Abstract
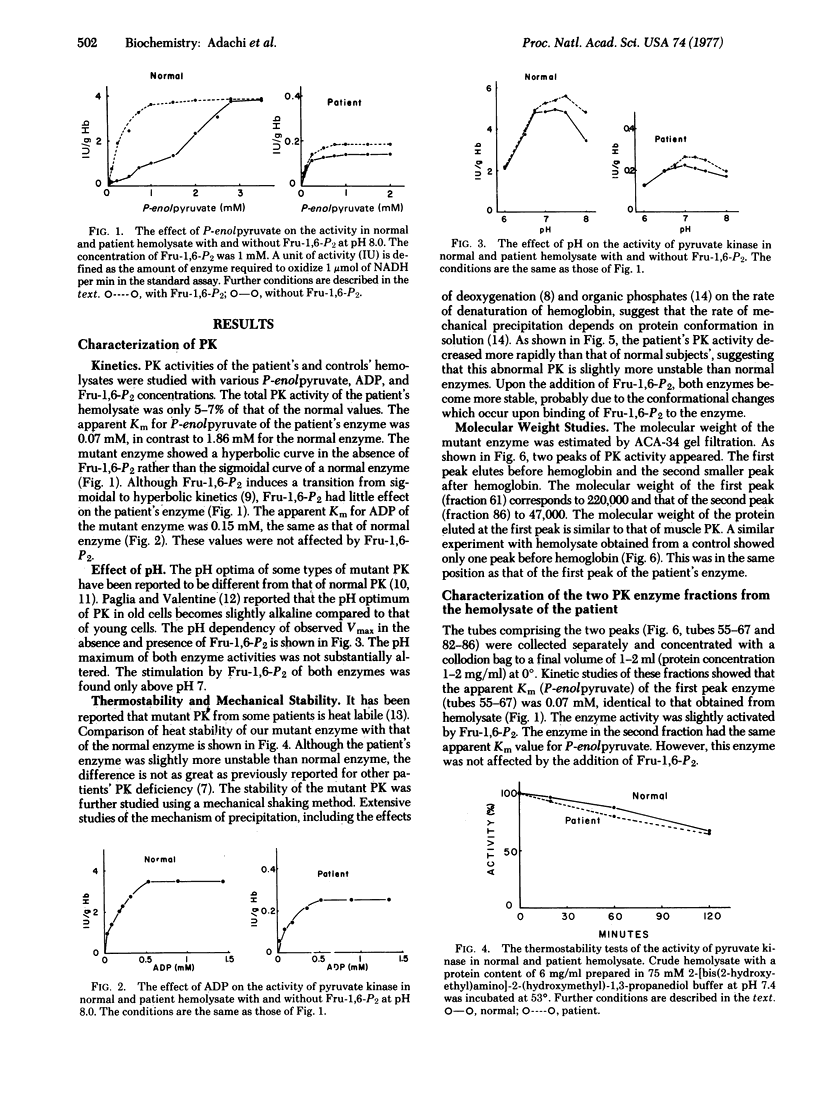
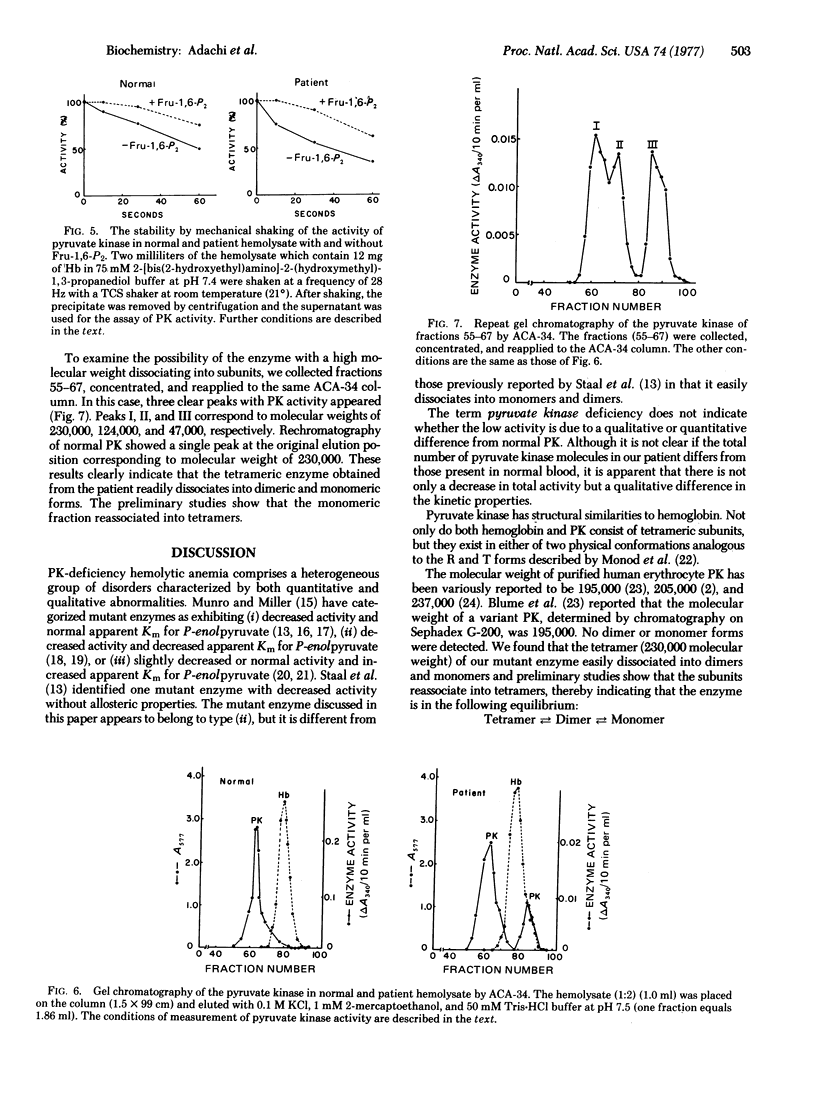
A mutant pyruvate kinase (ATP:pyruvate 2-O-phosphotransferase, EC 2.7.1.40) from human erythrocytes which is easily separated into monomers and dimers by gel chromatography is described. Tht mutant enzyme shows almost the same pH optimum and thermostability as normal enzyme, but has a decreased stability on shaking with air, a decreased Km for phosphoenolpyruvate and a loss of allosteric properties. The apparent Km values for phosphoenolpyruvate of tetramers and monomers were the same. The tetrameric enzyme was slightly activated by fructose-1,6-diphosphate but the monomeric form was not. The tetrameric enzyme was found to dissociate spontaneously to dimeric and monomeric forms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi K., Asakura T. Effect of 2,3-diphosphoglycerate and inositol hexaphosphate on the stability of normal sickle hemoglobins. Biochemistry. 1974 Nov 19;13(24):4976–4982. doi: 10.1021/bi00721a016. [DOI] [PubMed] [Google Scholar]
- Asakura T., Onishi T., Friedman S., Schwartz E. Abnormal precipitation of oxyhemoglobin S by mechanical shaking. Proc Natl Acad Sci U S A. 1974 May;71(5):1594–1598. doi: 10.1073/pnas.71.5.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume K. G., Arnold H., Lohr G. W., Beutler E. Additional diagnostic procedures for the detection of abnormal red cell pyruvate kinase. Clin Chim Acta. 1973 Feb 12;43(3):443–446. doi: 10.1016/0009-8981(73)90487-7. [DOI] [PubMed] [Google Scholar]
- Blume K. G., Hoffbauer R. W., Busch D., Arnold H., Löhr G. W. Purification and properties of pyruvate kinase in normal and in pyruvate kinase deficient human red blood cells. Biochim Biophys Acta. 1971 Feb 10;227(2):364–372. doi: 10.1016/0005-2744(71)90068-4. [DOI] [PubMed] [Google Scholar]
- Brandt N. J., Hanel H. K. Atypical pyruvate kinase in a patient with haemolytic anaemia. Scand J Haematol. 1971;8(2):126–133. doi: 10.1111/j.1600-0609.1971.tb01963.x. [DOI] [PubMed] [Google Scholar]
- Campos J. O., Koler R. D., Bigley R. H. Kinetic differences between human red cell and leucocyte pyruvate kinase. Nature. 1965 Oct 9;208(5006):194–195. doi: 10.1038/208194a0. [DOI] [PubMed] [Google Scholar]
- Ibsen K. H., Schiller K. W., Haas T. A. Interconvertible kinetic and physical forms of human erythrocyte pyruvate kinase. J Biol Chem. 1971 Mar 10;246(5):1233–1240. [PubMed] [Google Scholar]
- Koler R. D., Vanbellinghen P. The mechanism of precursor modulation of human pyruvate kinase I by fructose diphosphate. Adv Enzyme Regul. 1968;6:127–142. doi: 10.1016/0065-2571(68)90010-1. [DOI] [PubMed] [Google Scholar]
- Koster J. F., Staal G. E., van Milligan-Boersma L. The effect of urea and temperature on red blood cell pyruvate kinase. Biochim Biophys Acta. 1971 Apr 27;236(1):362–365. doi: 10.1016/0005-2795(71)90187-5. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Minakami S., Yoshikawa H. Studies on erythrocyte glycolysis. II. Free energy changes and rate limitings steps in erythrocyte glycolysis. J Biochem. 1966 Feb;59(2):139–144. doi: 10.1093/oxfordjournals.jbchem.a128274. [DOI] [PubMed] [Google Scholar]
- Miwa S., Nakashima K., Ariyoshi K., Shinohara K., Oda E. Four new pyruvate kinase (PK) variants and a classical PK deficiency. Br J Haematol. 1975 Jan;29(1):157–169. doi: 10.1111/j.1365-2141.1975.tb01809.x. [DOI] [PubMed] [Google Scholar]
- Munro G. F., Miller D. R. Mechanism of fructose diphosphate activation of a mutant pyruvate kinase from human red cells. Biochim Biophys Acta. 1970 Apr 22;206(1):87–97. doi: 10.1016/0005-2744(70)90085-9. [DOI] [PubMed] [Google Scholar]
- Oski F. A., Bowman H. A low Km phosphoenolpyruvate mutant in the Amish with red cell pyruvate kinase deficiency. Br J Haematol. 1969 Sep;17(3):289–297. doi: 10.1111/j.1365-2141.1969.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Oski F. A., Marshall B. E., Cohen P. J., Sugerman H. J., Miller L. D. The role of the left-shifted or right-shifted oxygen-hemoglobin equilibrium curve. Ann Intern Med. 1971 Jan;74(1):44–46. doi: 10.7326/0003-4819-74-1-44. [DOI] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N., Baughan M. A., Miller D. R., Reed C. F., McIntyre O. R. An inherited molecular lesion of erythrocyte pyruvate kinase. Identification of a kinetically aberrant isozyme associated with premature hemolysis. J Clin Invest. 1968 Aug;47(8):1929–1946. doi: 10.1172/JCI105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Evidence for molecular alteration of pyruvate kinase as a consequence of erythrocyte aging. J Lab Clin Med. 1970 Aug;76(2):202–212. [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N., Rucknagel D. L. Defective erythrocyte pyruvate kinase with impaired kinetics and reduced optimal activity. Br J Haematol. 1972 Jun;22(6):651–665. doi: 10.1111/j.1365-2141.1972.tb05713.x. [DOI] [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. Control of glycolysis in the human red blood cell. J Biol Chem. 1966 Nov 10;241(21):4848–4854. [PubMed] [Google Scholar]
- Staal G. E., Koster J. F., Kamp H., van Milligen-Boersma L., Veeger C. Human erythrocyte pyruvate kinase. Its purification and some properties. Biochim Biophys Acta. 1971 Jan 13;227(1):86–96. doi: 10.1016/0005-2744(71)90170-7. [DOI] [PubMed] [Google Scholar]
- Staal G. E., Koster J. F., van Milligen-Boersma L. Some properties of abnormal red blood cell pyruvate kinase. Biochim Biophys Acta. 1970 Dec 16;220(3):613–616. doi: 10.1016/0005-2744(70)90293-7. [DOI] [PubMed] [Google Scholar]
- Staal G. J., Koster J. F., Nijessen J. G. A new variant of red blood cell pyruvate kinase deficiency. Biochim Biophys Acta. 1972 Feb 28;258(2):685–687. doi: 10.1016/0005-2744(72)90262-8. [DOI] [PubMed] [Google Scholar]
- TANAKA K. R., VALENTINE W. N., MIWA S. Pyruvate kinase (PK) deficiency hereditary nonspherocytic hemolytic anemia. Blood. 1962 Mar;19:267–295. [PubMed] [Google Scholar]
- VALENTINE W. N., TANAKA K. R., MIWA S. A specific erythrocyte glycolytic enzyme defect (pyruvate kinase) in three subjects with congenital non-spherocytic hemolytic anemia. Trans Assoc Am Physicians. 1961;74:100–110. [PubMed] [Google Scholar]


