Abstract
The adipocyte-derived hormone adiponectin promotes fatty acid oxidation and improves insulin sensitivity and thus plays a key role in the regulation of lipid and glucose metabolism and energy homeostasis. Chronic cannabinoid type 1 (CB1) receptor blockade also increases lipid oxidation and improves insulin sensitivity in obese individuals or animals, resulting in reduced cardiometabolic risk. Chronic CB1 blockade reverses the obesity-related decline in serum adiponectin levels, which has been proposed to account for the metabolic effects of CB1 antagonists. Here, we investigated the metabolic actions of the CB1 inverse agonist rimonabant in high-fat diet (HFD)-induced obese adiponectin knockout (Adipo−/−) mice and their wild-type littermate controls (Adipo+/+). HFD-induced obesity and its hormonal/metabolic consequences were indistinguishable in the two strains. Daily treatment of obese mice with rimonabant for 7 days resulted in significant and comparable reductions in body weight, serum leptin, free fatty acid, cholesterol, and triglyceride levels in the two strains. Rimonabant treatment improved glucose homeostasis and insulin sensitivity to the same extent in Adipo+/+ and Adipo−/− mice, whereas it reversed the HFD-induced hepatic steatosis, fibrosis, and hepatocellular damage only in the former. The adiponectin-dependent, antisteatotic effect of rimonabant was mediated by reduced uptake and increased β-oxidation of fatty acids in the liver. We conclude that reversal of the HFD-induced hepatic steatosis and fibrosis by chronic CB1 blockade, but not the parallel reduction in adiposity and improved glycemic control, is mediated by adiponectin.
Keywords: cannabinoid type 1 antagonism, insulin resistance, hepatic steatosis and fibrosis, fatty acid uptake
adipose tissue is no longer considered a passive energy store but rather a complex and highly active metabolic and endocrine organ that produces and secretes biologically active substances known as “adipokines” (41). Among them, adiponectin, a 30-kDa polypeptide that circulates at high levels in the blood, has been implicated in the maintenance of energy homeostasis and the prevention of several metabolic diseases. Indeed, serum levels of adiponectin are reduced in individuals with obesity and type 2 diabetes (2, 4, 33, 34, 72, 76) and are positively correlated with insulin sensitivity (11, 16, 78), increased fatty acid oxidation in peripheral tissues (25, 66, 77, 79), and protection of the liver from nonalcoholic fatty liver disease (7, 40, 57, 75) and fibrosis (32).
Endocannabinoids are lipid mediators that interact with G protein-coupled cannabinoid type 1 (CB1) and 2 (CB2) receptors to produce a wide range of biological effects similar to those of marijuana (50). Activation of CB1 results in increased food intake (20), insulin resistance (13, 45, 48, 60), hepatic lipogenesis (36, 49, 74), and reduced fatty acid β-oxidation (22, 35), which points to the important role of the endocannabinoid/CB1 system in obesity and its metabolic sequealae. Accordingly, chronic CB1 blockade or genetic knockout of CB1 results in decreased food intake, body weight, and adiposity, increased insulin and leptin sensitivity, and improvements in glucose and lipid homeostasis, hepatic steatosis, and fibrosis in rodent models of obesity (9, 17, 24, 26, 36, 37, 47, 54, 55, 58, 62–64), and similar effects were reported in obese subjects treated with rimonabant (12, 18, 52, 67, 69, 73). Chronic treatment with either globally acting or peripherally restricted CB1 antagonists has been shown to reverse the obesity-induced reduction in plasma adiponectin and adiponectin mRNA expression in adipose tissue (10, 27, 42, 62, 63, 65), suggesting a role for adiponectin in the improved metabolic homeostasis caused by CB1 blockade. To test this hypothesis, we examined the metabolic effects of CB1 blockade in Adipo−/− and Adipo+/+ mice with high-fat diet (HFD)-induced obesity (DIO). The results indicate that the reversal of the HFD-induced hepatic steatosis and fibrosis by CB1 blockade requires intact adiponectin signaling, whereas the parallel reduction in body weight, improved hormonal homeostasis, and increased insulin sensitivity are independent of adiponectin.
MATERIALS AND METHODS
Animals.
The experimental protocol used was approved by the Institutional Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health (NIH). Male 6-wk-old Adipo−/− mice and their wild-type littermate C57Bl/6J controls (Adipo+/+) were obtained from the Jackson Laboratory (Bar Harbor, ME). The mice were maintained under a 12:12-h light-dark cycle and fed ad libitum either a HFD (D12492; 60% of calories from fat, 20% from protein, and 20% from carbohydrates; Research Diets) or mouse standard diet (STD; NIH no. 31 rodent diet) for 7 mo.
Experimental protocol.
HFD-fed obese Adipo−/− or Adipo+/+ mice received vehicle (1% Tween 80, 4% DMSO, and 95% saline) or rimonabant, 10 mg/kg daily, for 7 days by intraperitoneal (ip) injection. Body weight was monitored daily. Mice were euthanized by cervical dislocation, the livers were removed and weighed, and samples of each liver were either snap-frozen or fixed in 10% buffered formalin. Trunk blood was collected for determining endocrine and biochemical parameters. Adiposity index was defined as the ratio of the combined weight of the epididymal, retroperitoneal, and subcutaneous fat pads to total body weight (63).
Blood chemistry.
Blood was collected at the time the mice were euthanized. Serum alanine aminotransferases (ALT), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, and triglycerides (TGs) were quantified using AMS Vegasys Chemistry Analyzer (Diamond Diagnostics). Blood glucose was determined using the Elite (Bayer) glucometer. Serum insulin was determined using the Ultra-Sensitive Mouse Insulin ELISA kit (Crystal Chem). Serum leptin and adiponectin were determined by ELISA (B-Bridge International). Serum non-sterified free fatty acid (FFA) levels were measured using the HR Series NEFA kit (Wako Diagnostics).
Hepatic TG content.
Liver tissue was extracted as described previously (23) and its TG content determined using EnzyChrom Triglyceride Assay Kit (BioAssay Systems).
Histology.
Paraffin-embedded liver sections (5 μm) were stained with hematoxylin and eosin (H & E) for histological evaluation of hepatic steatosis and Sirius red for hepatic fibrosis. The amount of collagen deposition was quantified by measuring the proportion of Sirius red-stained area using color thresholding and measurement of area fraction with Image J software (NIH Public Domain). Images taken from five random 4× fields from the same liver lobe of each animal were measured by an individual blinded to the genotype/treatment of the animal (n = 4–5 mice/group). Representative images, presented in the figures at ×10 magnification, were taken from the animal with the median value for each group.
Glucose tolerance and insulin sensitivity tests.
Mice fasted overnight were injected with glucose (1.5 g/kg ip), followed by tail blood collection at 0, 15, 30, 45, 60, 90, and 120 min for determining blood glucose levels. On the following day, mice were fasted for 6 h before receiving insulin (0.75 U/kg ip; Eli Lilly), and blood glucose levels were determined at the same intervals as above.
Hyperinsulinemic euglycemic clamp.
Experiments were performed as described previously (6) with modifications. Briefly, 5 days before the experiment, the left common carotid artery and the right jugular vein of HFD-induced obese or lean control Adipo−/− and Adipo+/+ mice were catheterized under isofluorane anesthesia. Following a 14-h period of fasting, clamps were performed on unrestrained, conscious mice treated with rimonabant (10 mg·kg−1·day−1 ip) or vehicle for 7 days prior to the experiment. The clamp protocol consisted of a 120-min tracer equilibration period (from t = −120 to 0 min), followed by a 120-min clamp period (from t = 0 to 120 min). A 5-μCi bolus of [3-3H]glucose (Perkin Elmer) was given at t = −120 min, followed by a 0.05 μCi/min infusion for 2 h at a pump rate of 0.1 μl/min (CMA Microdialysis). The insulin clamp was begun at t = 0 min with a priming bolus (64 mU/kg) of human insulin (Humulin R; Eli Lilly), followed by an infusion (3.6 mU·kg−1·min−1) delivered at a pump rate of 0.1 μl/min from 0 to 120 min. The [3-3H]glucose infusion was increased to 0.1 μCi/min for the remainder of the experiment. Specific activity for individual time points did not vary by >15% from the average specific activity during the last 40 min of the clamp. Euglycemia (∼120–150 mg/dl) was maintained during clamps by measuring blood glucose every 10 min starting at t = 0 min and infusing 40% dextrose as necessary. Blood samples (60 μl) were taken every 10 min from t = 80 to 120 min and processed to determine glucose-specific activity. Mice received saline-washed erythrocytes from donors throughout the experimental period (4 μl/min) to prevent a fall of hematocrit by >5%. To estimate insulin-stimulated glucose fluxes in tissues, 2-deoxy-d-[1-14C]glucose (Perkin Elmer) was bolus administered (10 μCi) at t = 85 min, i.e., 45 min before the end of the experiment. At the end of the clamp, animals were anesthetized with intravenous injection of pentobarbital sodium. Within 5 min, gastrocnemius muscle from hindlimbs and liver and epididymal and subcutaneous fat were removed and frozen until analysis.
To determine [3-3H]glucose flux, plasma samples were deproteinized using barium hydroxide and zinc sulfate. The glucose production and disappearance rates were determined using Steele's non-steady-state equations (61). Clamp hepatic endogenous glucose production rate was determined by subtracting the glucose infusion rate (GIR) from total glucose turnover (Rd). The glucose uptake by tissues and glycogen synthesis rates were calculated as described previously (81).
Cell culture.
Human hepatoma HepG2 cells, purchased from the American Type Culture Collection, were plated in six-well plates at a density of 5 × 105 cells/ml and grown in Eagle's Modified Essential Medium (EMEM) with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate in a humidified atmosphere of 5% CO2 at 37°C. Medium was changed every 2–3 days until cells reached 80–90% confluence. Prior to adiponectin treatment, cells were starved in serum-free EMEM for 24 h.
Fatty acid uptake.
Palmitate uptake was initiated in HepG2 cells preincubated for 24 h with vehicle or adiponectin. Medium was replaced with an “incubation medium” containing serum-free EMEM supplemented with palmitic acid (final concentration: 500 μM) bound to fatty acid-free bovine serum albumin (BSA) at a 6:1 molar ratio and a trace amount (0.2 μCi) of radiolabeled [14C]palmitate (Perkin Elmer) at a specific activity of 2,220 MBq/mmol. Palmitate uptake was terminated after 1 h by removing the medium and washing twice with ice-cold PBS containing 0.5 mM MgCl2, 0.92 mM CaCl2, and 0.1% BSA. Then, cells were lysed with 1 M NaOH and centrifuged, and the supernatant was collected. [14C]palmitate was determined in the supernatant by liquid scintillation spectrometry (Beckman) and normalized for protein concentration. Results are reported as means ± SE of six samples/treatment from five independent experiments.
Real-time PCR.
Total mRNA was extracted using Trizol (for mouse liver; Invitrogen) or RNeasy Mini kit (for HepG2 cells; Qiagen), followed by DNase I treatment (Invitrogen), and reverse-transcribed using the Iscript cDNA kit (Bio-Rad). Real-time PCR was performed using the StepOnePlus Real-time PCR system (Life Technologies) and the QuantiTect primer assays QT00291151, QT00149240, QT00137984, QT00106820, QT00162204, QT01055516, QT00135758, QT00140119, QT00996282, and QT01058253 against mouse Scd1, Fasn, Ppara, Cpt1a, Col1α1, Col3α1, Fn1, Acta2 (α-SMA), Timp1, and Cd36, respectively, and the QuantiTect Primer Assays QT00020181 against human CD36. Normalization was performed with the QuantiTect primer assay against mouse 18s RNA (QT00164675) or human TATA box-binding protein (QT00000721).
Western blot analyses.
Whole cell or liver extracts were prepared by using RIPA buffer (Thermo Fisher Scientific) containing protein inhibitor cocktail (Roche), and Western blotting was performed as described previously (62). Antibodies specific for CD36 and β-actin (Abcam) were used to detect the corresponding proteins.
Materials.
Rimonabant was obtained from the National Institute of Drug Abuse Drug Supply Program. Human recombinant adiponectin was purchased from Enzo Life Sciences.
Statistics.
Values are expressed as mean ± SE. Unpaired two-tailed Student's t-test was used to determine differences between vehicle- and drug-treated groups. Time-dependent variables were analyzed, and results in multiple groups were compared by ANOVA followed by Bonferroni test. Significance was at P < 0.05.
RESULTS
Effects of rimonabant treatment on the metabolic profile of DIO mice.
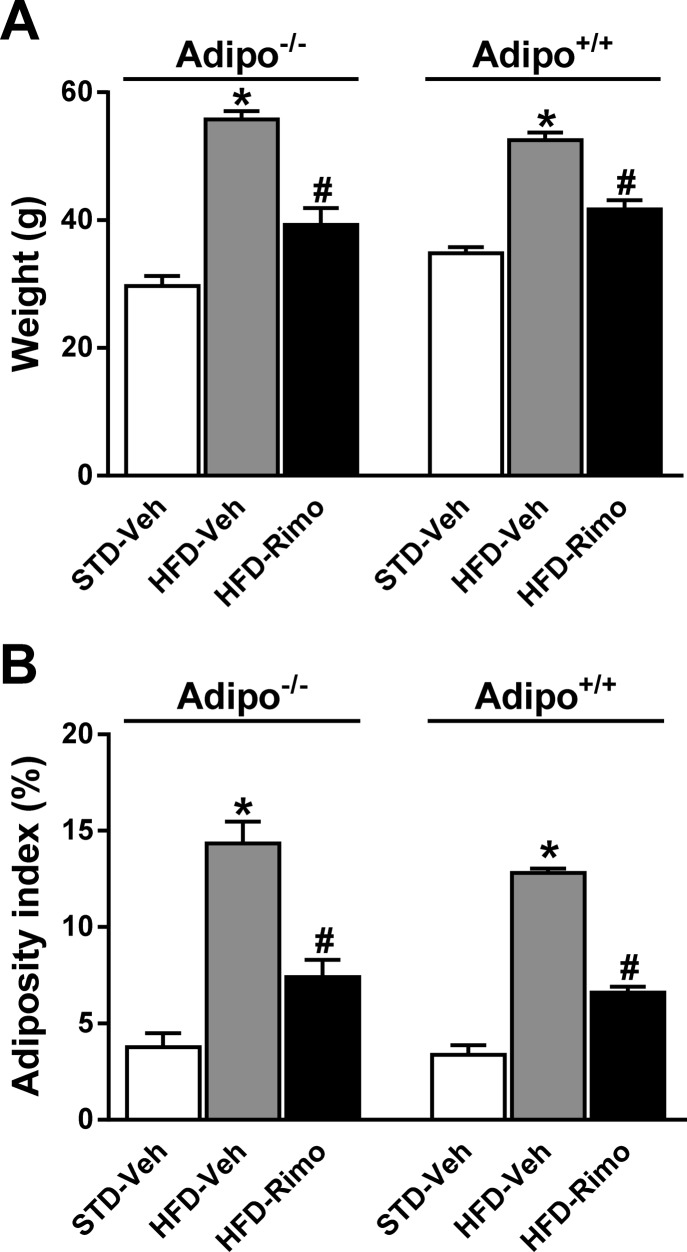
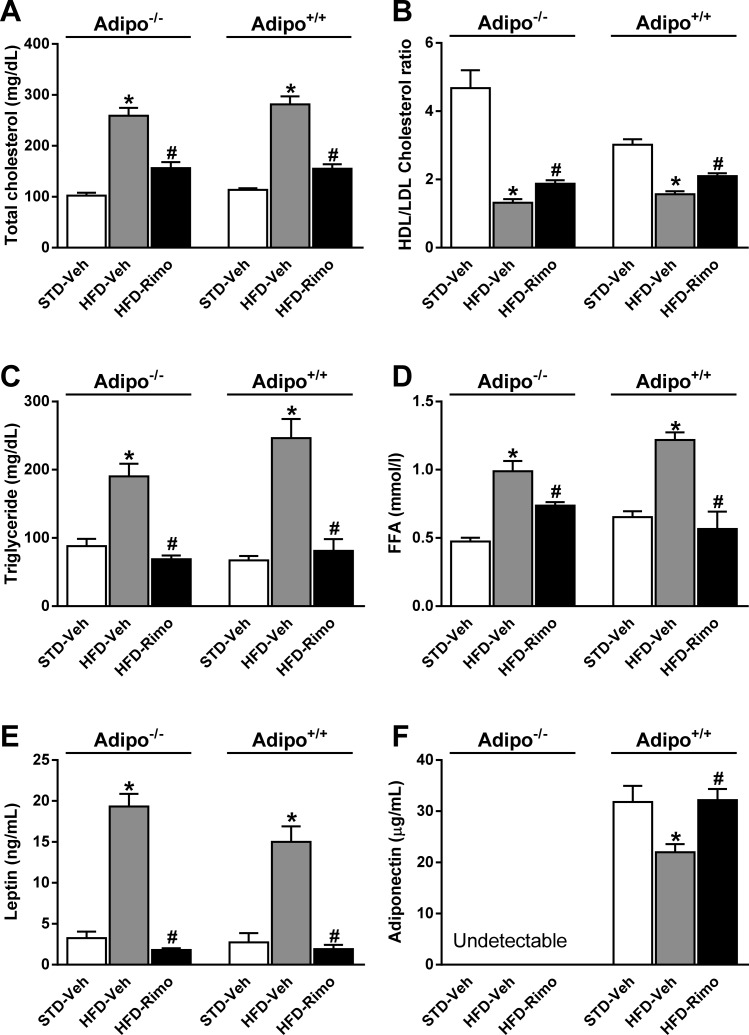
To examine the involvement of adiponectin in the improved metabolic profile by CB1 blockade, we tested the metabolic effects of short-term (7-day) treatment with 10 mg·kg−1·day−1 of rimonabant or vehicle in Adipo−/− and Adipo+/+ mice maintained on HFD for 7 mo, with age-matched lean controls providing baseline reference values. Preliminary experiments indicated that 10 mg/kg was the maximally effective dose of rimonabant in improving the metabolic parameters measured, and treatment for 7 days achieved ∼90% of the plateau response achieved by treatment for ≥2 wk. HFD feeding induced similar weight gain and adiposity in the two strains, and the reversal of these parameters by rimonabant was also similar (Fig. 1, A and B). In parallel, rimonabant treatment significantly reduced serum cholesterol, TG, FFA, and leptin levels and increased the HDL/LDL cholesterol ratio irrespective of the adiponectin genotype (Fig. 2, A–E). Adiponectin was undetectable in serum of Adipo−/− mice and, consistent with previous reports (10, 27, 42, 62, 63, 65), serum adiponectin levels were reduced by the HFD, and the reduction was reversed by rimonabant in Adipo+/+ mice (Fig. 2F).
Fig. 1.
Effects of high-fat diet (HFD) with or without cannibinoid type 1 (CB1) blockade on body weight and adiposity in Adipo+/+ and Adipo−/− mice. Obese Adipo−/− and Adipo+/+ mice were treated with rimonabant (Rimo), 10 mg·kg−1·day−1 ip, or vehicle for 7 days. Note a similar effect of rimonabant on body weight (A) and adiposity index (B) in both strains. Data represent means ± SE from 5 to 10 mice/group. *P < 0.05 relative to the corresponding vehicle (Veh) group on standard (STD) diet; #P < 0.05 relative to the corresponding vehicle group on HFD.
Fig. 2.
Metabolic effects of Rimo in obese Adipo−/− and Adipo+/+ mice. Serum levels of cholesterol (A), HDL/LDL cholesterol ratio (B), triglyceride (C), free fatty acid (FFA; D), leptin (E), and adiponectin (F) were measured in lean controls and in HFD-fed mice treated with Veh or Rimo for 7 days. Adipo−/− and Adipo+/+ mice maintained on HFD for 7 mo were treated with Rimo, 10 mg·kg−1·day−1 ip, or Veh for 7 days. Data represent means ± SE from 5 to 10 mice/group. *P < 0.05 relative to the corresponding Veh group on STD diet; #P < 0.05 relative to the corresponding Veh group on HFD.
Effects of CB1 antagonism on glycemic control.
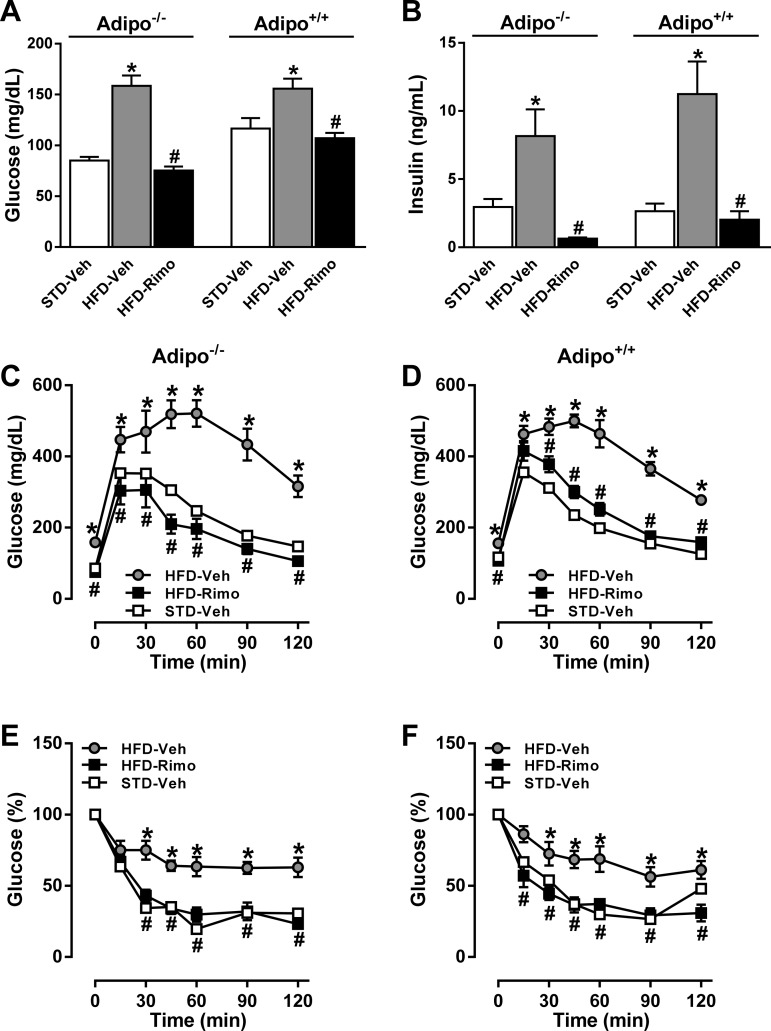
Fasting blood glucose and serum insulin levels were markedly increased by HFD feeding and were similarly reduced by rimonabant in the two strains (Fig. 3, A and B). In response to a glucose challenge, both Adipo−/− and Adipo+/+ mice on HFD showed a similar impairment of glucose tolerance, which was completely reversed by rimonabant treatment in both strains (Fig. 3, C and D). Similarly, rimonabant treatment improved insulin sensitivity to the same extent in the two strains (Fig. 3, E and F).
Fig. 3.
Effects of Rimo on glycemic control in HFD-fed Adipo−/− and Adipo+/+ mice. Adipo−/− and Adipo+/+ mice maintained on HFD for 7 mo were treated with 10 mg·kg−1·day−1 ip of Rimo or Veh for 7 days. Fasting blood glucose (A), plasma insulin (B), glucose tolerance (C and D), and insulin sensitivity (E and F) were measured at the end of the treatment period. Glucose tolerance was tested following the ip injection of 1.5 g/kg glucose (at 0 min) in overnight-fasted mice. On the following day, the mice were fasted for 6 h and then received 0.75 mU/g insulin ip at 0 min. Data represent means ± SE of serial blood glucose measurements at the indicated time points (n = 5–10 mice/group). *P < 0.05 between corresponding values in HFD-Veh vs. STD-Veh group; #P < 0.05 between corresponding values in HFD-Rimo vs. HFD-Veh group.
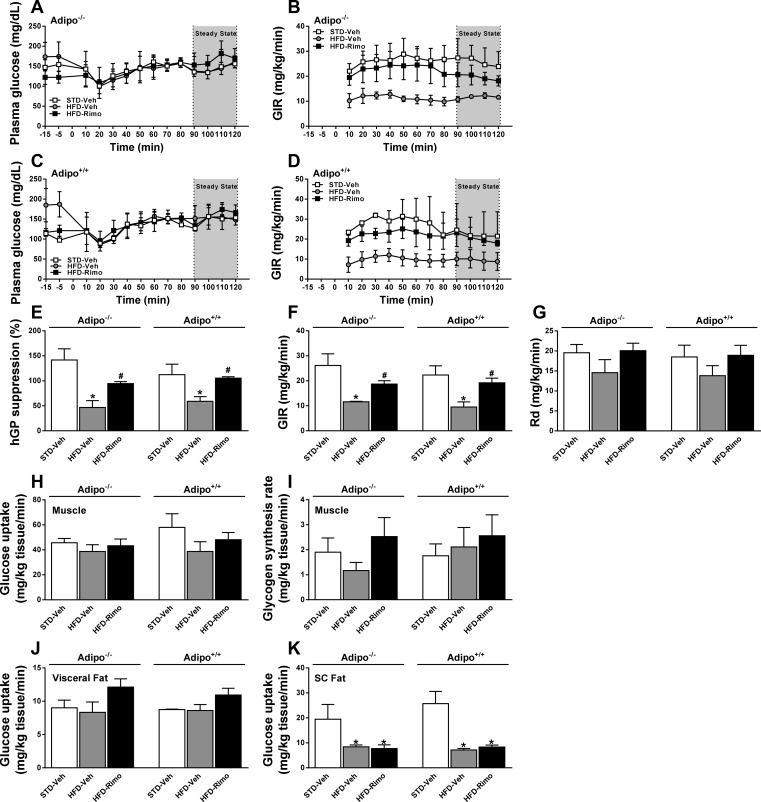
To analyze tissue-specific changes in insulin sensitivity, we performed hyperinsulinemic euglycemic clamp experiments in obese Adipo−/− and Adipo+/+ mice treated with rimonabant and in respective lean controls. Preclamp basal plasma glucose levels were higher, and glucose infusion rates during the clamp were correspondingly lower in HFD-fed than in lean control animals, and rimonabant treatment of HFD-fed mice of both strains normalized these parameters (Fig. 4, A–D). The insulin-induced suppression of hepatic glucose production was significantly attenuated by HFD, and rimonabant treatment restored this effect of insulin in both strains (hepatic glucose production; Fig. 4E), resulting in increased GIR (Fig. 4F). On the other hand, CB1 blockade was similarly ineffective in the two strains in altering whole body glucose clearance (Rd; Fig. 4G), glucose uptake (Fig. 4H), and glycogen synthesis by skeletal muscle (Fig. 4I) or glucose uptake by subcutaneous or visceral fat tissue (Fig. 4, J and K). These observations indicate that the primarily hepatic form of insulin resistance induced by HFD and its reversal by short-term CB1 blockade are independent of adiponectin.
Fig. 4.
Effects of CB1 blockade on tissue-specific insulin sensitivity. Hyperinsulinemic euglycemic clamps were performed on conscious, unrestrained obese Adipo−/− and Adipo+/+ mice treated with 10 mg·kg−1·day−1 ip of Rimo or Veh for 7 days and in respective lean controls. Time course of arterial glucose levels and glucose infusion rates (GIR) during the clamps for Adipo−/− (A and B) and Adipo+/+ mice (C and D). Levels of hepatic glucose production (hGP; E), GIR (F), and whole body glucose clearance (Rd; G) were measured during the steady-state period of the clamp. Glucose uptake into skeletal muscle (H) and its conversion to glycogen (I) and glucose uptake into visceral (J) and subcutaneous adipose tissue (K) were determined as described in materials and methods. Data represent means ± SE from 5 to 7 mice/group. *P < 0.05 relative to the corresponding values in STD-Veh group; #P < 0.05 relative to corresponding values in HFD-Veh group.
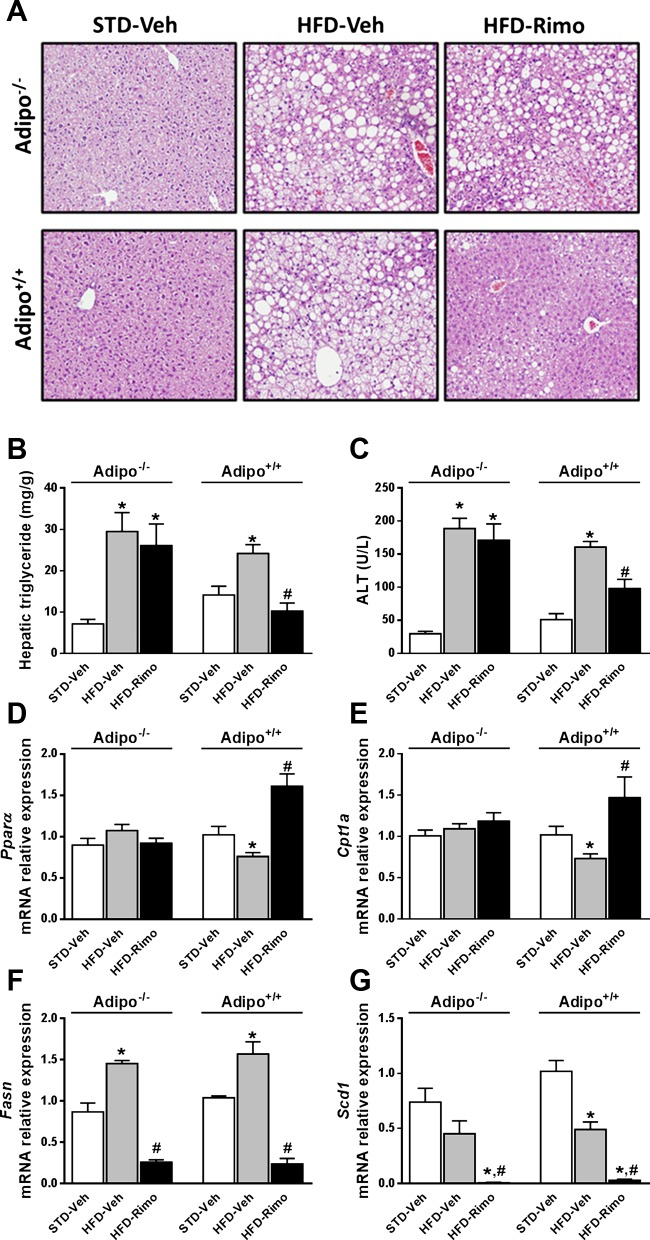
Effect of rimonabant treatment on HFD-induced hepatic steatosis and fibrosis.
We next analyzed the effect of rimonabant treatment on hepatic lipid parameters in Adipo−/− and Adipo+/+ mice with DIO. The HFD-induced fatty liver, as visualized by histology and reflected in the elevated hepatic TG content, as well as the associated hepatocellular damage indicated by increased plasma ALT levels were significantly reduced by rimonabant only in Adipo+/+ and not in Adipo−/− mice (Fig. 5, A–C). Similarly, rimonabant treatment increased the hepatic expression of lipid-mobilizing genes such as peroxisome proliferator-activated receptor-α (Pparα) and carnitine palmitoyl transferase-1 (Cpt-1) only in Adipo+/+ and not in Adipo−/− mice (Fig. 5, D and E). In contrast, the expression of the lipogenic genes fatty acid synthase (Fasn) and stearoyl coenzyme-A desaturase 1 (Scd1) was similarly reduced by rimonabant in the two strains (Fig. 5, F and G).
Fig. 5.
Rimo prevents hepatic steatosis and hepatocellular damage in Adipo+/+ but not in Adipo−/− mice. Obese Adipo−/− and Adipo+/+ mice were treated with 10 mg·kg−1·day−1 ip of Rimo or Veh for 7 days. Note that Rimo reduces the HFD-induced hepatic steatosis, as shown on hematoxylin and eosin-stained liver sections (A), and increases in hepatic TG (B) and serum alanine aminotransferase (ALT; C) levels in Adipo+/+ but not in Adipo−/− mice. Rimo treatment increases the mRNA levels of Pparα (D) and Cpt-1 (H) only in Adipo+/+ mice, whereas the hepatic mRNA levels of Fasn (F) and Scd1 (G) are reduced by Rimo in both strains. Data represent means ± SE from 5 to 10 mice/group. *P < 0.05 relative to the corresponding values in STD-Veh group; #P < 0.05 relative to corresponding values in HFD-Veh group.
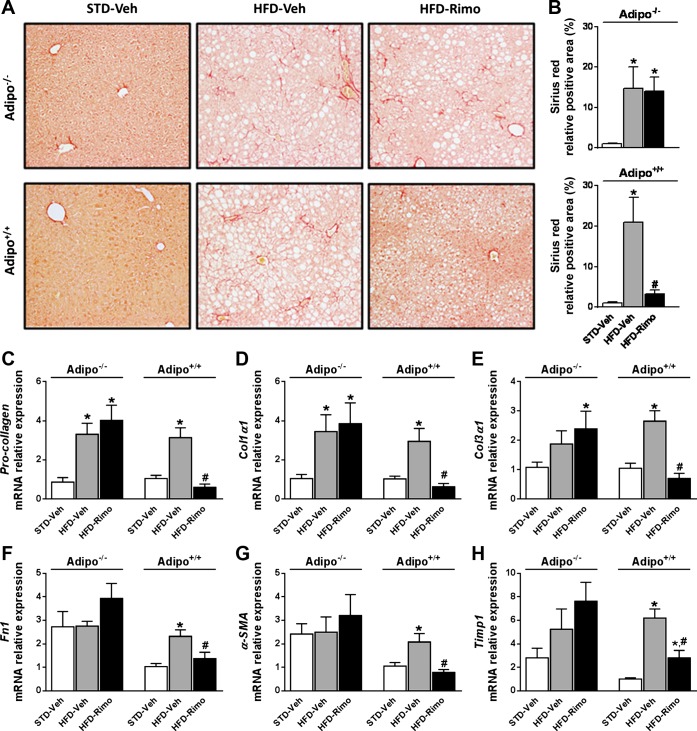
In general, short-term HFD feeding (≤14 wk) does not produce hepatic fibrosis (3), whereas long-term HFD feeding (28 wk or longer) may initiate it (70). Therefore, we evaluated the effect of rimonabant on hepatic fibrosis in Adipo−/− and Adipo+/+ mice maintained on HFD for 7 mo. Hepatic collagen deposition was visualized using Sirius red (Fig. 6A) and quantified using digital image analysis (Fig. 6B). Significant collagen staining was present in Adipo−/− and Adipo+/+ mice on HFD compared with STD-fed animals from the same strain. Similarly to its effect on hepatic steatosis, rimonabant treatment reduced collagen deposition in the liver only in Adipo+/+ and not in Adipo−/− mice (Fig. 6, A and B). Consistent with these findings, the mRNA expression of the fibrosis markers Pro-collagen, Col1α1, Col3α1, Fn1, α-SMA, and Timp1 was markedly increased in the liver of HFD-fed Adipo+/+ mice compared with their lean controls, and rimonabant treatment significantly attenuated these increases, whereas it had no such effect in Adipo−/− mice (Fig. 6, C–H).
Fig. 6.
Rimo reduces the long-term HFD-induced hepatic fibrosis in Adipo+/+ but not Adipo−/− mice. Obese Adipo−/− and Adipo+/+ mice were treated with 10 mg·kg−1·day−1 ip of Rimo or Veh for 7 days. Collagen deposition was evaluated by Sirius red staining (A) and quantified as described in materials and methods (B). Note the reduced deposition of collagen fibers in the liver of Adipo+/+ mice treated with Rimo as well as the normalized mRNA expression levels of the profibrogenic markers: pro-collagen (C), Col1α1 (D), Col3α1 (E), Fn1 (F), α-SMA (G), and Timp1 (H). Data represent means ± SE from 4 to 5 mice/group. *P < 0.05 relative to the corresponding values in STD-Veh group; #P < 0.05 relative to corresponding values in HFD-Veh group.
Adiponectin regulates hepatic FFA uptake.
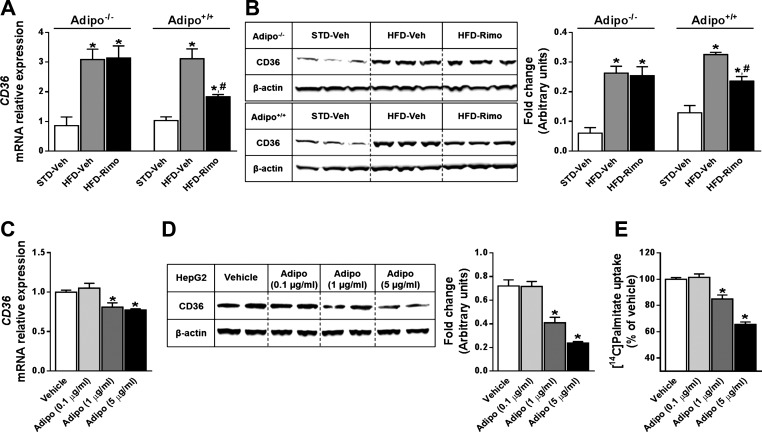
In addition to decreased fatty acid β-oxidation, HFD-induced steatosis also involves increased fatty acid uptake (28). Therefore, we explored the role of adiponectin and CB1 receptors in regulating hepatic fatty acid uptake. When compared with STD-fed lean animals, both HFD-fed Adipo−/− and Adipo+/+ mice showed a dramatic increase in the hepatic mRNA and protein levels of fatty acid translocase/CD36 (Fig. 7, A and B), a protein that mediates the uptake of FFA from the circulation to the liver (31), whereas rimonabant treatment reversed this increase only in Adipo+/+ and not in Adipo−/− mice.
Fig. 7.
Adiponectin reduces hepatic FFA uptake via CD36. A and B: 7-day treatment with Rimo (10 mg·kg−1·day−1 ip) significantly reduces the HFD-induced mRNA (A) and protein (B) expression levels of CD36 in the liver of Adipo+/+ but not Adipo−/− mice. Data represent means ± SE from 4 to 5 mice/group. *P < 0.05 relative to the corresponding values in STD-Veh group; #P < 0.05 relative to corresponding values in HFD-Veh group. C and D: adiponectin concentration dependently reduces CD36 mRNA (C) and protein expression levels (D) in HepG2 cells. Total RNA or cell lysates were harvested after 24 h of treatment with adiponectin. E: consistent with the reduced CD36 expression, adiponectin reduced palmitate uptake into HepG2 cells. Cells were incubated with increasing concentrations of adiponectin for 24 h and then exposed to 500 μM palmitate-BSA supplemented with a trace amount of [14C]palmitate for 1 h. *P < 0.05 relative to Veh group.
Additionally, we analyzed hepatic FFA uptake directly by measuring the uptake of [14C]palmitate into human hepatoma HepG2 cells, a suitable model for FFA uptake by hepatocytes (53), along with CD36 mRNA and protein levels. Exposure of HepG2 cells to increasing concentrations (0.1–5 μg/ml) of human recombinant adiponectin decreased CD36 mRNA and protein levels in a concentration-dependent manner (Fig. 7, C and D), with parallel reductions in the incorporation of [14C]palmitate into the cells (Fig. 7E).
DISCUSSION
The significant role of endocannabinoids acting through CB1 in appetite regulation, energy balance, and metabolic homeostasis is well established (50). Chronic blockade of CB1 has been shown to improve cardiometabolic risk associated with visceral obesity and its metabolic complications, and this was associated with the reversal of the obesity-related reduction in plasma adiponectin levels in both animals (62, 63) and humans (18, 19, 56, 68). In the present study, which was designed to explore the role of adiponectin in mediating the metabolic effects of CB1 antagonism in obesity, we demonstrate that the reversal of HFD-induced hepatic steatosis and fibrosis by CB1 blockade is adiponectin dependent and involves both reduced uptake and increased β-oxidation of FFA in the liver. On the other hand, the effects of CB1 blockade in reducing body weight and fat mass, improving hormonal and glucose metabolism, and increasing insulin sensitivity occur independently of adiponectin.
The HFD-induced accumulation of TGs in the liver is likely the result of the transfer of FFAs from adipose tissue (37, 44), with increased activity of hepatic CB1 playing only a minor role (45). On the other hand, global or peripheral CB1 blockade inhibits hepatic lipogenic gene expression and de novo lipogenesis, whereas it increases β-oxidation of FFAs, resulting in a complete reversal of obesity-induced fatty liver (36, 37, 49, 59, 63). Adiponectin, acting via its hepatic type 2 receptor (AdipoR2), also protects the liver from the HFD-induced TG accumulation, increased inflammation, and reduced fatty acid β-oxidation (7, 40, 57, 75, 77, 80). Indeed, the present findings indicate that chronic CB1 blockade reduces HFD-induced steatosis and fibrosis as well as hepatocellular injury by an adiponectin-dependent mechanism, as indicated by the absence of these effects in Adipo−/− mice (Figs. 5 and 6), despite the same degree of weight loss, reduced adiposity, and increased insulin sensitivity as in rimonabant-treated Adipo+/+ mice.
The decreased gene expression of the hepatic lipogenic enzymes Fasn and Scd1 by rimonabant was also similar in the two strains (Fig. 5, F and G). This indicates not only that these effects are independent of adiponectin but also that they do not contribute significantly to the alleviation of hepatic steatosis by rimonabant treatment. On the other hand, rimonabant treatment increased fatty acid β-oxidation only in Adipo+/+ and not in Adipo−/− mice, as documented by the increased gene expression of Pparα and its target Cpt-1, the rate-limiting enzyme in fatty acid oxidation (Fig. 5, D and E). This indicates a requirement for adiponectin in the antisteatotic effect of chronic CB1 blockade in DIO, which is also consistent with the well-established hepatoprotective function of adiponectin via stimulation of PPARα activity (77, 78, 80). A similar requirement for adiponectin in the antisteatotic effect of CB1 blockade has also been noted in a rare form of obesity due to the absence of leptin (71) and has been linked in humans to a polymorphism (G1359A) in the gene encoding the CB1 receptor (1).
An additional mechanism involved in the hepatoprotective effect of adiponectin is inhibition of FFA uptake into hepatocytes, as documented by the reduced uptake of palmitate (Fig. 7E), and a parallel reduction in CD36 expresssion in HepG2 cells exposed to adiponectin (Fig. 7, C and D). These findings are compatible with in vivo data on CD36 expression in the liver, which confirm the well-established upregulation of hepatic CD36 by HFD (28, 43) and also demonstrate that this effect of HFD present in both strains is reversed by rimonabant only in wild-type and not in Adipo−/− mice (Fig. 7, A and B). A similar reduction in the hepatic expression of CD36 has been demonstrated recently in DIO mice treated with the peripherally restricted CB1 antagonist JD 5037 (15), which also reverses the HFD-induced hepatic steatosis and reduction in adiponectin levels (62). Taken together, our results suggest that the antisteatotic effect of CB1 blockade is mediated indirectly by adiponectin, which acts directly on hepatocytes to reduce FFA uptake and increase fatty acid β-oxidation.
The antifibrogenic effect of adiponectin is also well documented in rodents (5, 32, 38, 39), and reduced serum adiponectin levels are associated with liver fibrosis in humans (8, 57). Long-term HFD feeding resulted in increased gene expression of fibrotic markers, with histological evidence of fibrosis in both Adipo+/+ and Adipo−/− mice (Fig. 6). There is evidence that endocannabinoids acting via hepatic CB1 exert profibrotic effects in the liver and that chronic CB1 blockade or knockdown ameliorates hepatic fibrosis (14, 17, 30, 64), but the underlying mechanisms are unclear. The ability of rimonabant to reverse HFD-induced fibrosis in wild-type but not in adiponectin-deficient mice, as documented here, clearly indicates the obligatory role of adiponectin in mediating the antifibrotic effect of CB1 blockade. Furthermore, the antifibrotic effect of rimonabant can be dissociated from the parallel reduction in body weight and the improved insulin sensitivity, which are present in both strains. Further studies should address whether the profibrotic effect of endocannabinoids/hepatic CB1 is due to reduced adiponectin signaling in the liver.
Compelling evidence indicates that adiponectin is a major insulin-sensitizing adipokine (11, 16, 72, 78). Chronic CB1 blockade, which alleviates obesity-induced insulin resistance via both central and peripheral mechanisms (13, 42, 45, 47, 48, 62, 63), also normalizes the reduced plasma levels of adiponectin in obese animals and humans (10, 18, 19, 27, 29, 42, 62, 63, 65), which could suggest adiponectin involvement in the reversal of insulin resistance by CB1 blockade. However, this is not supported by the present findings, which indicate that the insulin-sensitizing effect of rimonabant is similar in the presence or absence of adiponectin, as indicated by a number of parameters. Thus, the effects of rimonabant were similar in obese Adipo+/+ or Adipo−/− mice in reversing the fasting hyperglycemia and hyperinsulinemia (Fig. 3, A and B) and normalizing the glucose intolerance and insulin resistance, as tested by glucose tolerance test and insulin sensitivity test (Fig. 3, C–F). The effects of rimonabant on tissue-specific insulin sensitivity were also similar, as tested using a hyperinsulinemic euglycemic clamp. Namely, rimonabant treatment reversed the marked hepatic insulin resistance without significantly altering glucose uptake into muscle and fat in both strains. These findings are compatible with rimonabant directly targeting hepatocyte CB1, the activation of which has been shown to induce hepatic insulin resistance by inhibiting insulin signaling via insulin receptor substrate-1 and Akt-2 and causing a parallel reduction in insulin clearance via downregulating the insulin-degrading enzyme (45). Therefore, the increase in plasma adiponectin by short-term rimonabant treatment is not causally related to the parallel improvement of insulin resistance and may be a consequence of the improved metabolic profile.
In agreement with the present findings, the reduction of body weight and adiposity by CB1 antagonist treatment was found to be independent of adiponectin in leptin-deficient ob/ob mice (71) and in DIO mice (46). Similar to the DIO mice tested in the present study, the insulin resistance of ob/ob mice was due primarily to hepatic insulin resistance, which was improved by rimonabant in both the presence and absence of adiponectin. However, the improvement caused by rimonabant treatment was significantly less in the absence than in the presence of adiponectin, indicating an additional adiponectin-dependent mechanism (71). The presence of such a component in ob/ob but not in DIO mice may be related to the different obesity models (genetic vs. diet-induced) or the different duration of rimonabant treatment (3 vs. 1 wk).
The present findings also differ from the results of Migrenne et al. (46), who used a DIO mouse model similar to that in the present study but found that rimonabant treatment for 4 wk resulted in decreased hepatic glucose production and increased glucose utilization in adipose tissue during an insulin clamp in Adipo+/+ mice, whereas no such effects were observed in Adipo−/− mice, which suggested an adiponectin-dependent mechanism. There are a number of factors that may account for the discrepant results. The DIO mice in the earlier study had a body weight of <40 g (46) compared with the ∼50 g of body weight in the present study. It has been shown that, despite being an inbred strain, C57Bl6 mice maintained on a HFD have a clearly bimodal body weight distribution, with the low (<40 g) and high body weight groups (45–55 g) also displaying qualitative differences in their metabolic profile (21, 51). It is possible that the requirement for adiponectin for the glycemic response to CB1 blockade is limited to mice that are partially resistant to HFD-induced obesity. It is also possible that an adiponectin-dependent component in the insulin-sensitizing effect of CB1 blockade emerges only following more prolonged treatment, such as that used in the earlier study. However, in the same study, rimonabant was able to completely reverse the baseline hyperinsulinemia in both Adipo+/+ and Adipo−/− mice (46), which complicates interpretation of these findings as it argues against adiponectin involvement in the improved insulin sensitivity.
In summary, the present findings indicate a differential role of adiponectin in the beneficial metabolic effects of CB1 antagonism in obesity/metabolic syndrome. Namely, adiponectin appears to mediate the reversal of hepatic steatosis and fibrosis by CB1 antagonism through reducing FFA uptake into the liver and inducing a PPARα/CPT-1-mediated increase in hepatic fatty acid oxidation, whereas the reduction in body weight and adiposity and improved glucose and insulin homeostasis resulting from short-term CB1 blockade are independent from adiponectin.
GRANTS
This work was supported by intramural funds from the National Institute on Alcohol Abuse and Alcoholism, NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.T. and G.K. contributed to the conception and design of the research; J.T., G.G., B.J.E., and L.Z. performed the experiments; J.T., G.G., and B.J.E. analyzed the data; J.T., G.G., B.J.E., T.J., G.S., R.C., and G.K. interpreted the results of the experiments; J.T. prepared the figures; J.T. drafted the manuscript; J.T. and G.K. edited and revised the manuscript; J.T., G.G., L.Z., T.J., G.S., and G.K. approved the final version of the manuscript.
ACKNOWLEDGMENTS
Address for correspondence for J. Tam after June 2014: Obesity and Metabolism Laboratory, School of Pharmacy, Institute for Drug Research, Faculty of Medicine, The Hebrew University of Jerusalem, Jerusalem, 91120, Israel (e-mail: yossit@ekmd.huji.ac.il).
REFERENCES
- 1.Aller R, de Luis DA, Pacheco D, Velasco MC, Conde R, Izaola O, González Sagrado M. Influence of G1359A polimorphysm of the cannabinoid receptor gene (CNR1) on insulin resistance and adipokines in patients with non alcoholic fatty liver disease. Nutr Hosp 27: 1637–1642, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Altinova AE, Toruner F, Bukan N, Yasar DG, Akturk M, Cakir N, Arslan M. Decreased plasma adiponectin is associated with insulin resistance and HDL cholesterol in overweight subjects. Endocr J 54: 221–226, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol 87: 1–16, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79–83, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Asano T, Watanabe K, Kubota N, Gunji T, Omata M, Kadowaki T, Ohnishi S. Adiponectin knockout mice on high fat diet develop fibrosing steatohepatitis. J Gastroenterol Hepatol 24: 1669–1676, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bajaj M, Suraamornkul S, Piper P, Hardies LJ, Glass L, Cersosimo E, Pratipanawatr T, Miyazaki Y, DeFronzo RA. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab 89: 200–206, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Balmer ML, Joneli J, Schoepfer A, Stickel F, Thormann W, Dufour JF. Significance of serum adiponectin levels in patients with chronic liver disease. Clin Sci (Lond) 119: 431–436, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell-Anderson KS, Aouad L, Williams H, Sanz FR, Phuyal J, Larter CZ, Farrell GC, Caterson ID. Coordinated improvement in glucose tolerance, liver steatosis and obesity-associated inflammation by cannabinoid 1 receptor antagonism in fat Aussie mice. Int J Obes (Lond) 35: 1539–1548, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrie P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 63: 908–914, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7: 947–953, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Bergholm R, Sevastianova K, Santos A, Kotronen A, Urjansson M, Hakkarainen A, Lundbom J, Tiikkainen M, Rissanen A, Lundbom N, Yki-Järvinen H. CB(1) blockade-induced weight loss over 48 weeks decreases liver fat in proportion to weight loss in humans. Int J Obes (Lond) 37: 699–703, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Chanda D, Kim YH, Kim DK, Lee MW, Lee SY, Park TS, Koo SH, Lee CH, Choi HS. Activation of cannabinoid receptor type 1 (Cb1r) disrupts hepatic insulin receptor signaling via cyclic AMP-response element-binding protein H (Crebh)-mediated induction of Lipin1 gene. J Biol Chem 287: 38041–38049, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SW, Wu BY, Xu SP, Fan KX, Yan L, Gong Y, Wen JB, Wu DH. Suppression of CB1 cannabinoid receptor by lentivirus mediated small interfering RNA ameliorates hepatic fibrosis in rats. PLoS One 7: e50850, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cinar R, Godlewski G, Liu J, Tam J, Jourdan T, Mukhopadhyay B, Harvey-White J, Kunos G. Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology 59: 143–153, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest 108: 1875–1881, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLeve LD, Wang X, Kanel GC, Atkinson RD, McCuskey RS. Prevention of hepatic fibrosis in a murine model of metabolic syndrome with nonalcoholic steatohepatitis. Am J Pathol 173: 993–1001, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353: 2121–2134, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Després JP, Ross R, Boka G, Alméras N, Lemieux I; ADAGIO-Lipids Investigators Effect of rimonabant on the high-triglyceride/low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: the ADAGIO-Lipids trial. Arterioscler Thromb Vasc Biol 29: 416–423, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410: 822–825, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5: 181–194, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Flamment M, Gueguen N, Wetterwald C, Simard G, Malthiery Y, Ducluzeau PH. Effects of the cannabinoid CB1 antagonist rimonabant on hepatic mitochondrial function in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 297: E1162–E1170, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 24.Fong TM, Guan XM, Marsh DJ, Shen CP, Stribling DS, Rosko KM, Lao J, Yu H, Feng Y, Xiao JC, Van der Ploeg LH, Goulet MT, Hagmann WK, Lin LS, Lanza TJ, Jr, Jewell JP, Liu P, Shah SK, Qi H, Tong X, Wang J, Xu SS, Francis B, Strack AM, MacIntyre DE, Shearman LP. Antiobesity efficacy of a novel cannabinoid-1 receptor inverse agonist, N-[(1S,2S)-3-(4-chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-[[5-(trifluoromethyl)pyridin-2-yl]oxy]propanamide (MK-0364), in rodents. J Pharmacol Exp Ther 321: 1013–1022, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98: 2005–2010, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet-Trillou C, Chabbert M, Cruccioli N, Pfersdorff C, Roque C, Arnone M, Croci T, Soubrie P, Oury-Donat F, Maffrand JP, Scatton B, Lacheretz F, Le Fur G, Herbert JM, Bensaid M. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology 46: 122–129, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Gary-Bobo M, Elachouri G, Scatton B, Le Fur G, Oury-Donat F, Bensaid M. The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits cell proliferation and increases markers of adipocyte maturation in cultured mouse 3T3 F442A preadipocytes. Mol Pharmacol 69: 471–478, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Ge F, Zhou S, Hu C, Lobdell Ht, Berk PD. Insulin- and leptin-regulated fatty acid uptake plays a key causal role in hepatic steatosis in mice with intact leptin signaling but not in ob/ob or db/db mice. Am J Physiol Gastrointest Liver Physiol 299: G855–G866, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ge Q, Maury E, Rycken L, Gérard J, Noël L, Detry R, Navez B, Brichard SM. Endocannabinoids regulate adipokine production and the immune balance of omental adipose tissue in human obesity. Int J Obes (Lond) 37: 874–880, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Giannone FA, Baldassarre M, Domenicali M, Zaccherini G, Trevisani F, Bernardi M, Caraceni P. Reversal of liver fibrosis by the antagonism of endocannabinoid CB1 receptor in a rat model of CCl(4)-induced advanced cirrhosis. Lab Invest 92: 384–395, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest 109: 1381–1389, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handy JA, Fu PP, Kumar P, Mells JE, Sharma S, Saxena NK, Anania FA. Adiponectin inhibits leptin signalling via multiple mechanisms to exert protective effects against hepatic fibrosis. Biochem J 440: 385–395, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20: 1595–1599, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271: 10697–10703, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, Penarier G, Soubrie P, Le Fur G, Galiegue S, Casellas P. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J 19: 1567–1569, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Jourdan T, Demizieux L, Gresti J, Djaouti L, Gaba L, Verges B, Degrace P. Antagonism of peripheral hepatic cannabinoid receptor-1 improves liver lipid metabolism in mice: evidence from cultured explants. Hepatology 55: 790–799, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Jourdan T, Djaouti L, Demizieux L, Gresti J, Verges B, Degrace P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes 59: 926–934, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamada Y, Matsumoto H, Tamura S, Fukushima J, Kiso S, Fukui K, Igura T, Maeda N, Kihara S, Funahashi T, Matsuzawa Y, Shimomura I, Hayashi N. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J Hepatol 47: 556–564, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H, Okamoto Y, Kihara S, Miyagawa J, Shinomura Y, Funahashi T, Matsuzawa Y. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology 125: 1796–1807, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut 54: 117–121, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89: 2548–2556, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Kim SP, Woolcott OO, Hsu IR, Stefanoski D, Harrison LN, Zheng D, Lottati M, Kolka C, Catalano KJ, Chiu JD, Kabir M, Ionut V, Bergman RN, Richey JM. CB1 antagonism restores hepatic insulin sensitivity without normalization of adiposity in diet-induced obese dogs. Am J Physiol Endocrinol Metab 302: E1261–E1268, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koonen DP, Jacobs RL, Febbraio M, Young ME, Soltys CL, Ong H, Vance DE, Dyck JR. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes 56: 2863–2871, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Kunos G, Tam J. The case for peripheral CB(1) receptor blockade in the treatment of visceral obesity and its cardiometabolic complications. Br J Pharmacol 163: 1423–1431, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Zhou L, Xiong K, Godlewski G, Mukhopadhyay B, Tam J, Yin S, Gao P, Shan X, Pickel J, Bataller R, O'Hare J, Scherer T, Buettner C, Kunos G. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology 142: 1218–1228.e1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Migrenne S, Lacombe A, Lefèvre AL, Pruniaux MP, Guillot E, Galzin AM, Magnan C. Adiponectin is required to mediate rimonabant-induced improvement of insulin sensitivity but not body weight loss in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 296: R929–R935, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Nam DH, Lee MH, Kim JE, Song HK, Kang YS, Lee JE, Kim HW, Cha JJ, Hyun YY, Kim SH, Han SY, Han KH, Han JY, Cha DR. Blockade of cannabinoid receptor 1 improves insulin resistance, lipid metabolism, and diabetic nephropathy in db/db mice. Endocrinology 153: 1387–1396, 2012 [DOI] [PubMed] [Google Scholar]
- 48.O'Hare JD, Zielinski E, Cheng B, Scherer T, Buettner C. Central endocannabinoid signaling regulates hepatic glucose production and systemic lipolysis. Diabetes 60: 1055–1062, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 115: 1298–1305, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58: 389–462, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peyot ML, Pepin E, Lamontagne J, Latour MG, Zarrouki B, Lussier R, Pineda M, Jetton TL, Madiraju SR, Joly E, Prentki M. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes 59: 2178–2187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J; RIO-North America Study Group Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 295: 761–775, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Pohl J, Ring A, Stremmel W. Uptake of long-chain fatty acids in HepG2 cells involves caveolae: analysis of a novel pathway. J Lipid Res 43: 1390–1399, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 284: R345–R353, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrié P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 28: 640–648, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Rosenstock J, Hollander P, Chevalier S, Iranmanesh A; SERENADE Study Group SERENADE: the Study Evaluating Rimonabant Efficacy in Drug-naive Diabetic Patients: effects of monotherapy with rimonabant, the first selective CB1 receptor antagonist, on glycemic control, body weight, and lipid profile in drug-naive type 2 diabetes. Diabetes Care 31: 2169–2176, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savvidou S, Hytiroglou P, Orfanou-Koumerkeridou H, Panderis A, Frantzoulis P, Goulis J. Low serum adiponectin levels are predictive of advanced hepatic fibrosis in patients with NAFLD. J Clin Gastroenterol 43: 765–772, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Shearman LP, Stribling DS, Camacho RE, Rosko KM, Wang J, Tong S, Feng Y, Marsh DJ, Yu H, Guan X, Spann SK, Macneil DJ, Fong TM, Metzger JM, Goulet MT, Hagmann WK, Plummer CW, Finke PE, Mills SG, Shah SK, Truong Q, Van der Ploeg LH, Macintyre DE, Strack AM. Characterization of a novel and selective cannabinoid CB1 receptor inverse agonist, Imidazole 24b, in rodents. Eur J Pharmacol 579: 215–224, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Son MH, Kim HD, Chae YN, Kim MK, Shin CY, Ahn GJ, Choi SH, Yang EK, Park KJ, Chae HW, Moon HS, Kim SH, Shin YG, Yoon SH. Peripherally acting CB1-receptor antagonist: the relative importance of central and peripheral CB1 receptors in adiposity control. Int J Obes (Lond) 34: 547–556, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Song D, Bandsma RH, Xiao C, Xi L, Shao W, Jin T, Lewis GF. Acute cannabinoid receptor type 1 (CB1R) modulation influences insulin sensitivity by an effect outside the central nervous system in mice. Diabetologia 54: 1181–1189, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 187: 15–24, 1956 [DOI] [PubMed] [Google Scholar]
- 62.Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, Szanda G, Mukhopadhyay B, Chedester L, Liow JS, Innis RB, Cheng K, Rice KC, Deschamps JR, Chorvat RJ, McElroy JF, Kunos G. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab 16: 167–179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, Kunos G. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120: 2953–2966, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, Serriere-Lanneau V, Ledent C, Mallat A, Lotersztajn S. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med 12: 671–676, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Thornton-Jones ZD, Kennett GA, Benwell KR, Revell DF, Misra A, Sellwood DM, Vickers SP, Clifton PG. The cannabinoid CB1 receptor inverse agonist, rimonabant, modifies body weight and adiponectin function in diet-induced obese rats as a consequence of reduced food intake. Pharmacol Biochem Behav 84: 353–359, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 99: 16309–16313, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Topol EJ, Bousser MG, Fox KA, Creager MA, Despres JP, Easton JD, Hamm CW, Montalescot G, Steg PG, Pearson TA, Cohen E, Gaudin C, Job B, Murphy JH, Bhatt DL. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet 376: 517–523, 2010 [DOI] [PubMed] [Google Scholar]
- 68.Van Gaal L, Pi-Sunyer X, Despres JP, McCarthy C, Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care 31, Suppl 2: S229–S240, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Van Gaal LF, Scheen AJ, Rissanen AM, Rossner S, Hanotin C, Ziegler O. Long-term effect of CB1 blockade with rimonabant on cardiometabolic risk factors: two year results from the RIO-Europe Study. Eur Heart J 29: 1761–1771, 2008 [DOI] [PubMed] [Google Scholar]
- 70.VanSaun MN, Lee IK, Washington MK, Matrisian L, Gorden DL. High fat diet induced hepatic steatosis establishes a permissive microenvironment for colorectal metastases and promotes primary dysplasia in a murine model. Am J Pathol 175: 355–364, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watanabe T, Kubota N, Ohsugi M, Kubota T, Takamoto I, Iwabu M, Awazawa M, Katsuyama H, Hasegawa C, Tokuyama K, Moroi M, Sugi K, Yamauchi T, Noda T, Nagai R, Terauchi Y, Tobe K, Ueki K, Kadowaki T. Rimonabant ameliorates insulin resistance via both adiponectin-dependent and adiponectin-independent pathways. J Biol Chem 284: 1803–1812, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86: 1930–1935, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Wierzbicki AS, Pendleton S, McMahon Z, Dar A, Oben J, Crook MA, Botha AJ. Rimonabant improves cholesterol, insulin resistance and markers of non-alcoholic fatty liver in morbidly obese patients: a retrospective cohort study. Int J Clin Pract 65: 713–715, 2011 [DOI] [PubMed] [Google Scholar]
- 74.Wu HM, Yang YM, Kim SG. Rimonabant, a cannabinoid receptor type 1 inverse agonist, inhibits hepatocyte lipogenesis by activating liver kinase B1 and AMP-activated protein kinase axis downstream of Galpha i/o inhibition. Mol Pharmacol 80: 859–869, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112: 91–100, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001 [DOI] [PubMed] [Google Scholar]
- 79.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 55: 2562–2570, 2006 [DOI] [PubMed] [Google Scholar]
- 80.You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 42: 568–577, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Youn JH, Kim JK, Buchanan TA. Time courses of changes in hepatic and skeletal muscle insulin action and GLUT4 protein in skeletal muscle after STZ injection. Diabetes 43: 564–571, 1994 [DOI] [PubMed] [Google Scholar]