Abstract
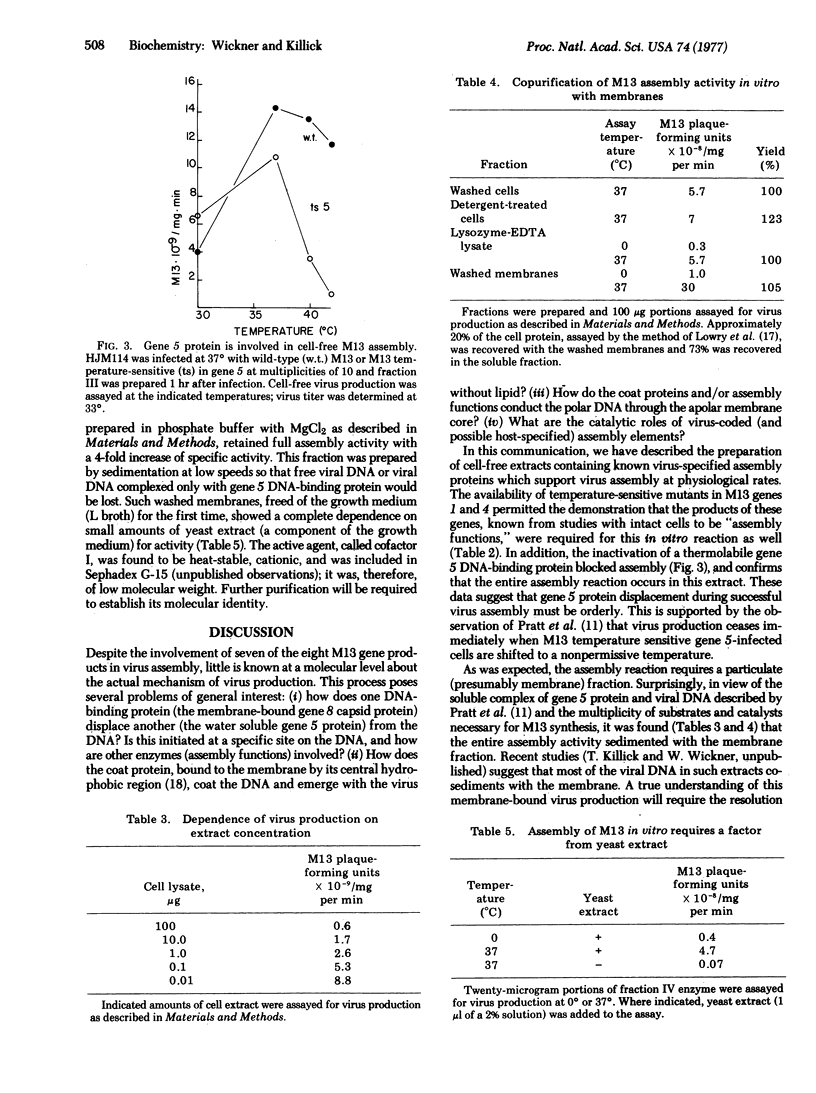
Assembly of coliphage M13 is known to occur as the viral DNA crosses the cytoplasmic membrane, shedding its virus-coded DNA unwinding protein and acquiring from the membrane approximately 2400 copies of the major coat protein. Conditions are described in which extracts of M13-infected E. coli and membranes prepared from such extracts will support virus assembly at a rate equivalent to that of intact cells. Extracts prepared from cells infected with temperature-sensitive M13 mutants in genes 1, 3, 4, or 5 are temperature-sensitive in this cell-free assembly reaction. Phage assembly in vitro requires magnesium and as yet an unidentified heat-stable cofactor of low molecular weight. The rate of virus assembly is approximately linear with respect to extract concentration over a 10(4)-fold range, consistent with the observation that the entire M13 assembly activity copurifies with the cell membrane fraction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbeck F., Beyreuther K., Köhler H., von Wettstein G., Braunitzer G. Virusproteine, IV. Die Konstitution des Hüllproteins des Phagen fd. Hoppe Seylers Z Physiol Chem. 1969 Sep;350(9):1047–1066. [PubMed] [Google Scholar]
- Berkowitz S. A., Day L. A. Mass, length, composition and structure of the filamentous bacterial virus fd. J Mol Biol. 1976 Apr 15;102(3):531–547. doi: 10.1016/0022-2836(76)90332-6. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Meier-Ewert H., Palese P. Assembly of lipid-containing viruses. J Supramol Struct. 1974;2(2-4):496–511. doi: 10.1002/jss.400020234. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., Marco R., Kornberg A. A coat protein of the bacteriophage M13 virion participates in membrane-oriented synthesis of DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):205–209. doi: 10.1073/pnas.70.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y., Konigsberg W. Reinvestigation of a region of the fd bacteriophage coat protein sequence. J Mol Biol. 1974 Sep 25;88(3):598–600. doi: 10.1016/0022-2836(74)90410-0. [DOI] [PubMed] [Google Scholar]
- Pratt D., Erdahl W. S. Genetic control of bacteriophage M13 DNA synthesis. J Mol Biol. 1968 Oct 14;37(1):181–200. doi: 10.1016/0022-2836(68)90082-x. [DOI] [PubMed] [Google Scholar]
- Pratt D., Laws P., Griffith J. Complex of bacteriophage M13 single-stranded DNA and gene 5 protein. J Mol Biol. 1974 Feb 5;82(4):425–439. doi: 10.1016/0022-2836(74)90239-3. [DOI] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Beaudoin J. Conditional lethal mutants of the small filamentous coliphage M13. II. Two genes for coat proteins. Virology. 1969 Sep;39(1):42–53. doi: 10.1016/0042-6822(69)90346-8. [DOI] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Erdahl W. S. Conditional lethal mutants of the small filamentous coliphage M13. I. Isolation, complementation, cell killing, time of cistron action. Virology. 1966 Nov;30(3):397–410. doi: 10.1016/0042-6822(66)90118-8. [DOI] [PubMed] [Google Scholar]
- Smilowitz H., Carson J., Robbins P. W. Association of newly synthesized major f1 coat protein with infected host cell inner membrane. J Supramol Struct. 1972;1(1):8–18. doi: 10.1002/jss.400010103. [DOI] [PubMed] [Google Scholar]
- Weiner J. H., Bertsch L. L., Kornberg A. The deoxyribonucleic acid unwinding protein of Escherichia coli. Properties and functions in replication. J Biol Chem. 1975 Mar 25;250(6):1972–1980. [PubMed] [Google Scholar]
- Woolford J. L., Jr, Webster R. E. Proteolytic digestion of the micellar complex of f1 coat protein and deoxycholate. J Biol Chem. 1975 Jun 10;250(11):4333–4339. [PubMed] [Google Scholar]


