Abstract
The phosphoinositide 3-kinase (PI3K) complex plays important roles in virtually all cells of the body. The enzymatic activity of PI3K to phosphorylate phosphoinositides in the membrane is mediated by a group of catalytic and regulatory subunits. Among those, the class I catalytic subunits, p110α, p110β, p110γ, and p110δ, have recently drawn attention in the neuroscience field due to their specific dysregulation in diverse brain disorders. While in non-neuronal cells these catalytic subunits may have partially redundant functions, there is increasing evidence that in neurons their roles are more specialized, and confined to distinct receptor-dependent pathways. This review will summarize the emerging role of class I PI3K catalytic subunits in neurotransmitter-regulated neuronal signaling, and their dysfunction in a variety of neurological diseases, including fragile X syndrome, schizophrenia, and epilepsy. We will discuss recent literature describing the use of PI3K subunit-selective inhibitors to rescue brain disease-associated phenotypes in in vitro and animal models. These studies give rise to the exciting prospect that these drugs, originally designed for cancer treatment, may be repurposed as therapeutic drugs for brain disorders in the future.
Keywords: PI3K signaling, neuronal signal transduction, autism, epilepsy, schizophrenia
INTRODUCTION
Signaling through phosphoinositide 3-kinases (PI3Ks) has diverse roles in the human body, regulating essential functions such as cell growth, migration, differentiation and survival. PI3K signaling is important for adequate immune response (Okkenhaug, 2013), hematopoiesis (Polak and Buitenhuis, 2012), and organ growth (Shioi et al., 2000). Mutations in PI3K catalytic subunits were found in primary immune deficiencies (Angulo et al., 2013) and in different forms of human cancer, including leukemia (Samuels et al., 2004; Gutierrez et al., 2009). Apart from a role in dividing cells, PI3K activity is also a key regulator of neuronal function. PI3K signaling transduces signals from cell surface receptors to the Akt/mTOR pathway and is essential for synapse and dendritic spine development (Jaworski et al., 2005; Chan et al., 2010; Cuesto et al., 2011; Lee et al., 2011) and for enduring forms of synaptic plasticity underlying learning and memory (Sanna et al., 2002; Man et al., 2003; Opazo et al., 2003; Sui et al., 2008; Hoeffer and Klann, 2010). Therefore, it is not surprising that an increasing body of evidence suggests dysregulated PI3K activity and downstream signaling as a key contributor and potential therapeutic target for mental disorders (Kalkman, 2006; Levitt and Campbell, 2009; Karam et al., 2010; Waite and Eickholt, 2010; Krueger et al., 2013).
SPLITTING THE WORK – NEURONAL PI3K ACTIVITY IS MEDIATED BY SEVERAL CATALYTIC SUBUNITS
In vertebrates, PI3K enzymatic activity is brought about by eight different catalytic subunits. These catalytic subunits are divided into class I, class II, and class III PI3K enzymes according to their protein structure, function and associated regulatory subunits (Hawkins et al., 2006). Here, we will focus on class I PI3K catalytic subunits, which are further sub-divided into class IA and IB. The class IA isoforms, p110α (PIK3CA), p110β (PIK3CB), and p110δ (PIK3CD), are associated with any one of the following regulatory (inhibitory) subunits, which are encoded by three different genes: p50α, p55α, p85α (PIK3R1); p85β (PIK3R2) and p55γ (PIK3R3). In contrast, the (sole) class IB subunit p110γ (PIK3CG) associates with p101 (PIK3R5) or p87 (a.k.a. p84, PIK3R6). Class I PI3Ks predominantly function as lipid kinases and catalyze the phosphorylation of the third hydroxyl group of the inositol ring of phosphatidylinositol (PI), PtdIns-4-phosphate (PI(4)P), and PtdIns-4,5-biphosphate (PI(4,5)P2). The PI3K products PI(3,4)P2 and PI(3,4,5)P3 recruit proteins that contain pleckstrin homology (PH) domains to the membrane, leading to their activation (Lemmon, 2007). These PI3K-regulated proteins can have diverse functions, for example as signal transduction molecules, including protein kinases and GTPase-modifying enzymes (Rodrigues et al., 2000; Fayard et al., 2010).
There are two major modes of activation of class I catalytic PI3K subunits by extracellular stimuli, namely via receptor tyrosine kinases (RTKs) and via G protein-coupled receptors (GPCRs). Activation of p110 subunits via RTKs is mediated through interaction of the SH2-domain of the regulatory subunits (e.g., p85a/β) with a phospho-tyrosine on the C-terminal tail of the RTKs or on RTK-associated proteins (Hawkins et al., 2006). Activation by GPCRs is mediated via heterotrimeric G-proteins or the scaffolding protein Homer and the PI3K enhancer PIKE-L (Rong et al., 2003; Hawkins et al., 2006). Association of p110 subunits with these receptors leads to their recruitment to the cell membrane where they are in close proximity to their substrates. Notably, the different p110 isoforms appear to have preferences for either one or the other type of receptor, implying isoform-specific PI3K activation (Guillermet-Guibert et al., 2008).
Earlier reports suggested some functional redundancy between the class I isoforms, specifically in their ability to maintain cell proliferation (Foukas et al., 2010). However, later work in non-neuronal cells has shown that the p110 isoforms can have distinct cellular functions, and are signaling downstream of specific membrane receptors (Vanhaesebroeck et al., 2010). This observation led to the development of subunit-selective antagonists as therapeutics for cancer (Zhao and Vogt, 2008), which are currently tested in clinical trials (Akinleye et al., 2013). Most recently, p110 subunit-specific functions and mechanisms have begun to be discovered in the brain. The different p110 isoforms appear to have unique roles in mediating distinct forms of neuronal function and synaptic plasticity, suggesting the use of subunit-selective p110 inhibitors for certain brain disorders. The importance of PI3K catalytic subunit-selective roles in neurons is illustrated by functional and genetic studies that have linked dysregulation or mutations of specific p110 isoforms with distinct brain disorders. Given the essential function of PI3K signaling in non-neuronal cells, a precise knowledge of the molecular mechanisms of neuron-specific PI3K enzyme regulation and dysregulation in disease is mandatory for the development of therapeutic strategies ameliorating brain disorders without compromising other essential functions of the body. Here, we will review and discuss recent progress and open questions in our understanding of how the specific class I PI3K catalytic isoforms p110α, p110β, p110γ, and p110δ are regulated in neurons and how their dysfunction might lead to mental diseases (summarized in Table 1 and Figure 1).
Table 1.
This table summarizes the current knowledge about neuron-specific signaling and function of class I PI3K catalytic subunits and lists available tools for their future study (transgenic mouse models and drugs).
| PI3K subunit (gene symbol) | Neuronal signaling pathway | Physiological function in the brain | Neurological disease | Transgenic mouse models | Antagonists | |
|---|---|---|---|---|---|---|
| Class IA | p110α(PIK3CA) | Insulin receptor | Insulin-dependent plasticity/LTD | megalencephaly, hemimegalencephaly Riviere etal. (2012) Epilepsy Alzheimer’s disease | →knockout (not viable) Bi etal. (2002) →transgenes with cancer mutations Koren and Bentires-Alj (2013) | INK1117a BYL719a A66 |
| p110β (PIK3CB) | mGlu1/5 S6, protein synthesis Rac, Rab5 | protein synthesis Gross and Bassell (2012) | FXS Gross etal. (2010) Autism Cusco etal. (2009) Alzheimer’s disease | →knockout (not viable) Bi etal. (2002) →conditional knockout (liver) Jia etal. (2008) | TGX-221b GSK2636771a AZD-6482a AZD8186a | |
| p110δ (PIK3CD) | Nrg1/ErbB4 RhoA | axon outgrowth and regeneration in sensory neurons Eickholt etal. (2007) | Schizophrenia Law etal. (2012) | →knockout Jou etal. (2002) →kinase-negative transgeneb Okkenhaug etal. (2002) | CAL-101a IC87114b TGR 1202a AMG319a PIK-294 | |
| Class IB | p110γ (PIK3CG) | NMDA Rap1, p38 PDE3B | NMDA-LTD, behavioral flexibility Kim etal. (2011) | Autism Serajee etal. (2003) Excitotoxicity/Brain ischemia/Epilepsy | →knockoutb Sasaki etal. (2000) →kinase-negative transgene Patrucco etal. (2004) | AS-605240b CZC24832 |
Italics point out that there is only indirect evidence to support the indicated roles.
ahas been or is currently being used in clinical trials (cancer)
bused to analyze neuronal phenotypes
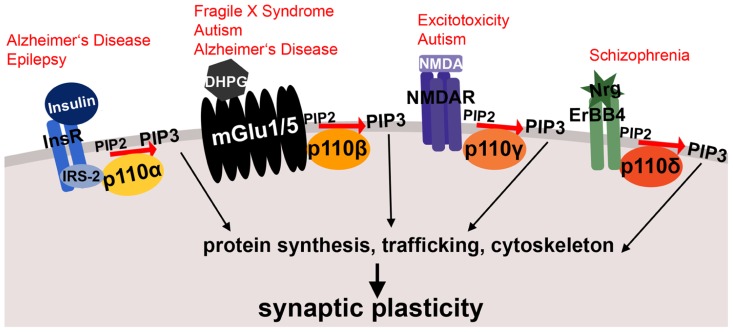
FIGURE 1.
Schematic illustrating membrane receptor-specific signaling of class I PI3K subunits and their potential link to mental disorders. Shown are examples of neuronal membrane receptors and the specific p110 catalytic subunits, through which they preferentially signal. All of these receptor-PI3K complexes were implicated in mental disorders, which are printed in red above the receptors. Both insulin signaling through p110α and metabotropic glutamate receptor signaling through p110β are affected in Alzheimer’s disease. Moreover, metabotropic glutamate receptor signaling through p110β is altered in fragile X syndrome and other autism spectrum disorders. The NMDA receptor complex (associated with p110γ-selective activity) plays a role in excitotoxicity and epilepsy, and p110δ-mediated signaling through ErbB4 is dysregulated in schizophrenia. InsR, insulin receptor; IRS-2, insulin receptor substrate 2; DHPG, dihydroxyphenylglycine, mGlu1/5 agonist; mGlu1/5, metabotropic glutamate receptor 1/5; NMDA, N-Methyl-D-aspartic acid; NMDAR, NMDA receptor; Nrg, Neuregulin; PIP2, phosphatidylinositol-4,5-biphosphate; PIP3, phosphatidylinositol-3,4,5-triphosphate.See text for references and further details.
p110α – insulin signaling to epilepsy and cognitive decline?
Each of the class I catalytic subunits has unique molecular features. P110α distinguishes itself from the other class I catalytic subunits by the absence of cell-transforming activity when overexpressed (Kang et al., 2006). Nevertheless, the majority of cancer-associated mutations in class I PI3K catalytic subunits were identified in the coding region of p110α. These mutations activate the enzymatic function and lead to oncogenic transformation (Samuels et al., 2004). In contrast, no oncogenic mutation in any of the other class I PI3K subunits has been reported so far. Interestingly, mutations in the kinase domain that activate p110α do not have an effect on p110β (Zhao et al., 2005) further corroborating the different modes of regulation of p110 catalytic subunits.
The important function of p110α in the brain is illustrated by enzyme-activating mutations in the p110α gene, PIK3CA that are associated with megalencephalies and hemimegalencephalies. These brain malformations lead to increased brain growth, developmental delay and epilepsy (Lee et al., 2012; Riviere et al., 2012). The p110α subunit is mainly activated by RTKs, and was shown to be a key mediator of insulin signaling in the liver (Sopasakis et al., 2010). Inhibitors of p110α but not p110β block insulin signaling in cultured cells (Knight et al., 2006). In the brain, insulin is important for cell survival and energy metabolism, but is also essential for PI3K-mediated regulation of synapse development (Lee et al., 2011) and enduring forms of synaptic plasticity (Zhao and Alkon, 2001). A brief exposure to insulin can induce long-term depression (LTD) at CA1 synapses that depends on PI3K signaling (Huang et al., 2003, 2004). It will be interesting to investigate if this form of LTD is mediated by p110α activity, whether it stimulates protein synthesis and how it might be affected by epilepsy-associated mutations in PIK3CA. Interestingly, correcting imbalances in insulin levels was suggested as therapeutic strategy for certain forms of epilepsy (Kim et al., 2013). Moreover, early stages of Alzheimer’s disease (AD) show signs of insulin resistance (Bosco et al., 2011), and insulin treatment is currently tested as a therapy in AD (de la Monte, 2013; Freiherr et al., 2013). Considering the predominant role of p110α in insulin signaling, selective manipulation of p110α activity may be beneficial to treat epilepsy or ameliorate cognitive decline in AD (Figure 1).
p110β – GPCRs, neuronal protein synthesis and autism
The p110β catalytic subunit is the predominant subunit associated with GPCRs (Guillermet-Guibert et al., 2008). This puts it in the unique position of being a key regulator of, e.g., metabotropic glutamate receptor 1/5 (mGlu1/5)-dependent forms of plasticity and protein synthesis in the brain. Interestingly, the regulatory subunits p85α and p85β have only reduced inhibitory effect toward p110β compared to other p110 subunits (Dbouk et al., 2010). RTKs activate PI3K signaling by releasing p85α/β-mediated inhibition of p110 subunits; lack of inhibition of p110β by p85α/β may thus contribute to the diminished stimulation of p110β signaling by RTKs (Kurosu et al., 1997; Guillermet-Guibert et al., 2008). Relatively low levels of p85α/β-mediated suppression of p110β may also cause the unusually high basal activity of p110β compared to other class I PI3K subunits.
The lack of this p85α/β-mediated inhibitory regulatory mechanism to suppress p110β activity under basal conditions suggests that increasing p110β protein levels through elevated p110β mRNA translation would directly lead to enhanced PI3K activity. In line with this assumption, the controlled expression of p110β appears to be an important mode of regulating p110β activity and PI3K-mediated protein synthesis in brain. Agonist-induced mGlu1/5 activation in mouse cortical synaptic fractions leads to increases in p110β protein levels and PI3K activity, which correlates with the PI3K-dependent stimulation of protein synthesis (Gross et al., 2010). p110β mRNA associates with and is translationally regulated by the fragile X mental retardation protein (FMRP), which is deficient in fragile X syndrome (FXS), the most common form of inherited intellectual disability and monogenic cause of autism (Gross et al., 2010; Sharma et al., 2010; Darnell et al., 2011). PI3K activity and protein synthesis are altered in FXS, and FXS mouse models and patient cells have increased p110β protein levels, which contributes to the observed elevated PI3K activity, downstream signaling and protein synthesis, and thus neuronal dysfunctions. Moreover, a duplication in the gene locus of p110β, PIK3CB, most likely leading to enhanced p110β-mediated PI3K activity, has been associated with autism (Cusco et al., 2009), further supporting an essential role of p110β expression in neuronal function. A p110β-selective inhibitor reduced the elevated protein synthesis rates in FXS mice and FXS patient cells suggesting that p110β has a crucial function to control neuronal protein synthesis (Gross and Bassell, 2012), and may be a promising therapeutic target for FXS and other autism spectrum disorders. However, more work is needed to assess the role of p110β and other p110 subunits in neuronal protein synthesis regulation and how this may be altered in human disease.
Defects in mGlu1/5-mediated signaling have not only been shown in FXS and other autism spectrum disorders (Williams, 2012), but also recently in AD (Ostapchenko et al., 2013; Um et al., 2013). The PI3K catalytic subunit p110β, similarly as discussed for p110α (see above), may thus also be a beneficial therapeutic target in certain forms of AD (Figure 1).
Signaling through p110β is unique, because it is not directly activated by the small GTPase Ras, as all other class I PI3K catalytic subunits (Zheng et al., 2012). Instead, it interacts with and is activated by Rac, a key regulator of the actin cytoskeleton (Fritsch et al., 2013), and by Rab5, a small GTPase essential for receptor-mediated endocytosis (Kurosu and Katada, 2001). The specific functions of Rac- and Rab5-mediated activation of p110β in neurons are unknown.
The phosphatase and tensin homologue (PTEN), a negative regulator of PI3K activity, which de-phosphorylates PI(3,4,5)P3, was shown to preferentially bind to p110β compared to other PI3K catalytic subunits in non-neuronal cells. P110β is thus a key treatment target in cancers associated with PTEN mutations (Shepherd and Denny, 2012). Of note, PTEN loss-of-function mutations lead to autism (Zhou and Parada, 2012), and PTEN was shown to inhibit axonal regeneration in adult neurons (Park et al., 2008; Christie et al., 2010; Liu et al., 2010); however, the role of p110β-regulation of PTEN in brain function still remains to be discovered. Considering the predominant role of p110β downstream of GPCRs, it will be interesting if PTEN mutations in autism preferentially lead to impaired GPCR signaling, as opposed to other forms of plasticity.
P110γ – a key mediator of NMDA-dependent plasticity
The PI3K subunit p110γ is categorized as class IB due to the specific regulatory subunits it is associated with (p101 and p87), which are different from those associating with p110α, p110β, and p110δ. While p110γ has been shown to play a role in the immune system and the heart several years ago (Okkenhaug et al., 2002; Oudit and Kassiri, 2007), its functions in the brain have just recently begun to be discovered. Using PIK3CG knockout mice as well as a p110γ-selective inhibitor (Camps et al., 2005), Kim and colleagues showed the requirement of p110γ for establishing NMDA-dependent LTD in the CA1 region of the hippocampus (Kim et al., 2011; Figure 1). In contrast, other forms of long-term plasticity, such as long-term potentiation, as well as mGlu5-dependent LTD were not affected by p110γ deletion or inhibition. Moreover, a p110α-selective inhibitor, and a broad-spectrum class IA inhibitor both did not affect NMDA-LTD, strongly suggesting a unique role of p110γ in NMDA-LTD in the hippocampus. The physiological role of p110γ for neuronal function was further corroborated by the observation that p110γ deletion led to impairments in reversal learning in mice.
NMDA receptor-mediated excitotoxicity depends on PI3K signaling (Brennan-Minnella et al., 2013). In view of the study by Kim et al. (2011), it will be interesting to examine if p110γ is critical for excitotoxicity and thus may have therapeutic potential to prevent excitotoxic events in the brain (Figure 1). P110γ associates with and activates phosphodiesterase 3B (PDE3B) in the heart, leading to increased cAMP levels in its absence (Patrucco et al., 2004). PDE3B is expressed throughout the brain (Reinhardt and Bondy, 1996) and up-regulated in cortical astrocytes and neurons after ischemic insult (Mitome-Mishima et al., 2013), but the function of p110γ-mediated regulation of PDE3B in neurons is unknown.
Corroborating an essential role of p110γ for neuronal plasticity, there is also a genetic link between p110γ dysfunction and mental disorders, particularly autism. The PIK3CG gene is located within the autism susceptibility locus AUTS1 on chromosome 7q22 (International Molecular Genetic Study of Autism Consortium, 2001; Kratz et al., 2002). Single nucleotide polymorphisms in PIK3CG, TSC1/2, which is mutated in the autism spectrum disorder tuberous sclerosis (TS), and INPP1, inositol polyphosphate-1-phosphatase, were shown to be in linkage disequilibrium in patients with autism (Serajee et al., 2003). This polymorphism was detected in the accessory domain (PIK domain) of p110γ, which is involved in substrate recognition (Domin and Waterfield, 1997). However, the polymorphism does not change the amino acid composition, and the effect it may have, e.g., on p110γ expression is unknown. Future work will have to show if p110γ dysregulation, either functional up- or down-regulation, can lead to autistic behavior in animal models.
P110δ – essential for developing axons and dysregulated in schizophrenia
The catalytic subunit p110δ was originally identified as key component of lymphocyte signaling (Okkenhaug, 2013) and a recent study reporting a specific enzyme-activating mutation in p110δ in humans with recurrent respiratory infections further supports an essential role of the p110δ subunit in the immune system (Angulo et al., 2013). In addition, a critical role for p110δ in neurons has become increasingly evident over the last years. A study using knockout mice and dominant negative forms of p110δ has shown that p110δ is essential for axonal outgrowth during development and in regenerating neurons (Eickholt et al., 2007).
More recently, increased p110δ mRNA expression and dysregulated p110δ-mediated signaling was associated with schizophrenia (Law et al., 2012), suggesting p110δ-selective inhibitors as a novel treatment strategy for schizophrenia and other psychotic diseases (Rico, 2012). Law and colleagues showed that p110δ is the major PI3K catalytic isoform signaling downstream of the neuregulin 1 (Nrg-1) receptor ErbB4 (Law et al., 2012; Figure 1). Both ErbB4, as well as Nrg-1 have been identified as risk genes for schizophrenia (Stefansson et al., 2002; Law et al., 2006; Norton et al., 2006; Silberberg et al., 2006). There are several isoforms of ErbB4, which have different capabilities of binding to, and activating PI3K catalytic subunits (Veikkolainen et al., 2011). Schizophrenia-associated polymorphisms lead to increased expression of the CYT-1 isoform of ErbB4, which is coupled to PI3K signaling (Law et al., 2007). These findings suggest dysregulation of the Nrg1-ErbB4-p110δ signaling complex as a risk factor for schizophrenia, and corroborate the importance of PI3K isoform-specific signaling mechanisms in neurons. It will be interesting to assess whether schizophrenia-associated mutations result in impairments in Nrg1-induced activation of p110δ-associated PI3K signaling and protein synthesis, suggesting parallels with p110β dysregulation in FXS.
Interestingly, ErbB4 was shown to be predominantly expressed in GABAergic interneurons in both the frontal cortex as well as the hippocampus (Vullhorst et al., 2009; Neddens et al., 2011). Using transgenic mice with cell type-specific ErbB4 deletions or overexpression, a recent study confirmed a major role of ErbB4 in dendritic spine morphology in parvalbumin-positive interneurons, but not pyramidal neurons (Yin et al., 2013). Studies in Drosophila corroborated the role of PI3K signaling in dendritic spine formation and synaptic plasticity in brain interneurons (Acebes et al., 2011, 2012); however, the role of p110δ or any other class I p110 subunit in vertebrate interneurons is unknown. To further elucidate the defects of ErbB4-p110δ signaling in schizophrenia it will be important to examine the specific roles of p110δ and other p110 isoforms in interneurons and other neuronal subtypes.
CHALLENGES AND OPEN QUESTIONS
The discussed studies are most likely just the tip of the iceberg illustrating the diverse and unique functions of the different class I p110 isoforms in the brain. These mechanisms of specialized PI3K signaling and regulation add to the variety of tools neurons utilize to achieve circuit-, cell-, synapse-, and stimulus-specificity. Future challenges will be to understand how receptor complex-specificity of the different PI3K subunits is achieved, how they are regulated developmentally and whether there are cell type- or brain circuit-specific differences in isoform signaling, as implied in the case of ErbB4 and p110δ. In particular, it will be interesting if distinct p110 subunits are selective transducers of mTOR-mediated protein synthesis regulation by different receptors in neurons. Possible mechanisms of p110-regulation may include control of local translation [as suggested by the presence of p110β mRNA in neuronal dendrites (Gross et al., 2010)] or the generation of local micro-domains of PI3K signaling by receptor and scaffold clustering (Gao et al., 2011).
As mentioned throughout this review, class I PI3K catalytic subunits were shown to be dysregulated in various forms of mental disorders. They seem to play important roles in the disease phenotypes, as shown by the therapeutic effect of isoform-selective inhibitors in preclinical studies. The discussed examples for p110δ in schizophrenia and p110β in FXS provide models, which are corroborated in mice and human patient cells. In the future, it will be interesting to determine if the disease phenotypes caused by p110 dysfunction are unique to specific p110 isoforms or if defects in the same isoform can lead to different types of brain diseases.
PI3K activates the mTOR pathway, which has been shown to be dysregulated in autism spectrum disorders of different etiologies, and was suggested as a therapeutic target (Wang and Doering, 2013). Targeting mTOR is an alternative approach to p110 subunit-modulating drugs that might be advantageous in some cases, because it might correct defects in several upstream pathways impinging on mTOR. The utility of mTOR inhibitors for TS has been shown in a mouse model (Tsai et al., 2012). In TS, the effected protein complex, TSC1/TSC2, lies almost directly upstream of mTOR (Inoki et al., 2002). A potential disadvantage of targeting mTOR is that it plays a crucial role in protein synthesis regulation in many different receptor pathways. In contrast, the specific manipulation of single PI3K catalytic subunits has the potential of being more selective to the receptor pathway that is primarily effected, and thus disease-targeted, leading to enhanced efficacy (Figure 1, Table 1). In the future, it will be interesting to investigate if mTOR is equally activated by all p110 subunits, or if specific p110 isoforms play more important roles than others, which could aid the development of future therapeutic strategies targeting mental disorders with impairments in mTOR.
Subunit-selective inhibitors potentially represent powerful therapeutic tools as they should not have deleterious effects on global PI3K activity, but rather only achieve selective inhibition of PI3K-activity coupled to specific receptors. Future research on the involvement of specific receptor-associated PI3K-signaling complexes may thus lead to the development of novel therapeutic strategies for autism, epilepsy or schizophrenia.
Conflict of Interest Statement
The authors are co-inventors on patent application PCT/US2010/055387, which suggests the use of (1) PI3K antagonists as a therapeutic treatment for fragile X syndrome and other autism spectrum disorders and (2) PI3K activity as a biomarker for these diseases.
Acknowledgments
This work was supported by a Pilot Grant from the Simons Foundation (SFARI #237324 to GJB and CG) and a NARSAD Distinguished Investigator Grant from the Brain and Behavior Research Foundation (to GJB). The authors apologize to all colleagues whose work on related topics was not discussed here due to the concise format of this Mini Review.
REFERENCES
- Acebes A., Devaud J. M., Arnes M., Ferrus A. (2012). Central adaptation to odorants depends on PI3K levels in local interneurons of the antennal lobe. J. Neurosci. 32 417–422 10.1523/JNEUROSCI.2921-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acebes A., Martin-Pena A., Chevalier V., Ferrus A. (2011). Synapse loss in olfactory local interneurons modifies perception. J. Neurosci. 31 2734–2745 10.1523/JNEUROSCI.5046-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinleye A., Avvaru P., Furqan M., Song Y., Liu D. (2013). Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J. Hematol. Oncol. 6 88 10.1186/1756-8722-6-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo I., Vadas O., Garcon F., Banham-Hall E., Plagnol V., Leahy T. R., et al. (2013). Phosphoinositide 3-Kinase delta gene mutation predisposes to respiratory infection and airway damage. Science 342 866–871 10.1126/science.1243292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi L., Okabe I., Bernard D. J., Nussbaum R. L. (2002). Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm. Genome 13 169–172 10.1007/s00335-001-2123-x [DOI] [PubMed] [Google Scholar]
- Bosco D., Fava A., Plastino M., Montalcini T., Pujia A. (2011). Possible implications of insulin resistance and glucose metabolism in Alzheimer’s disease pathogenesis. J. Cell. Mol. Med. 15 1807–1821 10.1111/j.1582-4934.2011.01318.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan-Minnella A. M., Shen Y., El-Benna J., Swanson R. A. (2013). Phosphoinositide 3-kinase couples NMDA receptors to superoxide release in excitotoxic neuronal death. Cell Death Dis. 4 e580 10.1038/cddis.2013.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M., Ruckle T., Ji H., Ardissone V., Rintelen F., Shaw J., et al. (2005). Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat. Med. 11 936–943 10.1038/nm1284 [DOI] [PubMed] [Google Scholar]
- Chan C.-B., Liu X., Ensslin M. A., Dillehay D. L., Ormandy C. J., Sohn P., et al. (2010). PIKE-A is required for prolactin-mediated STAT5a activation in mammary gland development. EMBO J. 29 956–968 10.1038/emboj.2009.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie K. J., Webber C. A., Martinez J. A., Singh B., Zochodne D. W. (2010). PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J. Neurosci. 30 9306–9315 10.1523/jneurosci.6271-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesto G., Enriquez-Barreto L., Carames C., Cantarero M., Gasull X., Sandi C., et al. (2011). Phosphoinositide-3-kinase activation controls synaptogenesis and spinogenesis in hippocampal neurons. J. Neurosci. 31 2721–2733 10.1523/JNEUROSCI.4477-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusco I., Medrano A., Gener B., Vilardell M., Gallastegui F., Villa O., et al. (2009). Autism-specific copy number variants further implicate the phosphatidylinositol signaling pathway and the glutamatergic synapse in the etiology of the disorder. Hum. Mol. Genet. 18 1795–1804 10.1093/hmg/ddp092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. C., Van Driesche S. J., Zhang C., Hung K. Y., Mele A., Fraser C. E., et al. (2011). FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146 247–261 10.1016/j.cell.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbouk H. A., Pang H., Fiser A., Backer J. M. (2010). A biochemical mechanism for the oncogenic potential of the p110beta catalytic subunit of phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. U.S.A. 107 19897–19902 10.1073/pnas.1008739107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S. M. (2013). Intranasal insulin therapy for cognitive impairment and neurodegeneration: current state of the art. Expert Opin. Drug Deliv. 10 1699–1709 10.1517/17425247.2013.856877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domin J., Waterfield M. D. (1997). Using structure to define the function of phosphoinositide 3-kinase family members. FEBS Lett. 410 91–95 10.1016/S0014-5793(97)00617-0 [DOI] [PubMed] [Google Scholar]
- Eickholt B. J., Ahmed A. I., Davies M., Papakonstanti E. A., Pearce W., Starkey M. L., et al. (2007). Control of axonal growth and regeneration of sensory neurons by the p110δ PI 3-kinase. PLoS ONE 2:e869 10.1371/journal.pone.0000869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayard E., Xue G., Parcellier A., Bozulic L., Hemmings B. A. (2010). Protein kinase B (PKB/Akt), a key mediator of the PI3K signaling pathway. Curr. Top. Microbiol. Immunol. 346 31–56 10.1007/82_2010_58 [DOI] [PubMed] [Google Scholar]
- Foukas L. C., Berenjeno I. M., Gray A., Khwaja A., Vanhaesebroeck B. (2010). Activity of any class IA PI3K isoform can sustain cell proliferation and survival. Proc. Natl. Acad. Sci. U.S.A. 107 11381–11386 10.1073/pnas.0906461107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiherr J., Hallschmid M., Frey W. H., II, Brunner Y. F., Chapman C. D., Holscher C., et al. (2013). Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs 27 505–514 10.1007/s40263-013-0076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch R., de krijger I., Fritsch K., George R., Reason B., Kumar MS., et al. (2013). RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell 153 1050–1063 10.1016/j.cell.2013.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Lowry P. R., Zhou X., Depry C., Wei Z., Wong G. W., et al. (2011). PI3K/Akt signaling requires spatial compartmentalization in plasma membrane microdomains. Proc. Natl. Acad. Sci. U.S.A. 108 14509–14514 10.1073/pnas.1019386108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C., Bassell G. J. (2012). Excess protein synthesis in FXS patient lymphoblastoid cells can be rescued with a p110beta-selective inhibitor. Mol. Med. 18 336–345 10.2119/molmed.2011.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C., Nakamoto M., Yao X., Chan C. B., Yim S. Y., Ye K., et al. (2010). Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J. Neurosci. 30 10624–10638 10.1523/JNEUROSCI.0402-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillermet-Guibert J., Bjorklof K., Salpekar A., Gonella C., Ramadani F., Bilancio A., et al. (2008). The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc. Natl. Acad. Sci. U.S.A. 105 8292–8297 10.1073/pnas.0707761105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A., Sanda T., Grebliunaite R., Carracedo A., Salmena L., Ahn Y., et al. (2009). High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood 114 647–650 10.1182/blood-2009-02-206722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. T., Anderson K. E., Davidson K., Stephens L. R. (2006). Signalling through Class I PI3Ks in mammalian cells. Biochem. Soc. Trans. 34 647–662 10.1042/BST0340647 [DOI] [PubMed] [Google Scholar]
- Hoeffer C. A., Klann E. (2010). mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 33 67–75 10.1016/j.tins.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-C., Lee C.-C., Hsu K.-S. (2004). An investigation into signal transduction mechanisms involved in insulin-induced long-term depression in the CA1 region of the hippocampus. J. Neurochem. 89 217–231 10.1111/j.1471-4159.2003.02307.x [DOI] [PubMed] [Google Scholar]
- Huang C.-C., You J.-L., Lee C.-C., Hsu K.-S. (2003). Insulin induces a novel form of postsynaptic mossy fiber long-term depression in the hippocampus. Mol. Cell. Neurosci. 24 831–841 10.1016/S1044-7431(03)00238-0 [DOI] [PubMed] [Google Scholar]
- Inoki K., Li Y., Zhu T., Wu J., Guan K.-L. (2002). TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4 648–657 10.1038/ncb839 [DOI] [PubMed] [Google Scholar]
- International Molecular Genetic Study of Autism Consortium. (2001). Further characterization of the autism susceptibility locus AUTS1 on chromosome 7q. Hum. Mol. Genet. 10 973–982 10.1093/hmg/10.9.973 [DOI] [PubMed] [Google Scholar]
- Jaworski J., Spangler S., Seeburg D. P., Hoogenraad C. C., Sheng M. (2005). Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. J. Neurosci. 25 11300–11312 10.1523/JNEUROSCI.2270-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S., Liu Z., Zhang S., Liu P., Zhang L., Lee S. H., et al. (2008). Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 454 776–779 10.1038/nature07091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou S.-T., Carpino N., Takahashi Y., Piekorz R., Chao J.-R., Carpino N., et al. (2002). Essential, nonredundant role for the phosphoinositide 3-kinase p110δ in signaling by the B-cell receptor complex. Mol. Cell. Biol. 22 8580–8591 10.1128/mcb.22.24.8580-8591.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkman H. O. (2006). The role of the phosphatidylinositide 3-kinase–protein kinase B pathway in schizophrenia. Pharmacol. Ther. 110 117–134 10.1016/j.pharmthera.2005.10.014 [DOI] [PubMed] [Google Scholar]
- Kang S., Denley A., Vanhaesebroeck B., Vogt P. K. (2006). Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. U.S.A. 103 1289–1294 10.1073/pnas.0510772103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam C. S., Ballon J. S., Bivens N. M., Freyberg Z., Girgis R. R., Lizardi-Ortiz J. E., et al. (2010). Signaling pathways in schizophrenia: emerging targets and therapeutic strategies. Trends Pharmacol. Sci. 31 381–390 10.1016/j.tips.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. I., Lee H. R., Sim S. E., Baek J., Yu N. K., Choi J. H., et al. (2011). PI3Kgamma is required for NMDA receptor-dependent long-term depression and behavioral flexibility. Nat. Neurosci. 14 1447–1454 10.1038/nn.2937 [DOI] [PubMed] [Google Scholar]
- Kim T. H., Petrou S., Reid C. A. (2013). Low glycaemic index diet reduces seizure susceptibility in a syndrome-specific mouse model of generalized epilepsy. Epilepsy Res. 10.1016/j.eplepsyres.2013.10.014 [DOI] [PubMed] [Google Scholar]
- Knight Z. A., Gonzalez B., Feldman M. E., Zunder E. R., Goldenberg D. D., Williams O., et al. (2006). A pharmacological map of the PI3-K family defines a role for p110 ± in insulin signaling. Cell 125 733–747 10.1016/j.cell.2006.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren S., Bentires-Alj M. (2013). Mouse models of PIK3CA mutations: one mutation initiates heterogeneous mammary tumors. FEBS J. 280 2758–2765 10.1111/febs.12175 [DOI] [PubMed] [Google Scholar]
- Kratz C. P., Emerling B. M., Bonifas J., Wang W., Green E. D., Beau M. M. L., et al. (2002). Genomic structure of the PIK3CG gene on chromosome band 7q22 and evaluation as a candidate myeloid tumor suppressor. Blood 99 372–374 10.1182/blood.V99.1.372 [DOI] [PubMed] [Google Scholar]
- Krueger D. A., Wilfong A. A., Holland-Bouley K., Anderson A. E., Agricola K., Tudor C., et al. (2013). Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann. Neurol. 74 679–687 10.1002/ana.23960 [DOI] [PubMed] [Google Scholar]
- Kurosu H., Katada T. (2001). Association of phosphatidylinositol 3-kinase composed of p110beta-catalytic and p85-regulatory subunits with the small GTPase Rab5. J. Biochem. 130 73–78 10.1093/oxfordjournals.jbchem.a002964 [DOI] [PubMed] [Google Scholar]
- Kurosu H., Maehama T., Okada T., Yamamoto T., Hoshino S., Fukui Y., et al. (1997). Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110beta is synergistically activated by the betagamma subunits of G proteins and phosphotyrosyl peptide. J. Biol. Chem. 272 24252–24256 10.1074/jbc.272.39.24252 [DOI] [PubMed] [Google Scholar]
- Law A. J., Kleinman J. E., Weinberger D. R., Weickert C. S. (2007). Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum. Mol. Genet. 16 129–141 10.1093/hmg/ddl449 [DOI] [PubMed] [Google Scholar]
- Law A. J., Lipska B. K., Weickert C. S., Hyde T. M., Straub R. E., Hashimoto R., et al. (2006). Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc. Natl. Acad. Sci. U.S.A. 103 6747–6752 10.1073/pnas.0602002103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law A. J., Wang Y., Sei Y., O’Donnell P., Piantadosi P., Papaleo F., et al. (2012). Neuregulin 1-ErbB4-PI3K signaling in schizophrenia and phosphoinositide 3-kinase-p110delta inhibition as a potential therapeutic strategy. Proc. Natl. Acad. Sci. U.S.A. 109 12165–12170 10.1073/pnas.1206118109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-C., Huang C.-C., Hsu K.-S. (2011). Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology 61 867–879 10.1016/j.neuropharm.2011.06.003 [DOI] [PubMed] [Google Scholar]
- Lee J. H., Huynh M., Silhavy J. L., Kim S., Dixon-Salazar T., Heiberg A., et al. (2012). De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat. Genet. 44 941–945 10.1038/ng.2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M. A. (2007). Pleckstrin homology (PH) domains and phosphoinositides. Biochem. Soc. Symp. 81–93 10.1042/BSS0740081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P., Campbell D. B. (2009). The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J. Clin. Invest. 119 747–754 10.1172/JCI37934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Lu Y., Lee J. K., Samara R., Willenberg R., Sears-Kraxberger I., et al. (2010). PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 13 1075–1081 10.1038/nn.2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man H.-Y., Wang Q., Lu W.-Y., Ju W., Ahmadian G., Liu L., et al. (2003). Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron 38 611–624 10.1016/S0896-6273(03)00228-9 [DOI] [PubMed] [Google Scholar]
- Mitome-Mishima Y., Miyamoto N., Tanaka R., Oishi H., Arai H., Hattori N., et al. (2013). Differences in phosphodiesterase 3A and 3B expression after ischemic insult. Neurosci. Res. 75 340–348 10.1016/j.neures.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Neddens J., Fish K. N., Tricoire L., Vullhorst D., Shamir A., Chung W., et al. (2011). Conserved interneuron-specific ErbB4 expression in frontal cortex of rodents, monkeys, and humans: implications for schizophrenia. Biol. Psychiatry 70 636–645 10.1016/j.biopsych.2011.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton N., Moskvina V., Morris D. W., Bray N. J., Zammit S., Williams N. M., et al. (2006). Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 141B 96–101 10.1002/ajmg.b.30236 [DOI] [PubMed] [Google Scholar]
- Okkenhaug K. (2013). Signaling by the phosphoinositide 3-kinase family in immune cells. Annu. Rev. Immunol. 31 675–704 10.1146/annurev-immunol-032712-095946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkenhaug K., Bilancio A., Farjot G., Priddle H., Sancho S., Peskett E., et al. (2002). Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science 297 1031–1034 10.1126/science.1073560 [DOI] [PubMed] [Google Scholar]
- Opazo P., Watabe A. M., Grant S. G, O’Dell T. J. (2003). Phosphatidylinositol 3-kinase regulates the induction of long-term potentiation through extracellular signal-related kinase-independent mechanisms. J. Neurosci. 23 3679–3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapchenko V. G., Beraldo F. H., Guimarães A. L. S., Mishra S., Guzman M., Fan J., et al. (2013). Increased prion protein processing and expression of metabotropic glutamate receptor 1 in a mouse model of Alzheimer’s disease. J. Neurochem. 127 415–425 10.1111/jnc.12296 [DOI] [PubMed] [Google Scholar]
- Oudit G. Y., Kassiri Z. (2007). Role of PI3 kinase gamma in excitation-contraction coupling and heart disease. Cardiovasc. Hematol. Disord. Drug Targets 7 295–304 10.2174/187152907782793545 [DOI] [PubMed] [Google Scholar]
- Park K. K., Liu K., Hu Y., Smith P. D., Wang C., Cai B., et al. (2008). Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322 963–966 10.1126/science.1161566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrucco E., Notte A., Barberis L., Selvetella G., Maffei A., Brancaccio M., et al. (2004). PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell 118 375–387 10.1016/j.cell.2004.07.017 [DOI] [PubMed] [Google Scholar]
- Polak R., Buitenhuis M. (2012). The PI3K/PKB signaling module as key regulator of hematopoiesis: implications for therapeutic strategies in leukemia. Blood 119 911–923 10.1182/blood-2011-07-366203 [DOI] [PubMed] [Google Scholar]
- Reinhardt R. R., Bondy C. A. (1996). Differential cellular pattern of gene expression for two distinct cGMP-inhibited cyclic nucleotide phosphodiesterases in developing and mature rat brain. Neuroscience 72 567–578 10.1016/0306-4522(95)00520-X [DOI] [PubMed] [Google Scholar]
- Rico B. (2012). Finding a druggable target for schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 109 11902–11903 10.1073/pnas.1209389109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere J. B., Mirzaa G. M., O’Roak B. J., Beddaoui M., Alcantara D., Conway R. L., et al. (2012). De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat. Genet. 44 934–940 10.1038/ng.2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G. A., Falasca M., Zhang Z., Ong S. H., Schlessinger J. (2000). A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell. Biol. 20 1448–1459 10.1128/MCB.20.4.1448-1459.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R., Ahn J. Y., Huang H., Nagata E., Kalman D., Kapp J. A., et al. (2003). PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat. Neurosci. 6 1153–1161 10.1038/nn1134 [DOI] [PubMed] [Google Scholar]
- Samuels Y., Wang Z., Bardelli A., Silliman N., Ptak J., Szabo S., et al. (2004). High frequency of mutations of the PIK3CA gene in human cancers. Science 304 554 10.1126/science.1096502 [DOI] [PubMed] [Google Scholar]
- Sanna P. P., Cammalleri M., Berton F., Simpson C., Lutjens R., Bloom F. E., et al. (2002). Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J. Neurosci. 22 3359–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Irie-Sasaki J., Jones R. G., Oliveira-Dos-Santos A. J., Stanford W. L., Bolon B., et al. (2000). Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science 287 1040–1046 10.1126/science.287.5455.1040 [DOI] [PubMed] [Google Scholar]
- Serajee F. J., Nabi R., Zhong H, Mahbubul Huq A. H. M. (2003). Association of INPP1, PIK3CG, and TSC2 gene variants with autistic disorder: implications for phosphatidylinositol signalling in autism. J. Med. Genet. 40 e119 10.1136/jmg.40.11.e119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Hoeffer C. A., Takayasu Y., Miyawaki T., Mcbride S. M., Klann E., et al. (2010). Dysregulation of mTOR signaling in fragile X syndrome. J. Neurosci. 30 694–702 10.1523/jneurosci.3696-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd P. R., Denny W. A. (2012). Beta-testing of PI3-kinase inhibitors: is beta better? Cancer Discov. 2 393–394 10.1158/2159-8290.cd-12-0122 [DOI] [PubMed] [Google Scholar]
- Shioi T., Kang P. M., Douglas P. S., Hampe J., Yballe C. M., Lawitts J., et al. (2000). The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 19 2537–2548 10.1093/emboj/19.11.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G., Darvasi A., Pinkas-Kramarski R., Navon R. (2006). The involvement of ErbB4 with schizophrenia: association and expression studies. Am. J. Med. Genet. Part B Neuropsychiat. Genet. 141B 142–148 10.1002/ajmg.b.30275 [DOI] [PubMed] [Google Scholar]
- Sopasakis V. R., Liu P., Suzuki R., Kondo T., Winnay J., Tran T. T., et al. (2010). Specific roles of the p110alpha isoform of phosphatidylinsositol 3-kinase in hepatic insulin signaling and metabolic regulation. Cell Metab. 11 220–230 10.1016/j.cmet.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H., Petursson H., Sigurdsson E., Steinthorsdottir V., Bjornsdottir S., Sigmundsson T., et al. (2002). Neuregulin 1 and susceptibility to schizophrenia. Am. J. Hum. Genet. 71 877–892 10.1086/342734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui L., Wang J., Li B. M. (2008). Role of the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex. Learn. Mem. 15 762–776 10.1101/lm.1067808 [DOI] [PubMed] [Google Scholar]
- Tsai P. T., Hull C., Chu Y., Greene-Colozzi E., Sadowski A. R., Leech J. M., et al. (2012). Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488 647–651 10.1038/nature11310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um J. W., Kaufman A. C., Kostylev M., Heiss J. K., Stagi M., Takahashi H., et al. (2013). Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer aβ oligomer bound to cellular prion protein. Neuron 79 887–902 10.1016/j.neuron.2013.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Guillermet-Guibert J., Graupera M., Bilanges B. (2010). The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 11 329–341 10.1038/nrm2882 [DOI] [PubMed] [Google Scholar]
- Veikkolainen V., Vaparanta K., Halkilahti K., Iljin K., Sundvall M., Elenius K. (2011). Function of ERBB4 is determined by alternative splicing. Cell Cycle 10 2647–2657 10.4161/cc.10.16.17194 [DOI] [PubMed] [Google Scholar]
- Vullhorst D., Neddens J., Karavanova I., Tricoire L., Petralia R. S., McBain C. J., et al. (2009). Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J. Neurosci. 29 12255–12264 10.1523/JNEUROSCI.2454-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite K., Eickholt B. J. (2010). The neurodevelopmental implications of PI3K signaling. Curr. Top. Microbiol. Immunol. 346 245–265 10.1007/822010_82 [DOI] [PubMed] [Google Scholar]
- Wang H., Doering L. C. (2013). Reversing autism by targeting downstream mTOR signaling. Front. Cell. Neurosci. 7:28 10.3389/fncel.2013.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. C. P. (2012). Drugs targeting mGluR5 receptor offer “fragile” hope for autism. Nat. Med. 18 840–840 10.1038/nm0612-840 [DOI] [PubMed] [Google Scholar]
- Yin D. M., Sun X. D., Bean J. C., Lin T. W., Sathyamurthy A., Xiong W. C., et al. (2013). Regulation of spine formation by ErbB4 in PV-positive interneurons. J. Neurosci. 33 19295–19303 10.1523/JNEUROSCI.2090-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. J., Liu Z., Wang L., Shin E., Loda M. F., Roberts T. M. (2005). The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 102 18443–18448 10.1073/pnas.0508988102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Vogt P. K. (2008). Class I PI3K in oncogenic cellular transformation. Oncogene 27 5486–5496 10.1038/onc.2008.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.-Q., Alkon D. L. (2001). Role of insulin and insulin receptor in learning and memory. Mol. Cell. Endocrinol. 177 125–134 10.1016/S0303-7207(01)00455-5 [DOI] [PubMed] [Google Scholar]
- Zheng W., Wang H., Zeng Z., Lin J., Little P. J., Srivastava L. K., et al. (2012). The possible role of the Akt signaling pathway in schizophrenia. Brain Res. 1470 145–158 10.1016/j.brainres.2012.06.032 [DOI] [PubMed] [Google Scholar]
- Zhou J., Parada L. F. (2012). PTEN signaling in autism spectrum disorders. Curr. Opin. Neurobiol. 22 873–879 10.1016/j.conb.2012.05.004 [DOI] [PubMed] [Google Scholar]