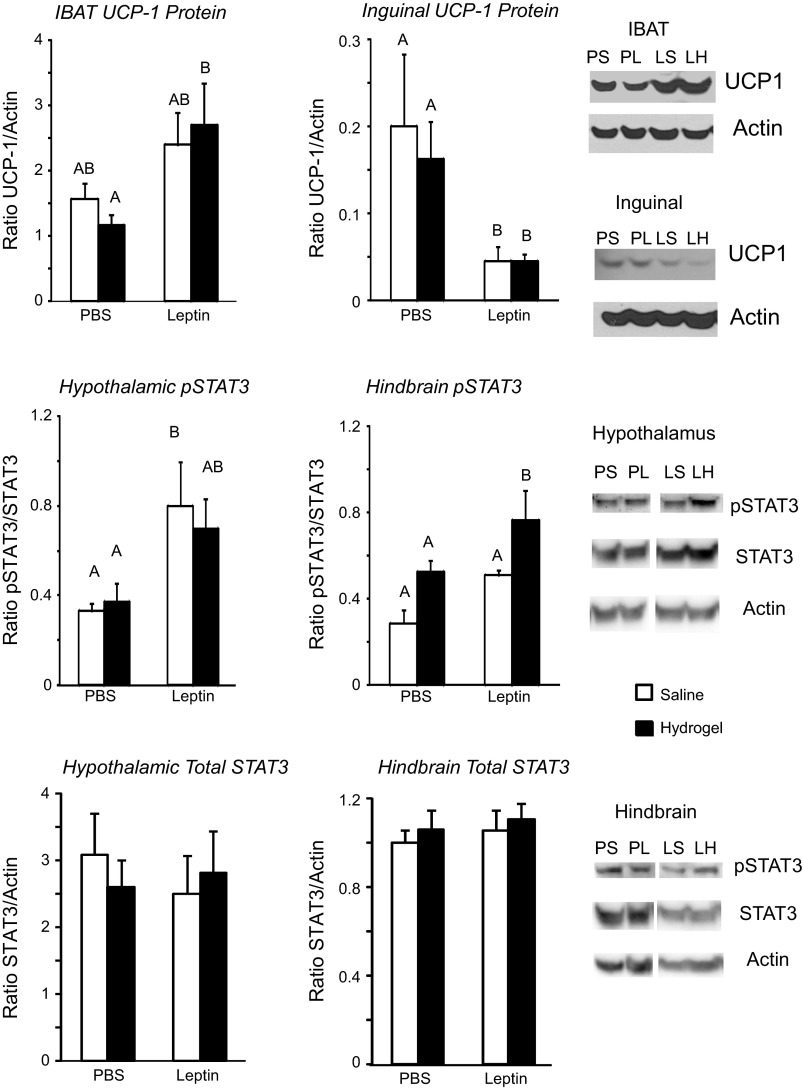
RER is an indirect measurement of the type of nutrient being oxidized for energy and followed changes in food intake. RER during the light phase was inhibited by leptin infusion and changed with time over the experiment, but there was no independent effect of hydrogel injection [P (F): leptin P < 0.0005 (15), hydrogel NS, day P < 0.0001 (13), leptin × day P < 0.0001 (9)]. Similar effects were found during the dark phase, when there also was an interaction between hydrogel injection and time [P (F): leptin P < 0.001 (13), hydrogel NS, day P < 0.0001 (5), leptin × day P < 0.0005 (4), hydrogel × day P < 0.05 (2)]. Post hoc analysis showed that although RER was lower in leptin-infused than in PBS-infused rats both before and after hydrogel injection (Table 1), there was no difference between saline-injected and hydrogel-injected rats within either the PBS- or leptin-treated groups. Total daily energy expenditure (Table 1) was not different between groups during the baseline period, and there was no consistent effect of third ventricle leptin infusion. Blocking the aqueduct with hydrogel inhibited energy expenditure in both PBS- and leptin-infused rats compared with their expenditure before hydrogel injection. However, there were no significant differences in expenditure of PBS-hydrogel rats compared with PBS-saline rats or between leptin-hydrogel rats and leptin-saline rats. Expenditure of leptin-saline rats was higher than that of PBS-hydrogel rats on day 6. [P (F): leptin P < 0.01 (7), hydrogel NS, day P < 0.0001 (5), leptin × day P < 0.02 (2), hydrogel × day P < 0.05 (2)]. There were no differences in total daily activity or in activity during the light or dark phase (data not shown) at any time during the experiment. Despite the similarities in energy expenditure, IBAT UCP1 protein measured at the end of the experiment was significantly increased in leptin-infused rats, but there was no effect of hydrogel injection (Fig. 3). Inguinal UCP1 protein showed the opposite response and was significantly inhibited in leptin-infused rats compared with those that received third ventricle infusions of PBS (Fig. 3).
Table 1.
Calorimetry measurements
| PBS |
Leptin |
|||
|---|---|---|---|---|
| Saline | Hydrogel | Saline | Hydrogel | |
| Light phase RER | ||||
| Day −1 | 0.90 ± 0.02 | 0.90 ± 0.01 | 0.91 ± 0.01 | 0.91 ± 0.01 |
| Day 4 | 0.86 ± 0.02a | 0.87 ± 0.02a | 0.79 ± 0.01b | 0.77 ± 0.01b |
| Day 7 | 0.87 ± 0.02a | 0.87 ± 0.01a,b | 0.82 ± 0.03b,c | 0.78 ± 0.01c |
| Dark phase RER | ||||
| Day −1 | 0.95 ± 0.02 | 0.94 ± 0.01 | 0.96 ± 0.01 | 0.95 ± 0.01 |
| Day 4 | 0.93 ± 0.03a | 0.93 ± 0.02a | 0.84 ± 0.01b | 0.84 ± 0.01b |
| Day 7 | 0.93 ± 0.02a | 0.92 ± 0.01a | 0.88 ± 0.02b | 0.85 ± 0.01b |
| Total daily energy expenditure, kcal·rat−1·24 h−1 | ||||
| Day −1 | 187 ± 12 | 183 ± 10 | 187 ± 14 | 208 ± 9 |
| Day 3 | 181 ± 12a,b | 172 ± 10b | 208 ± 13a,b | 216 ± 11a |
| Day 6 | 179 ± 11a,b | 163 ± 9b*# | 199 ± 8a | 191 ± 10a,b*# |
Data are means ± SE for groups of 7–9 rats. RER, respiratory exchange ratio. Values that do not share a common superscripted letter are significantly different at P < 0.05
Significant difference for a specific group between baseline and infusion periods;
significant difference for a specific group before and after placement of hydrogel.
Fig. 3.
Top: uncoupling protein 1 (UCP1) protein expressed as a ratio to actin in intrascapular brown fat (IBAT) and inguinal white fat collected at the end of the experiment. Middle: phosphorylated STAT3 (p-STAT3) in hypothalamic and hindbrain tissue blocks expressed as a ratio to total STAT3. Bottom: total STAT3 in hypothalamic and hindbrain tissue blocks expressed as a ratio to actin. Data are means + SE for groups of 7–9 rats. Values on a specific axis that do not share the same letter are significantly different at P < 0.05. PS, PBS-saline; PH, PBS-hydrogel; LS, leptin-saline; LH, leptin-hydrogel.
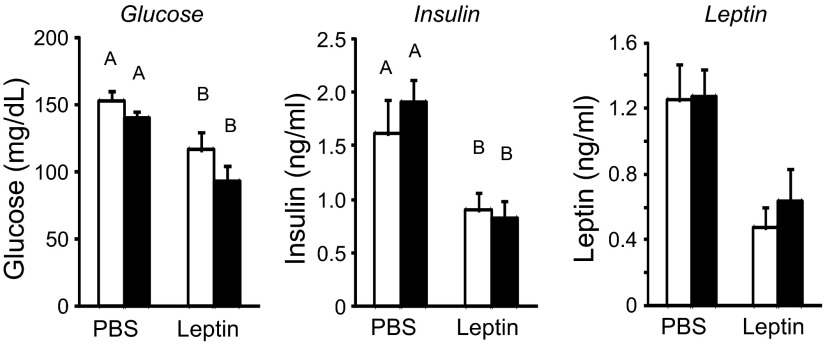
At the end of the experiment the leptin-infused rats weighed significantly less than those infused with PBS (Table 2). The difference in weight was associated with a 68% reduction in carcass fat and an 11% loss of protein (Table 2). The reduction in carcass fat was reflected in all of the fat depots that were weighed. Infusion of leptin reduced liver weight, with the biggest effect in the leptin-hydrogel rats (Table 2). Serum glucose and insulin were reduced in all rats that received third ventricle infusions of leptin, but there was no additional effect of hydrogel placement (Fig. 4). There were no significant effects of leptin infusion or hydrogel injection on serum leptin concentrations (Fig. 4). There were no significant differences in CSF leptin concentrations measured before perfusion of the second set of rats (data not shown).
Table 2.
Carcass composition
| PBS |
Leptin |
|||
|---|---|---|---|---|
| Saline | Hydrogel | Saline | Hydrogel | |
| Carcass weight, g | 277 ± 10a | 280 ± 8a | 254 ± 8b | 241 ± 6b |
| Carcass fat, g | 17.2 ± 1.7a | 15.9 ± 1.7a | 6.0 ± 0.8b | 4.5 ± 0.4b |
| Carcass protein, g | 65 ± 2a | 64 ± 2a | 58 ± 2b | 57 ± 2b |
| Liver weight, g | 12.2 ± 0.5a | 11.9 ± 0.7a | 9.0 ± 0.7b | 7.2 ± 0.5c |
| Inguinal fat, mg | 3,347 ± 342a | 3,519 ± 321a | 1,589 ± 207b | 1,309 ± 150b |
| Epididymal fat, mg | 2,262 ± 193a | 2,180 ± 310a | 1,005 ± 198b | 959 ± 178b |
| Retroperitoneal fat, mg | 744 ± 127a | 768 ± 116a | 135 ± 38b | 172 ± 8b |
| Mesenteric fat, mg | 997 ± 153a | 882 ± 123a | 257 ± 41b | 240 ± 26b |
| Total dissected fat, mg | 7,351 ± 738a | 7,350 ± 749a | 2,986 ± 456b | 2,680 ± 344b |
| IBAT, mg | 241 ± 20a,b | 265 ± 39a | 182 ± 19b,c | 151 ± 12c |
Data are means ± SE for groups of 7–9 rats. IBAT, intrascapular brown fat. Values that do not share a common superscripted letter are significantly different at P < 0.05.
Fig. 4.
Serum glucose, insulin, and leptin measured in trunk blood collected at the end of the experiment. Data are means + SE for groups of 7–9 rats. Values on a specific axis that do not share a common letter are significantly different at P < 0.05.
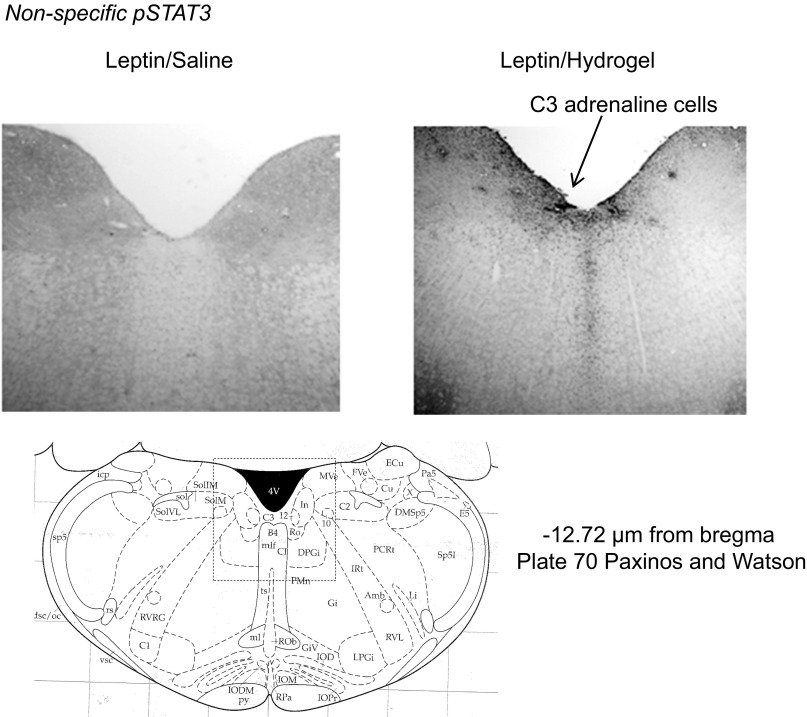
There were no differences in the levels of SOCS3 expression or in ERK1 or ERK2 activation in either the hypothalamus or hindbrain of the rats (data not shown). Phosphorylated STAT3 was increased in hypothalamic tissue of leptin-infused rats, with no additional effect of blocking the aqueduct with hydrogel (leptin P < 0.003, hydrogel NS, leptin × hydrogel NS; Fig. 3). Third ventricle leptin infusion produced an overall increase in hindbrain pSTAT3 (leptin P < 0.01, hydrogel P < 0.006, leptin × hydrogel NS), but this did not reach significance for saline-injected leptin-infused rats compared with saline-injected PBS-infused rats (P < 0.07). Blockade of the aqueduct with hydrogel also increased hindbrain p-STAT3, although this did not reach significance (P < 0.06) for the PBS-infused animals (Fig. 3). Immunohistochemistry indicated that the increase in p-STAT3 activation in the hypothalamus was essentially limited to the VMHDM (Fig. 5). There was no effect of leptin infusion on p-STAT3 in the ARC, and there was no effect of blocking of the aqueduct on p-STAT3 in either the VMHDM or ARC. In the hindbrain there was an interaction between leptin infusion and hydrogel injection on p-STAT3 levels in the medial NTS (leptin NS, hydrogel P < 0.05, leptin × hydrogel P < 0.002). The difference between PBS-saline and leptin-saline rats did not reach significance (P < 0.06), but p-STAT3 was lower in leptin-hydrogel rats than in leptin-saline rats. Blockade of the aqueduct with hydrogel increased p-STAT3 in the AP of PBS-infused rats but not those infused with leptin (Fig. 6). Casual observation indicated that injection of hydrogel into the aqueduct caused an increase in p-STAT3 in the hindbrain in areas that are not associated with high levels of Lepr expression. This was especially obvious on the floor of the fourth ventricle (Fig. 7) and may have contributed to the increase in hindbrain p-STAT3 that was detected by Western blot.
Fig. 5.
Immunohistochemistry for p-STAT3 in hypothalamic tissue. Data are means + SE for groups of 4 rats. p-STAT3 was measured as the no. of stained nuclei within a defined area. VMHDM, dorsomedial ventromedial hypothalamic nucleus; ArcM, medial arcuate nucleus; ArcL, lateral arcuate nucleus; ArcMP, medial posterior arcuate nucleus. Images have been adjusted for brightness and contrast.
Fig. 6.
Immunohistochemistry for p-STAT3 in hindbrain tissue. Data are means + SE for groups of 4 rats. p-STAT3 was measured as the no. of stained nuclei within a defined area. AP, area postrema; mNTS, medial nucleus of the solitary tract. Images have been adjusted for brightness and contrast.
Fig. 7.
Nonspecific phosphorylation of STAT3 (p-STAT3) on the floor of the 4th ventricle in a rat injected with saline (left) or hydrogel (right). Images have been adjusted for brightness and contrast.
DISCUSSION
The objective of this experiment was to determine whether activation of hindbrain Lepr contributed to or attenuated the catabolic effect of third ventricle infusions of leptin. It is important to note that this experiment utilized an experimental model to test a concept. Assuming that leptin is transported into the brain from the periphery, it is most unlikely that under normal circumstances leptin concentrations would be elevated in the forebrain without a simultaneous elevation in the periphery and hindbrain. The results suggest that leptin that drains into the fourth ventricle attenuates the response to activation of Lepr in the vicinity of the third ventricle because inhibition of food intake and weight loss were exaggerated in leptin-infused rats that had the cerebral aqueduct blocked with hydrogel. Immunohistochemistry showed a trend for an increase in p-STAT3, a marker of Lepr activation, in the medial NTS of leptin-infused rats and that this activation was reversed when the aqueduct was blocked. These observations suggest that prevention of a simultaneous stimulation of hindbrain Lepr by exogenous leptin exaggerated weight loss in rats receiving third ventricle infusions of leptin. It is possible that some of the exaggeration in weight loss of leptin-hydrogel rats was simply the result of a reduction in gut fill due to the suppression of food intake. Gut content represents ∼5% of body weight in a rat (19) and varies in direct proportion to daily food intake (20). Because rats eat a majority of their food at night and the animals in this study were weighed at the start of the light period, a suppression of food intake would result in a measureable change in gut fill and thus in body weight. A reduced food intake does not, however, account for all of the weight loss observed after hydrogel placement, because food intake of the leptin-infused rats gradually increased, but body weight continued to decline. This suggests that there would have been a significant difference in body composition of hydrogel and saline-injected rats if the experiment had continued for more than 3 days after the aqueduct was blocked.
Blocking diffusion of CSF from the third to the fourth ventricle of the rats potentially increases fluid accumulation in the forebrain and reduces the volume of CSF in the fourth ventricle. This in turn may modify function of the hindbrain if the washout of metabolites and the delivery of hormones are diminished. The increased expression of p-STAT3 on the floor of the fourth ventricle may have been indicative of inflammation as a secondary response to reduced fluid flow through the hindbrain. Inflammatory cytokines signal through pathways that include STAT3 (7). Therefore, the presence of proinflammatory cytokines could have increased p-STAT3 in the hindbrain tissue, whereas all of the localized activation of STAT3 in the hypothalamus could be attributed to the presence of Lepr. PBS-hydrogel rats acted as controls for the independent effect of hydrogel on behavior and energy balance of the rats. It is clear from the 2-day inhibition of food intake and 1-day inhibition of weight gain in these animals that placement of hydrogel was initially stressful and disrupted homeostasis. By the 3rd day of the experiment the PBS-hydrogel rats were not different from the PBS-saline rats, and for this reason we focused on the last day of each experimental period when testing for the specific effects of leptin in hydrogel-injected animals.
The results suggest that activation of hindbrain Lepr by leptin that drains from the third ventricle blunts the catabolic response mediated by forebrain Lepr. These data are consistent with the conclusions reached in a study in which body fat mass was increased in chronic decerebrate (CD) rats that received peripheral infusions of leptin (18). Decerebration involves a surgical transection to neurally isolate the caudal brainstem from the forebrain and has been used to demonstrate that many feeding and energetic responses in rats are intact in the absence of neural input from the forebrain (10, 11). The increase in adiposity of CD rats receiving peripheral infusions of leptin (18) suggested that activation of Lepr outside of the forebrain produced an anabolic response in the absence of neural feedback from the forebrain. Subsequently, we demonstrated that infusions of low doses of leptin into the fourth ventricle produced a small but significant increase in body fat (16), indicating that hindbrain, rather than peripheral Lepr, was responsible for the increase in body fat. The results from the CD rat study cannot be compared directly with the experiment described here because the CD rats received peripheral infusions of leptin, whereas this study examined the effect of central leptin infusion. The outcome of the CD study may, however, give some indication of the potential mechanism of communication between the forebrain and hindbrain that is required for an integrated response to central leptin in a normal animal. The increase in body fat of CD rats in the absence of direct neural connections between the forebrain and hindbrain implies that the opposing effects of forebrain and hindbrain leptin are not due to a direct neural or humoral inhibition of forebrain areas by the hindbrain. It is possible that hindbrain Lepr induces a change in peripheral metabolism that leads to increased fat deposition and that leptin-responsive neurons in the forebrain normally inhibit these hindbrain efferents. Alternatively, leptin-responsive forebrain and hindbrain neurons may project to a common area, and the change in body fat results from an integration of information at this site. Although the forebrain and hindbrain of decerebrate rats are neurally isolated, humoral communication between the two compartments of the brain is unchanged. Therefore, it is also possible that unidentified soluble factors were induced in the leptin-treated CD rats and that these were responsible for the increase in their body fat mass. One possible candidate is a change in the hypothalamic-pituitary-adrenal axis, but this axis did not account for the increased adiposity produced by leptin infusion because there was no effect of leptin on concentrations of corticosterone, adrenal weight, or adrenal norepinephrine content in the CD rats (18).
A number of studies in the literature suggest that hindbrain leptin receptors are essential for the normal regulation of body weight and that these receptors have an inhibitory effect on weight gain rather than the anabolic response indicated by the results of this experiment. Injection of leptin into the fourth ventricle (33) or the NTS (30) specifically suppresses food intake and inhibits weight gain. There are several possible reasons for the difference in results, including the method and dose of leptin administration. The amount of leptin required for a bolus fourth ventricle injection to suppress food intake ranged from 3 to 10 μg (24, 30, 33). These doses would have caused a much greater increase in CSF leptin than the nonsignificant doubling found in this study and may have activated different populations of receptors, including those in hypothalamic tissue. Ruiter et al. (30) have shown that injection of 3 μg of leptin into the fourth ventricle or 50 nmol directly into the NTS leads to a doubling of p-STAT3 in the arcuate, lateral, and ventromedial nuclei of the hypothalamus. This is a more widely distributed activation of p-STAT3 than we found with a continuous infusion of 0.3 μg leptin/24 h directly into the third ventricle.
Hayes et al. (21) downregulated hindbrain Lepr using AAV-shRNAi and reported hyperphagia and increased weight gain when the rats were fed a high-fat diet. During the first 10 days of the study, when the rats were fed chow, knockdown of hindbrain Lepr caused significant weight loss compared with control rats. These data are consistent with the notion that hindbrain Lepr attenuates the catabolic effect of forebrain leptin, at least for a short period of time. In a study of mice, Scott et al. (32) knocked down Lepr in hindbrain cells that expressed glucagon-like peptide-1 (GLP-1). These mice showed a small increase in weight gain, but at 28 wk of age there were no significant differences in body weight, fat content, or lean mass of the knockdown mice compared with controls, implying no major influence of hindbrain Lepr on body composition. These two studies are not directly comparable with the experiment described here because in both situations the number of hindbrain Lepr was reduced, and leptin was not administered either centrally or peripherally. By contrast, the hyrodgel injections in the study described here did not change Lepr number or hindbrain leptin signaling caused by endogenous leptin; it simply prevented exogenous leptin applied to the third ventricle from reaching the fourth ventricle. In addition, it may not be appropriate to compare mice with rats because Huo et al. (23) reported that leptin activated GLP-1-expressing cells in the NTS of mice, but not rats, and that there is a very different distribution of GLP-1 neurons in the two rodent species.
An alternative interpretation of data from this study is that blockade of the aqueduct increased activity of hypothalamic pathways that downregulate food intake and body weight. One obvious possibility is that accumulation of leptin in the third ventricle exaggerated the leptin response. This is unlikely, because we reported previously that 0.3 μg leptin/24 h produces a maximal inhibition of food intake and weight loss in rats (16). There was no difference between rats that received 0.3 μg leptin/24 h and those that received 0.9 μg leptin/24 h, and 0.9 μg is the total amount of leptin that could have accumulated in the third ventricle during the 3 days following hydrogel injection in this study. Another possibility is that accumulation of leptin infused into the third ventricle led to a downregulation of hypothalamic Lepr that increased the sensitivity of hindbrain Lepr to endogenous leptin and led to an inhibition of food intake and weight gain. Measurement of p-STAT3 by Western blot and immunohistochemistry as a marker of Lepr activation showed no change in p-STAT3 between leptin-saline and leptin-hydrogel rats, making it unlikely that hypothalamic Lepr was downregulated following hydrogel injection. Finally, because the PBS-infused rats did not show sustained weight loss after hydrogel injection, it could be argued that the exaggerated weight loss in leptin-hydrogel rats was a specific response to a change in leptin distribution rather than a nonspecific effect of the aqueduct being blocked.
Leptin-infused rats lost weight throughout the study, but energy expenditure was the same as that of PBS-infused controls. Normally, food-restricted rats in a state of negative energy balance conserve energy by reducing basal metabolic rate. The results of this experiment confirm previous observations that leptin maintains energy expenditure even when rats are hypophagic and losing weight (35). Leptin infusion stimulated IBAT UCP1 protein expression, which may be used as an index of brown fat nonshivering thermogenesis (27). Because blockade of the aqueduct did not prevent the increase in UCP1 protein and did not reduce energy expenditure, it appears that Lepr in the forebrain is primarily responsible for the increase in sympathetic outflow to brown adipose tissue and maintaining expenditure. Although energy expenditure was maintained, RER was reduced in leptin-infused rats, indicating that they were using more fat and protein as energy substrate than the PBS-infused controls that ate more of the high-carbohydrate chow diet.
Immunohistochemistry indicated that infusion of 0.3 μg leptin/24 h produced a limited distribution of Lepr activation. In the hypothalamus, the arcuate nucleus and VMHDM were the only areas that showed significant levels of p-STAT3 at the end of the study. This contrasts with the activation of multiple hypothalamic nuclei that has been reported for rats and mice treated with higher doses of leptin in single peripheral (9) or central injections (30). The difference may be due to the difference in dose of leptin used or because of the time interval between the start of leptin administration and detection of p-STAT3. We examined brains after 7 days of leptin infusion, when food intake of the leptin-treated rats was stabilized, and it is possible that additional sites of Lepr activation would have been detected if immunohistochemistry had been performed after only 1 or 2 days of leptin infusion. The arcuate nucleus showed significant levels of p-STAT3 in all rats, but leptin infusion did not increase this activation, and the VMHDM was the only hypothalamic site that responded to leptin infusion with an increase in p-STAT3. Neurons from the VMHDM project to various areas of the hindbrain, including the NTS (26), where they make contact with catecholaminergic neurons. The VMH has also been associated with control of blood glucose (2), consistent with the relative hypoglycemia of the leptin-infused rats. It is possible that p-STAT3 is not a good marker of Lepr activation; however, Bates et al. (3) reported that activation of the JAK-STAT3 pathway is essential for the energy balance effects of leptin, which were of primary interest in this experiment.
In summary, data from the study described here support previous observations that activation of Lepr in the vicinity of the fourth ventricle has the potential to attenuate the catabolic activity of leptin. This response is found with low-dose infusions of leptin, and it is possible that administration of higher doses would activate additional populations of Lepr and have a catabolic effect. Inhibition of food intake and body weight was exaggerated in rats in which leptin was infused into the third ventricle and was prevented from draining into the fourth ventricle. The increased weight loss was unlikely due to the accumulation of leptin in the third ventricle, as the dose of leptin used here was previously found to produce a maximal effect on food intake and body fat content of the rats (16). The response also was specific to leptin-infused rats because blockade of the aqueduct in control PBS-infused rats did not cause sustained weight loss. Further studies are needed to elucidate the pathways through which leptin activity in the forebrain and hindbrain is integrated.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-053903 (awarded to R. B. S. Harris).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.I.V., B.N.D., and R.B.H. contributed to the conception and design of the research; M.I.V., B.N.D., and R.B.H. performed the experiments; M.I.V., B.N.D., and R.B.H. interpreted the results of the experiments; M.I.V., B.N.D., and R.B.H. edited and revised the manuscript; M.I.V., B.N.D., and R.B.H. approved the final version of the manuscript; B.N.D. and R.B.H. analyzed the data; R.B.H. prepared the figures; R.B.H. drafted the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Larry L. Bellinger, Baylor College of Dentistry, for arranging the collaboration with Dr. Zhibing Hu, who unfortunately, passed away in 2012.
REFERENCES
- 1.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275: 14563–14572, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Barnes MB, Lawson MA, Beverly JL. Rate of fall in blood glucose and recurrent hypoglycemia affect glucose dynamics and noradrenergic activation in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 301: R1815–R1820, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421: 856–859, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Ciriello J, Moreau JM. Systemic administration of leptin potentiates the response of neurons in the nucleus of the solitary tract to chemoreceptor activation in the rat. Neuroscience 229: 88–99, 2013 [DOI] [PubMed] [Google Scholar]
- 5.de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific Lepr-b transgenes. J Clin Invest 115: 3484–3493, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai BN, Harris RB. Integrated effects of leptin in the forebrain and hindbrain of male rats. Endocrinology 154: 2663–2675, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devarajan E, Huang S. STAT3 as a central regulator of tumor metastases. Curr Mol Med 9: 626–633, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Ellacott KL, Halatchev IG, Cone RD. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology 147: 3190–3195, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci 31: 12189–12197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 16: 296–309, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol 23: 2–40, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143: 239–246, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA 94: 8878–8883, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haring SJ, Harris RB. The relation between dietary fructose, dietary fat and leptin responsiveness in rats. Physiol Behav 104: 914–922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris RB. Growth measurements in Sprague-Dawley rats fed diets of very low fat concentration. J Nutr 121: 1075–1080, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Harris RB. Leptin-induced increase in body fat content of rats. Am J Physiol Endocrinol Metab 304: E267–E281, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris RB, Apolzan JW. Changes in glucose tolerance and leptin responsiveness of rats offered a choice of lard, sucrose, and chow. Am J Physiol Regul Integr Comp Physiol 302: R1327–R1339, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris RB, Bartness TJ, Grill HJ. Leptin responsiveness in chronically decerebrate rats. Endocrinology 148: 4623–4633, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Harris RB, Hervey E, Hervey GR, Tobin G. Body composition of lean and obese Zucker rats in parabiosis. Int J Obes 11: 275–283, 1987 [PubMed] [Google Scholar]
- 20.Harris RB, Zhou J, Weigle DS, Kuijper JL. Recombinant leptin exchanges between parabiosed mice but does not reach equilibrium. Am J Physiol Regul Integr Comp Physiol 272: R1800–R1808, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11: 77–83, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Z, Xia X. Hydrogel nanoparticle dispersions with inverse thermreversible gelation. Adv Mat 16: 305–309, 2004 [Google Scholar]
- 23.Huo L, Gamber KM, Grill HJ, Bjørbaek C. Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology 149: 492–497, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huo L, Maeng L, Bjørbaek C, Grill HJ. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology 148: 2189–2197, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kramer PR, Guan G, Zhou J, Hu Z, Bellinger LL. Selective blockade of the rat brain aqueduct with thermogelling hydrogel nanoparticle dispersion. Physiol Behav 93: 546–552, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Lindberg D, Chen P, Li C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. J Comp Neurol 521: 3167–3190, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Nedergaard J, Golozoubova V, Matthias A, Shabalina I, Ohba K, Ohlson K, Jacobsson A, Cannon B. Life without UCP1: mitochondrial, cellular and organismal characteristics of the UCP1-ablated mice. Biochem Soc Trans 29: 756–763, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The Rat Brain. New York: Academic, 1998 [Google Scholar]
- 29.Peters JH, Simasko SM, Ritter RC. Modulation of vagal afferent excitation and reduction of food intake by leptin and cholecystokinin. Physiol Behav 89: 477–485, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Ruiter M, Duffy P, Simasko S, Ritter RC. Increased hypothalamic signal transducer and activator of transcription 3 phosphorylation after hindbrain leptin injection. Endocrinology 151: 1509–1519, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol 514: 518–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain GLP-1 neurons regulates food intake and energy balance in mice. J Clin Invest 121: 2413–2421, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology 150: 1705–1711, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tartaglia LA. The leptin receptor. J Biol Chem 272: 6093–6096, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Wang T, Hartzell DL, Rose BS, Flatt WP, Hulsey MG, Menon NK, Makula RA, Baile CA. Metabolic responses to intracerebroventricular leptin and restricted feeding. Physiol Behav 65: 839–848, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994 [DOI] [PubMed] [Google Scholar]