Abstract
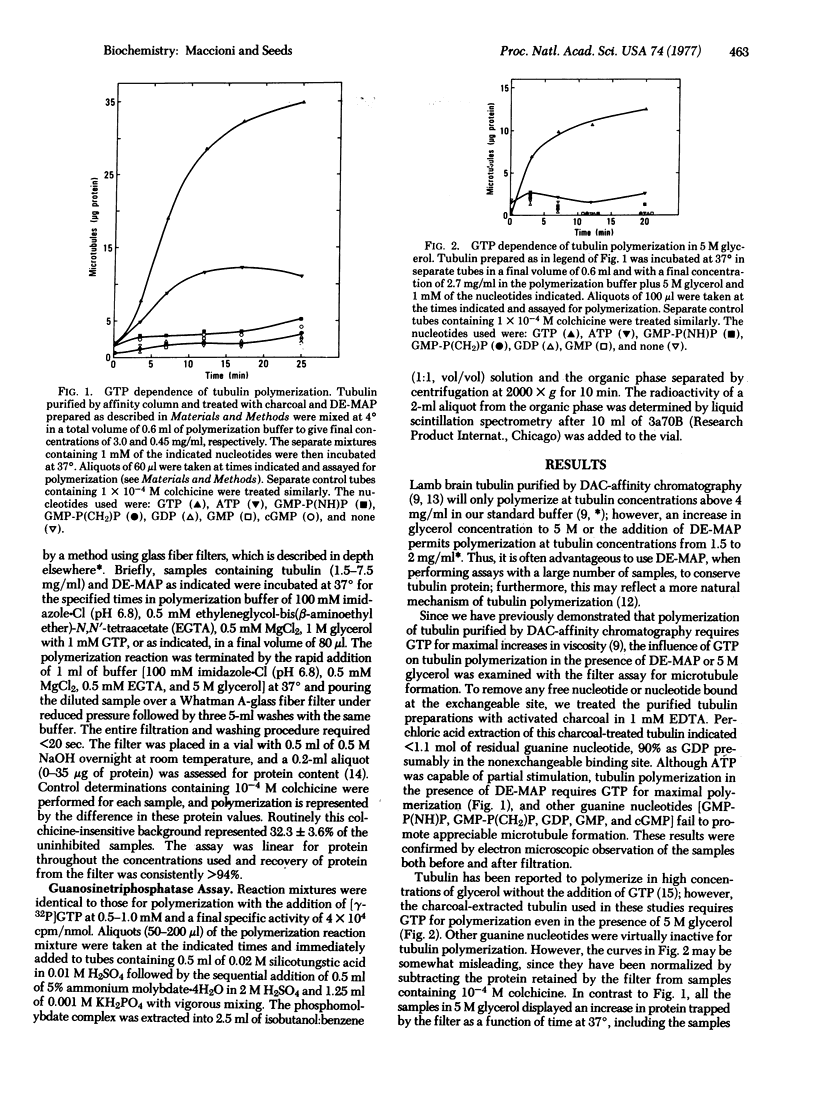
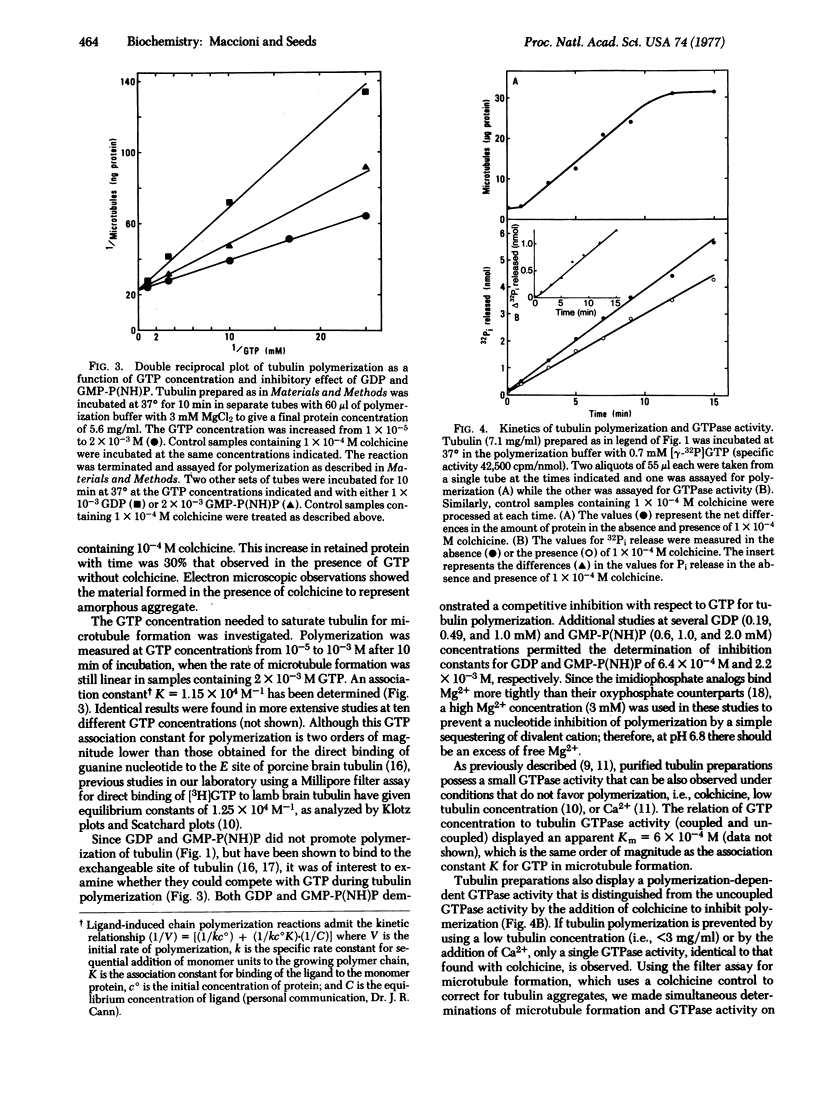
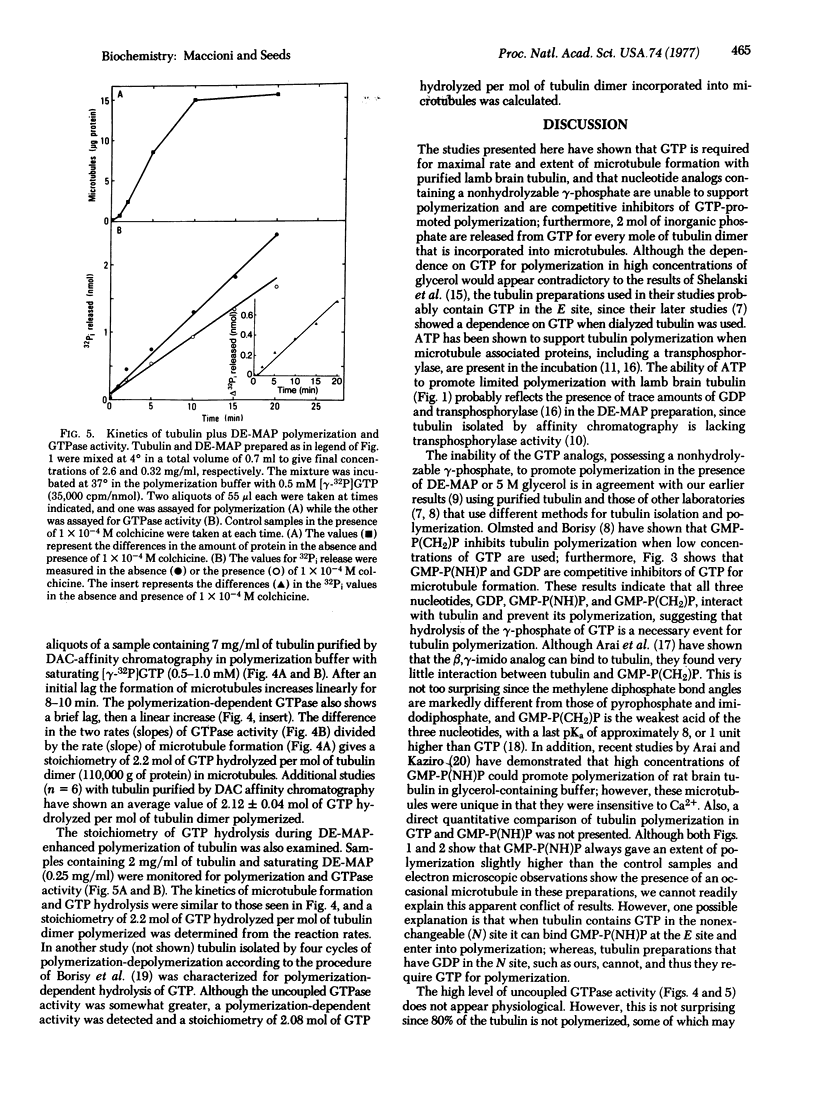
Microtubule formation from lamb brain tubulin isolated by affinity chromatography and freed of exchangeable nucleotide requires GTP for maximal rate and extent of polymerization. The nucleotide analogs guanylylmethylenediphosphate and guanylylimidodiphosphate fail to replace GTP; in addition, neither the presence of microtubule associated proteins nor 5 M glycerol relieves the GTP requirement. The relation of GTP concentration and microtubule formation shows an association constant K = 1 X 10(4) M-1; furthermore, GDP and guanylylimidodiphosphate are competitive inhibitors of GTP for polymerization. Using a rapid filter assay for microtubule formation that allows the quantitative analysis of early polymerization kinetics and correcting for GTP hydrolysis uncoupled from tubulin polymerization, a stoichiometry of two molecules of GTP hydrolyzed per mole of tubulin dimer incorporated into microtubules has been found.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Ihara Y., Arai K., Kaziro Y. Purification of tubulin from bovine brain and its interaction with guanine nucleotides. J Biochem. 1975 Mar;77(3):647–658. doi: 10.1093/oxfordjournals.jbchem.a130767. [DOI] [PubMed] [Google Scholar]
- Arai T., Kaziro Y. Effect of guanine nucleotides on the assembly of brain microtubles: ability of 5'-guanylyl imidodiphosphate to replace GTB in promoting the polymerization of microtubules in vitro. Biochem Biophys Res Commun. 1976 Mar 22;69(2):369–376. doi: 10.1016/0006-291x(76)90531-3. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Olmsted J. B., Marcum J. M., Allen C. Microtubule assembly in vitro. Fed Proc. 1974 Feb;33(2):167–174. [PubMed] [Google Scholar]
- Borisy G. G., Olmsted J. B. Nucleated assembly of microtubules in porcine brain extracts. Science. 1972 Sep 29;177(4055):1196–1197. doi: 10.1126/science.177.4055.1196. [DOI] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Hinman N. D., Morgan J. L., Seeds N. W., Cann J. R. Isolation of brain tubulin by affinity chromatography. Biochem Biophys Res Commun. 1973 Jun 8;52(3):752–758. doi: 10.1016/0006-291x(73)91001-2. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Caplow M. Microtubular protein reaction with nucleotides. Biochem Biophys Res Commun. 1976 Jan 12;68(1):127–135. doi: 10.1016/0006-291x(76)90019-x. [DOI] [PubMed] [Google Scholar]
- Jacobs M. Tubulin nucleotide reactions and their role in microtubule assembly and dissociation. Ann N Y Acad Sci. 1975 Jun 30;253:562–572. doi: 10.1111/j.1749-6632.1975.tb19229.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morgan J. L., Seeds N. W. Properties of tubulin prepared by affinity chromatography. Ann N Y Acad Sci. 1975 Jun 30;253:260–271. doi: 10.1111/j.1749-6632.1975.tb19205.x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Lipmann F. Comparison of guanosine triphosphate split and polypeptide synthesis with a purified E. coli system. Proc Natl Acad Sci U S A. 1966 Jan;55(1):212–219. doi: 10.1073/pnas.55.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Ionic and nucleotide requirements for microtubule polymerization in vitro. Biochemistry. 1975 Jul;14(13):2996–3005. doi: 10.1021/bi00684a032. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J. A., McIntosh J. R. Biochemistry and physiology of microtubules. Annu Rev Biochem. 1976;45:699–720. doi: 10.1146/annurev.bi.45.070176.003411. [DOI] [PubMed] [Google Scholar]
- Stephens R. E., Renaud F. L., Gibbons I. R., Stevens R. E. Guanine nucleotide associated with the protein of the outer fibers of flagella and cilia. Science. 1967 Jun 23;156(3782):1606–1608. doi: 10.1126/science.156.3782.1606. [DOI] [PubMed] [Google Scholar]
- Ventilla M., Cantor C. R., Shelanski M. A circular dichroism study of microtubule protein. Biochemistry. 1972 Apr 25;11(9):1554–1561. [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]


