Abstract
Background
Previous studies on membranoproliferative glomerulonephritis (MPGN) and cryoglobulinemic glomerulopathy (CG) were based upon case series that were performed before hepatitis C virus (HCV) infection was routinely investigated. Therefore, it remains unknown how far HCV contributes to MPGN or CG, and there have only been a few reports about HCV-negative idiopathic MPGN.
Patients and methods
Thirty-five patients with MPGN diagnosed by renal biopsy who underwent examination for HCV infection at our institute between 1990 and 2008 were recruited for this study. Patients with HCV infection at presentation were included, but patients with complications such as underlying lymphoproliferative disorders, autoimmune diseases like lupus nephritis, infection, and liver disease due to hepatitis B virus or alcohol abuse were excluded. A total of 35 patients were enrolled and they were divided into two groups according to the presence/absence of circulating cryoglobulins (cryo). The 23 patients who had cryo-negative and HCV-negative idiopathic MPGN were divided into subgroups with type 1 and type 3 disease.
Results
In the cryo-positive group (n = 9), 7 patients were positive for HCV infection, while 2 patients were negative. In the cryo-negative group (n = 26), 3 patients were positive for HCV infection, while 23 patients were negative (idiopathic MPGN). Compared with the cryo-negative group, the cryo-positive group had several characteristics such as more severe thrombocytopenia, higher serum immunoglobulin (Ig)G and IgM levels, lower levels of hemolytic complement (CH50) and complement component (C)4, predominant IgM staining, and type 1 histology. Patients with cryo-negative and HCV-negative ‘idiopathic’ MPGN showed predominant staining for IgG in both type 1 and type 3 cases, unlike the predominant staining for IgM in the cryo-positive group. Compared with type 3 cases, type 1 cases had a younger age, lower levels of CH50, C3 and C4, and less proteinuria. In the cryo-positive group, 4 patients (44.4 %) died, with death from B cell lymphoma and liver failure in 2 patients each, while 1 patient (8 %) developed end-stage renal failure requiring dialysis. In contrast, all patients in the cryo-negative group remained alive during follow-up, although 4 patients (2 type 1 cases and 2 type 3 cases) required dialysis.
Conclusion
Cryo-positive MPGN shows a close relationship with HCV infection and IgM, resulting in a poor prognosis. Cryo-negative and HCV-negative idiopathic MPGN has a close relationship with IgG staining, and type 1 cases feature characteristics such as a younger age, more severe hypocomplementemia, and less proteinuria than in type 3 cases.
Keywords: Membranoproliferative glomerulonephritis, Type 1 and type 3, Type 2, Cryoglobulin, Cryoglobulinemic glomerulopathy
Introduction
Cryoglobulins are serum proteins that are soluble at 37 °C, precipitate at lower temperatures, and dissolve again when heated. Renal disease in patients with cryoglobulinemia (cryo) is called cryoglobulinemic glomerulopathy (CG), and is usually the type 2 mixed form due to immune complexes formed by immunoglobulin (Ig)M directed against the Fc portion of polyclonal IgG. Cryo that is not secondary to lymphoproliferative disorders, autoimmune diseases such as systemic lupus erythematosus (SLE), or infection used to be called ‘essential’ [1–4]. However, Pascual et al. suggested an association between hepatitis C virus (HCV) and cryo in 1990 [5], after which Johnson et al. reported that chronic HCV infection is associated with cryo-positive membranoproliferative glomerulonephritis (MPGN) in 1993 [6]. Thus, many cases of CG that had been considered essential are now thought to be due to chronic HCV infection. However, Tervaert et al. [7] reported true essential CG of unknown etiology with negativity for HCV.
MPGN is histologically characterized by diffuse mesangial proliferation and thickening of the capillary walls, and three histopathological forms have been identified based upon electron microscopic findings. Type 1 features electron dense deposits (EDD) in the mesangium as well as in the subendothelial spaces, type 2 displays EDD on the glomerular basement membrane, and type 3 is characterized by EDD in the subepithelial spaces in addition to the mesangium and subendothelial spaces. Among these three types, type 1 is the most common [3, 8, 9].
A diagnosis of CG requires the histology of MPGN together with positivity for cryo, but histological findings specific to CG have also been reported [1–4]. Since textbook information on MPGN and CG is only based on case series and was acquired before testing could be performed routinely for HCV [10], the actual relationships among MPGN, CG, and HCV have not been fully elucidated.
In this study, MPGN was assessed in relation to the presence of cryo and HCV, and idiopathic MPGN without cryo or HCV infection was compared between type 1 and type 3.
Methods
Patients
Fifty-three patients were diagnosed as having MPGN by renal biopsy between 1990 and 2008 at our institution. Eighteen patients were excluded due to lymphoproliferative disorders, autoimmune diseases such as SLE, infectious diseases such as post-streptococcal glomerulonephritis, and liver disease due to hepatitis B virus infection or alcohol abuse. The remaining 35 patients (20 male, 15 female; age range 8−84 years), including 10 patients who showed positivity for HCV, were recruited for this study. The patients were divided into two groups according to the presence/absence of circulating cryoglobulins (cryo-positive and cryo-negative groups). The medical records of the subjects were reviewed retrospectively.
Study procedures
Histological evaluation
Renal biopsy specimens were processed for light microscopy (LM), immunofluorescence microscopy (IF), and electron microscopy (EM). Specimens for LM were fixed in 6 % formalin, embedded in paraffin, cut into 1–2 µm sections, and stained with hematoxylin and eosin (H&E), periodic acid Schiff (PAS), Weigert’s elastica-van Gieson, Masson trichrome, or periodic acid methanamine silver (PAM) stain. Specimens for IF were snap-frozen in a mixture of dry ice and acetone, and were cut into 3–4 µm sections on a Damon/IEC cryostat at −20 °C. After being fixed in acetone, the sections were incubated with fluorescein isothiocyanate-conjugated (FITC) rabbit antiserum directed against human IgG, IgA, and IgM, as well as complement component (C) 1q, C3, and C4 (Behringwerke, West Germany, and Fuji Zoki, Japan), in a moist chamber at 37 °C for 30 min. The slides were then examined under an Olympus fluorescence microscope (Japan) equipped with optimal excitation and barrier filters for FITC. For EM, renal biopsy cores were preserved in 3 % phosphate-buffered glutaraldehyde, diced into 1-mm cubes, rinsed in distilled water, transferred to 1 % aqueous osmium tetraoxide, and embedded in TAAB Emix resin. Sections were cut at 0.5 µm, mounted on glass slides, and stained with 1 % aqueous toluidine blue in 1 % sodium tetraborate for 15 s on a hot plate at 15 °C. After cooling, light microscopy was performed to find assessable glomeruli. The sections were then cut with a diamond knife on a Leica Ultracut E ultramicrotome, and were coated with gold particles of approximately 95 nm in diameter. Subsequently the sections were stained by immersion for 7 min in 50 % alcohol saturated uranyl water and 3 min in Reynolds lead citrate, followed by three washes in distilled water. The sections were then examined under a Philips 400 transmission electron microscope.
LM revealed MPGN with an increase of cellularity and capillary duplication showing a lobular pattern [3, 7, 8]. IF evaluated the presence of IgG, IgM, IgA and C3.
The type of MPGN was determined by EM—type 1 was diagnosed when EDD were detected mainly in the subendothelial spaces of the glomerular capillaries, while type 3 featured EDD in the subepithelial and subendothelial spaces. Type 2 (EDD largely replacing the lamina of the glomerular capillary basement membranes) was not included in this study.
Blood and urine analyses
Before renal biopsy, blood and urine samples were collected from all patients. Laboratory tests were performed to measure serum creatinine, hemoglobin, platelet count, rheumatoid factor, cryoglobulin, IgG, IgA, IgM, 50 % hemolytic complement (CH50) (normal range 32–47 U/mL), C3 (normal range 65–135 mg/dL), and C4 (normal range 13–35 mg/dL). Urine tests included assessment of 24-h protein excretion and assessment of hematuria [red blood cells per high-power field (RBC/HPF) in resuspended sediment—grade 1 (<1), grade 2 [1–5], grade 3 [6–10], grade 4 (11–30), and grade 5 (>30)]. Serum HCV antibody was evaluated by an enzyme-linked immunosorbent assay (ELISA; Abbott Diagnostics, Maidenhead, UK). Anti-HBV antibody was detected with a commercially available ELISA kit.
Detection of cryoglobulins
Each venous blood sample was promptly injected into a preheated glass test tube and maintained at 37 °C until the cells and serum were separated in the laboratory. The serum was then allowed to stand at 4 °C for at least 72 h in a hematocrit tube. If agglutination or gelation was detected and dissolution occurred on heating, the presence of cryoglobulins was confirmed. The precipitate/serum volume ratio was measured as the cryocrit [11]. The composition of the cryoprecipitate was characterized by immunofixation electrophoresis.
Statistical analysis
Statistical analysis was performed using the chi-squared test. Quantitative values were expressed as the mean ± SD, and differences were compared by Wilcoxon’s rank sum test. A probability value <0.05 was considered to indicate statistical significance. The SPSS software package (SPSS 11.0 for windows; SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
Comparison of the cryo-positive and cryo-negative groups
The 35 patients were divided into two groups based on positivity for cryo. Nine patients (25.7 %) were positive for MC and 26 patients (74.3 %) were negative for MC (Table 1).
Table 1.
Comparison of the cryo-positive and cryo-negative groups
| Cryo-positive group | Cryo-negative group | P value | |
|---|---|---|---|
| Number | 9 | 26 | |
| Age (years) |
54.5 ± 11.3 (27–69) |
37.5 ± 20.7 (8–84) |
<0.01 |
| Sex |
Male 4 Female 5 |
Male 16 Female 10 |
ns |
| Primary disease |
Idio (n = 2) HCV (n = 7) |
Idio (n = 23) HCV (n = 3) |
<0.01 |
| Observation period (years) |
6 ± 4.1 (3–17) |
8 ± 5.9 (3–22) |
ns |
| Serum creatinine (mg/dL) |
1.0 ± 0.6 (0.5–2.7) |
1.3 ± 0.9 (0.4–5.2) |
ns |
| Platelet (×103/μL) |
145.8 ± 66.4 (60–275) |
227.6 ± 69.2 (41–405) |
<0.001 |
| Serum IgG (mg/dL) |
1748.5 ± 1111.2 (552–4628) |
960.1 ± 459.8 (117–2139) |
<0.01 |
| Serum IgM (mg/dL) |
253.3 ± 145.7 (98–682) |
148.7 ± 82.6 (44.6–380) |
<0.01 |
| Serum IgA (mg/dL) |
264.5 ± 98.4 (110–513) |
255.1 ± 147.8 (53.3–718) |
ns |
| CH50 (U/mL) |
19.1 ± 14.5 (1–42.0) |
34.7 ± 13.1 (9–57) |
<0.001 |
| CH50 (% of patients with a decreased level <31) |
n = 7 (77.8 %) |
n = 10 (38.5 %) |
<0.01 |
| C3 (mg/dL) |
56.7 ± 36.2 (2–130) |
63.3 ± 27.6 (6.2–126) |
ns |
| C3 (% of patients with a decreased level <65) |
n = 6 (66.7 %) |
n = 15 (57.7 %) |
ns |
| C4 (mg/dL) |
13.6 ± 8.54 (3.9–33.6) |
24.5 ± 14.9 (3–64) |
<0.05 |
| C4 (% of patients with a decreased level <12) |
n = 7 (77.8 %) |
n = 5 (19.2 %) |
<0.001 |
| Proteinuria (g/day) |
4.5 ± 3.9 (0.16–11) |
3.99 ± 3.8 (0.21–18.6) |
ns |
| Hematuria (>10 RBC/HPF) |
2.8 ± 1.6 (1–5) |
3.1 ± 1.5 (1–5) |
ns |
Idio idiopathic, ns not significant
In the cryo-positive group, the age ranged from 27−69 years (mean ± SD, 54.5 ± 11.3). In the cryo-negative group, the age ranged from 8−84 years (mean ± SD, 37.5 ± 20.7). The mean age of the cryo-positive group was significantly higher than that of the cryo-negative group (P = 0.007).
In the cryo-positive group, purpura of the lower extremities specific to CG was noted in two patients with a cryocrit of >10 %. One patient showed leukocytoclastic vasculitis with positive IgM staining of the skin biopsy specimen. No symptoms specific to CG were noted in 7 patients with a cryocrit of <5 %. Purpura was not seen in the cryo-negative group.
In the cryo-positive group, 7 patients (78 %) were positive for HCV, while 2 patients (22 %) were negative for HCV and were considered to have idiopathic cryoglobulinemia because no primary disease causing MC was detected. In the cryo-negative group, 3 patients (10.7 %) were positive for HCV, while 23 patients (89.3 %) were negative and had idiopathic disease.
The white blood cell count and red blood cell count (including hemoglobin) showed no significant differences between the two groups, but the platelet count of the cryo-positive group was significantly lower than that of the cryo-negative group (145.8 ± 66.4 × 103/µL vs 227.6 ± 69.2 × 103/µL, P = 0.0009).
Serum IgG was significantly higher in the cryo-positive group than in the cryo-negative group (1749 ± 1111 mg/dL vs 960 ± 460 mg/dL, P < 0.007). Serum IgM was also significantly higher in the cryo-positive group than in the cryo-negative group (253 ± 145 mg/dL vs 149 ± 83 mg/dL, P < 0.006). Conversely, CH50 and C4 were significantly lower in the cryo-positive group than in the cryo-negative group (19.1 ± 14.5 U/mL and 13.6 ± 8.5 mg/dL vs 34.7 ± 13.1 U/mL and 24.5 ± 14.9 mg/dL, P < 0.001 and P < 0.05, respectively), while C3 showed no significant difference between the two groups. The percentage of patients with a low level of CH50 (<31 U/mL) or C4 (<12 mg/dL) was significantly higher in the cryo-positive group than in the cryo-negative group (77.8 and 77.8 % vs 38.5 and 19.2 %, P < 0.01 and P < 0.001, respectively), but the percentage of patients with a low level of C3 (<65 mg/dL) showed no significant difference between the two groups.
Histological findings (Tables 2 and 3)
Table 2.
EM findings between the cryo-positive and cryo-negative groups
| Cryo-positive group (n = 9) | Cryo-negative group (n = 26) |
|
|---|---|---|
|
Type 1 Mesangial and subendothelial deposits |
8 (HCV 6, idio 2) |
14 (HCV 3, idio11) |
|
Type 3 Subepithelial and subendothelial deposits |
1 (HCV 1) |
12 (idio) |
Idio idiopathic
Table 3.
IF findings between the cryo-positive and cryo-negative groups
| Cryo-positive group (n = 9) | Cryo-negative group (n = 26) | |
|---|---|---|
| IgG dominant |
1 (idio 1) Type 1 (n = 1) |
14 (idio 14) Type 1 (n = 5) Type 3 (n = 9) |
| IgM dominant |
6 (HCV 5, idio 1) Ttype 1 (n = 5) (HCV 4, idio 1) Type 3 (n = 1) (HCV1) |
1 (idio 1) Type 1 (n = 1) |
| IgA dominant | 0 |
2 (HCV 2) Type 1 (n = 2) |
|
IgG, IgM Equally |
2 (HCV2) Type 1 (n = 2) |
1 (idio 19) Type 1 (n = 1) |
| IgM, IgA equally | 0 |
2 (HCV1, idio 1) Type 1 (n = 2) |
| IgG, IgA | 0 |
2 (idio 2) Type 3 (n = 2) |
| Only C3 staining | 0 |
4 (idio 4) Type 1 (n = 3) Type 3 (n = 1) |
Idio idiopathic
In the cryo-positive group, 8 patients (89 %) had type 1 disease with subendothelial deposits, while 1 patient (11 %) had type 3 disease with both subendothelial and subepithelial deposits. Out of the 8 patients with type 1 disease, 6 were positive for HCV and the 1 patient with type 3 disease was also positive for HCV. In the cryo-negative group, 14 patients (53.8 %) were type 1 and 12 patients (46.2 %) were type 3. Out of the 14 patients with type 1 disease, 3 were positive for HCV and 11 patients were idiopathic. Among the patients with type 3 disease, all 12 were idiopathic (Table 2). Large thrombus-like deposits specific to CG were confirmed in 4 out of 9 patients from the cryo-positive group.
IF examination disclosed positive staining for C3 in all cryo-positive and cryo-negative patients (Table 3). In the cryo-positive group, 6 patients (87.8 %) were predominantly positive for IgM (Fig. 1), 1 patient showed predominant staining for IgG, and 2 patients showed equal staining for both IgG and IgM. In the cryo-negative group, 14 patients were predominantly positive for IgG (Fig. 2), 1 patient showed predominant staining for IgM, and 2 patients had predominant staining for IgA. In addition, 1 patient showed equal staining for IgG and IgM, 2 patients were equal for IgM and IgA, and 2 patients were equal for IgG and IgA. Four patients only showed positivity for c3. There were 3 cryo-negative and HCV-positive patients, among whom 2 were predominantly positive for IgA and 1 showed equal staining for IgA and IgM.
Fig. 1.
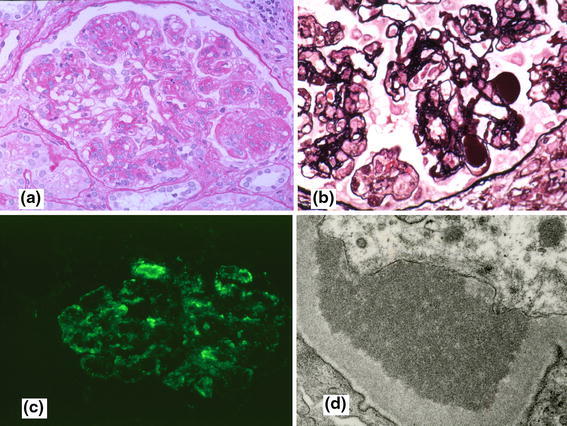
Histology of a 61-year-old female with cryo-positive type 1 MPGN. There is accentuation of glomerular lobulation (a), glomerular capillaries filled with thrombi (b), granular staining of the glomerular capillary walls for IgM (c), and subendothelial deposits with organized tubular structures (d). a PAS (×40). b PAM (×80). c IF (×40). d EM (×10,000)
Fig. 2.
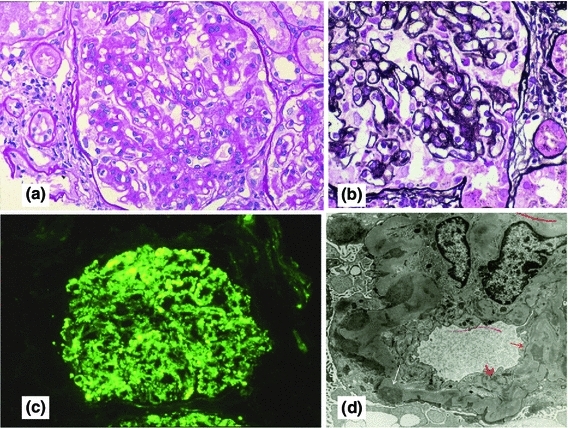
Histology of a 56-year-old female with cryo-negative idiopathic type 3 MPGN. There is a global increase of cellularity in the glomeruli with accentuation of the lobular pattern (a, b). Granular staining of the glomerular capillary walls for IgG (c). Subendothelial (red arrow) and subepithelial (white arrow) deposits with mesangial interposition (d). a PAS (×40), b PAM (×60), c IF (×40), d EM (×3,000)
In 5 out of 9 patients from the cryo-positive group, thick-walled microtubular structures were confirmed within the subendothelial EDD.
Idiopathic MPGN (type 1 vs type 3) (Tables 4 and 5)
Table 4.
IF findings between type 1 and type 2
| Type 1 (n = 11) | Type 3 (n = 12) | |
|---|---|---|
| IgG dominant | n = 5 | n = 9 |
| IgM dominant | 1 | 0 |
| IgG, IgA equally | 0 | 2 |
| IgA, IgM | 1 | 0 |
| IgG, IgM | 1 | 0 |
| Only C3 staining | 3 | 1 |
Table 5.
Clinical findings between type 1 and type 2
| Type 1 (n = 11) | Type 3 (n = 12) | P value | |
|---|---|---|---|
| Age |
30.1 ± 23.4 (8–75) |
49.7 ± 22.4 (8–84) |
<0.05 |
| Sex (M/F) | 8/3 | 9/3 | ns |
| CH50 |
27.9 ± 12.5 (9–47) |
39.6 ± 12.3 (14–52) |
<0.05 |
| CH50 (% of patients with a decreased level <31) |
n = 7 (63.6 %) |
n = 2 (16.7 %) |
<0.01 |
| C3 |
49 ± 26 (14–96) |
72 ± 25 (37–126) |
<0.05 |
| C3 (% of patients with a decreased level <65) |
n = 10 (90.9 %) |
n = 6 (50 %) |
<0.05 |
| C4 |
17.8 ± 12.6 (5–47) |
28.7 ± 13.2 (5–44) |
<0.05 |
| C4 (% of patients with a decreased level <12) |
n = 4 (36.4 %) |
n = 1 (8.3 %) |
<0.05 |
| Cre |
1.12 ± 0.5 (0.6–1.8) |
1.35 ± 0.78 (0.7–3.6) |
ns |
| U-pro |
2.8 ± 2.8 (0.48–9.5) |
4.29 ± 2.57 (0.86–7.72) |
<0.05 |
| Hematuria |
3.5 ± 1.4 (1–5) |
3.0 ± 1.0 (1–5) |
ns |
ns not significant, Cre creatinine, U-pro urine protein
Out of 26 cryo-negative patients, 3 HCV-positive patients were excluded, and the remaining 23 patients with idiopathic MPGN were investigated to determine the features of type 1 and type 3 disease (Table 4). Type 1 cases included 5 patients with predominant staining for IgG, 1 patient with predominance staining for IgM, 1 patient with equal staining for IgA and IgM, 1 patient with equal staining for IgG and IgM, and 3 patients who only showed staining for C3 without any staining for IgG, IgA, or IgM. Type 3 cases included 9 patients with predominance staining for IgG, 2 patients with equal staining for IgG and IgA, and 1 patient who only had C3 staining.
Next, the clinical features of type 1 and type 3 cases were compared. Compared with type 3 cases, type 1 cases were younger (49.7 ± 22.4 vs 30.1 ± 23.4 years), and 5 out of 11 type 1 patients were <20 years versus 2 out of 12 type 3 patients. Serum complement levels were significantly lower in type 1 than in type 3 (CH50: 27.9 ± 12.5 vs 39.6± 12.3; C3: 49 ± 26 vs 72 ± 25; and C4: 17.8 ± 12.6 vs 28.7 ± 13.2, P < 0.05, respectively). The percentage of patients with reduced serum complement levels was significantly higher in type 1 than in type 3 (CH50: 63.6 vs 16.7 %; C3: 90.9 vs 50.0 %; and C4: 36.4 vs 8.3 %, P < 0.01, P < 0.05, and P < 0.05, respectively). Urinary protein excretion was also lower in type 1 than in type 3 (2.8 ± 2.8 vs 4.29 ± 2.57, P < 0.05, respectively).
Outcome
The outcome after the diagnosis of MPGN was evaluated over an average observation period of 7.7 ± 5.3 years (range 3–20). The cryo-positive group was followed for a mean period of 6 ± 4.1 years (range 3–17) and the cryo-negative group was followed for mean period of 8 ± 5.9 years (range 3–22). Among 9 patients in the cryo-positive group, 4 patients (44.4 %) died, with death being due to B cell lymphoma and liver failure in 2 patients each. One patient (11 %) developed end-stage renal failure requiring dialysis. In contrast, all of the patients from the cryo-negative group remained alive during follow-up, although 4 patients (2 with type 1 and 2 with type 3) required dialysis.
Discussion
The histological findings specific to CG can be summarized as follows [1, 10]. LM shows glomerular lobulation with infiltration of monocytes into the capillary spaces and large deposits (referred to as thrombi). On IF, staining for IgM is often more intense than that for IgG. EM reveals EDD in the subendothelial and mesangial areas that are characterized by thick-walled microtubular or annular structure measuring 30 nm in diameter. In the present study, large thrombus-like deposits specific to CG were confirmed in 4 out of 9 patients from the cryo-positive group, and thick-walled microtubular structures were seen in the EDD of 5 patients. IgM-dominant staining was also seen, consistent with previous reports. Eight out of 9 patients were type 1, and 1 patient was type 3.
There has been little information available about the differences between type 1 and type 3 MPGN. The majority of patients with MPGN are reported to be children between the ages of 8 and 16 years, and type 1 occupies 90 % of MPGN [3, 8, 9]. Type 3 MPGN has been reported to occur in a small number of children and young adults, and it has clinical features quite similar to those of type 1 MPGN. The characteristic IF pattern of type 1 MPGN is peripheral granular to band-like staining for C3, with staining for immunoglobulins such as IgG, IgM, and IgA also being seen. Type 3 MPGN has similar features to type 1 MPGN. The above-mentioned features of MPGN are based upon reports published before testing for HCV was routine [3, 8, 9], and there have only been a few detailed studies of true HCV-negative MPGN [12]. In the present study, patients with type 1 idiopathic MPGN were younger, had more severe hypocomplementemia, and had less proteinuria compared with type 3 patients.
Recently, Nasr et al. reported a novel disease entity that is termed proliferative glomerulonephritis with monoclonal IgG deposits (PGNMID). Some of the immune-complex glomerulonephritides such as MPGN with IgG deposition are monoclonal, and staining reveals only a single subclass of IgG and a single light-chain isotype, which is most commonly IgG3 kappa. However, the majority of patients do not have an M-spike or a plasma cell dyscrasia. This type of monoclonal disease affects adults and is more common in white females [13]. In the future, when the position of PGNMID in relation to idiopathic MPGN is reviewed, accumulation of more information about idiopathic MPGN without cryo or HCV positivity may lead to re-evaluation of the relationship between these diseases.
Sethi et al. and Bomback, and Appel proposed a new classification of MPGN according to whether it was immunoglobulin-positive or -negative by IF [14, 15]. Immunoglobulin-positive MPGN suggests activation of the classical pathway and they divided it into infections (including HCV), immune complex diseases including lupus nephritis, neoplasms, and others based on the underlying cause of antigenemia. Immunoglobulin-negative C3-positive MPGN is due to dysregulation of the alternative pathway, and this was divided into dense deposit disease (DDD) and C3 glomerulonephritis (C3GN). DDD and C3GN are distinguishable by the appearance and localization of deposits on electron microscopy. However, their report did not discuss the significance of detecting different types of immunoglobulin, including IgG and IgM, and CG was also not mentioned.
In summary, when underlying diseases (including lymphoproliferative disorders, autoimmune diseases, infectious diseases such as post-streptococcal glomerulonephritis, and liver disease due to hepatitis B or alcohol abuse) are excluded, MPGN diagnosed by LM and EM can be divided into cases with deposition of C3 plus immunoglobulin (IgM dominant or IgG dominant) and cases with C3 deposition only. IgM-dominant deposition occurs in cryo-positive CG, which is either HCV-positive or HCV-negative (‘essential’). In contrast, the IgG-dominant type is cryo-negative and can be classified as PGNMID or ‘idiopathic’. If there is deposition of C3 only, the disease is classified as DDD or C3GN.
Conflict of interest
None.
References
- 1.D’Amico G, Colasanti G, Ferrario F, Sinico RA. Renal involvement in essential mixed cryoglobulinemia. Kidney Int. 1989;35:1004–14. [DOI] [PubMed]
- 2.Herrera GA, Picken MM. Cryoglobulinemic nephropathy. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall’s pathology of the kidney, 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 896–900.
- 3.Schena FP, Alpers CE. Membranoproliferative glomerulonephritis and cryoglobulinemic glomerulopathy. In: Feehally J, Floege J, Johnson RJ, editors. Comprehensive clinical nephropathy. 4. Mosby Elsevier: Philadelphia; 2010. pp. 260–269. [Google Scholar]
- 4.Appel GB, D’Agati VD. Secondary glomerular disease. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AL, Brenner BM, editors. Brenner & Rector’s The Kidney. 9. Elsevier Saunders: Philadelphia; 2012. pp. 1192–1277. [Google Scholar]
- 5.Pascual M, Perrin L, Giostra E, Schifferli JA. Hepatitis C virus in patients with cryoglobulinemia type II. J Infect Dis. 1990;162(2):569–570. doi: 10.1093/infdis/162.2.569. [DOI] [PubMed] [Google Scholar]
- 6.Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, Couser WG, Corey L, Wener MH, Alpers CE, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med. 1993;328(7):465–470. doi: 10.1056/NEJM199302183280703. [DOI] [PubMed] [Google Scholar]
- 7.Tervaert JW, Van Paassen P, Damoiseaux J. Type II cryoglobulinemia is not associated with hepatitis C infection: the Dutch experience. Ann N Y Acad Sci. 2007;1107:251–258. doi: 10.1196/annals.1381.027. [DOI] [PubMed] [Google Scholar]
- 8.Zhou XJ, Silva FG. Membranproliferative glomerulonephritis. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall’s pathology of the kidney; 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 253–307.
- 9.Nachman PH, Jennette C, Falk RJ. Primary glomerular disease. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AL, Brenner BM, editors. Brenner & Rector’s The Kidney. 9. Elsevier Saunders: Philadelphia; 2012. pp. 1100–1191. [Google Scholar]
- 10.Rennke HG. Secondary membranoproliferative glomerulonephritis. Kidney Int. 1995;47(2):643–656. doi: 10.1038/ki.1995.82. [DOI] [PubMed] [Google Scholar]
- 11.Ferri C, Sebastiani M, Giuggioli D, Cazzato M, Longombardo G, Antonelli A, Puccini R, Michelassi C, Zignego AL. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33(6):355–374. doi: 10.1016/j.semarthrit.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Yamabe H, Johnson RJ, Gretch DR, Fukushi K, Osawa H, Miyata M, Inuma H, Sasaki T, Kaizuka M, Tamura N, et al. Hepatitis C virus infection and membranoproliferative glomerulonephritis in Japan. J Am Soc Nephrol. 1995;6(2):220–223. doi: 10.1681/ASN.V62220. [DOI] [PubMed] [Google Scholar]
- 13.Nasr SH, Satoskar A, Markowitz GS, Valeri AM, Appel GB, Stokes MB, Nadasdy T, D’Agati VD. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20(9):2055–2064. doi: 10.1681/ASN.2009010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sethi S, Nester CM, Smith RJ. Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int. 2012;81(5):434–441. doi: 10.1038/ki.2011.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bomback AS, Appel GB. Pathogenesis of the C3 glomerulopathies and reclassification of MPGN. Nat Rev Nephrol. 2012;8(11):634–642. doi: 10.1038/nrneph.2012.213. [DOI] [PubMed] [Google Scholar]


