Abstract
Papillon–Lefevre syndrome (PLS) is a very rare, autosomal recessive syndrome characterized by palmar–plantar hyperkeratosis and severe destructive periodontitis. Most patients present with PLS harbor mutations in the cathepsin C gene, but recent studies have identified individuals with classic PLS symptoms without such mutations. This suggests more genetic heterogeneity for PLS than previously thought. Here we present an individual’s manifesting characteristic clinical features of PLS with no mutations in the coding sequence of cathepsin C. We suggest there must be alternative genetic causes for such forms of PLS.
Keywords: Hyperkeratosis, Papillon–Lefevre syndrome, Periodontitis
1. Introduction
Papillon–Lefevre syndrome (PLS) is characterized by palmoplantar hyperkeratosis, precocious, rapidly progressive periodontal disease (Papillon and Lefevre, 1924; Haneke, 1979), and calcification of the duramater (Gorlin et al., 1964). PLS is inherited in an autosomal recessive fashion, and most parents of PLS patients are consanguineous (first cousins) (Inaloz et al., 2001). PLS occurs in approximately 1–4 cases per million, and the carrier rate is approximately 2–4 per 1000 persons (Angel et al., 2002). Most patients with PLS have a mutation in the cathepsin C gene, located on chromosome 11q14–q21 (Hart et al., 1999; Toomes et al., 1999). It is not understood how defects in cathepsin C cause periodontal disease. There have been five reported cases of late-onset PLS without cathepsin C mutations, making the genetic etiology of this condition even more complex (Pilger et al., 2003). Some reports have described impairment in the chemotactic and phagocytic function of polymorphonuclear leukocytes (PMNs) in PLS patients (Fqratlq et al., 1996; Ghaffer et al., 1999; Liu et al., 2000; Schroeder et al., 1983). Here we report a classic case of PLS with no detected mutation in the cathepsin C gene, along with a brief review of the literature. This research was approved by the ethics committees of the Government Dental College, Srinagar, India and the subject’s parents provided informed consent.
2. Case report
A 12 year old female presented to the Department of Periodontics, Government Dental College & Hospital, Srinagar, India, complaining of loose teeth, pain on mastication, and spontaneous gingival bleeding. Her history included recurrent pus exudation from the gums and early loss of deciduous teeth. She also complained of persistent scabbing and thickening of the skin of her soles and palms, which aggravated in the winter. Her parents were non consanguineous. Scaly, keratotic, coalescent patches affecting the skin of her palms and soles and spreading onto the dorsal aspects of her feet were present. No deformity was observed in her nails or hair. Well-delineated, reddish, scabby patches were present on both knees (Fig. 1a–e). Severe gingival inflammation, spontaneous bleeding from the gums, abscess formation, deep periodontal pockets, furcation involvement, and generalized gingival recession were noted. All teeth were mobile with extensive deposits of plaque and calculus (Fig. 1f). The panographic radiogram showed generalized and severe loss of alveolar bone. The mandibular posterior teeth were almost out of socket with severe loss of bone in mandibular anteriors as well (Fig. 2). Routine bloodwork showed values within normal range for serum acid and alkaline phosphatases, hemoglobin, total leukocyte count, differential leukocyte count, and thyroid profile. A blood sample was sent for DNA sequencing of the cathepsin C gene and short tandem repeat (STR) analysis of the surrounding locus (MACROGEN, Seoul, Korea). No mutations were detected within the coding regions of the cathepsin C gene. Analysis of STRs surrounding the cathepsin C locus showed no common haplotypes (Figs. 3–5). Despite an inconclusive mutation analysis, the patient was diagnosed with PLS based on her consistent clinical findings.
Figure 1.
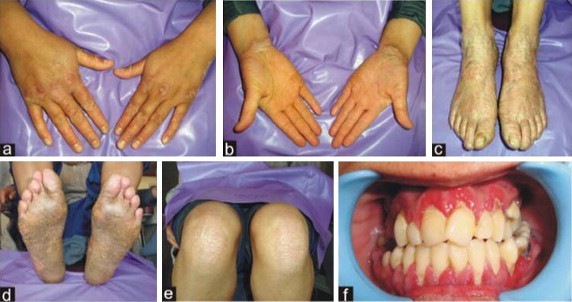
(a and b) Characteristic palmer keratosis; (c and d) keratotic plaques affecting the soles extending onto the dorsal surfaces of the feet; (e) circumscribed, erythematous, scaly plaques on the knees bilaterally; (f) intra-oral examination revealing severe gingival inflammation, spontaneous bleeding from the gums, abscess formation, deep periodontal pockets, furcation involvement and generalized gingival recession.
Figure 2.
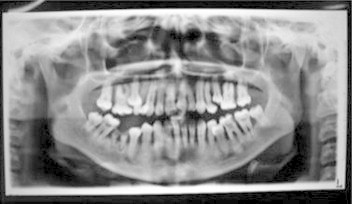
Orthopantomographic (OPG) examination showing generalized and severe loss of alveolar bone and premature eruption of mandibular 3rd molars probably due to precocious exfoliation of primary molars.
Figure 3.
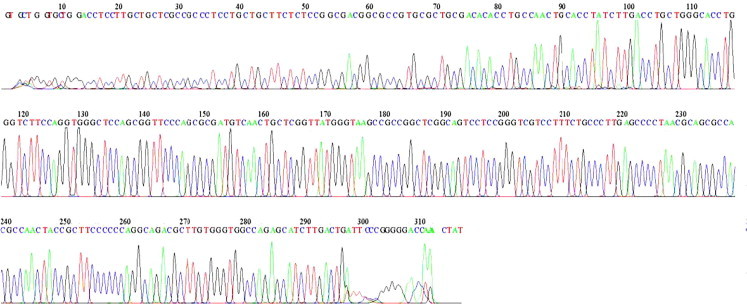
Sequencing and mutational analysis of exon 1 of the cathepsin C (CTSC) gene (18 Base spacing: 14.641174 318 bases in 3941 scans). No mutation is revealed in the chromatogram. There were no mis-spaced peaks, no noticeable baseline noise, and the spacing between the basecall letters at the top was regular.
Figure 4.
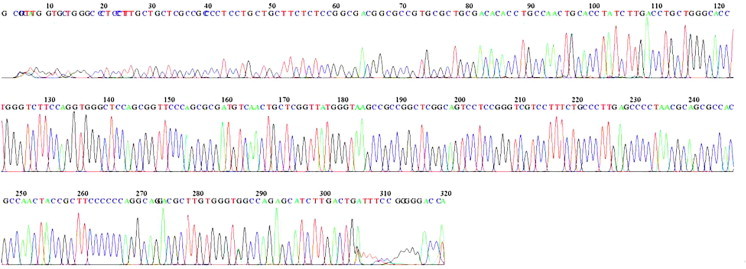
Sequencing and mutational analysis of exon 3 of the CTSC gene (45 Base spacing: 14.621844 320 bases in 3907 scans). No mutation revealed.
Figure 5.
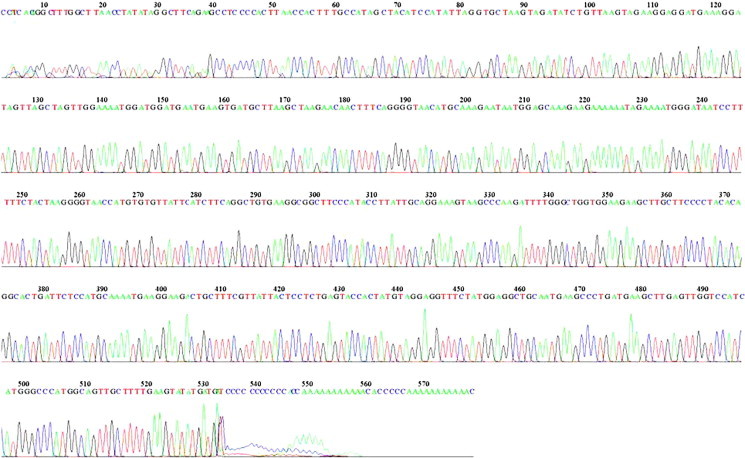
Sequencing and mutational analysis of exon 7 of the CTSC gene (41 Base spacing: 14.614846 579 bases in 6957 scans). No mutation revealed in the chromatogram.
3. Discussion
PLS is an inherited, autosomal recessive disorder with palmoplantar keratosis, psoriasiform scabies of elbows and knees, and periodontitis that manifests at 1–4 years of age (Haneke, 1979; Hart and Shapira, 1994). Prevalence is estimated in 1–4 cases per million persons across all races and genders (Cury et al., 2002). The keratotic lesions observed in our patient were consistent with PLS. Patients may also demonstrate reduced neutrophil, lymphocyte, or monocyte activities with resultant increased susceptibility to recurrent pyogenic skin infections (Giansanti et al., 1973).
In patients with PLS, the eruption pattern and developmental process of deciduous teeth are normal but is followed by severe gingival inflammation and rapid periodontal destruction. Conventional modes of therapy fail to resolve the aggressive periodontitis and results in premature exfoliation of primary dentition usually by the age of 4 years. The gingival inflammation resolves after exfoliation of teeth. However, as the permanent teeth erupt the process of gingivitis and periodontitis recommences, resulting in early loss of permanent teeth as well. PLS patients often display periodontitis as well as hyperkeratosis. Cathepsin C is expressed in the epithelium of tissues affected by PLS and mutations in the gene are found in many patients. However, the genetic etiology of PLS is sometimes ambiguous, with several reported cases having no cathepsin C gene mutations, including the patient presented here. It is possible that in such cases there are mutations in intronic or regulatory regions of the cathepsin C gene that could result in aberrant splicing of the transcript or reduced gene expression.
The skin lesions and dental problems associated with PLS can have an adverse effect on a child’s psychological and social development. Therefore, regular dental care and parent counseling are important for PLS children. Management of PLS requires multidisciplinary involvement of dentist, dermatologist, and pediatrician. Oral retinoids such as acitretin and isotretinoin can be beneficial in treating dental and skin lesions of PLS. Retinoid treatment is recommended during the eruption of permanent dentition until the completion of normal developmental process (Al-Khenaizan, 2002).
Conflict of interest
None.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Khenaizan S. Papillon–Lefèvre syndrome: the response to acitretin. Int. J. Dermatol. 2002;41:938–941. doi: 10.1046/j.1365-4362.2002.01664_3.x. [DOI] [PubMed] [Google Scholar]
- Angel T.A., Hsu S., Kornbleuth S.I., Kornbleuth J., Kramer E.M. Papillon–Lefevre syndrome: a case report of four affected siblings. J. Am. Acad. Dermatol. 2002;46:8–10. doi: 10.1067/mjd.2002.104968. [DOI] [PubMed] [Google Scholar]
- Cury V.F., Costa J.E., Gomez R.S., Boson W.L., Loures C.G., De M.L. A novel mutation of the cathepsin C gene in Papillon Lefevre syndrome. Periodontol. 2002;73(3):307–312. doi: 10.1902/jop.2002.73.3.307. [DOI] [PubMed] [Google Scholar]
- Fqratlq E., Gurel N., Efeoglu A., Badur S. Clinical and immunological findings in two siblings with Papillon Lefevre syndrome. J. Periodontol. 1996;67:1210–1215. doi: 10.1902/jop.1996.67.11.1210. [DOI] [PubMed] [Google Scholar]
- Ghaffer K.A., Zahran F.M., Fahmy H.M., Brown R.S. Papillon Lefevre syndrome. Neutrophil function in 15 cases from 4 families in Egypt. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999;88:320–325. doi: 10.1016/s1079-2104(99)70036-3. [DOI] [PubMed] [Google Scholar]
- Giansanti J.S., Hrabak R.P., Waldron C.A. Palmar plantar hyperkeratosis and concomitant periodontal destruction (Papillon–Lefevre syndrome) Oral Surg. Oral Med. Oral Pathol. 1973;36(1):40–48. doi: 10.1016/0030-4220(73)90265-x. [DOI] [PubMed] [Google Scholar]
- Gorlin R.J., Sedano H., Anderson V.E. The syndrome of palmar plantar hyperkeratosis and premature periodontal destruction of the teeth: a clinical and genetic analysis of the Papillon Lefevre syndrome. Pediatrics. 1964;65:895–908. doi: 10.1016/s0022-3476(64)80014-7. [DOI] [PubMed] [Google Scholar]
- Haneke E. The Papillon Lefevre syndrome: keratosis palmoplantaris with periodontopathy: report of a case and review of the cases in the literature. Hum. Genet. 1979;51:1–35. doi: 10.1007/BF00278288. [DOI] [PubMed] [Google Scholar]
- Hart T.C., Hart P.S., Bowden D.W. Mutations of the cathepsin C gene are responsible for Papillon Lefevre syndrome. J. Med. Genet. 1999;36:881–887. [PMC free article] [PubMed] [Google Scholar]
- Hart T.C., Shapira L. Papillon Lefevre syndrome. Periodontol. 1994;2000(6):88–100. doi: 10.1111/j.1600-0757.1994.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Inaloz H.S., Harman M., Akdeniz S., Inaloz S.S., Isik A.G. Atypical familial Papillon Lefevre syndrome. Eur. Acad. Dermatol. Venereol. 2001;15:48–50. doi: 10.1046/j.1468-3083.2001.00121.x. [DOI] [PubMed] [Google Scholar]
- Liu R., Cao C., Meng H., Tang Z. Leukocyte function in 2 cases of Papillon Lefevre syndrome. J. Clin. Periodontol. 2000;27:69–73. doi: 10.1034/j.1600-051x.2000.027001069.x. [DOI] [PubMed] [Google Scholar]
- Papillon M.M., Lefevre P. Two cases of symmetrical, familial (Meledas malady) palmar and plantar keratosis of brother and sister: coexistence in two cases with serious dental changes (in French) Bull. Soc. Fr. Dermatol. Syphilgir. 1924;31:82–87. [Google Scholar]
- Pilger U., Hennies H.C., Truschnegg A., Aberer E. Late-onset Papillon–Lefèvre syndrome without alteration of the cathepsin C gene. J. Am. Acad. Dermatol. 2003;49(5 Suppl.):S240–S243. doi: 10.1016/s0190-9622(03)01558-5. [DOI] [PubMed] [Google Scholar]
- Schroeder H.E., Seger R.A., Keller H.U., Rateitschak-pluss E.M. Behaviour of neutrophilic granulocytes in a case of Papillon Lefevre syndrome. J. Clin. Periodontal. 1983;10:618–635. doi: 10.1111/j.1600-051x.1983.tb01300.x. [DOI] [PubMed] [Google Scholar]
- Toomes C., James J., Wood A.J. Loss of function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nat. Genet. 1999;23:421–424. doi: 10.1038/70525. [DOI] [PubMed] [Google Scholar]



