Abstract
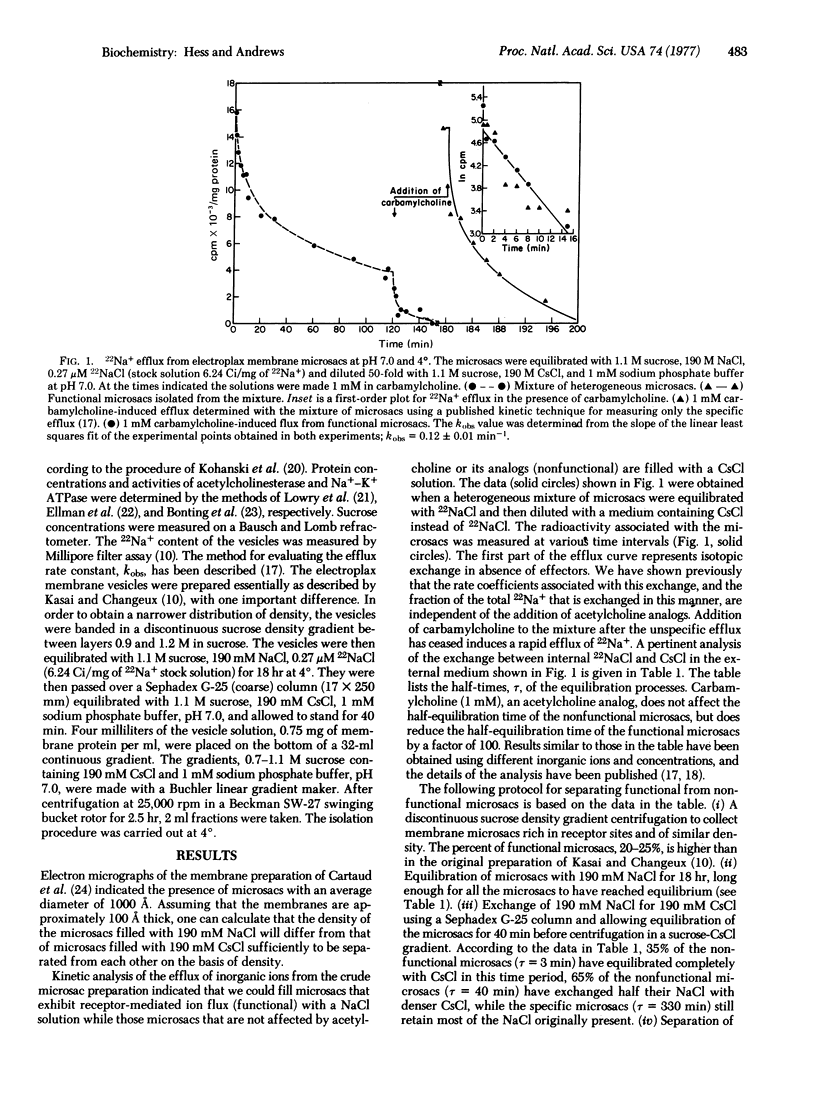
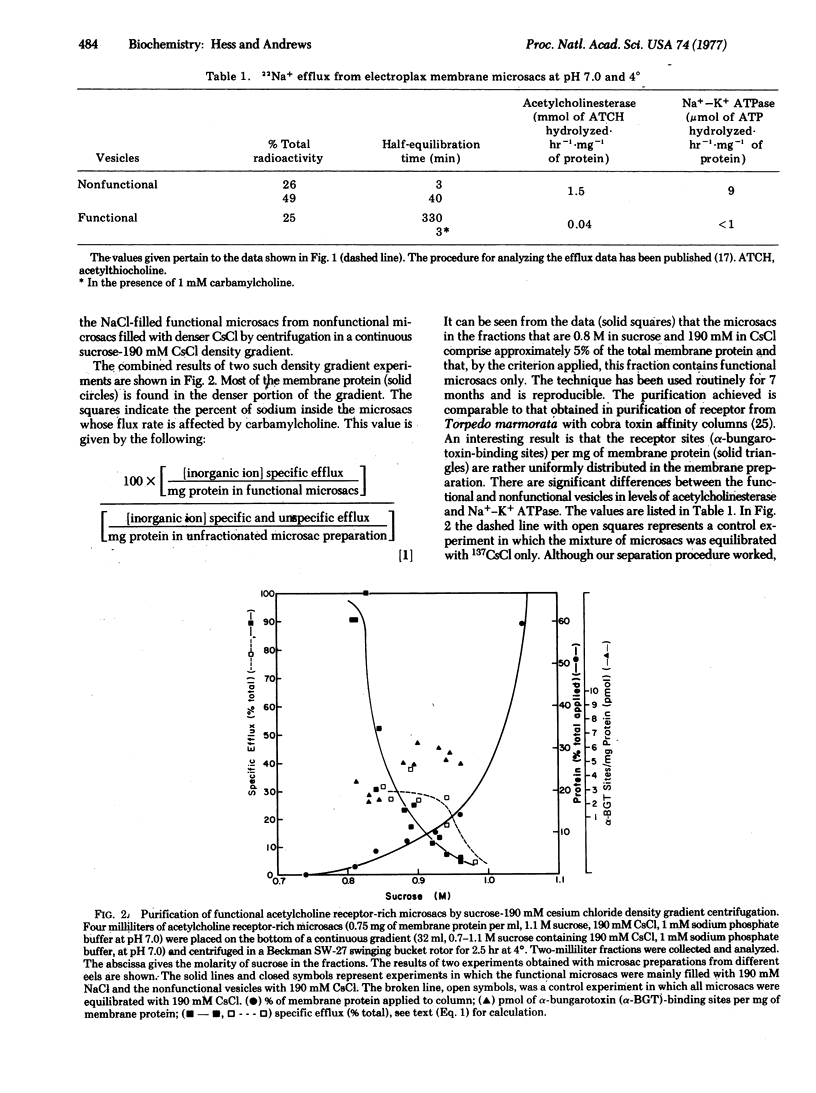
Kinetic analysis of the flux of sodium ions in a heterogeneous population of acetylcholine receptor-rich microsacs (vesicles) formed by membrane fragments of electroplax indicated that functional microsacs, which on average comprise only 15% of the preparation, can be filled with 190 mM sodium chloride while nonfunctional microsacs are filled by 190 mM cesium chloride. The functional microsacs have then been successfully separated from nonfunctional microsacs on the basis of their density differences with a continuous sucrose-190 mM cesium chloride density gradient. In the presence of acetylcholine analogs all the internal sodium ions in these microsacs rapidly exchange with external ions. The efflux of sodium ions follows a single exponential decay. The isolation of functional microsacs opens up at least two new avenues of investigation of the molecular mechanism of receptor-mediated processes. The first deals with the efficiency of the process, and the second with the characterization of membrane components important in this process. The conclusions reached so far are: (i) The efficiency of the receptor-mediated process that allows inorganic ions to equilibrate across the membranes of the microsacs can adequately account for electrophysiological results obtained with muscle and nerve cells. (ii) In the receptor-rich heterogeneous population of microsacs the concentration of receptor sites in functional and nonfunctional microsacs is about the same and is therefore not the only factor determining functionality. Significant differences between functional and nonfunctional microsacs have been found so far in the concentrations of acetylcholinesterase and Na+-K+ ATPase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONTING S. L., SIMON K. A., HAWKINS N. M. Studies on sodium-potassium-activated adenosine triphosphatase. I. Quantitative distribution in several tissues of the cat. Arch Biochem Biophys. 1961 Dec;95:416–423. doi: 10.1016/0003-9861(61)90170-9. [DOI] [PubMed] [Google Scholar]
- Bulger J. E., Hess G. P. Evidence for separate initiation and inhibitory sites in the regulation of membrane potential of electroplax. I. Kinetic studies with alpha-bungarotoxin. Biochem Biophys Res Commun. 1973 Sep 18;54(2):677–684. doi: 10.1016/0006-291x(73)91476-9. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Sodium transport by the acetylcholine receptor of cultured muscle cells. J Biol Chem. 1975 Mar 10;250(5):1776–1781. [PubMed] [Google Scholar]
- Changeux J. P., Benedetti L., Bourgeois J. P., Brisson A., Cartaud J., Devaux P., Grünhagen H., Moreau M., Popot J. L., Sobel A. Some structural properties of the cholinergic receptor protein in its membrane environmental relevant to its function as a pharmacological receptor. Cold Spring Harb Symp Quant Biol. 1976;40:211–230. doi: 10.1101/sqb.1976.040.01.023. [DOI] [PubMed] [Google Scholar]
- Donner D. B., Fu J. J., Hess G. P. Equilibrium dialysis of the membrane-bound acetylcholine receptor: a simple method to avoid common errors. Anal Biochem. 1976 Oct;75(2):454–463. doi: 10.1016/0003-2697(76)90100-7. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Eldefraw M. E., Eldefrawi M. E. Molecular and functional properties of the acetylcholine-receptor. Ann N Y Acad Sci. 1975 Dec 30;264:183–202. doi: 10.1111/j.1749-6632.1975.tb31483.x. [DOI] [PubMed] [Google Scholar]
- Eldefrawi A. T., Eldefrawi M. E. Identification of a calcium-binding subunit of the acetylcholine receptor. Biochem Biophys Res Commun. 1976 Jun 7;70(3):1020–1027. doi: 10.1016/0006-291x(76)90694-x. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T. Purification and molecular properties of the acetylcholine receptor from Torpedo electroplax. Arch Biochem Biophys. 1973 Nov;159(1):362–373. doi: 10.1016/0003-9861(73)90462-1. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J. L., Donner D. B., Hess G. P. Half-of-the-sites reactivity of the membrane-bound Electrophorus electricus acetylcholine receptor. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1072–1080. doi: 10.1016/0006-291x(74)90422-7. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Andrews J. P., Struve G. E. Apparent cooperative effects in acetylcholine receptor-mediated ion flux electroplax membrane preparations. Biochem Biophys Res Commun. 1976 Apr 5;69(3):830–837. doi: 10.1016/0006-291x(76)90950-5. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Andrews J. P., Struve G. E., Goombs S. E. Acetylcholine-receptor-mediated ion flux in electroplax membrane preparations. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4371–4375. doi: 10.1073/pnas.72.11.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G. P., Bulger J. E., Fu J. J., Hindy E. F., Silberstein R. J. Allosteric interactions of the membrane-bound acetylcholine reception: kinetic studies with alpha-bungarotoxin. Biochem Biophys Res Commun. 1975 Jan 2;64(3):1018–1027. doi: 10.1016/0006-291x(75)90149-7. [DOI] [PubMed] [Google Scholar]
- Karlin A., Weill C. L., McNamee M. G., Valderrama R. Facets of the structures of acetylcholine receptors from Electrophorus and Torpedo. Cold Spring Harb Symp Quant Biol. 1976;40:203–210. doi: 10.1101/sqb.1976.040.01.022. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee C. Y. Chemistry and pharmacology of polypeptide toxins in snake venoms. Annu Rev Pharmacol. 1972;12:265–286. doi: 10.1146/annurev.pa.12.040172.001405. [DOI] [PubMed] [Google Scholar]
- Maelicke A., Reich E. On the interaction between cobra alpha-neurotoxin and the acetylcholine receptor. Cold Spring Harb Symp Quant Biol. 1976;40:231–235. doi: 10.1101/sqb.1976.040.01.024. [DOI] [PubMed] [Google Scholar]
- NACHMANSOHN D. Metabolism and function of the nerve cell. Harvey Lect. 1953;49:57–99. [PubMed] [Google Scholar]
- Raftery M. A., Vandlen R. L., Reed K. L., Lee T. Characterization of Torpedo californica acetylcholine receptor: its subunit composition and ligand-binding properties. Cold Spring Harb Symp Quant Biol. 1976;40:193–202. doi: 10.1101/sqb.1976.040.01.021. [DOI] [PubMed] [Google Scholar]
- Rang H. P. Acetylcholine receptors. Q Rev Biophys. 1974 Jul;7(3):283–399. doi: 10.1017/s0033583500001463. [DOI] [PubMed] [Google Scholar]
- Rübsamen H., Hess G. P., Eldefrawi A. T., Eldefrawi M. E. Interaction between calcium and ligand-binding sites of the purified acetylcholine receptor studied by use of a fluorescent lanthanide. Biochem Biophys Res Commun. 1976 Jan 12;68(1):56–63. doi: 10.1016/0006-291x(76)90009-7. [DOI] [PubMed] [Google Scholar]


