Abstract
Alcoholism is associated with acute and long-term cognitive dysfunction including memory impairment, resulting in substantial disability and cost to society. Thus, understanding how ethanol impairs cognition is essential for developing treatment strategies to dampen its adverse impact. Memory processing is thought to involve persistent, use-dependent changes in synaptic transmission, and ethanol alters the activity of multiple signaling molecules involved in synaptic processing, including modulation of the glutamate and gamma-aminobutyric acid (GABA) transmitter systems that mediate most fast excitatory and inhibitory transmission in the brain. Effects on glutamate and GABA receptors contribute to ethanol-induced changes in long-term potentiation (LTP) and long-term depression (LTD), forms of synaptic plasticity thought to underlie memory acquisition. In this paper, we review the effects of ethanol on learning-related forms of synaptic plasticity with emphasis on changes observed in the hippocampus, a brain region that is critical for encoding contextual and episodic memories. We also include studies in other brain regions as they pertain to altered cognitive and mental function. Comparison of effects in the hippocampus to other brain regions is instructive for understanding the complexities of ethanol’s acute and long-term pharmacological consequences.
Keywords: alcohol, long-term potentiation, long-term depression, NMDA receptors, GABA receptors, neurosteroids, acetaldehyde
Introduction
Alcohol intoxication and addiction are major public health problems. In the United States, about 15% of adults have an alcohol-related disorder at some point in their life, and alcohol abuse costs the economy more than $220 billion per year in medical care and lost productivity (Bouchery, Harwood, Sacks, Simon, & Brewer, 2011; Hasin, Stinson, Ogburn, & Grant, 2007). A high percentage of motor vehicle accidents and violent crimes also involve alcohol use, adding to overall societal burden, and alcohol abuse is associated with medical co-morbidities affecting numerous body systems, including the brain and central nervous system (CNS).
Among the major adverse effects of ethanol is its ability to cause short- and long-term cognitive dysfunction. During milder intoxication (blood alcohol levels [BAL] in the 10–20 mM range), most individuals exhibit motor incoordination and diminished reaction times. With higher BAL (20–40 mM, ~100–200 mg/dL), slowed thinking and altered cognitive processing is observed, and at BAL of 40 mM and above some individuals develop memory “blackouts” (White, 2003). The latter are periods in which individuals perform complex behaviors but have no subsequent recollection. A blackout reflects an acute defect in new memory formation and is a state that can have devastating consequences. In addition to these acute effects, chronic alcohol abuse is associated with persistent impairments in memory and cognition, referred to as “alcoholic dementia” (Oslin & Cary, 2003). Chronic memory problems can also result from nutritional deficiencies associated with ethanol use. Together, these findings strongly support the idea that ethanol is a major risk factor for cognitive dysfunction.
Understanding how ethanol produces its direct effects on memory and cognition is important for developing prevention and treatment strategies to dampen the public health impact of alcoholism. While mechanisms underlying memory are not completely understood, present evidence supports a role for persistent, use-dependent changes in glutamate-mediated excitatory neurotransmission (Martin, Grimwood, & Morris, 2000), with long-term potentiation (LTP) and long-term depression (LTD) being leading candidates as synaptic memory mechanisms (Malenka & Bear, 2004). In this review, we will focus on the effects of ethanol on LTP and LTD. While our emphasis is on acute effects of ethanol, we will also highlight studies that provide insights into longer-term consequences, including studies examining the effects of repeated ethanol intoxication and withdrawal. Because much of the work to date has been done in the hippocampus, a brain region critical for declarative memory formation, we will highlight studies in this region, but we will also describe important results from other brain regions that highlight the complexity of ethanol’s CNS actions.
Ethanol and the CNS
Ethanol is an intriguing drug with low molecular weight and solubility in both water and lipid (Deitrich, Dunwiddie, Harris, & Erwin, 1989). The lipid solubility led to early hypotheses that ethanol acts by perturbing cell membranes with secondary effects on cellular proteins. Ethanol thus initially shared a “membrane fluidity hypothesis” with other CNS depressants including general anesthetics (Samson & Harris, 1992). As information about direct effects on proteins evolved and greater information about the molecular structure of proteins became available, emphasis shifted to describing actions on specific signaling systems. It is now clear that ethanol directly alters the activity of numerous ion channels, receptors, and enzymes, and these effects contribute to changes in synaptic function and plasticity (Harris, 1999; Vengeliene, Bilbao, Molander, & Spanagel, 2008).
Among the diverse effects of ethanol, present evidence suggests that changes in glutamatergic and GABAergic neurotransmission, the major fast excitatory and inhibitory transmitter systems in the CNS, determine many of ethanol’s acute and long-term properties (Chandler, Harris, & Crews, 1998). There is considerable evidence that ethanol augments the actions of GABA at certain GABAA receptors (GABAARs) and inhibits the effects of glutamate at N-methyl-D-aspartate receptors (NMDARs) and kainate receptors (KARs), with additional effects on AMPA type receptors (AMPARs) and metabotropic glutamate receptors (mGluRs). Effects on glutamatergic and GABAergic systems are likely to drive changes in cognition and will be a major focus of this review.
Effects of ethanol on GABA receptors
GABA gates chloride channels (called GABAARs) to provide fast inhibitory transmission in the CNS (Sieghart, 1995). GABAARs are major sites of action of important sedative, anticonvulsant, and anesthetic drugs including benzodiazepines, barbiturates, neuroactive steroids, and volatile anesthetics. GABAARs are pentameric proteins that belong to the cys-loop family of ion channels with 4 membrane-spanning regions per subunit. Multiple GABAAR subunits in several subfamilies (α, β, γ, δ, ε, π, ρ, θ) have been identified, and at least 12 different subunits are expressed in the hippocampus alone, providing substrate for a large array of possible receptor subtypes (Olsen & Sieghart, 2009; Sieghart, 1995). Fortunately, a limited number of subunit combinations appear to dominate brain GABAAR expression, making this a more tractable problem (Olsen & Sieghart, 2008, 2009). Acting at different GABAARs, GABA provides two major types of inhibition – fast phasic inhibition associated with release at synapses and slower, more persistent tonic inhibition mediated by extrasynaptic GABAARs. Synaptic and extrasynaptic GABAARs express different, but sometimes overlapping subunits. For example, synaptic receptors typically contain γ2 subunits, while δ subunits, in the restricted cell populations that express them, are found exclusively in extrasynaptic receptors; γ2 subunits, however, can also be expressed outside of synapses (Farrant & Nusser, 2005; Glykys & Mody, 2007).
Different combinations of subunits markedly influence physiological and pharmacological properties, and ethanol enhances the actions of GABA at some GABAARs. Early studies suggested that effects of ethanol are most prominent at receptors expressing γ2L subunits that contain a site for phosphorylation by protein kinase C (PKC) (Wafford et al., 1991), and mice with targeted deletions of the γ isoform of PKC have diminished ethanol sensitivity (Harris et al., 1995). The importance of γ2L has not been observed in all studies and the α1 subunit may be important in determining ethanol effects at some GABAARs (Criswell et al., 1993; Mihic, Whiting, & Harris, 1994). Additional subtypes of GABAARs may also be highly sensitive to ethanol. These include extrasynaptic receptors expressing α4β3δ and α6β3δ subunits (Criswell & Breese, 2005). In the hippocampus, the α4β3δ subtype (or perhaps α4β2δ) is expressed extrasynaptically in dentate gyrus and contributes to tonic inhibition (Herd et al., 2008; Mody, 2001). This GABAAR was initially reported to be potentiated by ethanol at low mM concentrations (Wallner, Hanchar, & Olsen, 2003), but this result has not been replicated by other groups (Borghese & Harris, 2007; Borghese et al., 2006). Nonetheless, ongoing work has shown the importance of extrasynaptic GABAARs, particularly those expressing δ subunits (Meera, Olsen, Otis, & Wallner, 2010). Effects of ethanol on extrasynaptic receptors may also involve modulation by PKCδ (Choi et al., 2008) and changes in receptor surface expression (Suryanarayanan et al., 2011).
Whether effects on GABAARs result from direct actions on receptors or from release of endogenous modulators that act alone or in concert with ethanol is uncertain, and may vary by brain region. There is evidence that some ethanol effects are mediated indirectly by increases in GABA-potentiating neurosteroids (Morrow et al., 1999; VanDoren et al., 2000). These neurosteroids include 5α-reduced pregnanes such as allopregnanolone (3α-hydroxy-5αpregnan-20-one, 3α5αP, alloP) and 3α,5α-3,21-dihydroxypregnan-20-one (3α5α-THDOC), agents that are potent and highly effective GABAAR modulators (Belelli & Lambert, 2005; Zorumski, Paul, Izumi, Covey, & Mennerick, 2013). Synthesis of these GABAergic steroids accounts for at least some of ethanol’s effects on inhibitory transmission and neuronal firing rates in the CA1 hippocampal region (Roberto et al., 2006; Sanna et al., 2004; Siggins, Roberto, & Nie, 2005; Tokunaga, McDaniel, Morrow, & Matthews, 2003). Furthermore, ethanol and 5α-reduced neuroactive steroids can act synergistically at GABAARs to enhance ion channel function (Akk & Steinbach, 2003). Ethanol also promotes the synthesis of neurosteroids locally in the brain, with increases in steroid levels in the hippocampus within minutes following exposure to 50 mM ethanol (Sanna et al., 2004); both excitatory neurons (Agís-Balboa et al., 2006) and glia (King et al., 2002) contribute to local 5α-reduced steroid synthesis. Presynaptic effects of ethanol also contribute to enhanced inhibition, although this appears to vary across brain regions (Criswell, Ming, Kelm, & Breese, 2008; Roberto et al., 2006; Siggins et al., 2005). At inhibitory synapses onto pyramidal neurons in the hippocampus, the net effect of ethanol is to augment phasic inhibition (Sanna et al., 2004). Interestingly, ethanol may differentially enhance GABAergic synapses onto hippocampal pyramidal neuron cell bodies relative to distal dendritic synapses (Weiner, Gu, & Dunwiddie, 1997). Additionally, both ethanol and neurosteroids may have greater and more potent effects on extrasynaptic GABAARs that mediate regional tonic inhibition (Wallner et al., 2003).
Effects of ethanol on glutamate receptors (GluRs)
Glutamate is the brain’s major fast excitatory transmitter, and acts at 4 families of receptors. These include ligand-gated ion channels (NMDARs, KARs, and AMPARs) that serve as principal mediators of fast neurotransmission (Dingledine, Borges, Bowie, & Traynelis, 1999; Traynelis et al., 2010), and a group of G-protein-coupled metabotropic receptors (Anwyl, 1999). NMDARs are a site of action of multiple psychoactive drugs including ketamine, phencyclidine (PCP), and nitrous oxide. Native NMDARs typically express GluN1 (NR1) subunits in combination with GluN2 (GluN2A-D, previously called NR2A-D) subunits (Ogden & Traynelis, 2011; Traynelis et al., 2010). At low millimolar concentrations (5–10 mM), similar to levels achieved following 1 or 2 standard drinks (each with ~12 g of alcohol), ethanol non-competitively inhibits NMDARs (Lovinger, White, & Weight, 1989, 1990), affecting ion channel gating rather than agonist binding (Wright, Peoples, & Weight, 1996). Ethanol, however, is a weak NMDAR antagonist and even at very high concentrations (60 mM and above) is only a partial inhibitor. While ethanol is not selective for any particular NMDAR subtype, some evidence suggests preferential inhibition of receptors expressing GluN1/GluN2B subunits (Masood, Wu, Brauneis, & Weight, 1994), a class that is highly sensitive to block by ifenprodil-type agents (Williams, 1993). Other studies indicate that ethanol inhibits receptors containing GluN2A or GluN2B with less effect on those containing GluN2C or GluN2D (Tsai & Coyle, 1998). Ethanol’s site of action on NMDARs is not certain, although amino acids in the 3rd and 4th membrane spanning domains in GluN1 and GluN2A are important (Ren, Honse, & Peoples, 2003; Ronald, Mirshahi, & Woodward, 2001). Recent studies in GluN1/GluN2A indicate that 4 pairs of residues in the 3rd and 4th membranespanning domains at the interface between subunits interact with each other to regulate ethanol sensitivity (Ren, Zhao, Dwyer, & Peoples, 2012), with phenylalanine 636 in GluN2A playing a key role (Ren, Zhao, Wu, & Peoples, 2013). This latter residue also influences agonist affinity and channel gating. Evidence does not support actions at sites for other major modulators including Mg2+, glycine, polyamine, and phencyclidine/MK-801 (Chu, Anantharam, & Treistman, 1995). The degree of inhibition, however, may depend on the presence of extracellular Mg2+ (Calton, Wilson, & Moore, 1998).
Ethanol also inhibits KARs (Dildy-Mayfield & Harris, 1995; Valenzuela, Bhave, Hoffman, & Harris, 1998). KARs are glutamate-gated channels prominently expressed in hippocampus that participate in transmission at certain synapses. Among other effects, KARs regulate GABA release, and KAR inhibition by low concentrations of ethanol may contribute to ethanol-mediated disinhibition in the CA1 region (Carta, Ariwodola, Weiner, & Valenzuela, 2003). Other evidence suggests that acute and chronic ethanol alters the function of mGluRs linked to phosphoinositide (PI) turnover and mobilization of intracellular Ca2+ (Minami, Gereau, Minami, Heinemann, & Harris, 1998; Simonyi, Christian, Sun, & Sun, 2004).
NMDARs and Synaptic Plasticity in the Hippocampus
A potentially important consequence of GluR inhibition is the effect that this has on long-term synaptic plasticity. NMDARs and Group I mGluRs (mGluR1 and mGluR5) play important roles in the induction and modulation of LTP and LTD in the CA1 region (Anwyl, 1999; Hölscher, Gigg, & O’Mara, 1999), an area that is critical for declarative memory formation. LTP refers to a lasting enhancement of transmission that typically follows brief high-frequency activation of glutamate synapses (Bliss & Collingridge, 1993; Malenka & Bear, 2004). Although LTP has been described at glutamate synapses in many brain regions, the process has been most intensively studied in the CA1 Schaffer collateral pathway. Changes underlying LTP last for hours in vitro and for days to weeks in vivo. These persisting changes make it attractive to view LTP as a synaptic memory mechanism because synapses “remember” that they have experienced certain patterns of activation; that is, these synapses show persistently enhanced responses to an invariant stimulus following particular patterns of activation. Whether an LTP-like process occurs during memory formation in vivo is not completely certain. There is evidence that drugs that alter LTP also affect learning (Martin et al., 2000) and experiments using transgenic mice with targeted alterations in key proteins involved in LTP have provided strong correlative, but not universal, support for the hypothesis (Chen & Tonegawa, 1997; Malenka & Bear, 2004; Martin et al., 2000). Work by Bear and colleagues has provided particularly compelling findings in a one-trial inhibitory avoidance-learning paradigm (Whitlock, Heynen, Shuler, & Bear, 2006). Similar considerations exist for LTD, with some evidence suggesting a role for this form of plasticity in novelty processing and one-trial forms of spatial learning (Kemp & Manahan-Vaughan, 2007; Manahan-Vaughan & Braunewell, 1999).
NMDARs play complex roles in synaptic plasticity. Depending on timing and pattern of activation, NMDARs not only promote LTP but also induce homosynaptic LTD or dampen the ability to generate LTP. The latter effect is referred to as “metaplasticity” (modulation of synaptic plasticity) (Abraham & Tate, 1997; Zorumski & Izumi, 2012). When CA1 synapses are activated at 1 Hz for 10–15 min, persisting homosynaptic LTD typically ensues (Dudek & Bear, 1992). Additionally, synapses that have previously undergone LTP can be “depotentiated” by 1 Hz stimulation (Fujii, Saito, Miyakawa, Ito, & Kato, 1991), providing a mechanism for synaptic resetting. The induction of both LTD and LTP-depotentiation (LTP-D), like LTP, is inhibited by NMDAR antagonists and requires Ca2+ influx into postsynaptic neurons (Mulkey & Malenka, 1992). Whether synapses exhibit LTP or LTD appears to depend on the degree and timing of increases in postsynaptic Ca2+, and ultimately on the Ca2+-dependent messengers that are activated (Lisman, 1989; Malenka & Bear, 2004). Greater increases in intracellular Ca2+ and activation of protein kinases contribute to early phases of LTP whereas activation of protein phosphatases contributes to the initiation of LTD and LTP-D (Mulkey, Endo, Shenolikar, & Malenka, 1994; O’Dell & Kandel, 1994). Specific subtypes of NMDARs may participate in LTP and LTD. Early studies suggested that LTP requires GluN1/GluN2A receptors while LTD requires GluN1/GluN2B (Liu et al., 2004; Massey et al., 2004). More recent studies indicate that LTP involves multiple NMDAR subtypes, including GluN1/GluN2A and GluN1/GluN2B (Berberich et al., 2005; Volianskis et al., 2013). GluN2B-expressing receptors have been more consistently linked to LTD (Brigman et al., 2010), but even here not all studies are consistent (Paoletti, Bellone, & Zhou, 2013). These results suggest that agents with differential effects on NMDAR subtypes may differentially modulate LTP and LTD. Further complicating things, triheteromeric NMDARs with GluN1, GluN2A, and GluN2B subunits are expressed at mature synapses, and this has implications for interpreting the effects of subtype selective antagonists (Paoletti et al., 2013).
Studies outlined above indicate that excitatory synapses, particularly those in area CA1, operate over a range of efficacy and that NMDARs help to determine the effective range. Furthermore, these studies are consistent with the idea that the threshold for synaptic plasticity is dynamic and subject to modulation (Bienenstock, Cooper, & Munro, 1982). In hippocampal slices from young rodents, Dudek and Bear (1992) found that the frequency at which a fixed number of stimuli are delivered to Schaffer collateral inputs determines whether CA1 synapses show LTP, LTD, or no change. When 900 pulses are administered at 1–5 Hz, LTD ensues. The same number of pulses at ~10 Hz produces no lasting change in synaptic efficacy, whereas 900 pulses at 30 Hz or greater produces LTP. This suggests that 10 Hz is a frequency “threshold” for synaptic change. This threshold can be shifted over the course of postnatal development and by specific neuromodulators (Katsuki, Izumi, & Zorumski, 1997).
Acute Effects of Ethanol on Hippocampal Synaptic Plasticity
As noted, an interesting feature of ethanol is its ability to cause acute memory “blackouts” (Nelson et al., 2004; White, 2003). The importance of NMDARs in synaptic plasticity and ethanol’s inhibitory actions on these receptors, make it likely that NMDARs mediate at least some of ethanol’s effects on memory (Morrisett & Swartzwelder, 1993; White & Swartzwelder, 2004). Although results have been variable, it appears that concentrations of ethanol in the range of 5 to 60 mM inhibit both NMDAR responses and LTP in the CA1 region of hippocampal slices (Table 1) (Chandler et al., 1998). Interestingly, block of NMDARs is only partial at ethanol concentrations that inhibit LTP, and similar partial inhibition of synaptic NMDARs by more selective NMDAR antagonists is insufficient to block LTP (Izumi, Nagashima, Murayama, & Zorumski, 2005c). For reference, a concentration of 50 mM is about 0.2% ethanol (200 mg/dL), a highly intoxicating blood level in most humans (legal intoxication is ~0.08%).
Table 1.
Acute Ethanol & CA1 Hippocampal LTP in vitro
| LTP Depressed or Blocked Completely | ||||||
|---|---|---|---|---|---|---|
| Study | Age | Ca/Mg | K | Stimulus | Intensity | [Ethanol] |
| Blitzer (1990) | P50–90 | 2.5/1.5 | 5.0 | 100Hz × 1s × 2 | 10% max | 5 mM |
| Sugiura (1995) | P56–63 | 2.4/1.3 | 6.2 | 100Hz × 0.5s | 50% max | 50–75 mM |
| Swartzw’r (1995) | P15–25 | 2.0/1.0 | 3.3 | 100Hz × .04s × 10 | 25% max | 60 mM |
| Schummers (1997) | P40–60 | 2.5/1.0 | 5.0 | 100Hz × 1s or TBS | 40–50% max | 50 mM |
| Pyapali (1999) | P30 | 2.4/2.0 | 3.25 | TBS | 50% max | 10–30 mM |
| Izumi (2005c) | P30 | 2.0/2.0 | 5.0 | 100Hz × 1 s | 50% max | 60 mM |
| Izumi (2007) | P30 | 2.0/2.0 | 5.0 | 100Hz × 1 s | 50% max | 40–60 mM |
| Fujii (2008) | ~P120 | 2.5/2.0 | 5.0 | 100Hz × 0.25 s | 40–60% max | 8.6 mM |
| Tokuda (2011) | P30 | 2.0/2.0 | 5.0 | 100Hz × 1 s | 50% max | 60 mM |
| LTP Not Inhibited | ||||||
|---|---|---|---|---|---|---|
| Study | Age | Ca/Mg | K | Stimulus | Intensity | [Ethanol] |
| Swartzw’r (1995) | P70–100 | 2.0/1.0 | 3.3 | 100Hz × .04s × 10 | 25% max | 60 mM |
| Randall (1995) | P40–50 | 2.2/2.0 | 3.5 | 100Hz × 1s × 2 | 40% max | 22 mM |
| Pyapali (1999) | P90 | 2.4/2.0 | 3.25 | TBS | 50% max | 10–30 mM |
| Izumi (2005c) | P30 | 2.0/2.0 | 5.0 | 100Hz × 1 s | 50% max | 18 mM |
| Izumi (2007) | P30 | 2.0/2.0 | 5.0 | 100Hz × 1 s | 50% max | 20 mM |
| Fujii (2008) | ~P120 | 2.5/2.0 | 5.0 | 100Hz × 0.25s | 40–60% max | 4.3 mM |
Abbreviations: Age (animal age at which studies were done); P (postnatal day); Ca/Mg (concentrations of extracellular calcium and magnesium, in mM); K (extracellular potassium concentration, in mM); [Ethanol] (concentration of ethanol that was studied or that was required for significant effect on LTP); TBS (theta burst stimulation)
In some cases, the degree of LTP was diminished, but not completely blocked by this concentration of ethanol. In Schummers et al. (1997), TBS consisted of 100 Hz × 4 pulses × 10 every 0.2 s. In Izumi et al. (2007), 40 mM ethanol produced a partial block of LTP with complete block requiring 60 mM. Morrisett & Swartzwelder (1993) examined LTP n the dentate gyrus and found that 75 mM ethanol blocked LTP.
A challenge in interpreting the acute effects of ethanol on LTP is that studies have varied widely in terms of alcohol concentration, age of the animals studied, and recording conditions. Experimental variables (age, ionic conditions, and stimulus parameters) are important because they influence the ease with which LTP can be generated. Table 1 presents an overview of studies examining acute ethanol and LTP in the CA1 region. LTP inhibition typically requires high concentrations of ethanol (in the 40 to 60 mM range), although Blitzer, Gil, & Landau (1990) observed effects at 5 mM in slices from young adult rodents (postnatal day [P] 50–90), and Fujii, Yamazaki, Sugihara, & Wakabayashi (2008) reported dampening at 8.6 mM in adult rats. Pyapali, Turner, Wilson, & Swartzwelder (1999) found that LTP was diminished by 10–30 mM ethanol in slices from P30 (juvenile) rats, but not P90 rats. Other studies report that 18–22 mM ethanol does not block LTP in slices from P30–50 animals (Izumi et al., 2007; Izumi, Nagashima, et al., 2005c; Randall, Lee, Meyer, Wittenberg, & Gruol, 1995). Variables that could contribute to these discrepancies, including brain slice condition, are discussed below and highlighted in Table 1. It is important to consider differences in experimental paradigms because it seems unlikely that low alcohol (< 10 mM) routinely causes profound defects in LTP; otherwise, memory blackouts would be a regular occurrence with only a few standard drinks, something not usually observed. Nonetheless, a dampening of plasticity could contribute to milder dysfunction with low level intoxication, and prior history of alcohol use could modulate these effects through tolerance.
In contrast to LTP, there is much less information about effects of ethanol on hippocampal LTD. Some work suggests that despite inhibitory effects on NMDARs, ethanol may acutely enhance LTD in the CA1 region (Hendricson, Miao, Lippmann, & Morrisett, 2002). As discussed below, this latter observation could involve metaplastic effects of ethanol. It is also important to consider that LTD, like LTP, is mechanistically complex with several distinct induction mechanisms (Izumi & Zorumski, 2012). These include forms of LTD involving Group I mGluRs, particularly mGluR5. While most studies of ethanol have focused on NMDAR-mediated LTD, Overstreet, Pasternak, Colley, Slater, and Trommer (1997) found that acute ethanol also blocks mGluR-dependent LTD in the hippocampus.
In a series of studies, we examined acute effects of ethanol on NMDAR-dependent LTP and LTD in the CA1 region of P30 rats (Izumi et al., 2007; Izumi, Nagashima, et al., 2005c; Tokuda, Izumi, & Zorumski, 2011). We found that ethanol inhibits both LTP and LTD when administered for 15–30 min prior to and during standard stimuli used to induce LTP and LTD. Effects on LTD mirrored the block of synaptic NMDARs, and ethanol appeared to preferentially inhibit ifenprodil-sensitive (GluN2B-expressing) NMDARs. LTD was significantly diminished by 18 mM ethanol with complete block at 60 mM. Importantly, effects of 60 mM ethanol on LTD, like effects on synaptic NMDARs, were reversible after washout. Effects on LTP were more complex. First, it required ~60 mM ethanol to completely inhibit CA1 LTP during 15–30 min administration, and the effects were slow to reverse (up to several hours) following washout (Fig. 1). Second, LTP block, including the longer-lived inhibition, was overcome by picrotoxin, a GABAAR antagonist. This suggests that ethanol-mediated effects on inhibitory transmission in addition to, or instead of ethanol-mediated effects on NMDARs contribute to LTP block. These latter results are consistent with other studies showing that effects of ethanol on LTP may not result simply from NMDAR block (Schummers, Bentz, & Browning, 1997; Schummers & Browning, 2001). Recent studies suggest that generation of endogenous GABA-potentiating neurosteroids contributes to effects on LTP (Izumi et al., 2007). Interestingly, 60 mM ethanol also blocked an NMDAR-independent form of LTP that involves L-type voltage-activated calcium channels (Izumi, Nagashima, et al., 2005ca), supporting the idea of more complex effects than NMDAR inhibition alone.
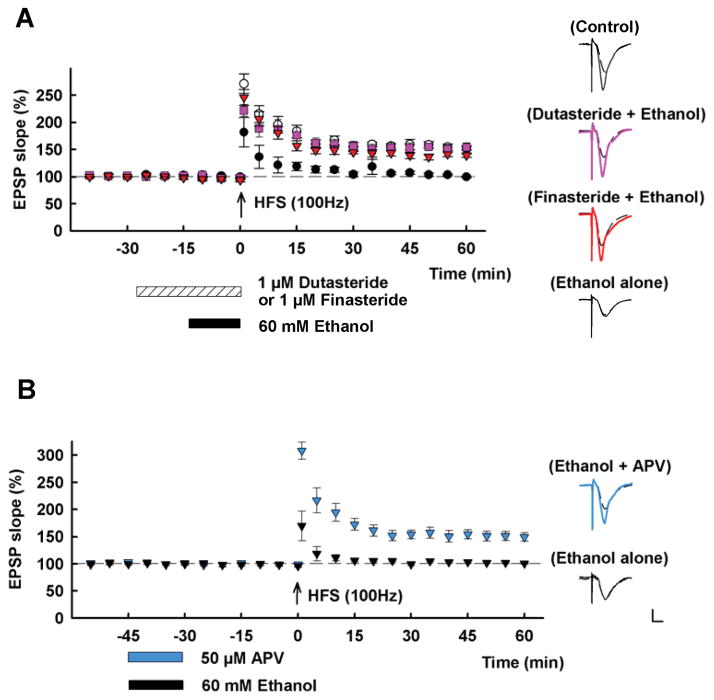
Figure 1.
This figure highlights several features of ethanol’s acute effects on LTP in the CA1 region of hippocampal slices from P30 rats. A. The graph shows the ability of 60 mM ethanol administered for 15 min (black bar) to block LTP in the Schaffer collateral pathway (black circles). This LTP inhibition is overcome by pre-treatment with 1 μM finasteride (red triangles) or 1 μM dutasteride (purple squares). Finasteride and dutasteride block the synthesis of alloP and other 5α-reduced neurosteroids. Control LTP is shown in white circles. B. The graph shows that 60 mM ethanol inhibits LTP for at least 30 min following washout (black triangles) and that full block of NMDARs with 50 μM APV during ethanol administration overcomes LTP inhibition (blue triangles) when both drugs are washed out 30 min prior to high frequency stimulation (HFS, arrow; 100 Hz × 1 s tetanus). Traces to the right show EPSPs recorded during baseline (dashed traces) and 60 min following HFS (solid traces). Calibration: 1 mV, 5 ms. This figure is reproduced from Tokuda et al., Journal of Neuroscience, 2011.
Acute ethanol tolerance and synaptic plasticity
The effects of ethanol outlined above typically result from a rapid increase in ethanol levels to fixed concentrations that are sustained for varying periods (often 15–30 min before and during tetanic stimulation). It is also known that during acute ingestion certain behavioral effects of ethanol diminish despite the presence of high BAL, a phenomenon known as “acute tolerance” (Batista, Prediger, Morato, & Takahashi, 2005; Silveri & Spear, 2004). Acute tolerance may be important in determining susceptibility to alcoholism and its long-term consequences, and has been observed for effects on NMDARs and GABAARs. Acute tolerance of NMDARs may involve phosphorylation of GluN2B subunits by the tyrosine kinase, Fyn (Miyakawa et al., 1997). During acute exposure, ethanol causes dissociation of the scaffolding protein RACK1 from Fyn, allowing Fyn to phosphorylate GluN2B subunits and enhance function (Yaka, Phamluong, & Ron, 2003). Additionally, acute tolerance of GABAARs may reflect changes in receptor phosphorylation (Morrow, VanDoren, Penland, & Matthews, 2001), although changes in presynaptic release of GABA (Ariwodola & Weiner, 2004) and changes in the release of neurosteroids by ethanol (Barbosa & Morato, 2001) may also contribute.
Another factor determining the effects of ethanol involves the rate of rise of ethanol concentration. Clinical manifestations of ethanol intoxication are determined not only by the levels of alcohol achieved but also by the rate of change in ethanol concentration. In most synaptic studies, ethanol is administered at fixed concentrations fairly rapidly and for sustained periods. Thus, relevance to effects observed clinically is uncertain. It is estimated that a single standard drink increases BAL into the low mM range. This makes it unlikely that during a bout of oral intoxication a rise to 50 mM or greater will occur within minutes even during binge consumption. Tokuda, Zorumski, & Izumi (2007) examined the effects of slower changes in ethanol concentration by increasing ethanol levels in 10 mM increments every 15 min to achieve a final level of 60 mM, a concentration that routinely blocks LTP when administered acutely. Under these circumstances, 60 mM ethanol no longer completely inhibited CA1 LTP. Furthermore, LTP induced under these conditions had unique features, including lack of block by a full NMDAR antagonist, a broad-spectrum mGluR antagonist, or an inhibitor of L-type calcium channels. Interestingly, “ethanol-tolerant LTP” was blocked by inhibiting calcium release from intracellular stores, and the generation of this form of tolerance was prevented by complete NMDAR antagonism during the period of escalating ethanol administration. The latter finding suggests that during ethanol exposure, activation of unblocked NMDARs modulates neuronal function and has consequences for synaptic plasticity.
GABA-enhancing neurosteroids and acute effects of ethanol on LTP
Ethanol can enhance GABA responses in the hippocampus, and changes in inhibitory transmission likely contribute to effects on LTP (Izumi, Nagashima, et al., 2005c; Morrisett & Swartzwelder, 1993; Schummers et al., 1997; Schummers & Browning, 2001). It is not certain which effects of ethanol on GABAergic inhibition contribute to acute LTP block, but there is evidence that ethanol may preferentially augment a form of inhibition in the CA1 region resulting from activation of GABAARs near the pyramidal cell body layer (Izumi et al., 2007; Weiner et al., 1997). This proximal inhibition is well positioned to regulate excitability and output of pyramidal neurons and influence synaptic plasticity.
Because other studies found that ethanol promotes synthesis of neurosteroids (Sanna et al., 2004), we examined whether allopregnanolone (alloP), a 5α-reduced neurosteroid that is an effective and potent enhancer of GABAARs (Akk et al., 2007; Chisari, Eisenman, Covey, Mennerick, & Zorumski, 2010), altered the effects of ethanol. When administered at levels that had no effect on paired-pulse depression (PPD) of CA1 population spike firing, alloP enhanced PPD in the presence of ethanol (Murayama, Zorumski, & Izumi, 2006). This suggests that GABAergic neurosteroids may contribute to ethanol’s ability to modulate local inhibition. Consistent with this, a concentration of alloP that alone had no effect on LTP facilitated ethanol-mediated LTP inhibition (Izumi et al., 2007). In the absence of exogenous alloP, a high acute concentration of ethanol (60 mM) is required to block LTP. In the presence of alloP, significant effects were observed at 20 mM, with complete LTP block at 40 mM. Similarly, Talani, Biggio, & Sanna (2011) found that behavioral stressors associated with increased endogenous neurosteroids (Paul & Purdy, 1992) acutely enhanced sensitivity of LTP to ethanol by a mechanism involving neurosteroid synthesis.
Supporting a role for endogenous neurosteroids in the effects of ethanol on LTP, other studies have found that agents that inhibit alloP synthesis prevent the effects of ethanol on LTP in hippocampal slices (Izumi et al., 2007; Tokuda et al., 2011) (Fig. 1). Similarly, inhibiting the actions of neurosteroids with 17-PA (3α,5α-17-phenyladrost-16-en-ol), a synthetic steroid that blocks the effects of 5α-reduced steroids on GABAARs (Mennerick et al., 2004), or removing neurosteroids with a cyclodextrin also overcomes acute LTP inhibition (Izumi et al., 2007). Cyclodextrins are cyclic oligosaccharides that can serve as molecular sponges to scavenge neurosteroids and terminate their actions on GABAARs (Shu et al., 2004). Effects of ethanol on proximal inhibition in the CA1 region are also prevented by inhibitors of neurosteroids (Izumi et al., 2007). Taken together, these studies indicate that GABAergic neurosteroids facilitate the effects of ethanol on both local CA1 inhibition and LTP, and that high concentrations of ethanol promote the synthesis of GABAergic neurosteroids contributing to effects on inhibition and LTP. Because neurosteroid levels can be manipulated by a variety of agents and stressors (Tokuda, O’Dell, Izumi, & Zorumski, 2010; Zorumski et al., 2013), these findings have implications for interpreting the concentrations of ethanol that acutely modulate synaptic function. Under conditions in which basal neurosteroid levels are elevated, ethanol is more potent against LTP.
Do metaplastic effects contribute to acute LTP inhibition?
Studies implicating a role for GABAergic steroids in acute ethanol-mediated LTP inhibition strongly suggest that NMDAR block does not account for all effects on LTP. Even at 60 mM, block of synaptic NMDARs by ethanol is only partial (~50% block) and appears to involve ifenprodil-sensitive (GluN2B) NMDARs in the CA1 region of juvenile rats (Izumi, Nagashima, et al., 2005c). This implies that a large fraction of NMDARs remains unblocked, and similar partial inhibition of synaptic NMDARs with ifenprodil or 2-amino-5-phosophovalerate (APV) is insufficient to mimic ethanol’s effects on LTP. Furthermore, and differing from effects of ethanol on LTD, LTP inhibition by 60 mM ethanol persists following drug washout (Izumi, Nagashima, et al., 2005c). These observations, coupled with the finding that complete NMDAR block during ethanol administration overcame acute tolerance (Tokuda et al., 2007), led to experiments examining whether complete NMDAR block during ethanol exposure could also overcome effects on LTP. Taking advantage of the prolonged block of LTP following ethanol washout and the fact that APV can be washed out rapidly, Tokuda et al. (2011) found that co-administration of 60 mM ethanol with APV allowed LTP 30 min after both drugs were removed, whereas ethanol alone resulted in persistent LTP inhibition (Fig. 1). Furthermore, the ability of ethanol to stimulate neurosteroid synthesis in CA1 pyramidal neurons was prevented by complete NMDAR block during ethanol administration. Effects of ethanol on neurosteroid immunostaining mirrored the concentration-dependence of LTP inhibition, with no effect at 20 mM but enhanced staining at 60 mM (Tokuda et al., 2011).
Prior studies have shown that low-level tonic NMDAR activation prior to tetanic stimulation can persistently block LTP (Zorumski & Izumi, 2012). This effect is observed with low concentrations of NMDA (e.g., 1 μM × 5 min) that produce no change in basal AMPAR-mediated synaptic transmission (Izumi, Clifford, & Zorumski, 1992a). Persistent LTP block is also achieved by mildly stressful metabolic conditions including low glucose (Izumi & Zorumski, 1997), brief hypoxia (Izumi, Katsuki, Benz, & Zorumski, 1998), and ammonia (Izumi, Izumi, Matsukawa, Funatsu, & Zorumski, 2005a), and following behavioral stress (Kim, Foy, & Thompson, 1996; Yang, Yang, Huang, & Hsu, 2008). In all of these examples, an NMDAR antagonist administered during the stressor paradoxically promotes LTP, implicating untimely, low-level NMDAR activation in LTP inhibition. Interestingly, and of potential relevance to ethanol, antagonists with preference for GluN2A but not GluN2B receptors overcome this LTP inhibition (Izumi, Auberson, & Zorumski, 2006). The effect of the various stressors involves a form of “metaplasticity” and reflects a shift in the relative ease with which LTP can be induced. Furthermore, blocking neurosteroid synthesis with finasteride (or dutasteride) during low NMDA administration (Tokuda et al., 2011) or ammonia (Izumi, Svrakic, O’Dell, & Zorumski, 2013), overcomes LTP inhibition and the ability of these stressors to enhance neurosteroid staining. Metaplastic effects of mild NMDAR activation involve several signaling systems including calcium, calcineurin (and other serine phosphatases), nitric oxide, and p38 MAP kinase (Izumi, Clifford, & Zorumski, 1992b; Izumi, Tokuda, & Zorumski, 2008; Kato, Li, & Zorumski, 1999). Whether ethanol shares this signaling is unknown. Other work has demonstrated a role for tyrosine phosphatases, particularly striatal-enriched protein tyrosine phosphatase (STEP), in ethanol’s effects on GluN2B receptors and LTP (Hicklin et al., 2011).
A curious feature of ethanol’s acute effects on LTP and neurosteroids is that these actions require high concentrations in naïve hippocampus. As noted, 15–30 min of acute administration of 20 mM ethanol neither blocks LTP nor promotes neurosteroid synthesis, whereas 60 mM ethanol has both effects (Izumi, Nagashima, et al., 2005c; Tokuda et al., 2011). Coupled with the finding that 20 mM ethanol in the presence of exogenous alloP diminishes LTP (Izumi et al., 2007), we have proposed that ethanol has two effects on LTP – one mediated by lower concentrations (likely involving partial NMDAR antagonism), and the other requiring high ethanol concentrations (Tokuda et al., 2011; Tokuda, Izumi, & Zorumski, 2013a) (Fig. 2). This hypothesis led to studies examining whether a metabolite of ethanol, particularly acetaldehyde, is generated locally in the hippocampus during exposure to high ethanol. Prior studies have suggested that brain-generated acetaldehyde contributes to several effects of ethanol (Correa et al., 2012; Deng & Dietrich, 2008; Quertemont, Tambour, & Tirelli, 2005). Ethanol can be metabolized to acetaldehyde by 3 pathways — alcohol dehydrogenase (ADH), catalase, and cytochrome P450 2E1 (CYP2E1) (Correa et al., 2012; Quertemont et al., 2005). Using inhibitors of these enzymes, we found that ADH, but not catalase or CYP2E1, contributes to effects of 60 mM ethanol on LTP and neurosteroid synthesis in hippocampal slices (Tokuda, Izumi, & Zorumski, 2013a, b). Effects of exogenous acetaldehyde on LTP and neurosteroids were overcome by a full NMDAR antagonist, suggesting that acetaldehyde may trigger ethanol’s metaplastic effects. Furthermore, ethanol shows enhanced potency to block LTP in the presence of exogenous acetaldehyde (Tokuda et al., 2013b). The brain expresses several ADH variants (Galter, Carmine, Buervenich, Duester, & Olson, 2003), and hippocampal pyramidal neurons have a variant with low ethanol affinity (Haseba & Ohno, 2010; Mori et al., 2000), consistent with the requirement for high concentrations for effects on LTP and neurosteroids. These findings are also consistent with prior studies demonstrating that acetaldehyde can serve as a trigger for neurosteroid synthesis (Boyd, O’Buckley, & Morrow, 2008), and that an inhibitor of aldehyde dehydrogenase facilitates ethanol’s block of LTP in the dentate gyrus in vivo (Abe, Yamaguchi, Sugiura, & Saito, 1999).
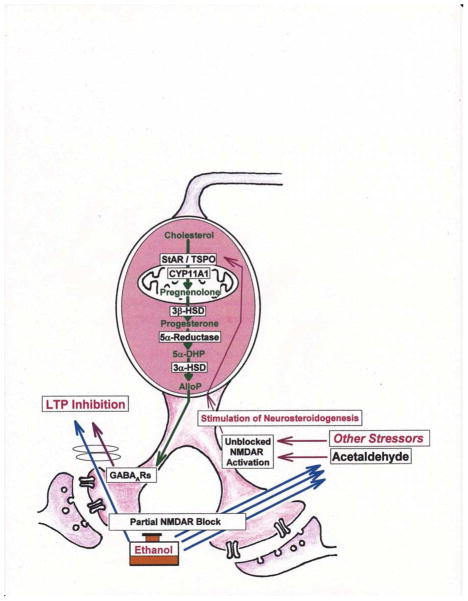
Figure 2.
The diagram depicts a scheme for acute block of LTP by ethanol in the CA1 region. Acute LTP inhibition requires high concentrations of ethanol (triple blue arrows) that partially inhibit NMDARs. Perhaps via local metabolism to acetaldehyde (Tokuda et al., 2013a, b; right side of figure), high concentrations of ethanol paradoxically promote activation of unblocked NMDARs, perhaps through elevation of glutamate levels. This untimely NMDAR activation, in turn, promotes local synthesis of GABA-enhancing neurosteroids (alloP) and enhanced GABAAR function, resulting in dampened LTP induction. The diagram also depicts key steps in the synthesis of alloP from cholesterol. The effects of high ethanol can be mimicked by lower ethanol in combination with exogenous alloP. The effects of low ethanol (single blue arrow) are not completely known, but include partial NMDAR antagonism (Izumi, Nagashima, et al., 2005c). Importantly, low ethanol alone does not enhance endogenous alloP production (Tokuda et al., 2011; left side of figure). Some actions of ethanol can be mimicked by other stressors that promote NMDAR activation. These include behavioral and metabolic stresses and can be mimicked, in part, by other drugs that enhance neurosteroid synthesis (Zorumski & Izumi, 2012). These latter conditions enhance the potency of ethanol to block LTP.
Chronic effects of ethanol on hippocampal plasticity
Studies outlined above focus on acute effects of ethanol on neuronal function and synaptic plasticity. Because alcoholism is a chronic illness, it is also important to consider longer-term consequences on brain function. It is estimated that over 50% of alcoholics, including detoxified drinkers, exhibit some form of memory problem or cognitive dysfunction (Vetreno, Hall, & Savage, 2011), and alcoholism is recognized as a cause of dementia (Oslin & Cary, 2003). Multiple factors contribute to adverse neuropsychiatric effects of alcoholism, including chronic use, repeated bouts of intoxication and withdrawal, nutritional deficiencies, head injuries, and concurrent medical disorders (Vetreno et al., 2011).
Studies in rodents provide clear evidence that chronic ethanol has adverse effects on synaptic function and synaptic plasticity in the hippocampus and cortex, among other areas (Lovinger & Roberto, 2013; McCool, 2011), and impaired plasticity correlates with defects in learning and memory, particularly spatial learning (Vetreno et al., 2011). Hippocampal slices prepared from rodents treated with ethanol over a prolonged period have diminished ability to generate LTP even when ethanol is withdrawn for one month or more in some studies (Durand & Carlen, 1984; Tremwel & Hunter, 1994). Interestingly, some evidence suggests that a calcium channel blocker overcomes effects of chronic ethanol on LTP (Ripley & Little, 1995), suggesting that intracellular calcium may trigger longer-term consequences.
Important variables in determining the effects of chronic exposure include the dose of ethanol, the pattern of exposure, and drug withdrawal. A chronic intermittent ethanol (CIE) paradigm in rodents has been extremely helpful in exploring long-term binge-like ethanol consumption (Olsen, Liang, Cagetti, & Spigelman, 2005). In this model, there are persistent changes in both glutamate- and GABA-mediated transmission (Nelson, Ur, & Gruol, 2005). Specific changes include diminished function of GABAARs with dampened expression of specific receptor subunits (Cagetti, Liang, Spigelman, & Olsen, 2003). Changes in glutamate transmission include increased expression of GluN2A and GluN2B subunits following withdrawal (Nelson et al., 2005; Qiang, Denny, & Ticku, 2007). These effects are associated with diminished LTP and can recover following ethanol abstinence (Roberto, Nelson, Ur, & Gruol, 2002). The latter changes reflect, in part, altered signaling through the mitogen-activated protein kinase (MAPK) system (Roberto et al., 2003). The CIE model does not result in significant neurodegeneration in the hippocampus or cortex (Nelson et al., 2005). In prefrontal cortex, CIE leads to increased GluN2B expression and altered LTP, possibly contributing to defects in executive function and decision making (Kroener et al., 2012). Adolescent rats exposed to CIE from 30 to 50 days of age exhibit tolerance to ethanol-induced spatial learning defects and exhibit changes in neurosteroid levels in hippocampus and cortex at P50 that reverse by early adulthood (Silvers et al., 2006). Interestingly, a form of ethanol-tolerant, APV-insensitive LTP has been described in rats exposed chronically to ethanol (Fujii et al., 2008), possibly contributing to the behavioral tolerance noted in adolescent animals.
An alternative approach for studying longer-term consequences of alcohol involves continuous exposure models in which animals receive daily ethanol at varying doses over weeks to months (Savage, Candon, & Hohmann, 2000). Unlike CIE, chronically treated rodents show morphological changes and neuronal loss in hippocampus and cortex (Farr, Scherrer, Banks, Flood, & Morley, 2005; Mandyam, 2013; Walker, Barnes, Zornetzer, Hunter, & Kubanis, 1980). Chronic ethanol treatment diminishes LTP in the hippocampus and, again, this correlates with deficits in spatial learning (Hu, Walker, Vickroy, & Peris, 1999; Peris et al., 1997). Effects on LTD have received less attention, but chronic exposure appears to dampen this form of plasticity as well (Thinschmidt, Walker, & King, 2003). Effects on synaptic plasticity are associated with changes in several neurotransmitter systems including changes in GABAergic inhibition as well as diminished acetylcholine signaling and alterations in neurotrophins, particularly brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) (Vetreno et al., 2011). There are also compensatory increases in synaptic NMDARs without effects on extrasynaptic NMDARs (Carpenter-Hyland, Woodward, & Chandler, 2004). Models using chronic ethanol exposure also demonstrate changes in small and large conductance calcium-activated potassium (K+) channels that contribute to longer-term ethanol tolerance and adaptive plasticity. These latter changes include disrupted interactions between small conductance calcium-activated K+ (SK) channels and NMDARs (Mulholland et al., 2009).
Ethanol and synaptic plasticity in the amygdala
Lateral/basolateral amygdala
Ethanol has prominent effects on mood and anxiety (McCool, Christian, Diaz, & Läck, 2010). Thus, there is considerable interest in understanding how ethanol modulates function in the amygdala, a brain region intimately involved in networks underlying emotional processing (Johansen, Cain, Ostroff, & LeDoux, 2011). In rodents, long-term forms of synaptic plasticity in the amygdala play key roles in classical fear conditioning, one of the best understood paradigms for emotional learning (Ehrlich et al., 2009). Furthermore, the amygdala has strong connections with the hippocampus where synaptic interactions contribute to contextual aspects of fear learning. The amygdala also has extensive connectivity with other subcortical structures including the bed nucleus of the stria terminalis (BNST) and the striatum. These connections help to regulate anxiety, habits, and motivation. Connections from prefrontal cortex are involved in top-down control over emotions (Johansen et al., 2011).
The amygdala is a group of nuclei that play different roles in emotion (Ehrlich et al., 2009; Johansen et al., 2011). The basolateral amygdala (BLA), particularly the lateral (LA) nucleus, is a major input station that processes information from primary sensory systems in thalamus and cortex, and sends excitatory projections to the central nucleus and BNST. In the LA, LTP of AMPAR-mediated synaptic transmission is associated with fear conditioning, resulting in enhanced behavioral responses to previously innocuous stimuli (Johansen et al., 2011). LA LTP, like CA1 LTP, involves a form of Hebbian plasticity triggered by NMDARs and results in increased expression of synaptic AMPARs and enhanced efficacy of excitatory transmission (Johansen et al., 2011; McCool, 2011). LA LTP is modulated by monoamines, GABA, and glutamate acting at mGluRs, particularly mGluR5 (Ehrlich et al., 2009; McCool, 2011; Roberto, Gilpin, & Siggins, 2012). Ethanol, both acutely and chronically, dampens LA LTP. Acute LTP inhibition involves NMDAR antagonism, although enhanced GABAergic inhibition also likely contributes (Roberto et al., 2012). Chronic ethanol exposure upregulates NMDARs and is associated with enhanced AMPAR synaptic responses (Christian, Alexander, Diaz, Robinson, & McCool, 2012). McCool (2011) has argued that these effects of ethanol usurp mechanisms involved in BLA LTP and reflect a form of ethanol-mediated plasticity that occludes standard LTP. Such effects could account for the ability of ethanol to dampen acute fear conditioning while also resulting in emotional dysregulation during chronic exposure and withdrawal. Importantly, the ability of chronic ethanol and withdrawal to enhance basal AMPAR transmission appears to differ from effects observed in the hippocampus, and mechanisms underlying these changes are not yet certain.
Central amygdala
The central amygdala (CEA) nucleus is another key region in emotional processing. The CEA gets strong excitatory input from BLA and provides the major output of the amygdala, sending GABAergic projections to brain regions involved in emotional expression, including hypothalamus and brainstem (Ehrlich et al., 2009). CEA neurons express receptors for multiple neuromodulators involved in the effects of ethanol, including receiving input from the hypothalamus via CRF (Roberto et al., 2012). Ethanol acutely enhances GABA transmission in the CEA and chronic administration dampens AMPAR- and NMDAR-mediated excitatory responses. Effects of ethanol on NMDARs in CEA may preferentially involve receptors expressing GluN2B subunits (Roberto et al., 2004). Chronic ethanol upregulates GluN2B expression in CEA but not BLA (Roberto et al., 2012), and ethanol withdrawal is associated with enhanced NMDAR responses. Interestingly, chronic ethanol results in enhanced ethanol sensitivity of synaptic NMDARs in CEA, an effect also not observed in BLA. Long-term forms of synaptic plasticity have been described in CEA, including a form of LTP that involves presynaptic NMDARs (Samson & Paré, 2005). Little information is presently available about acute and chronic effects of ethanol on CEA plasticity.
Bed nucleus of the stria terminalis
The amygdala is part of an extended network that includes the striatum and BNST. Like the amygdala, the BNST has subnuclei that play important roles in emotion, including the expression of anxiety (rather than fear). Importantly, the BNST is positioned between amygdala and stress circuitry in the hypothalamic-pituitary-adrenal (HPA) axis and helps to regulate stress responses, including those associated with alcohol intoxication and withdrawal. The BNST is sensitive to modulation by acute and chronic ethanol and plays a role in the behavioral effects of intoxication and withdrawal (Wills & Winder, 2013). As in other regions, glutamate acting at AMPARs and NMDARs plays a key role in synaptic function and plasticity. In multiple subregions of the BNST, ethanol is an effective inhibitor of NMDARs, particularly altering the function of NMDARs containing GluN2B subunits (Wills et al., 2012). NMDAR inhibition dampens LTP as it does in other brain regions. Similarly, chronic ethanol exposure and withdrawal upregulate NMDAR responses in the BNST (Kash, Baucum, Conrad, Colbran, & Winder, 2009), and this is accompanied by increased expression of GluN2B subunits, a recurring theme seen in other brain regions. Recent work has also shown that the excitability of BNST neurons is dampened in animals withdrawn from chronic ethanol, altering the ability of this region to modulate CeA (Szücs, Berton, Sanna, & Francesconi, 2012).
Effects of ethanol on long-term plasticity in the striatum
Dorsal striatum
The dorsal striatum is the major input to the basal ganglia and regulates initiation and timing of movements, including skilled motor behaviors. This region also plays a key role in instrumental learning and habit formation (Lovinger, 2010). The principal output cells of the striatum are GABAergic medium spiny neurons (MSNs) whose firing is under control of glutamatergic inputs from other brain regions, particularly cortex. Synaptic plasticity in the dorsal striatum has unique features compared to hippocampus, cortex, and amygdala, and this plasticity helps to regulate MSN activity, and motor and habit learning. Although early studies had difficulty demonstrating LTP in the dorsal striatum, it is now clear that cortical glutamatergic inputs onto MSNs exhibit both LTP and LTD (Lovinger, 2010). As is true in many regions, LTP involves NMDARs (Calabresi, Pisani, Mercuri, & Bernardi, 1992), but these receptors are supplemented by D1 and D2 dopamine receptors (D1Rs and D2Rs) and adenosine type 2A receptors (A2ARs) in the direct and indirect pathways (Calabresi et al., 2000; Lovinger, Partridge, & Tang, 2003; Shen, Flajolet, Greengard, & Surmeier, 2008). The direct and indirect pathways refer to different components of the striatum based on inputs and projections. MSNs in the direct pathway project (directly) to substantia nigra pars reticulata and express D1Rs but not D2Rs. In contrast, indirect pathway MSNs express D2Rs and A2ARs, but not D1Rs, and project to the internal segment of the globus pallidus (Grueter, Rothwell, & Malenka, 2012). The cellular and synaptic signaling that drives LTP in both direct and indirect dorsal striatal pathways increases intracellular calcium and activates CamKII, as in other brain regions, with contributions from cyclic AMP via modulatory receptors. The end result, as in other regions, is increased expression of AMPARs at postsynaptic sites on MSNs. Interpreting the roles of specific receptors in LTP is complicated by the fact that the dorsal striatum is not a homogeneous structure, and dorsomedial and dorsolateral aspects of the structure differ in their involvement in skill learning and instrumental conditioning, and use different modulating transmitters to effect synaptic changes. The dorsomedial striatum participates in early skill learning using LTP that involves NMDARs and D1Rs, while the dorsolateral striatum contributes to later phases of this learning and is modulated by CB1 cannabinoid receptors (CB1Rs) and D2Rs. Similarly, the dorsomedial striatum contributes to early phases of instrumental learning using NMDARs and D1Rs, while the dorsolateral region contributes to late phases, perhaps via LTD and the involvement of A2ARs, CB1Rs, and D2Rs (Lovinger, 2010).
LTD in the dorsal striatum also has interesting features. Here LTD induction involves Group I mGluRs (likely both mGluR1 and mGluR5) as well as postsynaptic depolarization. Whereas glutamate release and postsynaptic depolarization typically trigger NMDAR activation in most brain regions, in dorsal striatum, postsynaptic depolarization drives the opening of Cav 1.3 (L-type) voltage-activated calcium channels (VACCs) (Adermark & Lovinger, 2007). The combination of calcium influx via Cav 1.3, calcium release from intracellular stores, and activation of calcium-dependent second messengers results in synthesis and release of an endocannabinoid (EC) (Adermark, Talani, & Lovinger, 2009; Kreitzer & Malenka, 2005). The EC then traverses glutamate synapses in a retrograde fashion to activate presynaptic CB1Rs. At presynaptic terminals, CB1Rs dampen release of glutamate and produce a persistent decrease in glutamate-mediated transmission. The latter effect may involve altered presynaptic calcium channel function and/or changes in the trafficking of synaptic vesicles. Dopamine plays important roles in LTD in both the indirect and direct dorsal striatal pathways. In the indirect pathway, activation of D2Rs helps to promote LTD (Kreitzer & Malenka, 2005, 2007, 2008), while LTD in the direct pathway requires block of D1Rs (Lüscher & Huber, 2010; Shen et al., 2008). Further complicating LTD in the dorsal striatum, this plasticity is modulated by D2Rs on cholinergic interneurons and a dampening of muscarinic (M1) receptor activation (Wang et al., 2006). Similar to other brain regions, acute ethanol dampens LTP in dorsal striatum in a concentration-dependent fashion. Interestingly, block of LTP can convert LTP into LTD depending on the ethanol concentration and how ethanol is administered (Yin, Park, Adermark, & Lovinger, 2007). A low concentration of ethanol (2 mM) diminishes the magnitude of LTP and 10 mM blocks LTP completely. Thus, dorsal striatal LTP appears to be exquisitely sensitive to modulation by ethanol compared to hippocampus. Furthermore, at 50 mM ethanol, a stimulus that usually results in LTP now produces robust LTD. The timing of ethanol exposure also appears to be important. When 50 mM ethanol is washed out immediately following tetanic stimulation, LTP is blocked and no LTD is observed. When ethanol is washed out 10 min after stimulation, LTD ensues. The mechanisms contributing to these effects are complex because 50 mM ethanol only inhibits synaptic NMDARs by about 25% at striatal synapses, and ethanol-promoted LTD involves both CB1Rs and D2Rs. These results are consistent with other work showing that acute ethanol does not block striatal LTD (Clarke & Adermark, 2010; see also McCool, 2011). Other studies have found that acute effects of ethanol on striatal LTP involve a dampening of second messenger pathways, including ERK, a MAP kinase (Xie et al., 2009). Interestingly, longer-term ethanol dampens striatal LTD, and this effect is associated with altered ERK activity (Cui et al., 2011). Recent data demonstrate that chronic intermittent ethanol also downregulates CB1R signaling and eliminates CB1R-dependent LTD in dorsal striatum, while priming the striatum to play an enhanced role in learning (Depoy et al., 2013). Repeated cycles of ethanol exposure and withdrawal upregulate GluN2B expression and are associated with facilitated LTP that can be blocked by antagonists of GluN2B-expressing NMDARs (Wang et al., 2012; Xia et al., 2006).
Ventral striatum/nucleus accumbens
Like dorsal striatum, the ventral striatum/nucleus accumbens integrates information from multiple brain regions to regulate motor output. Here neural processing plays a key role in motivated behaviors and in the expression of reward signals (Stuber, Hopf, Tye, Chen, & Bonci, 2010). The ventral striatum has high connectivity to limbic and neocortical regions involved in emotion and, in turn, influences plasticity and learning in those other regions. The ventral striatum receives glutamatergic inputs from multiple cortical and subcortical areas, including hippocampus and amygdala, as well as strong innervation from dopamine neurons in the ventral tegmental area (VTA). The various inputs regulate the principal projection neurons in the region, which, like dorsal striatum, are GABAergic MSNs. The nucleus accumbens consists of two major regions – a core region involved in sensorimotor function, reward, and motivation, and a shell area involved in emotion and motor processing. Like dorsal striatum, the ventral striatum has direct and indirect paths, with indirect MSNs expressing higher levels of NMDARs than direct pathway MSNs (Grueter et al., 2012). Virtually all drugs of abuse, including alcohol, modulate activity in the ventral striatum and there is considerable interest in understanding how neuroadaptations in this region contribute to substance abuse and dependence, particularly the propensity to long-term use. Altered plasticity and function in the ventral striatum and the associated VTA contribute strongly to the concept of alcoholism (and drug abuse) as states of aberrant learning (Stuber et al., 2010). Importantly, acute administration of a variety of abused drugs, including ethanol, enhances dopamine accumulation in the ventral striatum, and this is thought to contribute to rewarding effects of these agents (Koob & Volkow, 2010).
Both direct and indirect pathways in the nucleus accumbens exhibit NMDAR-dependent LTD (Grueter et al., 2012). Stimulation of inputs to the nucleus accumbens from prelimbic cortex also drives mGluR-dependent LTD (Robbe, Kopf, Remaury, Bockaert, & Manzoni, 2002) that involves mGluR 2/3 and changes in the activity of P/Q calcium channels involved in regulating neurotransmitter release (Robbe, Alonso, Chaumont, Bockaert, & Manzoni, 2002). In the core region of the accumbens, indirect pathway MSNs exhibit two forms of mGluR5-dependent LTD (Grueter et al., 2012), a form involving endocannabinoids and another involving activation of postsynaptic transient receptor potential vanilloid type 1 channels (TRPV1). The latter form of LTD has both presynaptic and postsynaptic components (Grueter, Brasnjo, & Malenka, 2010).
In the shell region of the nucleus accumbens, ethanol acutely inhibits NMDAR-dependent LTD in a concentration-dependent fashion with complete block at 40 mM. Interestingly, lower (20 mM) and higher (60 mM) concentrations inhibit LTD to a lesser extent but to the same degree (Jeanes, Buske, & Morrisett, 2011), suggesting a U-type relationship. LTD block is mimicked by NMDAR antagonists with selectivity for GluN2B type receptors. Further complicating things, effects of ethanol on LTD are occluded by a D1R antagonist. Interestingly, exposure to chronic intermittent ethanol (CIE) followed by 24 h of withdrawal resulted in a loss of LTD and conversion to NMDAR-dependent LTP. By 72 h after withdrawal from a 3-day bout of CIE, however, both LTD and LTP are absent (Jeanes et al., 2011).
Other studies in an acute slice preparation indicate that 50 mM ethanol inhibits LTP in the nucleus accumbens via effects on group I mGluRs (mGluR1 and 5) and dampens dopamine release (Mishra, Zhang, & Chergui, 2012). Chronic ethanol also dampens NMDAR function in the nucleus accumbens and depresses NMDAR-dependent LTP. These latter effects correlate with locomotor sensitization to ethanol and enhanced ethanol consumption (Abrahao et al., 2013). Withdrawal from chronic ethanol increases the expression of GluN1 and GluN2B subunits in the nucleus accumbens, along with increased mGluR1. Thus, altered expression of NMDAR subunits (particularly GluN2B) following withdrawal from chronic ethanol appears to be a common theme across multiple brain regions involved in learning, emotion, and motivation.
Ventral tegmental area
The VTA plays a major role in motivation and reward, and, together with the nucleus accumbens, is a key part of the mesolimbic dopamine system (Stuber et al., 2010). The VTA provides major dopaminergic innervation to the ventral striatum and modulates MSN function and synaptic plasticity. This dopamine input is thought to provide critical signals underlying motivation and reward (Kelley, 2004; Wise, 2004). Using a measure of synaptic efficacy based on changes in the ratio of AMPAR to NMDAR synaptic currents, Saal, Dong, Bonci, & Malenka (2003) found that a single in vivo exposure to ethanol (20 mg/kg) was sufficient to enhance AMPAR-mediated glutamatergic transmission in midbrain dopamine neurons for at least 24 h (reminiscent of LTP). This use of changes in the AMPAR/NMDAR ratio as an index of synaptic plasticity is based on the trafficking of AMPARs into and out of synapses during LTP and LTD, respectively, and followed earlier work showing that acute cocaine induced a form of LTP (increased AMPAR/NMDAR ratio) at excitatory synapses in the VTA (Ungless, Whistler, Malenka, & Bonci, 2001), while chronic cocaine induced LTD at prefrontal cortical synapses in the nucleus accumbens (Thomas, Beurrier, Bonci, & Malenka, 2001). The augmented responses observed by Saal et al. (2003) were mimicked by acute in vivo exposures to other addictive drugs including cocaine, morphine, and nicotine, and acute behavioral stress, but were not mimicked by fluoxetine (an antidepressant) or carbamazepine (an anticonvulsant). Interestingly, changes in VTA neurons may involve only a specific subtype of neurons that also show increases in dendritic spine density (Sarti, Borgland, Kharazia, & Bonci, 2007). Thus, acute plastic and persisting changes in VTA function may help to determine the addictive potential of certain drugs and to regulate stress-related behavior and learning (Borgland, Malenka, & Bonci, 2004). Other work has shown that chronic ethanol increases susceptibility to NMDAR-dependent LTD in VTA dopamine neurons (Bernier, Whitaker, & Morikawa, 2011). The latter effect involves at least 2 signaling systems including inositol trisphosphate (IP3) and protein kinase A. Taken together these findings support the idea that ethanol and other abused drugs induce their own forms of plasticity (“learning”) in dopamine neurons, and that this may increase the probability of drug dependence.
Effects of ethanol on cerebellar plasticity
The cerebellum is involved in motor function and helps to control coordination, balance, and posture. This brain region is also involved in motor learning, and may participate in higher cognitive function. GABAergic Purkinje cells are principal neurons and their output regulates the function of deep cerebellar nuclei. Purkinje cell activity is modulated by several forms of synaptic plasticity at different glutamate inputs. LTD is particularly important in cerebellar activity and serves as an underpinning for specific forms of motor learning (Valenzuela, Lindquist, & Zamudio-Bulcock, 2010).
Peripheral inputs to the cerebellum arrive via the mossy fibers that make excitatory connections with glutamatergic granule cells. Granule cells in turn send excitatory inputs to Purkinje cells via the parallel fibers. Purkinje cells also receive excitatory inputs from the inferior olivary nucleus (ION) via the climbing fibers. Both parallel fiber and climbing fiber inputs exhibit robust LTD. Repeated concurrent activation of the parallel and climbing fibers with low frequency stimulation (e.g., 1 Hz × 5 min or more) results in parallel fiber LTD that has 3 temporal components, involving interactions among receptors and intracellular messengers (Ogasawara, Doi, & Kawato, 2008). Early parallel fiber LTD involves dual activation of AMPARs and mGluR1. mGluR1 acts via Gq-type G-proteins to activate phospholipase C-beta (PLCβ), which drives production of diacylglycerol and inositol 1,4,5-trisphosphate (IP3) to activate protein kinase C-alpha (PKCα) and stimulate release of calcium from intracellular stores (Aida et al., 1994). Transient receptor potential channels 1/3 (TRP 1/3) also contribute and help to activate voltage-gated calcium channels that provide a further calcium signal (Hartmann et al., 2008). Activation of PKC results in phosphorylation of GluA2 AMPAR subunits and internalization of AMPARs (Wang & Linden, 2000). An intermediate phase of parallel fiber LTD involves more persistent AMPAR endocytosis and results from activation of MAP kinase and phospholipase A2 (PLA2), with synthesis of arachidonic acid, activation of cyclooxygenase 2 (COX2), and production of prostaglandins D2 and E2 (Tanaka & Augustine, 2008). This second phase of LTD involves feedback that more persistently depresses AMPARs at these synapses. A late phase of parallel fiber LTD that persists for weeks or longer in vivo involves changes in protein synthesis (Linden, 1996).
Parallel fibers also exhibit a form of presynaptic LTP (Shibuki & Okada, 1992). This synaptic enhancement involves protein kinase A (PKA)-mediated phosphorylation of proteins involved in glutamate release from presynaptic terminals, including RIM-1α. A form of postsynaptic LTP involving insertion of AMPARs into synapses has also been described (Lev-Ram, Wong, Storm, & Tsien, 2002). Postsynaptic LTP results from 1 Hz stimulation of parallel fibers without concurrent activation of climbing fibers (Coesmans, Weber, De Zeeuw, & Hansel, 2004; Jörntell & Hansel, 2006).
Climbing fiber inputs onto Purkinje cells also exhibit LTD (Hansel & Linden, 2000). Here, repeated stimulation at 5 Hz for 30 sec results in persistent depression of AMPAR-mediated transmission. This form of LTD shares mechanisms with parallel fiber LTD and involves mGluR1, increases in intracellular calcium, and activation of PKC. Other modulators, including corticotrophin releasing factor, also participate.
Ethanol has long been known to depress cerebellar function, as manifested by motor incoordination and gait disturbances during acute intoxication. Longer-term alcoholism can result in chronic cerebellar dysfunction with persistent problems with coordination and gait, and cerebellar degeneration. Furthermore, ethanol impairs motor learning, and both parallel fiber and climbing fiber LTD are inhibited by ethanol (Valenzuela et al., 2010). While climbing fiber LTD may be more sensitive to ethanol than parallel fiber LTD, both are blocked acutely by 50 mM ethanol. Effects of ethanol on both forms of LTD are likely mediated by inhibition of mGluR1 and calcium channels (Belmeguenai et al., 2008; Su, Sun, & Shen, 2010). The greater sensitivity of climbing fiber LTD to ethanol may reflect greater potency of ethanol for inhibiting mGluR1-dependent signaling at these Purkinje cell inputs (Carta, Mameli, & Valenzuala, 2006). It is less certain, however, whether the block of LTD results from direct effects on mGluR1 or changes in complex downstream signaling pathways involved in cerebellar plasticity; effects on climbing fiber NMDAR inputs may also be involved (He, Titley, Grasselli, Piochon, & Hansel, 2013). Chronic effects of ethanol on cerebellar plasticity have been less extensively studied, but there are likely to be changes with repeated exposures that contribute to persistent dysfunction.
Ethanol and use-dependent plasticity of GABA synapses
We have focused on effects of ethanol on glutamate-mediated synaptic plasticity because these forms of plasticity have been most clearly linked to learning and memory and because mechanisms underlying these synaptic changes are reasonably well understood. There is also evidence that GABAergic synapses undergo long-term use-dependent forms of enhancement that modulate regional and interregional brain networks (referred to as LTPGABA) (Nugent & Kauer, 2008). LTPGABA involves heterosynaptic changes at GABA synapses evoked by activation of postsynaptic NMDARs followed by increases in presynaptic GABA release. While there is considerable information about effects of acute and chronic ethanol on GABA synapses, less information is available about LTPGABA. In the VTA, Guan and He (2010) found that 40 mM ethanol acutely inhibited LTPGABA by a mechanism involving mu (μ) opiate receptors. Interestingly, exposure to ethanol in vivo 1 day prior to the study also resulted in inhibition of LTPGABA. How these changes affect behavior and learning are less certain, although changes in GABA plasticity in the VTA could influence drug-seeking behaviors and addiction. Several other abused drugs share ethanol’s ability to dampen LTPGABA in the VTA (Niehaus, Murali, & Kauer, 2010). In dorsolateral striatum, GABAergic inhibition also undergoes a form of persistent depression (a form of LTDGABA) in rats exposed to intermittent ethanol (Adermark, Jonsson, Ericson, & Söderpalm, 2011).
Developmental ethanol exposure and long-term synaptic plasticity
Although alcoholism is largely a problem of adolescence and adulthood, children born to mothers who abuse alcohol during pregnancy can exhibit a cluster of features known as “fetal alcohol spectrum disorders” (FASD) (Valenzuela, Morton, Diaz, & Topper, 2012). Individuals with FASD, particularly those with the most severe form of the disorder, fetal alcohol syndrome (FAS), have a high incidence of cognitive problems, including deficits in learning and memory (Lebel et al., 2012; Streissguth et al., 1994). These individuals also have increased risk of developing major psychiatric disorders including substance use, mood disorders, and psychotic disorders as they mature to adulthood (Famy, Streissguth, & Unis, 1998). Although mechanisms underlying FAS are incompletely understood, alcohol exposure during the early postnatal period in rodents, a time corresponding to late pregnancy and early postnatal life in humans, greatly increases apoptotic neuronal death throughout the CNS (Ikonomidou et al., 2000), and there is evidence that exposure of prenatal and neonatal rodents to ethanol results in diminished brain mass and loss of neurons in the hippocampus (Barnes & Walker, 1981). Ethanol-induced developmental apoptosis appears to result from combined effects on NMDARs and GABARs, and can be mimicked by other abused (Farber & Olney, 2003) and therapeutic drugs (Jevtovic-Todorovic et al., 2003). Agents that are particularly noxious are CNS depressants that dampen neural excitability during synaptogenesis (Mennerick & Zorumski, 2000).
Consistent with changes in hippocampal structure, early ethanol exposure alters hippocampal physiology as measured by changes in synaptic strength, inhibition, and short- and long-term forms of synaptic plasticity (Berman & Hannigan, 2000). Animals with developmental ethanol exposure also exhibit defects in hippocampal-dependent learning (Kim et al., 1997; Lilliquist, Highfield, & Amsel, 1999). A variety of alcohol delivery paradigms in utero or in the early postnatal development result in synaptic and behavioral changes. These include chronic exposures throughout pregnancy, shorter binge-like exposures, and even single-day exposures during the early postnatal period (Berman & Hannigan, 2000; Ikonomidou et al., 2000).
Early ethanol exposure is associated with changes in the expression of receptors that could contribute to defects in behavior and memory. In particular, exposure to high ethanol levels during development results in decreased NMDAR number and function (Costa, Savage, & Valenzuela, 2000). Interestingly, there is evidence that Mg2+ block of NMDA channels may be augmented by early ethanol, making it more difficult for these channels to participate in synaptic plasticity (Hughes, Kim, Randall, & Leslie, 1998; Morrisett, Martin, Wilson, Savage, & Swartzwelder, 1989). Early ethanol exposure also diminishes the function of PI-linked mGluRs and protein kinases (Mahadev & Vemuri, 1999; Perrone-Bizzozero et al., 1998; Queen, Sanchez, Lopez, Paxton, & Savage, 1993; Rhodes, Cai, & Zhu, 1994) that contribute to hippocampal synaptic plasticity. There is less information about longer-term changes in AMPA/kainate-type glutamate receptors. Some evidence suggests that GABA-mediated inhibition may be altered by early ethanol exposure with augmented effects of benzodiazepines and GABA-potentiating neuroactive steroids in the hippocampus later in life (Allan, Wu, Paxton, & Savage, 1998).
Although variable results are reported, hippocampal slices prepared from rodents treated with ethanol in the prenatal/early postnatal period show diminished ability to generate LTP in adolescence and adulthood (Bellinger, Bedi, Wilson, & Wilce, 1999; Chepkova et al, 1995; Izumi, Kitabayashi, et al., 2005b; Krahl, Berman, & Hannigan, 1999; Richardson, Byrnes, Brien, Reynolds, & Dringenberg, 2002; Swartzwelder, Farr, Wilson, & Savage, 1988; Tan, Berman, Abel, & Zajac, 1990; but see Titterness & Christie, 2012) and defects in hippocampal homosynaptic LTD (Izumi, Kitabayashi, et al., 2005b). Deficits in multiple transmitter systems likely contribute to these changes (Valenzuela et al., 2012) and some evidence suggests long-term changes in specific NMDAR subtypes, including dampened transmission mediated by GluN2B-expressing receptors (Izumi, Kitabayashi, et al., 2005b). Other work shows changes in GABAergic interneurons that contribute to defective brain network function (Miller, 2006; Zucca & Valenzuela, 2010). Early ethanol exposure also disrupts neurogenesis in the adult brain and this could contribute to learning and psychiatric problems as animals mature (Ieraci & Herrara, 2007). Interestingly, some studies in mice indicate that learning defects are more prominent in juveniles following early postnatal ethanol exposure and diminish with maturation to adulthood (Wozniak et al., 2004). How this functional recovery occurs is uncertain, but likely reflects ongoing plasticity of the maturing brain.
While much work on early alcohol exposure has focused on changes in hippocampus, defects in synaptic plasticity and function affect multiple brain regions including neocortex, cerebellum, and striatum, among other areas (Valenzuela et al., 2012). A major concern in interpreting the existing literature and translating effects in rodents to humans is that studies vary considerably in timing of ethanol exposure and ethanol dose, with a number of studies using very high doses. Nonetheless, there is evidence that even moderate doses of ethanol during early development causes neuronal damage (Young & Olney, 2006) and has adverse effects on synaptic and behavioral outcomes (Valenzuela et al., 2012).
Summary and discussion
There is little doubt that ethanol impairs learning, memory, and other cognitive functions. While mechanisms underlying memory are not completely understood, available data support a role for long-term, use-dependent synaptic change, including LTP and LTD. These forms of plasticity are observed throughout the brain, although mechanisms underlying synaptic change can differ among regions. Evidence reviewed here indicates that ethanol has both acute and chronic adverse effects on learning-related plasticity across brain regions, and this likely contributes to cognitive dysfunction and disability. Effects of ethanol on synaptic plasticity are complex and depend upon the age of the animals, the dose and mode of ethanol administration, and the impact of ethanol withdrawal following chronic exposure. Sex and hormonal status are also likely important, but have received less systematic attention.
Ethanol’s effects on learning-related plasticity involve multiple neurotransmitters and a host of receptors and intracellular and intercellular signaling systems (Lovinger & Roberto, 2013). Many of ethanol’s effects on these systems are inhibitory, but it is also clear that some signaling pathways are activated by ethanol; this includes ethanol-induced stimulation of neurosteroids and other stress-related modulators. We have focused heavily upon ethanol’s actions in the CA1 region of the hippocampus. In part this is because of the key role of the hippocampus in learning and memory, but also because numerous mechanistic studies have been conducted in this region. Thus, findings in the hippocampus are instructive for understanding ethanol’s diverse actions, although effects in the hippocampus do not necessarily translate to all brain regions. Given the key role that NMDARs play in LTP and LTD, the ability of ethanol to block NMDARs is a natural starting point for describing effects on synaptic plasticity. As highlighted in Table 1, acute block of CA1 LTP typically involves high ethanol concentrations (usually 30 mM and above). At these concentrations, ethanol only partially inhibits NMDARs and may preferentially block a particular subtype expressing GluN2B subunits (Izumi, Nagashima, et al., 2005c). Similar degrees of NMDAR block by other antagonists, including subtype selective GluN2B antagonists, do not reproduce ethanol’s effects on LTP, although GluN2B antagonists can block LTD. This has led to a search for other mechanisms and has raised the possibility that partial NMDAR antagonism may result in metaplastic actions as a result of glutamate’s ability to stimulate residual unblocked NMDARs. Metaplastic changes, in turn, open up more complex intracellular signaling, including local production of GABA-enhancing neurosteroids within CA1 pyramidal neurons (Tokuda et al., 2011). Importantly, the facilitating effects of neurosteroids in LTP inhibition provide a model for considering how ethanol interacts with various stressors and other drugs (e.g. benzodiazepines) to impair memory. Stressful conditions or drugs that elevate neurosteroid levels would be expected to enhance the adverse effects of ethanol on LTP and learning, rendering ethanol more potent than in naïve tissue (Talani et al., 2011).
The findings with neurosteroids support the concept that glutamatergic neurons have the machinery to enhance their own GABAergic inhibitory responses in a paracrine or autocrine fashion when exposed acutely to certain stimuli or stresses (Tokuda et al., 2010). How this occurs with ethanol is not certain, but recent studies suggest that high concentrations of ethanol may be metabolized locally in the hippocampus to acetaldehyde, providing a trigger for neurosteroid synthesis and altered plasticity (Tokuda et al., 2013a, b). Given the known neurotoxicity of acetaldehyde (Correa et al., 2012; Quertemont et al., 2005), this raises an intriguing possibility – acute synaptic dysfunction in the context of severe ethanol intoxication, and perhaps memory blackouts themselves, may reflect homeostatic neuroprotective adaptations on the part of glutamatergic neurons. How the acute effects of ethanol relate to longer-term deleterious effects also remains uncertain, but such studies must account for ethanol-induced neurodegeneration in the mature brain.
We have focused on ethanol’s ability to inhibit traditional models of synaptic plasticity. It is also clear, however, that ethanol induces its own lasting synaptic changes, and that these neuroadaptations likely contribute to addiction and to adverse psychiatric outcomes associated with alcoholism. Of note, a single in vivo administration of ethanol results in lasting synaptic changes in the VTA, reflecting a prominent drug effect on the brain’s motivation system (Saal et al., 2003). Other work from McCool and colleagues (2010) highlights persistent LTP-like changes in the amygdala, consistent with the ability of ethanol to alter mood and anxiety. Understanding mechanisms underlying these changes could help in the development of more effective treatment and prevention strategies. Furthermore, it is possible to conceptualize alcoholism and other drug addictions as involving intertwined sets of neurocircuitry changes that initiate in the VTA-ventral striatal system (the point of attack of abused substances), and that progress over time to involve emotional and cognitive systems as these illnesses cycle through repeated bouts of intoxication, withdrawal, and chronic use, in the end becoming progressively more complex from a psychiatric perspective (Koob & Volkow, 2010). Thus, alcoholism, like other major psychiatric disorders, should be viewed as a neurocognitive illness reflecting, at least in part, drug-induced synaptic changes. In this scenario, adverse effects of ethanol on traditional learning-related synaptic plasticity outlined here are seen as contributing to illness progression by hampering the brain’s ability to correct circuitry errors via new experiences and learning.
The current hope in psychiatry is to understand mental illnesses as disorders of neural networks (Zorumski & Rubin, 2011). Such efforts will be a major challenge in most psychiatric disorders. Because of the better-defined acute mechanisms of action of alcohol and other abused drugs, efforts to disentangle network dysfunction may be more tractable in substance use syndromes, providing instructive starting points for understanding the involvement of brain circuitry in other mental illnesses. Ultimately, prevention of persistent dysfunction and disability, including cognitive impairment, must be a therapeutic goal in order to dampen the public health impact of these illnesses.
Acknowledgments
Work in the authors’ laboratories is supported by grants MH077791, GM47969, AA017413, MH078823, and DA032915 from the National Institutes of Health, and the Bantly Foundation. CFZ serves on the Scientific Advisory Board of Sage Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Yamaguchi S, Sugiura M, Saito H. The ethanol metabolite acetaldehyde inhibits the induction of long-term potentiation in the rat dentate gyrus in vivo. British Journal of Pharmacology. 1999;127:1805–1810. doi: 10.1038/sj.bjp.0702738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Progress in Neurobiology. 1997;52:303–323. doi: 10.1016/s0301-0082(97)00018-x. [DOI] [PubMed] [Google Scholar]
- Abrahao KP, Ariwodola OJ, Butler TR, Rau AR, Skelly MJ, Carter E, et al. Locomotor sensitization to ethanol impairs NMDA receptor-dependent synaptic plasticity in the nucleus accumbens and increases ethanol self-administration. The Journal of Neuroscience. 2013;33:4834–4842. doi: 10.1523/JNEUROSCI.5839-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Jonsson S, Ericson M, Söderpalm B. Intermittent ethanol consumption depresses endocannabinoid-signaling in the dorsolateral striatum of rat. Neuropharmacology. 2011;61:1160–1165. doi: 10.1016/j.neuropharm.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Adermark L, Lovinger DM. Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induce striatal long-term depression. The Journal of Neuroscience. 2007;27:6781–6787. doi: 10.1523/JNEUROSCI.0280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Talani G, Lovinger DM. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. The European Journal of Neuroscience. 2009;29:32–41. doi: 10.1111/j.1460-9568.2008.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, et al. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, et al. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. Mechanisms of neurosteroid interactions with GABA(A) receptors. Pharmacology & Therapeutics. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Steinbach JH. Low doses of ethanol and a neuroactive steroid positively interact to modulate rat GABA(A) receptor function. The Journal of Physiology. 2003;546:641–646. doi: 10.1113/jphysiol.2002.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AM, Wu H, Paxton LL, Savage DD. Prenatal ethanol exposure alters the modulation of the gamma-aminobutyric acidA1 receptor-gated chloride ion channel in adult rat offspring. The Journal of Pharmacology and Experimental Therapeutics. 1998;284:250–257. [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Research Brain Research Reviews. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. The Journal of Neuroscience. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa AD, Morato GS. Influence of neurosteroids on the development of rapid tolerance to ethanol in mice. European Journal of Pharmacology. 2001;431:179–188. doi: 10.1016/s0014-2999(01)01337-1. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Walker DW. Prenatal ethanol exposure permanently reduces the number of pyramidal neurons in rat hippocampus. Brain Research. 1981;227:333–340. doi: 10.1016/0165-3806(81)90071-7. [DOI] [PubMed] [Google Scholar]
- Batista LC, Prediger RD, Morato GS, Takahashi RN. Blockade of adenosine and dopamine receptors inhibits the development of rapid tolerance to ethanol in mice. Psychopharmacology. 2005;181:714–721. doi: 10.1007/s00213-005-0014-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nature Reviews Neuroscience. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Bedi KS, Wilson P, Wilce PA. Ethanol exposure during the third trimester equivalent results in long-lasting decreased synaptic efficacy but not plasticity in the CA1 region of the rat hippocampus. Synapse. 1999;31:51–58. doi: 10.1002/(SICI)1098-2396(199901)31:1<51::AID-SYN7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Belmeguenai A, Botta P, Weber JT, Carta M, De Ruiter M, De Zeeuw CI, et al. Alcohol impairs long-term depression at the cerebellar parallel fiber-Purkinje cell synapse. Journal of Neurophysiology. 2008;100:3167–3174. doi: 10.1152/jn.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby Ø, et al. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. The Journal of Neuroscience. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bernier BE, Whitaker LR, Morikawa H. Previous ethanol experience enhances synaptic plasticity of NMDA receptors in the ventral tegmental area. The Journal of Neuroscience. 2011;31:5205–5212. doi: 10.1523/JNEUROSCI.5282-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. The Journal of Neuroscience. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Gil O, Landau EM. Long-term potentiation in rat hippocampus is inhibited by low concentrations of ethanol. Brain Research. 1990;537:203–208. doi: 10.1016/0006-8993(90)90359-j. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Harris RA. Studies of ethanol actions on recombinant delta-containing gamma-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41:155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Stórustovu Sí, Ebert B, Herd MB, Belelli D, Lambert JJ, et al. The delta subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. The Journal of Pharmacology and Experimental Therapeutics. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. The Journal of Neuroscience. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. American Journal of Preventive Medicine. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Boyd KN, O’Buckley TK, Morrow AL. Role of acetaldehyde in ethanol-induced elevation of the neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one in rats. Alcoholism: Clinical and Experimental Research. 2008;32:1774–1781. doi: 10.1111/j.1530-0277.2008.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, et al. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. The Journal of Neuroscience. 2010;30:4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Molecular Pharmacology. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Gubellini P, Centonze D, Picconi B, Bernardi G, Chergui K, et al. Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. The Journal of Neuroscience. 2000;20:8443–8451. doi: 10.1523/JNEUROSCI.20-22-08443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term potentiation in the striatum is unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels. The European Journal of Neuroscience. 1992;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Calton JL, Wilson WA, Moore SD. Magnesium-dependent inhibition of N-methyl-D-aspartate receptor-mediated synaptic transmission by ethanol. The Journal of Pharmacology and Experimental Therapeutics. 1998;287:1015–1019. [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. The Journal of Neuroscience. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Ariwodola OJ, Weiner JL, Valenzuela CF. Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6813–6818. doi: 10.1073/pnas.1137276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuala CF. Alcohol potently modulates climbing fiber-Purkinje neuron synapses: role of metabotropic glutamate receptors. The Journal of Neuroscience. 2006;26:1906–1912. doi: 10.1523/JNEUROSCI.4430-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ, Harris RA, Crews FT. Ethanol tolerance and synaptic plasticity. Trends in Pharmacological Sciences. 1998;19:491–495. doi: 10.1016/s0165-6147(98)01268-1. [DOI] [PubMed] [Google Scholar]
- Chen C, Tonegawa S. Molecular genetic analysis of synaptic plasticity, activity-dependent neural development, learning, and memory in the mammalian brain. Annual Review of Neuroscience. 1997;20:157–184. doi: 10.1146/annurev.neuro.20.1.157. [DOI] [PubMed] [Google Scholar]
- Chepkova AN, Doreulee NV, Trofimov SS, Gudasheva TA, Ostrovskaya RU, Skrebitsky VG. Nootropic compound L-pyroglutamyl-D-alanine-amide restores hippocampal long-term potentiation by exposure to ethanol in rats. Neuroscience Letters. 1995;188:163–166. doi: 10.1016/0304-3940(95)11421-r. [DOI] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. The sticky issue of neurosteroids and GABA(A) receptors. Trends in Neurosciences. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, et al. Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. The Journal of Neuroscience. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA. Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking. Neuropharmacology. 2012;62:2430–2439. doi: 10.1016/j.neuropharm.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B, Anantharam V, Treistman SN. Ethanol inhibition of recombinant heteromeric NMDA channels in the presence and absence of modulators. Journal of Neurochemistry. 1995;65:140–148. doi: 10.1046/j.1471-4159.1995.65010140.x. [DOI] [PubMed] [Google Scholar]
- Clarke RB, Adermark L. Acute ethanol treatment prevents endocannabinoid-mediated long-lasting disinhibition of striatal output. Neuropharmacology. 2010;58:799–805. doi: 10.1016/j.neuropharm.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;44:691–700. doi: 10.1016/j.neuron.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Correa M, Salamone JD, Segovia KN, Pardo M, Longoni R, Spina L, et al. Piecing together the puzzle of acetaldehyde as a neuroactive agent. Neuroscience and Biobehavioral Reviews. 2012;36:404–430. doi: 10.1016/j.neubiorev.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Costa ET, Savage DD, Valenzuela CF. A review of the effects of prenatal or early postnatal ethanol exposure on brain ligand-gated ion channels. Alcoholism: Clinical and Experimental Research. 2000;24:706–715. [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Kelm MK, Breese GR. Brain regional differences in the effect of ethanol on GABA release from presynaptic terminals. The Journal of Pharmacology and Experimental Therapeutics. 2008;326:596–603. doi: 10.1124/jpet.107.135418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Simson PE, Duncan GE, McCown TJ, Herbert JS, Morrow AL, et al. Molecular basis for regionally specific action of ethanol on gamma-aminobutyric acidA receptors: generalization to other ligand-gated ion channels. The Journal of Pharmacology and Experimental Therapeutics. 1993;267:522–537. [PubMed] [Google Scholar]
- Cui SZ, Wang SJ, Li J, Xie GQ, Zhou R, Chen L, et al. Alteration of synaptic plasticity in rat dorsal striatum induced by chronic ethanol intake and withdrawal via ERK pathway. Acta Pharmacologica Sinica. 2011;32:175–181. doi: 10.1038/aps.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitrich RA, Dunwiddie TV, Harris RA, Erwin VG. Mechanism of action of ethanol: initial central nervous system actions. Pharmacological Reviews. 1989;41:489–537. [PubMed] [Google Scholar]
- Deng XS, Deitrich RA. Putative role of brain acetaldehyde in ethanol addiction. Current Drug Abuse Reviews. 2008;1:3–8. doi: 10.2174/1874473710801010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, et al. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dildy-Mayfield JE, Harris RA. Ethanol inhibits kainate responses of glutamate receptors expressed in Xenopus oocytes: role of calcium and protein kinase C. The Journal of Neuroscience. 1995;15:3162–3171. doi: 10.1523/JNEUROSCI.15-04-03162.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacological Reviews. 1999;51:7–61. [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D, Carlen PL. Impairment of long-term potentiation in rat hippocampus following chronic ethanol treatment. Brain Research. 1984;308:325–332. doi: 10.1016/0006-8993(84)91072-2. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Famy C, Streissguth AP, Unis AS. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. The American Journal of Psychiatry. 1998;155:552–554. doi: 10.1176/ajp.155.4.552. [DOI] [PubMed] [Google Scholar]
- Farber NB, Olney JW. Drugs of abuse that cause developing neurons to commit suicide. Brain Research Developmental Brain Research. 2003;147:37–45. doi: 10.1016/j.devbrainres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Farr SA, Scherrer JF, Banks WA, Flood JF, Morley JE. Chronic ethanol consumption impairs learning and memory after cessation of ethanol. Alcoholism: Clinical and Experimental Research. 2005;29:971–982. doi: 10.1097/01.alc.0000171038.03371.56. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nature Reviews Neuroscience. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fujii S, Saito K, Miyakawa H, Ito K, Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Research. 1991;555:112–122. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- Fujii S, Yamazaki Y, Sugihara T, Wakabayashi I. Acute and chronic ethanol exposure differentially affect induction of hippocampal LTP. Brain Research. 2008;1211:13–21. doi: 10.1016/j.brainres.2008.02.052. [DOI] [PubMed] [Google Scholar]
- Galter D, Carmine A, Buervenich S, Duester G, Olson L. Distribution of class I, III and IV alcohol dehydrogenase mRNAs in the adult rat, mouse and human brain. European Journal of Biochemistry. 2003;270:1316–1326. doi: 10.1046/j.1432-1033.2003.03502.x. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nature Neuroscience. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Current Opinion in Neurobiology. 2012;22:545–551. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan YZ, Ye JH. Ethanol blocks long-term potentiation of GABAergic synapses in the ventral tegmental area involving mu-opioid receptors. Neuropsychopharmacology. 2010;35:1841–1849. doi: 10.1038/npp.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel C, Linden DJ. Long-term depression of the cerebellar climbing fiber--Purkinje neuron synapse. Neuron. 2000;26:473–482. doi: 10.1016/s0896-6273(00)81179-4. [DOI] [PubMed] [Google Scholar]
- Harris RA. Ethanol actions on multiple ion channels: which are important? Alcoholism: Clinical and Experimental Research. 1999;23:1563–1570. [PubMed] [Google Scholar]
- Harris RA, McQuilkin SJ, Paylor R, Abeliovich A, Tonegawa S, Wehner JM. Mutant mice lacking the gamma isoform of protein kinase C show decreased behavioral actions of ethanol and altered function of gamma-aminobutyrate type A receptors. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3658–3662. doi: 10.1073/pnas.92.9.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, et al. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseba T, Ohno Y. A new view of alcohol metabolism and alcoholism--role of the high-Km Class III alcohol dehydrogenase (ADH3) International Journal of Environmental Research and Public Health. 2010;7:1076–1092. doi: 10.3390/ijerph7031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on alcohol and related conditions. Archives of General Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- He Q, Titley H, Grasselli G, Piochon C, Hansel C. Ethanol affects NMDA receptor signaling at climbing fiber-Purkinje cell synapses in mice and impairs cerebellar LTD. Journal of Neurophysiology. 2013;109:1333–1342. doi: 10.1152/jn.00350.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricson AW, Miao CL, Lippmann MJ, Morrisett RA. Ifenprodil and ethanol enhance NMDA receptor-dependent long-term depression. The Journal of Pharmacology and Experimental Therapeutics. 2002;301:938–944. doi: 10.1124/jpet.301.3.938. [DOI] [PubMed] [Google Scholar]
- Herd MB, Haythornthwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, et al. The expression of GABAA beta subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. The Journal of Physiology. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicklin TR, Wu PH, Radcliffe RA, Freund RK, Goebel-Goody SM, Correa PR, et al. Alcohol inhibition of the NMDA receptor function, long-term potentiation, and fear learning requires striatal-enriched protein tyrosine phosphatase. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6650–6655. doi: 10.1073/pnas.1017856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher C, Gigg J, O’Mara SM. Metabotropic glutamate receptor activation and blockade: their role in long-term potentiation, learning and neurotoxicity. Neuroscience and Biobehavioral Reviews. 1999;23:399–410. doi: 10.1016/s0149-7634(98)00045-1. [DOI] [PubMed] [Google Scholar]
- Hu DW, Walker DW, Vickroy TW, Peris J. Chronic ethanol exposure increases 3H-GABA release in rat hippocampus by presynaptic muscarinic receptor modulation. Alcoholism: Clinical and Experimental Research. 1999;23:1587–1595. [PubMed] [Google Scholar]
- Hughes PD, Kim YN, Randall PK, Leslie SW. Effect of prenatal ethanol exposure on the developmental profile of the NMDA receptor subunits in rat forebrain and hippocampus. Alcoholism: Clinical and Experimental Research. 1998;22:1255–1261. [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Single alcohol exposure in early life damages hippocampal stem/progenitor cells and reduces adult neurogenesis. Neurobiology of Disease. 2007;26:597–605. doi: 10.1016/j.nbd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Auberson YP, Zorumski CF. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. The Journal of Neuroscience. 2006;26:7181–7188. doi: 10.1523/JNEUROSCI.1258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Low concentrations of N-methyl-D-aspartate inhibit the induction of long-term potentiation in rat hippocampal slices. Neuroscience Letters. 1992a;137:245–248. doi: 10.1016/0304-3940(92)90414-3. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Inhibition of long-term potentiation by NMDA-mediated nitric oxide release. Science. 1992b;257:1273–1276. doi: 10.1126/science.1519065. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Izumi M, Matsukawa M, Funatsu M, Zorumski CF. Ammonia-mediated LTP inhibition: effects of NMDA receptor antagonists and L-carnitine. Neurobiology of Disease. 2005a;20:615–624. doi: 10.1016/j.nbd.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Katsuki H, Benz AM, Zorumski CF. Oxygen deprivation produces delayed inhibition of long-term potentiation by activation of NMDA receptors and nitric oxide synthase. Journal of Cerebral Blood Flow and Metabolism. 1998;18:97–108. doi: 10.1097/00004647-199801000-00010. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Kitabayashi R, Funatsu M, Izumi M, Yuede C, Hartman RE, et al. A single day of ethanol exposure during development has persistent effects on bi-directional plasticity, N-methyl-D-aspartate receptor function and ethanol sensitivity. Neuroscience. 2005b;136:269–279. doi: 10.1016/j.neuroscience.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Murayama K, Tokuda K, Krishnan K, Covey DF, Zorumski CF. GABAergic neurosteroids mediate the effects of ethanol on long-term potentiation in rat hippocampal slices. The European Journal of Neuroscience. 2007;26:1881–1888. doi: 10.1111/j.1460-9568.2007.05809.x. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Nagashima K, Murayama K, Zorumski CF. Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience. 2005c;136:509–517. doi: 10.1016/j.neuroscience.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Svrakic N, O’Dell K, Zorumski CF. Ammonia inhibits long-term potentiation via neurosteroid synthesis in hippocampal pyramidal neurons. Neuroscience. 2013;233:166–173. doi: 10.1016/j.neuroscience.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Tokuda K, Zorumski CF. Long-term potentiation inhibition by low-level N-methyl-D-aspartate receptor activation involves calcineurin, nitric oxide, and p38 mitogen-activated protein kinase. Hippocampus. 2008;18:258–265. doi: 10.1002/hipo.20383. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. Involvement of nitric oxide in low glucose-mediated inhibition of hippocampal long-term potentiation. Synapse. 1997;25:258–262. doi: 10.1002/(SICI)1098-2396(199703)25:3<258::AID-SYN4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. NMDA receptors, mGluR5, and endocannabinoids are involved in a cascade leading to hippocampal long-term depression. Neuropsychopharmacology. 2012;37:609–617. doi: 10.1038/npp.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA. In vivo chronic intermittent ethanol exposure reverses the polarity of synaptic plasticity in the nucleus accumbens shell. The Journal of Pharmacology and Experimental Therapeutics. 2011;336:155–164. doi: 10.1124/jpet.110.171009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. The Journal of Neuroscience. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, 2nd, Conrad KL, Colbran RJ, Winder DG. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2009;34:2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Li ST, Zorumski CF. Modulation of long-term potentiation induction in the hippocampus by N-methyl-D-aspartate-mediated presynaptic inhibition. Neuroscience. 1999;92:1261–1272. doi: 10.1016/s0306-4522(99)00080-9. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Izumi Y, Zorumski CF. Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. Journal of Neurophysiology. 1997;77:3013–3020. doi: 10.1152/jn.1997.77.6.3013. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends in Neurosciences. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kim CK, Kaynchuk LE, Kornecook TJ, Mumby DG, Dadgar NA, Pinel JP, et al. Object-recognition and spatial learning and memory in rats prenatally exposed to ethanol. Behavioral Neuroscience. 1997;111:985–995. doi: 10.1037//0735-7044.111.5.985. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, et al. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. The Journal of Neuroscience. 2002;22:10613–10620. doi: 10.1523/JNEUROSCI.22-24-10613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahl SE, Berman RF, Hannigan JH. Electrophysiology of hippocampal CA1 neurons after prenatal ethanol exposure. Alcohol. 1999;17:125–131. doi: 10.1016/s0741-8329(98)00043-3. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. The Journal of Neuroscience. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannibinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic ethanol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7(5):e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, et al. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal process of brain development. The Journal of Neuroscience. 2012;32:15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Ram V, Wong ST, Storm DR, Tsien RY. A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8389–8393. doi: 10.1073/pnas.122206399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilliquist MW, Highfield DA, Amsel A. Effects of early postnatal alcohol exposure on learning in the developing rat: replication with intubation method of delivery. Alcoholism: Clinical and Experimental Research. 1999;23:1085–1093. [PubMed] [Google Scholar]
- Linden DJ. A protein synthesis-dependent late phase of cerebellar long-term depression. Neuron. 1996;17:483–490. doi: 10.1016/s0896-6273(00)80180-4. [DOI] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Partridge JG, Tang KC. Plastic control of striatal glutamatergic transmission by ensemble actions of several neurotransmitters and targets for drugs of abuse. Annals of the New York Academy of Sciences. 2003;1003:226–240. doi: 10.1196/annals.1300.014. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Roberto M. Synaptic effects induced by alcohol. Current Topics in Behavioral Neurosciences. 2013;13:31–86. doi: 10.1007/7854_2011_143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. The Journal of Neuroscience. 1990;10:1372–1379. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadev K, Vemuri MC. Effect of pre- and postnatal ethanol exposure on protein tyrosine kinase activity and its endogenous substrates in rat cerebral cortex. Alcohol. 1999;17:223–229. doi: 10.1016/s0741-8329(98)00052-4. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD. The interplay between the hippocampus and amygdala in regulating aberrant hippocampal neurogenesis during protracted abstinence from alcohol dependence. Frontiers in Psychiatry. 2013;4:61. doi: 10.3389/fpsyt.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annual Review of Neuroscience. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Masood K, Wu C, Brauneis U, Weight FF. Differential ethanol sensitivity of recombinant N-methyl-D-aspartate receptor subunits. Molecular Pharmacology. 1994;45:324–329. [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, et al. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. The Journal of Neuroscience. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacology. 2011;61:1097–1108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Christian DT, Diaz MR, Läck AK. Glutamate plasticity in the drunken amygdala: the making of an anxious synapse. International Review of Neurobiology. 2010;91:205–233. doi: 10.1016/S0074-7742(10)91007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Olsen RW, Otis TS, Wallner M. Alcohol- and alcohol antagonist-sensitive human GABAA receptors: tracking δ subunit incorporation into functional receptors. Molecular Pharmacology. 2010;78:918–924. doi: 10.1124/mol.109.062687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, He Y, Jiang X, Manion BD, Wang M, Shute A, et al. Selective antagonism of 5alpha-reduced neurosteroid effects at GABA(A) receptors. Molecular Pharmacology. 2004;65:1191–1197. doi: 10.1124/mol.65.5.1191. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Neural activity and survival in the developing nervous system. Molecular Neurobiology. 2000;22:41–54. doi: 10.1385/MN:22:1-3:041. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Whiting PJ, Harris RA. Anaesthetic concentrations of alcohols potentiate GABAA receptor-mediated currents: lack of subunit specificity. European Journal of Pharmacology. 1994;268:209–214. doi: 10.1016/0922-4106(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effect of prenatal exposure to ethanol on glutamate and GABA immunoreactivity in macaque somatosensory and motor cortices: critical timing of exposure. Neuroscience. 2006;138:97–107. doi: 10.1016/j.neuroscience.2005.10.060. [DOI] [PubMed] [Google Scholar]
- Minami K, Gereau RW, 4th, Minami M, Heinemann SF, Harris RA. Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus laevis oocytes. Molecular Pharmacology. 1998;53:148–156. doi: 10.1124/mol.53.1.148. [DOI] [PubMed] [Google Scholar]
- Mishra D, Zhang X, Chergui K. Ethanol disrupts the mechanisms of induction of long-term potentiation in the mouse nucleus accumbens. Alcoholism: Clinical and Experimental Research. 2012;36:2117–2125. doi: 10.1111/j.1530-0277.2012.01824.x. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, et al. Fyn-kinase as determinant of ethanol sensitivity: relation to NMDA-receptor function. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochemical Research. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Mori O, Haseba T, Kameyama K, Shimizu H, Kudoh M, Ohaki O, et al. Histological distribution of class III alcohol dehydrogenase in human brain. Brain Research. 2000;852:186–190. doi: 10.1016/s0006-8993(99)02201-5. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Martin D, Wilson WA, Savage DD, Swartzwelder HS. Prenatal exposure to ethanol decreases the sensitivity of the adult rat hippocampus to N-methyl-D-aspartate. Alcohol. 1989;6:415–420. doi: 10.1016/0741-8329(89)90013-x. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Swartzwelder HS. Attenuation of hippocampal long-term potentiation by ethanol: a patch-clamp analysis of glutamatergic and GABAergic mechanisms. The Journal of Neuroscience. 1993;13:2264–2272. doi: 10.1523/JNEUROSCI.13-05-02264.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, et al. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcoholism: Clinical and Experimental Research. 1999;23:1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Research Brain Research Reviews. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Hopf FW, Bukiya AN, Martin GE, Liu J, Dopico AM, et al. Sizing up ethanol-induced plasticity: the role of small and large conductance calcium-activated potassium channels. Alcoholism: Clinical and Experimental Research. 2009;33:1125–1135. doi: 10.1111/j.1530-0277.2009.00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Murayama K, Zorumski CF, Izumi Y. Effects of neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one on ethanol-mediated paired-pulse depression of population spikes in the CA1 region of rat hippocampal slices. Neuroscience Letters. 2006;394:28–32. doi: 10.1016/j.neulet.2005.09.062. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Heath AC, Bucholz KK, Madden PA, Fu Q, Knopik V, et al. Genetic epidemiology of alcohol-induced blackouts. Archives of General Psychiatry. 2004;61:257–263. doi: 10.1001/archpsyc.61.3.257. [DOI] [PubMed] [Google Scholar]
- Nelson TE, Ur CL, Gruol DL. Chronic intermittent ethanol exposure enhances NMDA-receptor-mediated synaptic responses and NMDA receptor expression in hippocampal CA1 region. Brain Research. 2005;1048:69–79. doi: 10.1016/j.brainres.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Niehaus JL, Murali M, Kauer JA. Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. The European Journal of Neuroscience. 2010;32:108–117. doi: 10.1111/j.1460-9568.2010.07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS, Kauer JA. LTP of GABAergic synapses in the ventral tegmental area and beyond. The Journal of Physiology. 2008;586:1487–1493. doi: 10.1113/jphysiol.2007.148098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell TJ, Kandel ER. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learning & Memory. 1994;1:129–139. [PubMed] [Google Scholar]
- Ogasawara H, Doi T, Kawato M. Systems biology perspectives on cerebellar long-term depression. Neurosignals. 2008;16:300–317. doi: 10.1159/000123040. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Liang J, Cagetti E, Spigelman I. Plasticity of GABAA receptors in brains of rats treated with chronic intermittent ethanol. Neurochemical Research. 2005;30:1579–1588. doi: 10.1007/s11064-005-8836-6. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacological Reviews. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden KK, Traynelis SF. New advances in NMDA receptor pharmacology. Trends in Pharmacological Sciences. 2011;32:726–733. doi: 10.1016/j.tips.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Cary MS. Alcohol-related dementia: validation of diagnostic criteria. The American Journal of Geriatric Psychiatry. 2003;11:441–447. [PubMed] [Google Scholar]
- Overstreet LS, Pasternak JF, Colley PA, Slater NT, Trommer BL. Metabotropic glutamate receptor mediated long-term depression in developing hippocampus. Neuropharmacology. 1997;36:831–844. doi: 10.1016/s0028-3908(97)00031-2. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nature Reviews Neuroscience. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB Journal. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Peris J, Anderson KJ, Vickroy TW, King MA, Hunter BE, Walker DW. Neurochemical basis of disruption of hippocampal long term potentiation by chronic alcohol exposure. Frontiers in Bioscience. 1997;2:309–316. doi: 10.2741/a193. [DOI] [PubMed] [Google Scholar]
- Perrone-Bizzozero NI, Isaacson TV, Keidan GM, Eriqat C, Meiri KF, Savage DD, et al. Prenatal ethanol exposure decreases GAP-43 phosphorylation and protein kinase C activity in the hippocampus of adult rat offspring. Journal of Neurochemistry. 1998;71:2104–2111. doi: 10.1046/j.1471-4159.1998.71052104.x. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Qiang M, Denny AD, Ticku MK. Chronic intermittent ethanol treatment selectively alters N-methyl-D-aspartate receptor subunit surface expression in cultured cortical neurons. Molecular Pharmacology. 2007;72:95–102. doi: 10.1124/mol.106.033043. [DOI] [PubMed] [Google Scholar]
- Queen SA, Sanchez CF, Lopez SR, Paxton LL, Savage DD. Dose- and age-dependent effects of prenatal ethanol exposure on hippocampal metabotropic-glutamate receptor-stimulated phosphoinositide hydrolysis. Alcoholism: Clinical and Experimental Research. 1993;17:887–893. doi: 10.1111/j.1530-0277.1993.tb00859.x. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Tambour S, Tirelli E. The role of acetaldehyde in the neurobehavioral effects of ethanol: a comprehensive review of animal studies. Progress in Neurobiology. 2005;75:247–274. doi: 10.1016/j.pneurobio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Randall RD, Lee SY, Meyer JH, Wittenberg GF, Gruol DL. Acute alcohol blocks neurosteroid modulation of synaptic transmission and long-term potentiation in the rat hippocampal slice. Brain Research. 1995;701:238–248. doi: 10.1016/0006-8993(95)01007-9. [DOI] [PubMed] [Google Scholar]
- Ren H, Honse Y, Peoples RW. A site of alcohol action in the fourth membrane-associated domain of the N-methyl-D-aspartate receptor. The Journal of Biological Chemistry. 2003;278:48815–48820. doi: 10.1074/jbc.M302097200. [DOI] [PubMed] [Google Scholar]
- Ren H, Zhao Y, Dwyer DS, Peoples RW. Interactions among positions in the third and fourth membrane-associated domains at the intersubunit interface of the N-methyl-D-aspartate receptor forming sites of alcohol action. The Journal of Biological Chemistry. 2012;287:27302–27312. doi: 10.1074/jbc.M111.338921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Zhao Y, Wu M, Peoples RW. A novel alcohol-sensitive position in the N-methyl-D-aspartate receptor GluN2A subunit M3 domain regulates agonist affinity and ion channel gating. Molecular Pharmacology. 2013;84:501–510. doi: 10.1124/mol.113.085993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes PG, Cai Z, Zhu N. Prenatal ethanol exposure reduces phosphoinositide hydrolysis stimulated by quisqualate in rat cerebellar granule cell cultures. Molecular and Chemical Neuropathology. 1994;23:63–76. doi: 10.1007/BF02858507. [DOI] [PubMed] [Google Scholar]
- Richardson DP, Byrnes ML, Brien JF, Reynolds JN, Dringenberg HC. Impaired acquisition in the water maze and hippocampal long-term potentiation after chronic prenatal ethanol exposure in the guinea-pig. The European Journal of Neuroscience. 2002;16:1593–1598. doi: 10.1046/j.1460-9568.2002.02214.x. [DOI] [PubMed] [Google Scholar]
- Ripley TL, Little HJ. Nitrendipine prevents the decrease caused by chronic ethanol intake in the maintenance of tetanic long-term potentiation. Experimental Brain Research. 1995;103:1–8. doi: 10.1007/BF00241959. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Chaumont S, Bockaert J, Manzoni OJ. Role of p/q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses. The Journal of Neuroscience. 2002;22:4346–4356. doi: 10.1523/JNEUROSCI.22-11-04346.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, Siggins GR. The central amygdala and alcohol: role of γ-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harbor Perspectives in Medicine. 2012;2:ao12195. doi: 10.1101/cshperspect.a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, Brunelli M, Sanna PP, Gruol DL. The transient depression of hippocampal CA1 LTP induced by chronic intermittent ethanol exposure is associated with an inhibition of the MAP kinase pathway. The European Journal of Neuroscience. 2003;17:1646–1654. doi: 10.1046/j.1460-9568.2003.02614.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, Gruol DL. Long-term potentiation in the rat hippocampus is reversibly depressed by chronic intermittent ethanol exposure. Journal of Neurophysiology. 2002;87:2385–2397. doi: 10.1152/jn.2002.87.5.2385. [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. The Journal of Neuroscience. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Treistman SN, Pietrzykowski AZ, Weiner J, Galindo R, Mameli M, et al. Actions of acute and chronic ethanol on presynaptic terminals. Alcoholism: Clinical and Experimental Research. 2006;30:222–232. doi: 10.1111/j.1530-0277.2006.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald KM, Mirshahi T, Woodward JJ. Ethanol inhibition of N-methyl-D-aspartate receptors is reduced by site-directed mutagenesis of a transmembrane domain phenylalanine residue. The Journal of Biological Chemistry. 2001;276:44729–44735. doi: 10.1074/jbc.M102800200. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Samson HH, Harris RA. Neurobiology of alcohol abuse. Trends in Pharmacological Sciences. 1992;13:206–211. doi: 10.1016/0165-6147(92)90065-e. [DOI] [PubMed] [Google Scholar]
- Samson RD, Paré D. Activity-dependent synaptic plasticity in the central nucleus of the amygdala. The Journal of Neuroscience. 2005;25:1847–1855. doi: 10.1523/JNEUROSCI.3713-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, et al. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. The Journal of Neuroscience. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarti F, Borgland SL, Kharazia VN, Bonci A. Acute cocaine exposure alters spine density and long-term potentiation in the ventral tegmental area. The European Journal of Neuroscience. 2007;26:749–756. doi: 10.1111/j.1460-9568.2007.05689.x. [DOI] [PubMed] [Google Scholar]
- Savage LM, Candon PM, Hohmann HL. Alcohol-induced brain pathology and behavioral dysfunction: using an animal model to examine sex differences. Alcoholism: Clinical and Experimental Research. 2000;24:465–475. [PubMed] [Google Scholar]
- Schummers J, Bentz S, Browning MD. Ethanol’s inhibition of LTP may not be mediated solely via direct effects on the NMDA receptor. Alcoholism: Clinical and Experimental Research. 1997;21:404–408. doi: 10.1111/j.1530-0277.1997.tb03783.x. [DOI] [PubMed] [Google Scholar]
- Schummers J, Browning MD. Evidence for a role for GABA(A) and NMDA receptors in ethanol inhibition of long-term potentiation. Brain Research Molecular Brain Research. 2001;94:9–14. doi: 10.1016/s0169-328x(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K, Okada D. Cerebellar long-term potentiation under suppressed postsynaptic Ca2+ activity. Neuroreport. 1992;3:231–234. doi: 10.1097/00001756-199203000-00003. [DOI] [PubMed] [Google Scholar]
- Shu HJ, Eisenman LN, Jinadasa D, Covey DF, Zorumski CF, Mennerick S. Slow actions of neuroactive steroids at GABAA receptors. The Journal of Neuroscience. 2004;24:6667–6675. doi: 10.1523/JNEUROSCI.1399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacological Reviews. 1995;47:181–234. [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacology & Therapeutics. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on acute and rapid tolerance to ethanol during ontogeny. Alcoholism: Clinical and Experimental Research. 2004;28:884–894. doi: 10.1097/01.alc.0000128221.68382.ba. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, O’Buckley T, Morrow AL, Matthews DB. Chronic intermittent ethanol exposure during adolescence reduces the effect of ethanol challenge on hippocampal allopregnanolone levels and Morris water maze task performance. Alcohol. 2006;39:151–158. doi: 10.1016/j.alcohol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Christian MR, Sun AY, Sun GY. Chronic ethanol-induced subtype- and subregion-specific decrease in the mRNA expression of metabotropic glutamate receptors in rat hippocampus. Alcoholism: Clinical and Experimental Research. 2004;28:1419–1423. doi: 10.1097/01.alc.0000139825.35438.a4. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Sampson PD, Olson HC, Bookstein FL, Barr HM, Scott M, et al. Maternal drinking during pregnancy: attention and short-term memory in 14-year-old offspring--a longitudinal prospective study. Alcoholism: Clinical and Experimental Research. 1994;18:202–218. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Tye KM, Chen BT, Bonci A. Neuroplastic alterations in the limbic system following cocaine or alcohol exposure. Current Topics in Behavioral Neurosciences. 2010;3:3–27. doi: 10.1007/7854_2009_23. [DOI] [PubMed] [Google Scholar]
- Su LD, Sun CL, Shen Y. Ethanol acutely modulates mGluR1-dependent long-term depression in cerebellum. Alcoholism: Clinical and Experimental Research. 2010;34:1140–1145. doi: 10.1111/j.1530-0277.2010.01190.x. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Shoyama Y, Saito H, Abe K. The effects of ethanol and crocin on the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Japanese Journal of Pharmacology. 1995;67:395–397. doi: 10.1254/jjp.67.395. [DOI] [PubMed] [Google Scholar]
- Suryanarayanan A, Liang J, Meyer EM, Lindemeyer AK, Chandra D, Homanics GE, et al. Subunit compensation and plasticity of synaptic GABA(A) receptors induced by ethanol in α4 subunit knockout mice. Frontiers in Neuroscience. 2011;5:110. doi: 10.3389/fnins.2011.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Farr KL, Wilson WA, Savage DD. Prenatal exposure to ethanol decreases physiological plasticity in the hippocampus of the adult rat. Alcohol. 1988;5:121–124. doi: 10.1016/0741-8329(88)90008-0. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcoholism: Clinical and Experimental Research. 1995;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Szücs A, Berton F, Sanna PP, Francesconi W. Excitability of jcBNST neurons is reduced in alcohol-dependent animals during protracted alcohol withdrawal. PLoS One. 2012;7:e42313. doi: 10.1371/journal.pone.0042313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talani G, Biggio G, Sanna E. Enhanced sensitivity to ethanol-induced inhibition of LTP in CA1 pyramidal neurons of socially isolated C57BL/6J mice: role of neurosteroids. Frontiers in Endocrinology. 2011;2:56. doi: 10.3389/fendo.2011.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SE, Berman RF, Abel EL, Zajac CS. Prenatal alcohol exposure alters hippocampal slice electrophysiology. Alcohol. 1990;7:507–511. doi: 10.1016/0741-8329(90)90040-j. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Augustine GJ. A positive feedback signal transduction loop determines timing of cerebellar long-term depression. Neuron. 2008;59:608–620. doi: 10.1016/j.neuron.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinschmidt JS, Walker DW, King MA. Chronic ethanol treatment reduces the magnitude of hippocampal LTD in the adult rat. Synapse. 2003;48:189–197. doi: 10.1002/syn.10203. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nature Neuroscience. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Titterness AK, Christie BR. Prenatal ethanol exposure enhances NMDAR-dependent long-term potentiation in the adolescent female dentate gyrus. Hippocampus. 2012;22:69–81. doi: 10.1002/hipo.20849. [DOI] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. The Journal of Neuroscience. 2011;31:9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF. Locally-generated acetaldehyde is involved in ethanol-mediated LTP inhibition in the hippocampus. Neuroscience Letters. 2013a;537:40–43. doi: 10.1016/j.neulet.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF. Locally-generated acetaldehyde contributes to the effects of ethanol on neurosteroids and long-term potentiation in the hippocampus. Neurology and Clinical Neuroscience. 2013b;1:138–147. doi: 10.1111/ncn3.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, O’Dell KA, Izumi Y, Zorumski CF. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. The Journal of Neuroscience. 2010;30:16788–16795. doi: 10.1523/JNEUROSCI.4101-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, Zorumski CF, Izumi Y. Modulation of hippocampal long-term potentiation by slow increases in ethanol concentration. Neuroscience. 2007;146:340–349. doi: 10.1016/j.neuroscience.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga S, McDaniel JR, Morrow AL, Matthews DB. Effect of acute ethanol administration and acute allopregnanolone administration on spontaneous hippocampal pyramidal cell neural activity. Brain Research. 2003;967:273–280. doi: 10.1016/s0006-8993(02)04266-x. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacological Reviews. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremwel MF, Hunter BE. Effects of chronic ethanol ingestion on long-term potentiation remain even after a prolonged recovery from ethanol exposure. Synapse. 1994;17:141–148. doi: 10.1002/syn.890170210. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annual Review of Medicine. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Bhave S, Hoffman P, Harris RA. Acute effects of ethanol on pharmacologically isolated kainate receptors in cerebellar granule neurons: comparison with NMDA and AMPA receptors. Journal of Neurochemistry. 1998;71:1777–1780. doi: 10.1046/j.1471-4159.1998.71041777.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Lindquist B, Zamudio-Bulcock PA. A review of synaptic plasticity at Purkinje neurons with a focus on ethanol-induced cerebellar dysfunction. International Review of Neurobiology. 2010;91:339–372. doi: 10.1016/S0074-7742(10)91011-8. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Morton RA, Diaz MR, Topper L. Does moderate drinking harm the fetal brain? Insights from animal models. Trends in Neurosciences. 2012;35:284–292. doi: 10.1016/j.tins.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. The Journal of Neuroscience. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. British Journal of Pharmacology. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Hall JM, Savage LM. Alcohol-related amnesia and dementia: animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiology of Learning and Memory. 2011;96:596–608. doi: 10.1016/j.nlm.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volianskis A, Bannister N, Collett VJ, Irvine MW, Monaghan DT, Fitzjohn SM, et al. Different NMDA receptor subtypes mediate induction of long-term potentiation and two forms of short-term potentiation at CA1 synapses in rat hippocampus in vitro. The Journal of Physiology. 2013;591:955–972. doi: 10.1113/jphysiol.2012.247296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Burnett DM, Leidenheimer NJ, Burt DR, Wang JB, Kofuji P, et al. Ethanol sensitivity of the GABAA receptor expressed in Xenopus oocytes requires 8 amino acids contained in the gamma 2L subunit. Neuron. 1991;7:27–33. doi: 10.1016/0896-6273(91)90071-7. [DOI] [PubMed] [Google Scholar]
- Walker DW, Barnes DE, Zornetzer SF, Hunter BE, Kubanis P. Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science. 1980;209:711–713. doi: 10.1126/science.7394532. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ben Hamida S, Darcq E, Zhu W, Gibb SL, Lanfranco MF, et al. Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. The Journal of Neuroscience. 2012;32:15124–15132. doi: 10.1523/JNEUROSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Weiner LJ, Gu C, Dunwiddie TV. Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. Journal of Neurophysiology. 1997;77:1306–1312. doi: 10.1152/jn.1997.77.3.1306. [DOI] [PubMed] [Google Scholar]
- White AM. What happened? Alcohol, memory blackouts, and the brain. Alcohol Research & Health. 2003;27:186–196. [PMC free article] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Annals of the New York Academy of Sciences. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Molecular Pharmacology. 1993;44:851–859. [PubMed] [Google Scholar]
- Wills T, Klug JR, Silberman Y, Baucum AJ, Weitlauf C, Colbran RJ, et al. GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E278–E287. doi: 10.1073/pnas.1113820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Winder DG. Ethanol effects on N-methyl-D-aspartate receptors in the bed nucleus of the stria terminalis. Cold Springs Harbor Perspectives in Medicine. 2013;3:a012161. doi: 10.1101/cshperspect.a012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nature Reviews Neuroscience. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, et al. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiology of Disease. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Wright JM, Peoples RW, Weight FF. Single-channel and whole-cell analysis of ethanol inhibition of NMDA-activated currents in cultured mouse cortical and hippocampal neurons. Brain Research. 1996;738:249–256. doi: 10.1016/s0006-8993(96)00780-9. [DOI] [PubMed] [Google Scholar]
- Xia JX, Li J, Zhou R, Zhang XH, Ge YB, Ru Yuan X. Alterations of rat corticostriatal synaptic plasticity after chronic ethanol exposure and withdrawal. Alcoholism: Clinical and Experimental Research. 2006;30:819–824. doi: 10.1111/j.1530-0277.2006.00095.x. [DOI] [PubMed] [Google Scholar]
- Xie GQ, Wang SJ, Li J, Cui SZ, Zhou R, Chen L, et al. Ethanol attenuates the HFS-induced, ERK-mediated LTP in a dose-dependent manner in rat striatum. Alcoholism: Clinical and Experimental Research. 2009;33:121–128. doi: 10.1111/j.1530-0277.2008.00818.x. [DOI] [PubMed] [Google Scholar]
- Yaka R, Phamluong K, Ron D. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. The Journal of Neuroscience. 2003;23:3623–3632. doi: 10.1523/JNEUROSCI.23-09-03623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PC, Yang CH, Huang CC, Hsu KS. Phosphatidylinositol 3-kinase activation is required for stress protocol-induced modification of hippocampal synaptic plasticity. The Journal of Biological Chemistry. 2008;283:2631–2643. doi: 10.1074/jbc.M706954200. [DOI] [PubMed] [Google Scholar]
- Yin HH, Park BS, Adermark L, Lovinger DM. Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. The European Journal of Neuroscience. 2007;25:3226–3232. doi: 10.1111/j.1460-9568.2007.05606.x. [DOI] [PubMed] [Google Scholar]
- Young C, Olney JW. Neuroapoptosis in the infant mouse brain triggered by a transient small increase in blood alcohol concentration. Neurobiology of Disease. 2006;22:548–554. doi: 10.1016/j.nbd.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Izumi Y. NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neuroscience and Biobehavioral Reviews. 2012;36:989–1000. doi: 10.1016/j.neubiorev.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S. Neurosteroids, stress and depression: potential therapeutic opportunities. Neuroscience and Biobehavioral Reviews. 2013;37:109–122. doi: 10.1016/j.neubiorev.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Rubin EH. Psychiatry and Clinical Neuroscience: A Primer. New York: Oxford University Press; 2011. [Google Scholar]
- Zucca S, Valenzuela CF. Low concentrations of alcohol inhibit BDNF-dependent GABAergic plasticity via L-type Ca2+ channel inhibition in developing CA3 hippocampal pyramidal neurons. The Journal of Neuroscience. 2010;30:6776–6781. doi: 10.1523/JNEUROSCI.5405-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]