Abstract
Bioactive glass is a novel material that dissolves and forms a bond with bone when exposed to body fluids. Bioactive glasses are silicate-based, with calcium and phosphate in identical proportions to those of natural bone; therefore, they have high biocompatibility. Bioactive glasses have wide-ranging clinical applications, including the use as bone grafts, scaffolds, and coating materials for dental implants. This review will discuss the effects of ions on the various compositions of bioactive glasses, as well as the clinical applications of bioactive glasses in medicine and dentistry.
Keywords: Bioactive glass, Ions, Clinical applications, Medicine, Dentistry
1. Introduction
According to Hench et al. (1971), a material can be classified as bioactive if it evokes a specific biological response that results in bond formation between the material and a tissue (e.g., bone). Biomaterials were originally designed to be inert, and the discovery of bioactive glasses by Hench initiated an exciting new era in the field. In SiO2, CaO, Na2O, and P2O5 systems, certain compositions were observed to resist removal and to form a strong bond with the bone after implantation (Hench and Wilson, 1993). These silicate-based bioactive glasses have a high degree of biocompatibility, with calcium and phosphorus in proportions identical to those of natural bone. Bioactive glasses have also been used to bind to tooth components (Hench, 2006).
The purpose of this review was to analyze the effect of ions in the properties of bioactive glasses and to summarize their major clinical applications in the field of medicine and dentistry reported in the literature. In preparing this review, all English-language articles published between 1971 (the first report of bioactive glass) and 2013 were accessed electronically using automated searches. The PubMed database and Google search engine were searched with keywords, including: bioactive glass, bioactive glass ions, clinical applications of bioactive glasses, and bioactive glasses in medicine and dentistry. We reviewed the abstracts of over 100 articles and short-listed 50 articles and scientific proceedings on the basis of their relevance to the review topic. Articles reporting similar findings were excluded. The final articles were printed and studied in detail.
2. Effects of ions on the composition of bioactive glasses
The first bioactive glass, Bioglass™, was discovered by Hench. The original formulation, commonly called 45S5, contained 45 wt.% SiO2, 24.5 wt.% Na2O and CaO, and 6 wt.% P2O5 (Kobayashi et al., 2010). Most current research focuses on changing the structure of 45S5 by adding or removing ions to make the material more compatible for different clinical applications.
2.1. Effect of fluoride
The addition of fluoride can provide numerous advantages to bioactive glasses and ceramics (Hench et al., 1988). Fluoride prevents dental decay by inhibiting the demineralization of enamel and dentin, enhancing remineralization, and inhibiting bacterial enzymes (Thuy et al., 2008). Fluoride also forms fluorapatite (FAP) instead of carbonated hydroxyapatite, which is more acid-resistant. Thus, adding fluoride to bioactive glass can improve oral health (Brauer et al., 2009).
2.2. Effect of phosphate
Phosphate can be present in bioactive glasses as orthophosphate (Elgayar et al., 2005). When the bioactive glass is exposed to body fluids, a layer of hydroxycarbonate apatite is formed (Wallace et al., 1999). Hydroxycarbonate apatite layer significantly increases the biocompatibility of these bioactive glasses (Olmo et al., 2003). The resulting bioactive glass can be used to treat dentin hypersensitivity (DH) by occluding dentinal tubules (Litkowski et al., 1997). However, FAP is superior to hydroxycarbonate apatite in terms of acid resistance (Lynch et al., 2012). Increasing the P2O5 and cation contents in fluoride-containing glasses aids in maintaining the network connectivity and favors the formation of FAP, rather than fluorite, with apatite also being formed at low pH. These conditions (increasing P2O5 and cation content in fluoride containing glasses) are more favorable for clinical applications of dentistry and orthopedics (Brauer et al., 2010).
2.3. Effect of strontium
Strontium is a bone-seeking agent like calcium that is naturally found in the liver, physiological fluids, muscles, and bones (Patrick et al., 1997). Strontium can favorably impact bone cells. Strontium ranelate and strontium chloride have been used in the treatment of osteoporosis (Dahl et al., 2001). Strontium can be substituted for calcium in bioactive glass, resulting in better bone bonding and osteoblast stimulation, with anabolic and anti-catabolic properties (Fredholm el al., 2012). Strontium-substituted Bioglass™ promotes osteoblast proliferation and decreases osteoclast activity in cell culture (Gentleman et al., 2010). Strontium in a silica-based dentifrice was observed to be clinically effective in treating DH (Addy et al., 1987).
2.4. Effect of zinc
Zinc can improve the ability of glass to bond with bone (Aina et al., 2007). Zinc is a fundamental ion that controls cell growth, differentiation, and development, but the biomechanical mechanisms involved in these processes are not entirely understood (Brandao et al., 1995). Zinc is essential for DNA replication and stimulates protein synthesis (Tang et al., 2001). Zinc deficiency slows skeletal growth and causes alterations in bone calcification (Holloway et al., 1996). Zinc can activate bone formation and inhibit bone resorption (Yamaguchi and Yamaguchi, 1986). Dentifrices with 2% zinc citrate have been used in the treatment of poor gingival health, as they have antiinflammatory and antimicrobial properties (Williams et al., 1998).
3. Clinical applications of bioactive glasses in medicine and dentistry
Bioactive glasses have a wide range of clinical applications in both medicine and dentistry (Melek et al., 2013). The first reported clinical application of bioactive glass was the treatment of conductive hearing loss for the reconstruction of the bony ossicular chain of the middle ear (Greenspan, 1999). Bioactive silicate glass has also been used for implant coatings, as a bone graft (Towler et al., 2002), in dentifrices (Tai et al., 2006), and as air-abrasive particles to remove carious enamel and dentin (Farooq et al., 2012). Goudouri et al. (2011) indicated that bioactive glass could be used as a dental material to improve the bonding of the restorative material to dentin.
3.1. Application as a bone graft
Bone grafts can impact osteogenesis, osteoconduction, and osteoinduction (Lindhe et al., 2008). Bioglass™ has been used clinically as a synthetic bone graft material for over 10 years under two different product names: Novabone™ for orthopedics (Ahmed, 2006) and Perioglass™ for maxillofacial surgery (Fetner et al., 1994). Bioglass™ was approved by the US Food and Drug Administration (FDA) in 2005 for osteostimulation (Hench, 1998). It functions as an osteoconductive scaffold and has an osteostimulatory effect (Boccaccini et al., 2010), which traditional calcium phosphate and calcium sulfate osteoconductive bioceramics do not have (Gerhardt and Boccaccini, 2010) (Table 1).
Table 1.
Comparison of HA/TCP and bioactive glasses as bone graft substitutes (Valimaki and Aro, 2006).
| HA and HA/TCP | Bioactive glasses | |
|---|---|---|
| Chemical composition | One or two chemical components (Hydroxyapatite, tricalcium phosphate, or both) | Several components at least four components Original; four-component system of SiO2, Na2O, CaO and P2O5Hench et al. (1971) (4). Modified system; Na2O–K2O–MgO–CaO–B2O3–P2O5–SiO2Brink et al. (1997) (6) |
| Physical forms | Porous blocks or granules | Granules or sintered porous blocks, fibers and Woven structures |
| Basic mechanism | Serves as osteoconductive surface | Forms chemical bonding with ongrowing new Bone. Osteopromotive |
| Molecular mechanism of action in vivo | Not defined | Induce high local bone turnover |
| Regulation of bioactivity | Based only on HA/TCP ratio | Can be regulated by modifying the chemical composition |
| Resorption rate | TCP is resorbed fast (months) HA is slowly/very slowly resorbed | Resorption can be highly regulated [from weeks to years] by modifying the chemical composition |
| Mechanism of resorption | Involves chemical dissolution and Osteoclastic resorption | Chemical dissolution |
| Antimicrobial properties | Not reported | Inhibition of bacterial growth in vitro, dependent on the chemical composition |
3.2. Application as a coating material for dental implants
Although hydroxyapatite can be sprayed on the surface of dental implants as a bioactive coating material, its use has unavoidable drawbacks (Eberhardt et al., 1992). Implant coatings require good adherence to the metal substrate. Titanium implant surfaces are routinely coated with hydroxyapatite to produce this rough surface and improve osseointegration, but their adherence to the metal is not ideal (Whitehead et al., 1993). Consequently, researchers have studied components to improve physical compatibility with titanium (Pazo et al., 1998). Bioglass™ may be used as an alternate osteoproductive, abrasive surface material for implants (Koller et al., 2007). However, more research is needed for bioactive glasses to be used clinically as a coating material for dental implants.
3.3. Application as a disinfectant
Bioactive glasses can serve as topical endodontic disinfectants with no effects on dentin stability (Doyon et al., 2005). Bioactive glass can raise the pH of an aqueous environment to produce its antimicrobial effects (Allan et al., 2001). Bioglass™ can also mineralize, which may be advantageous in endodontics, but further research is required to support this idea. When implanted in areas of periodontal defects, Bioglass™ can inhibit bacterial colonization at the surgical site (Allan et al., 2002) by increasing the pH and calcium levels (Allan et al., 2001). Calcium hydroxide can be used as an intracanal dressing to form a hard apical barrier in young, traumatized teeth, but its alkaline nature can weaken the dentin structure by dissolving the acidic bonding agents in the organic dentin matrix (Andreasen et al., 2002). Calcium hydroxide has a better antimicrobial effect than bioactive glass (Zehnder et al., 2006).
3.4. Application in bone regeneration
Bioactive glass can promote bone regeneration, with osteostimulatory effects in vitro (Hench, 2013). Similarly, in primate models, bioactive glass filled bony defects by stimulating osteoproduction (Wilson et al., 1987). Felipe et al. (2009) reported that bioactive glass particles were able to treat periodontal defects and triggered the development of mineralized bone in dogs.
3.5. Application in treating DH
The similarity between bone and dentin provides a rationale for testing bioactive glass in treating DH (Lynch et al., 2012). Bioglass™ 45S5, for example, can be used for remineralizing dentifrices for DH treatment (Farooq et al., 2013) (Fig. 1). However, there is no commercially available material for treating DH via permanent blockade of the dentinal tubules. Therefore, further research is needed to identify materials that form intimate bonds with the tooth structure and permanently block the tubules. Permanent bonding would reduce the incidence of dentinal tubule reopening resulting from oral fluid exposure.
Figure 1.
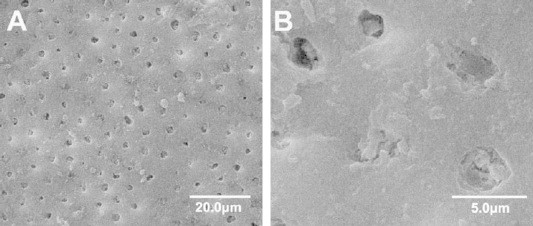
SEM-micrographs showing dentine surface brushed with Novamin™ (with 45S5) toothpaste for 2 min demonstrating tubule occlusion (Wang et al., 2010). (A) Bioactive glass particles on the dentine surfaces (at 1500×). (B) Bioactive glass particles embedded inside the dentinal tubules (at 7500×).
4. Conclusion
Bioactive glasses have many applications in dentistry and medicine. Altering the composition of the bioactive glass by adding different ions changes its suitability for specific clinical applications. Considering the existing applications, there is a strong rationale for additional use in medicine and dentistry, and further research is needed to explore further applications.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Addy M., Mostafa P., Newcombe R.G. Dentine hypersensitivity: a comparison of five toothpastes used during a 6-week period. BDJ. 1987;163:45–50. doi: 10.1038/sj.bdj.4806185. [DOI] [PubMed] [Google Scholar]
- Ahmed E. Correction of craniofacial skeleton contour defects using bioactive glass particles. Egypt J. Plast. Reconstr. Surg. 2006;30(2):113–119. [Google Scholar]
- Aina V. Cytotoxicity of zinc-containing bioactive glasses in contact with human osteoblasts. Chem. Biol. Interact. 2007;167(3):207–218. doi: 10.1016/j.cbi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Allan I., Newman H., Wilson M. Antibacterial activity of particulate bioglass against supra- and subgingival bacteria. Biomaterials. 2001;22:1683–1687. doi: 10.1016/s0142-9612(00)00330-6. [DOI] [PubMed] [Google Scholar]
- Allan I., Wilson M., Newman H. Particulate Bioglass® reduces the viability of bacterial biofilms formed on its surface in an in-vitro model. Clin. Oral Impl. Res. 2002;13:53–58. doi: 10.1034/j.1600-0501.2002.130106.x. [DOI] [PubMed] [Google Scholar]
- Andreasen J.O., Farkik B., Munksgaard E.C. Long-term, calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent. Traumatol. 2002;18:134–137. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- Boccaccini A.R. Polymer/bioactive glass nanocomposites for biomedical applications: a review. Compos. Sci. Technol. 2010;70(13):1764–1776. [Google Scholar]
- Brandao N.J., Stefan V., Mendonca B.B., Bloise W., Castro A.V.V. The essential role of zinc in growth. Nutr. Res. 1995;15:335–358. [Google Scholar]
- Brauer D.S., Karpukhina N., O’Donnell M.D., Law R., Hill R.G. Structure of fluoride-containing bioactive glasses. J. Mater. Chem. 2009;19:5629–5636. [Google Scholar]
- Brauer D., Karpukhina N., O’Donnell M.D., Law R.V., Hill R.G. Fluoride-containing bioactive glasses: effect of glass design and structure on degradation, pH and apatite formation in simulated body fluid. Acta Biomater. 2010;6:3275–3282. doi: 10.1016/j.actbio.2010.01.043. [DOI] [PubMed] [Google Scholar]
- Brink M., Turunen T., Happonen R.P., Yli-Urpo A. Compositional dependence of bioactivity of glasses in the system Na2O-K2O-MgO-CaO-B2O3-P2O5-SiO2. J Biomed Mater Res. 1997;37:114–121. doi: 10.1002/(sici)1097-4636(199710)37:1<114::aid-jbm14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Dahl S. Incorporation and distribution of strontium in bone. Bone. 2001;28(4):446–453. doi: 10.1016/s8756-3282(01)00419-7. [DOI] [PubMed] [Google Scholar]
- Doyon G.E., Dumsha T., VonFraunhofer J.A. Fracture resistance of human root dentin exposed to intracanal calcium hydroxide. J. Endodont. 2005;31:895–897. doi: 10.1097/01.don.0000194542.02521.af. [DOI] [PubMed] [Google Scholar]
- Eberhardt A.W., Zhou C., Rigney E.D. Proceedings of the Seventh National Spray conference. Boston MA; USA: 1992. Bending and thermal stresses in fatigue experiments of hydroxyapatite coated titanium rods; pp. 165–169. [Google Scholar]
- Elgayar I., Aliev A.E., Boccaccini A.R., Hill R.G. Structural analysis of bioactive glasses. J. Non-Cryst. Solids. 2005;351:173–183. [Google Scholar]
- Farooq I., Imran Z., Farooq U., Leghari A., Ali H. Bioactive glass: a material for the future. World J. Dent. 2012;3(2):199–201. [Google Scholar]
- Farooq I., Tylkowski M., Muller S., Janicki T., Brauer D., Hill R. Influence of sodium content on the properties of bioactive glasses for use in air abrasion. Biomed. Mater. 2013;8:065008. doi: 10.1088/1748-6041/8/6/065008. [DOI] [PubMed] [Google Scholar]
- Felipe Potential of bioactive glass particles of different size ranges to affect bone formation in interproximal periodontal defects in dogs. J. Periodontol. 2009;80(5):808–815. doi: 10.1902/jop.2009.080583. [DOI] [PubMed] [Google Scholar]
- Fetner A.E., Hartigan M.S., Low S.B. Periodontal repair using PerioGlas® in non-human primates. Clin. Histol. Obs. Compendium. 1994;15:932–938. [PubMed] [Google Scholar]
- Fredholm Y.C., Karpukhina N., Brauer D.S., Jones J.R., Law R.V., Hill R.G. Influence of strontium for calcium substitution in bioactive glasses on degradation, ion release and apatite formation. J. R. Soc. Interf. 2012 May 7;9(70):880–889. doi: 10.1098/rsif.2011.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman E., Fredholm Y., Jell G., Lotfibakhshaiesh N., O’Donnell M., Hill R., Stevens M. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts, in vitro. Biomaterials. 2010;31:3949–3956. doi: 10.1016/j.biomaterials.2010.01.121. [DOI] [PubMed] [Google Scholar]
- Gerhardt L.C., Boccaccini A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials. 2010;3:3867–3910. doi: 10.3390/ma3073867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudouri O.M. Dental ceramics/bioactive glass composites: characterization and mechanical properties investigation. Bioceramics Develop. Appl. 2011;1:1–4. [Google Scholar]
- Greenspan D.C. Medical Device and Diagnostics Industry; 1999. Developments in Biocompatible Glass Compositions. p. 150. [Google Scholar]
- Hench L.L. Bioceramics. J. Am. Ceram. Soc. 1998;81(7):1705–1728. [Google Scholar]
- Hench L.L. The story of Bioglass™. J. Mater. Sci.: Mater. Med. 2006;17:967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- Hench L.L. Chronology of bioactive glass development and clinical applications. New J. Glass Ceram. 2013;3(2):67–73. [Google Scholar]
- Hench L.L., Wilson J. World Scientific Publishing; Singapore: 1993. An Introduction to Bioceramics. [Google Scholar]
- Hench L.L., Splinter R.J., Allen W.C., Greenlee T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. 1971;5(6):117–141. [Google Scholar]
- Hench LL, Spilman DB, Hench JW, inventors; University of Florida, assignee. Fluoride-modified bioactive glass (Bioglass) and its use as implant material. US patent 4775646; 1988.
- Holloway W.R., Collier F.M., Herbt R.E., Hodge J.M., Nicolson G.C. Osteoblast – mediated effects of zinc on isolated rat osteoclasts: inhibition of bone resorption and enhancement of osteoclast number. Bone. 1996;19:137–142. doi: 10.1016/8756-3282(96)00141-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi Masahiro, Saito Hiroaki, Mase Takatsune, Sasaki Taketo, Wang Wei, Tanaka Yumi. Polarization of hybridized calcium phosphoaluminosilicates with 45S5-type bioglasses. Biomed. Mater. 2010;5(2):25001. doi: 10.1088/1748-6041/5/2/025001. [DOI] [PubMed] [Google Scholar]
- Koller G., Cook R., Thompson I., Watson T., DiSilvio L. Surface modifications of titanium implants using bioactive glasses with air abrasion technologies. J. Mater. Sci.: Mater. Med. 2007;18:2291–2296. doi: 10.1007/s10856-007-3137-z. [DOI] [PubMed] [Google Scholar]
- Lindhe J., Lang N.P., Karring T. Clinical Periodontology and Implant Dentistry. fifth ed. Vol. 1. 2008. 93. [Google Scholar]
- Litkowski L.J., Hack G.D., Sheaffer H.B., Greenspan D.C. Occlusion of dentin tubules by 45S5 Bioglass® Bioceramics 10. In: Sedel L., Rey C., editors. Proceedings of the 10th International Symposium on Ceramics in Medicine. France; Paris: Oct 1997. [Google Scholar]
- Lynch E., Brauer D., Karpukhina N., Gillam D., Hill R. Multi-component bioactive glasses of varying fluoride content for treating dentin hypersensitivity. Dental Mater. 2012;28:168–178. doi: 10.1016/j.dental.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Melek E.T., Zheng Kai, Boccaccini Aldo R. Novel bioactive glasses in medical applications. Int. J. Appl. Glass Sci. 2013:1–13. [Google Scholar]
- Olmo N., Martin A.L.., Salinas A.J., Vallet-Regi M.A., Turnay J. Bioactive sol–gel glasses with and without a hydroxycarbonate apatite layer as substrates for osteoblast cell adhesion and proliferation. Biomaterials. 2003;24(20):3383–3393. doi: 10.1016/s0142-9612(03)00200-x. [DOI] [PubMed] [Google Scholar]
- Patrick C.D. Measurement of strontium in serum, urine, bone, and soft tissues by Zeeman atomic absorption spectrometry. Clin. Chem. 1997;43(1):121–128. [PubMed] [Google Scholar]
- Pazo A., Saiz E., Tomsia P. Silicate glass coatings on Ti-based implants. Acta Mater. 1998;46:2551–2558. [Google Scholar]
- Tai B.J., Bian Z., Jiang H., Greenspan D.C., Zhong J., Clark A.E., Du M.Q. Anti-gingivitis effect of a dentifrice containing bioactive glass (NovaMin) particulate. J. Clin. Periodontol. 2006 Feb;33(2):86–91. doi: 10.1111/j.1600-051X.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- Tang Z.L., Wasserloos K., Croix C.M., St., Pitt B.R. Role of zinc in pulmonary endothelial cell response to oxidative stress. Am. J. Physiol. (Lung) 2001;281:243–249. doi: 10.1152/ajplung.2001.281.1.L243. [DOI] [PubMed] [Google Scholar]
- Thuy T.T., Nakagaki H., Kato K., Hung P.A., Inukai J., Tsuboi S., Hirose M.N., Igarashi S., Robinson C. Effect of strontium in combination with fluoride on enamel demineralization in vitro. Arch. Oral. Biol. 2008;53:1017–1022. doi: 10.1016/j.archoralbio.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Towler M.R., Crowley C.M., Murphy D., O’Callaghan A. A preliminary study of aluminum free glass poly alkenote cement. J. Mater. Sci. Let. 2002;21:1123. [Google Scholar]
- Valimaki V., Aro T. Molecular basis for action of bioactive glasses as bone graft substitute. Scandinavian J. Surg. 2006;95:95–102. doi: 10.1177/145749690609500204. [DOI] [PubMed] [Google Scholar]
- Wallace K.E., Hill R.G., Pembroke J.T., Brown C.J., Hatton P.V. Influence of sodium oxide content on bioactive glass properties. J. Mater. Sci.: Mater. Med. 1999;10:697–701. doi: 10.1023/a:1008910718446. [DOI] [PubMed] [Google Scholar]
- Wang Z., Sa Y., Sauro S., Chen H., Xing W., Ma X., Jiang T., Wang Y. Effect of densensitizing toothpastes on dentinal tubule occlusion: a dentine permeability measurement and SEM in vitro study. J. Dent. 2010;38:400–410. doi: 10.1016/j.jdent.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Whitehead R.Y., Lacefield W.R., Lucas L.C. Structure and integrity of a plasma sprayed hydroxylapatite coating on titanium. J. Biomed. Mater. Res. 1993;27:1501–1507. doi: 10.1002/jbm.820271206. [DOI] [PubMed] [Google Scholar]
- Williams C. Efficacy of a dentifrice containing zinc citrate for the control of plaque and gingivitis: a 6-month clinical study in adults. Compend Contin Educ Dent. 1998;19(Suppl 2):4–15. [PubMed] [Google Scholar]
- Wilson J., Low S., Fetner A., Hench L.L. Bioactive materials for periodontal treatment: a comparative study. In: Pizzoferrato A., Marchetti P.G., Ravaglioli A., Lee A.J.C., editors. Biomaterials and Clinical Applications. Vol. 5. Elsevier Science Publishers BV; Amsterdam: 1987. pp. 223–228. [Google Scholar]
- Yamaguchi M., Yamaguchi R. Action of zinc on bone metabolism in rats. Increases in alkaline phosphatise activity and DNA content. Biochem. Pharmacol. 1986;35:773–777. doi: 10.1016/0006-2952(86)90245-5. [DOI] [PubMed] [Google Scholar]
- Zehnder M., Luder H.U., Schätzle M., Kerosuo E., Waltimo T. A comparative study on the disinfection potentials of bioactive glass S53P4 and calcium hydroxide in contra-lateral human premolars ex-vivo. Int. Endodontic J. 2006;39:952–958. doi: 10.1111/j.1365-2591.2006.01173.x. [DOI] [PubMed] [Google Scholar]



