Abstract
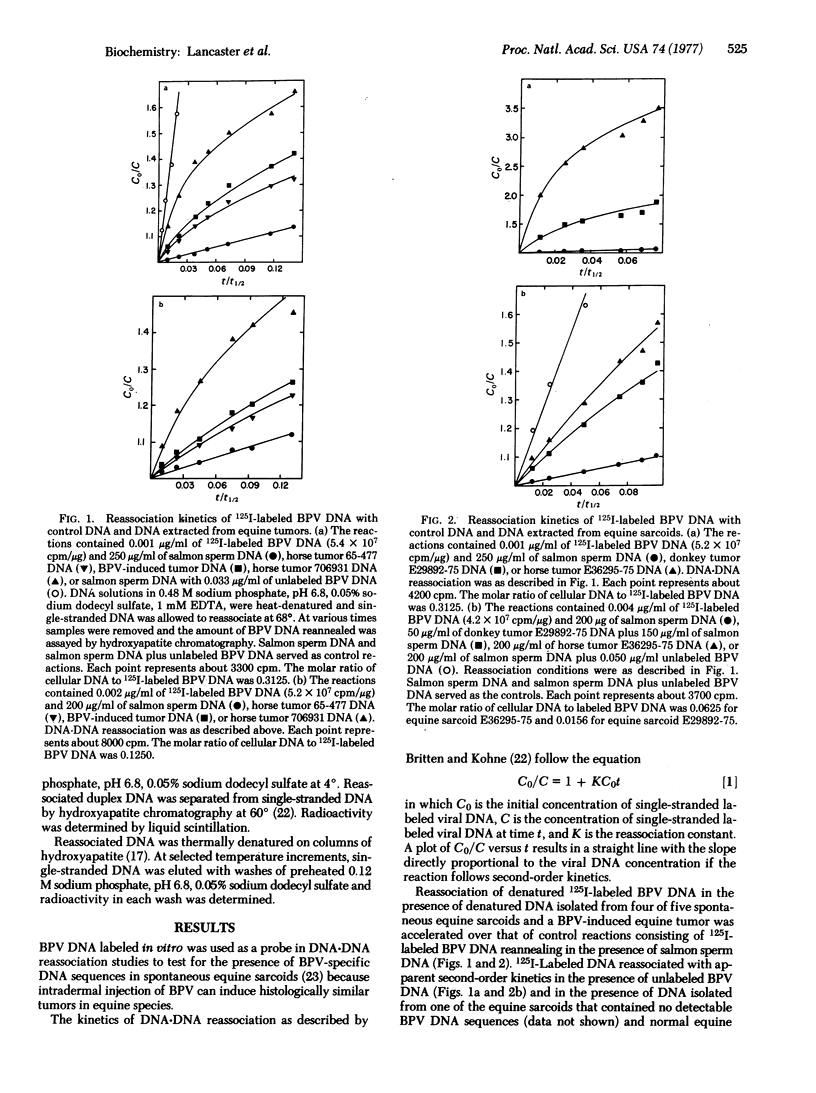
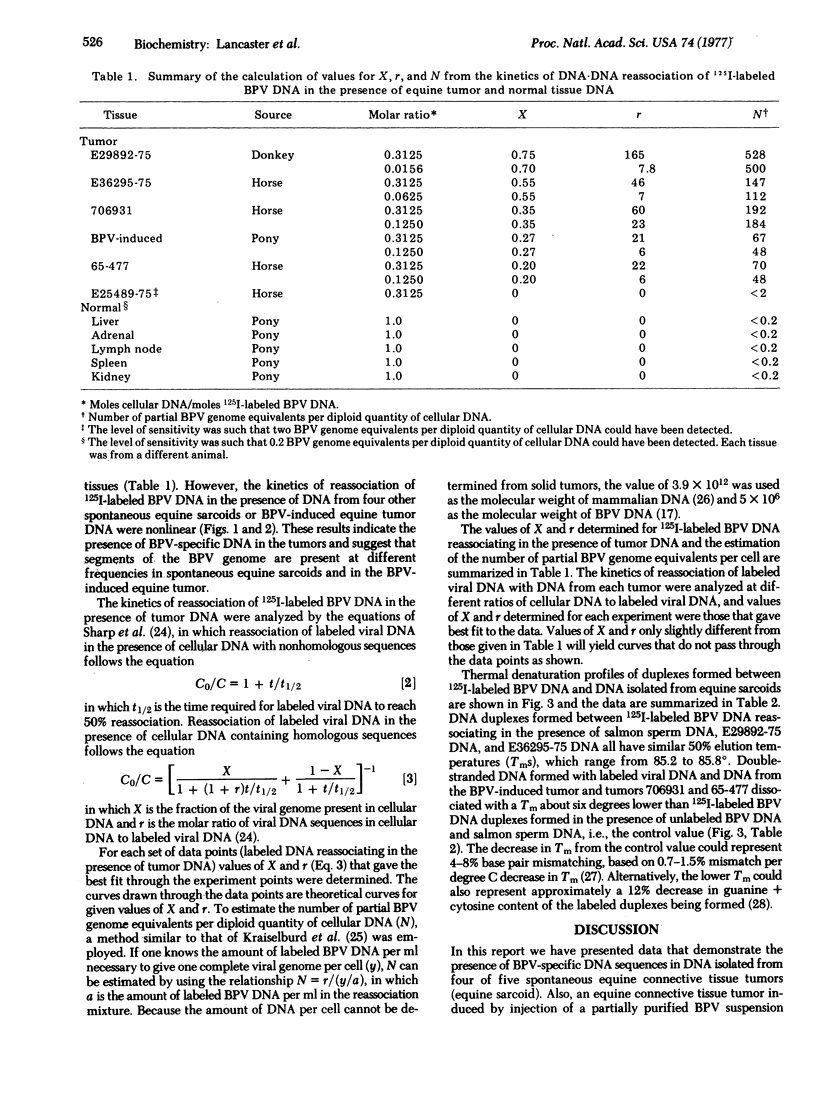
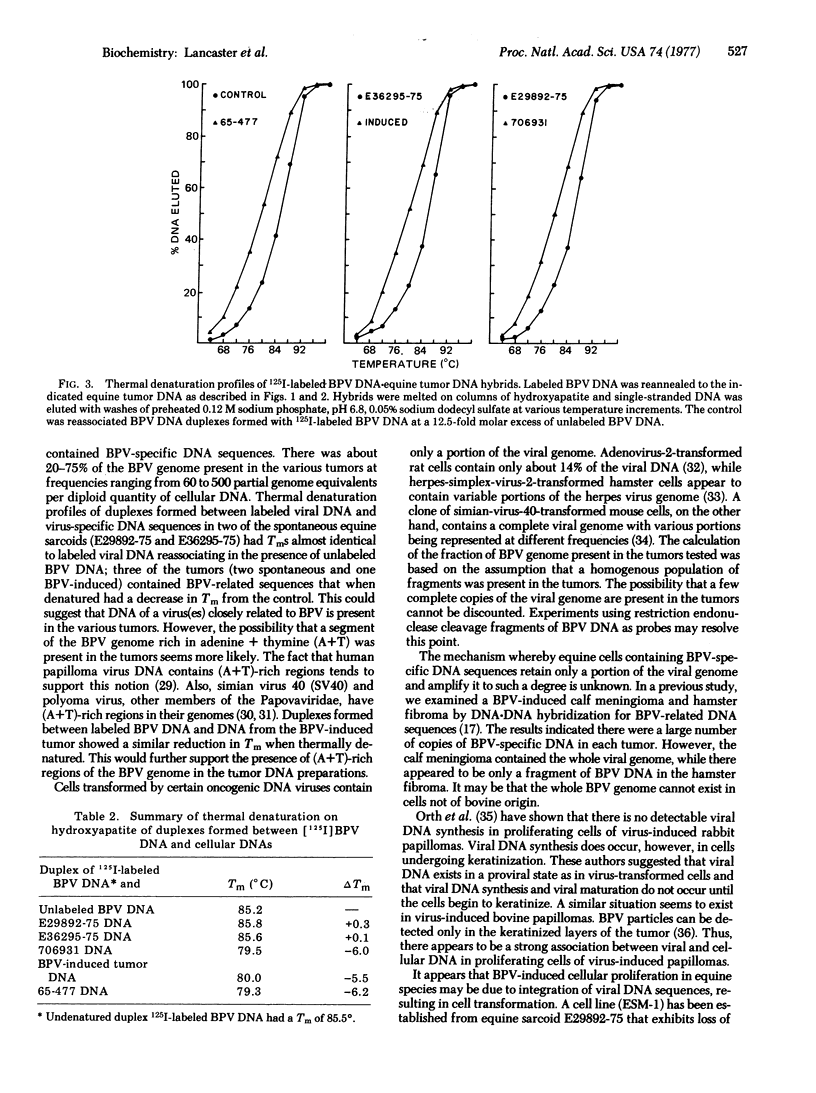
Four of five spontaneous benign equine connective tissue tumors of unknown etiology and a bovine papilloma virus (BPV)-induced equine tumor contained BPV-specific DNA sequences as determined by DNA-DNA hybridization of DNA from tumors with BPV DNA labeled in vitro. Analysis of the kinetics of reassociation indicated that 20-75% of the BPV genome was present in the various tumors. The number of partial BPV genome equivalents ranged from 60 to 500 copies per diploid quantity of cellular DNA. Thermal denaturation profiles of duplexes formed between labeled BPV DNA and DNA from tumor cells indicated two tumors contained viral DNA with base sequences identical to BPV DNA. Three tumors (including DNA from the BPV-induced tumor) contained BPV-related DNA sequences that were less thermally stable. The decrease in thermal denaturation temperature may be due to the presence of (adenine + thymine)-rich regions of the BPV genome in the tumor cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK P. H., HARTLEY J. W., ROWE W. P., HUEBNER R. J. TRANSFORMATION OF BOVINE TISSUE CULTURE CELLS BY BOVINE PAPILLOMA VIRUS. Nature. 1963 Sep 7;199:1016–1018. doi: 10.1038/1991016a0. [DOI] [PubMed] [Google Scholar]
- BOIRON M., LEVY J. P., THOMAS M., FRIEDMANN J. C., BERNARD J. SOME PROPERTIES OF BOVINE PAPILLOMA VIRUS. Nature. 1964 Jan 25;201:423–424. doi: 10.1038/201423a0. [DOI] [PubMed] [Google Scholar]
- BOIRON M., THOMAS M., CHENAILLE P. A BIOLOGICAL PROPERTY OF DEOXYRIBONUCLEIC ACID EXTRACTED FROM BOVINE PAPILLOMA VIRUS. Virology. 1965 May;26:150–153. doi: 10.1016/0042-6822(65)90037-1. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Botchan M., Ozanne B., Sugden B., Sharp P. A., Sambrook J. Viral DNA in transformed cells. III. The amounts of different regions of the SV40 genome present in a line of transformed mouse cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4183–4187. doi: 10.1073/pnas.71.10.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Dumas L. B., Darby G., Sinsheimer R. L. The replication of bacteriophage phi X174 DNA in vitro. Temperature effects on repair synthesis and displacement synthesis. Biochim Biophys Acta. 1971 Jan 28;228(2):407–422. [PubMed] [Google Scholar]
- England J. J., Watson R. E., Jr, Larson K. A. Virus-like particles in an equine sarcoid cell line. Am J Vet Res. 1973 Dec;34(12):1601–1603. [PubMed] [Google Scholar]
- Follett E. A., Crawford L. V. Electron microscope study of the denaturation of human papilloma virus DNA. II. The specific location of denatured regions. J Mol Biol. 1967 Sep 28;28(3):461–467. doi: 10.1016/s0022-2836(67)80096-2. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Locker H., Cox B., Roizman B., Rapp F. Herpes simplex virus DNA in transformed cells: sequence complexity in five hamster cell lines and one derived hamster tumor. J Virol. 1976 Jun;18(3):885–893. doi: 10.1128/jvi.18.3.885-893.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y., Olson C. The fine structure of the bovine wart. Pathol Vet. 1966;3(6):659–684. doi: 10.1177/030098586600300607. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Gordon D. E., Olson C. Meningiomas and fibroblastic neoplasia in calves induced with the bovine papilloma virus. Cancer Res. 1968 Dec;28(12):2423–2431. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kelly R. B., Cozzarelli N. R., Deutscher M. P., Lehman I. R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem. 1970 Jan 10;245(1):39–45. [PubMed] [Google Scholar]
- Kraiselburd E., Gage L. P., Weissbach A. Presence of a herpes simplex virus DNA fragment in an L cell clone obtained after infection with irradiated herpes simplex virus I. J Mol Biol. 1975 Oct 5;97(4):533–542. doi: 10.1016/s0022-2836(75)80057-x. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Meinke W. Persistence of viral DNA in human cell cultures infected with human papilloma virus. Nature. 1975 Jul 31;256(5516):434–436. doi: 10.1038/256434a0. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C., Meinke W. Quantitation of bovine papilloma viral DNA in viral-induced tumors. J Virol. 1976 Mar;17(3):824–831. doi: 10.1128/jvi.17.3.824-831.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure B., Yaniv M. Denaturation map of polyoma DNA. J Virol. 1975 Sep;16(3):720–724. doi: 10.1128/jvi.16.3.720-724.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAZAWA Y., THOMAS C. A., Jr NUCLEOTIDE COMPOSITION OF SHORT SEGMENTS OF DNA MOLECULES. J Mol Biol. 1965 Feb;11:223–237. doi: 10.1016/s0022-2836(65)80053-5. [DOI] [PubMed] [Google Scholar]
- Meinke W., Goldstein D. A., Hall M. R. Rapid isolation of mouse DNA from cells in tissue culture. Anal Biochem. 1974 Mar;58(1):82–88. doi: 10.1016/0003-2697(74)90444-8. [DOI] [PubMed] [Google Scholar]
- Meinke W., Goldstein D. A. Reassociation and dissociation of cytoplasmic membrane-associated DNA. J Mol Biol. 1974 Jul 15;86(4):757–773. doi: 10.1016/0022-2836(74)90352-0. [DOI] [PubMed] [Google Scholar]
- OLSON C., Jr, COOK R. H. Cutaneous sarcoma-like lesions of the horse caused by the agent of bovine papilloma. Proc Soc Exp Biol Med. 1951 Jun;77(2):281–284. doi: 10.3181/00379727-77-18750. [DOI] [PubMed] [Google Scholar]
- Orth G., Jeanteur P., Croissant O. Evidence for and localization of vegetative viral DNA replication by autoradiographic detection of RNA-DNA hybrids in sections of tumors induced by Shope papilloma virus. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1876–1880. doi: 10.1073/pnas.68.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland W. L., Keown G. H., Gorham J. R. An epizootic of equine sarcoid. Nature. 1966 Jun 25;210(5043):1399–1399. doi: 10.1038/2101399a0. [DOI] [PubMed] [Google Scholar]
- Ragland W. L., Spencer G. R. Attempts to relate bovine papilloma virus to the cause of equine sarcoid: immunity to bovine papilloma virus. Am J Vet Res. 1968 Jul;29(7):1363–1366. [PubMed] [Google Scholar]
- Robl M. G., Olson C. Oncogenic action of bovine papilloma virus in hamsters. Cancer Res. 1968 Aug;28(8):1596–1604. [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Shaw J. E., Huang E. S., Pagano J. S. Iodination of herpesvirus nucleic acids. J Virol. 1975 Jul;16(1):132–140. doi: 10.1128/jvi.16.1.132-140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS M., BOIRON M., TANZER J., LEVY J. P., BERNARD J. IN VITRO TRANSFORMATION OF MICE CELLS BY BOVINE PAPILLOMA VIRUS. Nature. 1964 May 16;202:709–710. doi: 10.1038/202709a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss J. L. Transmission of equine sarcoid. Am J Vet Res. 1969 Feb;30(2):183–191. [PubMed] [Google Scholar]
- Yoshiike K., Furuno A., Suzuki K. Denaturation maps of complete and defective simian virus 40 DNA molecules. J Mol Biol. 1972 Oct 14;70(3):415–423. doi: 10.1016/0022-2836(72)90549-9. [DOI] [PubMed] [Google Scholar]


