Summary
We describe the labeling of adult neural stem cells (aNSCs) in the mouse and human dentate gyrus (DG) by the combinatorial expression of glial fibrillary acidic protein (GFAP) and Prominin1, as revealed by immunohistochemistry. Split-Cre-based genetic fate mapping of these double-positive cells in the adult murine DG reveals their NSC identity, as they are self-renewing and contribute to neurogenesis over several months. Their progeny reacts to stimuli such as voluntary exercise with increased neurogenesis. Prominin1+/GFAP+ cells also exist in the adult human DG, the only region in the human brain for which adult neurogenesis has been consistently reported. Our data, together with previous evidence of such double-positive NSCs in the developing murine brain and in neurogenic regions of vertebrates with widespread neurogenesis, suggest that Prominin1- and GFAP-expressing cells are NSCs in a wide range of species in development and adulthood.
Highlights
-
•
Prominin1 is expressed in radial and nonradial NSCs in the adult hippocampus
-
•
Fate mapping reveals the long-term neurogenic lineage of Prominin1+/hGFAP+ NSCs
-
•
Prominin1 labels GFAP+ radial glia processes in the adult human hippocampus
The combinatorial expression of GFAP and Prominin1 labels adult neural stem cells (NSCs) in the mouse dentate gyrus as revealed by immunohistochemistry and split-Cre-based fate mapping. Additionally, Prominin1+/GFAP+ cells exist in the adult human dentate gyrus. Such double-positive cells also represent NSCs in the embryonic forebrain and the adult subependymal zone, suggesting Prominin1+/GFAP+ expression as a general property of NSCs.
Introduction
Since the discovery of adult neural stem cells (aNSCs), the extent to which stem cells have common hallmarks, either in the same tissue from development into adulthood or across different adult tissues, has been a key issue. The transcription factor SOX2, the intermediate filament protein Nestin, and the membrane protein Prominin1 have been suggested as common molecular markers for NSCs across different regions and ontogenies (Lendahl et al., 1990; Suh et al., 2007; Weigmann et al., 1997). However, these markers are rather widespread in the adult brain, with SOX2 being expressed in all astrocytes (Suh et al., 2007) and Nestin and Prominin1 present in all ependymal cells lining the ventricle (Beckervordersandforth et al., 2010; Coskun et al., 2008).
To overcome the problems associated with the promiscuity of a single marker, we previously applied a combinatorial approach to detect and isolate radial glial cells (i.e., NSCs) in developing and adult mouse brains (Götz and Huttner, 2005; Kriegstein and Alvarez-Buylla, 2009) by their glial identity (e.g., GLAST (Ninkovic et al., 2007), GFAP (Morshead et al., 2003), and human GFAP [hGFAP]-eGFP) and coexpression of Prominin1 (Pinto et al., 2008; Beckervordersandforth et al., 2010), a membrane-associated glycoprotein that is expressed in neurogenic regions of other vertebrates with widespread neurogenesis (Jászai et al., 2013). This approach allowed not only purification of NSCs in the developing brain (Pinto et al., 2008) but also enrichment of aNSCs from the subependymal zone (SEZ; Beckervordersandforth et al., 2010).
Little is known about Prominin1 localization on adult NSCs in the dentate gyrus (DG), the only region where neurogenesis appears to persist prominently in the adult human brain (Sanai et al., 2011; Spalding et al., 2013). Although Prominin1 is present in neurosphere-forming cells from the adult murine hippocampus (Walker et al., 2013), the relationship of Prominin1 with GFAP+ radial and horizontal astrocytes that act as NSCs in this region (Lugert et al., 2010) is less well understood. Here, we examined the extent of Prominin1 and GFAP coexpression in aNSCs in murine and human DG, and monitored the progeny of these cells by genetic fate mapping.
Results
Prominin1 Is Expressed in Radial and Nonradial Glial Cells in the Subgranular Zone of the Adult Hippocampus
Prominin1 maps to loci that promote neurogenesis in the DG (Kempermann et al., 2006), and was recently shown to be expressed in most neurosphere-forming cells of the hippocampus (Walker et al., 2013). To examine the colocalization of Prominin1 with GFAP, we immunostained adult hippocampal sections of hGFAP-eGFP transgenic mice. Interestingly, we found that Prominin1 immunoreactivity was restricted to the DG (Figure 1A), where it localized along the radial process and soma of radial astrocytes (Figure 1A), horizontal astrocytes in the subgranular zone (SGZ), and some astrocytes in the hilus. Prominin1+ radial astrocytes showed high levels of hGFAP-eGFP (Figure 1A) and coexpressed endogenous GFAP (Figure 1B), Nestin (Figure 1C), and SOX2 (Figure 1E). All Nestin-immunoreactive radial astrocytes were positive for Prominin1, while 40% of the radial astrocytes expressed both Prominin1 and hGFAP-eGFP. Very few Prominin1+/hGFAP-eGFP+ cells were positive for S100β, which labels postmitotic astrocytes, and no colocalization with the intermediate progenitor (IP) marker TBR2 (Figure S1A available online) or the neuroblast (NB) marker Doublecortin (DCX) was detectable (Figure 1D; summarized in Figure 1F). hGFAP-eGFP+ cells that lacked Prominin1 comprised many other cell types, such as parenchymal astrocytes in the molecular layer (ML), the granular zone (GZ), and the hilus (GFAP in Figure 1B), and were S100β immunoreactive (Figure 1F), consistent with a more mature astrocyte identity (Raponi et al., 2007). A small proportion of DCX+ NBs and immature neurons (Figure 1D) were Prominin1− and had weaker GFP signals than radial and other astrocytes, consistent with the idea that they inherited the GFP from their ancestors but had turned off the activity of the hGFAP promoter (Beckervordersandforth et al., 2010; Pinto et al., 2008). We also observed Prominin1+ radial cells that were negative for hGFAP-eGFP and DCX, but positive for GFAP (Figure 1B), Nestin (Figure 1C), and SOX2 (Figure 1F). In the SGZ, Prominin1+/hGFAP-eGFP+ cells consisted of radial and horizontal astrocytes coexpressing aNSC proteins such as SOX2 and Nestin, whereas the population that was positive for only one of the markers comprised many more cell types within and beyond the DG.
Figure 1.
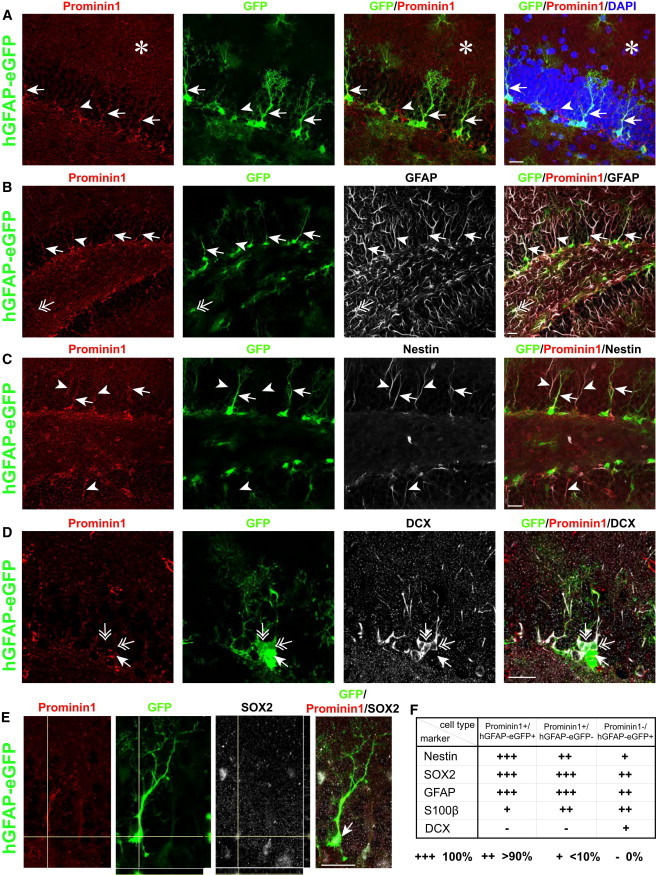
Characterization of Cell Types Expressing Prominin1 and hGFAP-eGFP
(A) Expression of Prominin1 (red) in processes of radial astrocytes (arrowheads), including hGFAP-eGFP+ cells (green; arrows). Prominin1 is not specifically expressed outside the DG (asterisk).
(B) Colocalization of Prominin1 with GFAP (white) in the SGZ (arrowhead), including those positive for hGFAP-eGFP+ (arrows). Most hGFAP-eGFP+-only cells also express GFAP (double-headed arrow).
(C) Colocalization of Prominin1 with Nestin (white) in the DG reveals coexpression in all Prominin1+/hGFAP-eGFP+ (arrows) and Prominin1+-only cells (arrowheads).
(D) Example of Prominin1+/hGFAP-eGFP+ cells negative for DCX (arrow), surrounded by DCX+ cells (white) that weakly express hGFAP-eGFP and are negative for Prominin1 (double-headed arrows).
(E) Prominin1+/hGFAP-eGFP+ cell (arrow) immunoreactive for SOX2 (white).
(F) Table summarizing the expression of molecular markers in Prominin1+/hGFAP-eGFP+, Prominin1+/hGFAP-eGFP−, and Prominin1−/hGFAP-eGFP+ cells (three or more animals analyzed per marker). Scale bars, 20 μm.
See also Figure S1.
Prominin1+/hGFAP-eGFP+ Cells Are Bromodeoxyuridine-Label Retaining in the Adult Murine DG
Next, we examined the extent to which the Prominin1+/hGFAP-eGFP+ cells were bromodeoxyuridine (BrdU)-label retaining, which is a property of slow-dividing aNSCs (Lugert et al., 2010). BrdU was provided in drinking water for hGFAP-eGFP mice (2 weeks to 2 months old) to label the cells that divided in this time window, followed by a 2 week BrdU-free chase period. In this paradigm, slow-dividing aNSCs and postmitotic progeny such as neurons are labeled, but the latter can be distinguished by costaining for neuronal markers. Indeed, most of the BrdU-labeled cells were positive for DCX (76%, n = 242 BrdU+ cells, 4 animals), indicative of an NB or immature neuron identity (Figure 2A), whereas some BrdU+ cells expressed GFAP (Figure 2B). We found that 3% of the BrdU-label-retaining cells (n = 845 BrdU+ cells, 4 animals) were Prominin1+/hGFAP-eGFP+ (Figures 2C and 2D). Conversely, 29.8% of the Prominin1+/hGFAP-eGFP+ radial astrocytes retained the BrdU label, consistent with their slow mode of cell division (n = 120 Prominin1+/hGFAP-eGFP+ cells, 4 animals). Some BrdU+ cells were colabeled with either Prominin1 or hGFAP-eGFP alone (3.9% and 4.4%, respectively; Figure 2C; n = 845 BrdU+ cells, 4 animals). The latter category comprised DCX+ cells as well as GFAP+ astrocytes, consistent with the finding that some dividing aNSCs can give rise to astrocytes (Encinas et al., 2011). Few BrdU-label-retaining cells coexpressed the cell-cycle marker KI67, implying that they reentered the cell cycle (Figure 2E). Since these cells were hGFAP-eGFP+ with long radial processes that were always labeled with Prominin1 (Figure 1A), we conclude that this population is BrdU-label retaining and represents self-renewing aNSCs.
Figure 2.

Prominin1+/hGFAP-eGFP+ Cells Proliferate In Vivo
(A–E) Confocal images of hGFAP-eGFP in the BrdU pulse-chase paradigm.
(A) Colocalization of DCX (white) with BrdU in hGFAP-eGFP mice. Note that NBs comprise the majority of all BrdU-label-retaining cells (arrows).
(B) Example of a GFAP+, BrdU-label-retaining cell (white, arrow).
(C) BrdU-label-retaining cells in the DG expressing Prominin1 and hGFAP-eGFP (arrows), Prominin1+ only (arrowhead), or hGFAP-eGFP+ only (double-headed arrow).
(D) Magnified image of a Prominin1+/hGFAP-eGFP+ BrdU-label-retaining cell (arrow labels the soma, arrowhead labels the process).
(E) Image of a cell positive for hGFAP-eGFP and coexpressing BrdU and the cell-cycle marker KI67 (white; arrow labels the soma, arrowheads label the process). Scale bars, 20 μm.
In Vivo Targeting of aNSCs in the DG by a Split-Cre Approach
Next, we performed a fate-mapping analysis of Prominin1+/hGFAP+ cells to assess lineage and self-renewal. For this purpose, we employed the split-Cre approach (Hirrlinger et al., 2009), in which the C-terminal half of the Cre recombinase is driven by the Prominin1 P2 promoter and inserted into one lentiviral vector, while the N-terminal half of Cre is driven by hGFAP promoter elements and inserted into a second lentiviral vector (Beckervordersandforth et al., 2010). We injected the two lentiviruses into the DG of 2-month-old CAG CAT GFP reporter mice and assessed the identity of the recombined cells by immunohistochemistry along with morphological criteria and localization. The recombined cells were analyzed shortly after the injection (3 days postinjection [dpi]) to determine their identity and at later time points (7 dpi, and 1 and 3 months postinjection [mpi]) to monitor their progeny. At 3 dpi, the majority of the reporter-positive cells were GFAP+ (88.2%; n = 272 GFP+ cells in 3 different animals). In the SGZ, the majority of GFP+/GFAP+ cells had a radial or horizontal morphology (Figures 3A and 3B; Table S1) consistent with the suggestion that the DG contains morphologically distinct aNSC populations (Lugert et al., 2010). We also observed some GFP+ astrocytes in the hilus and GZ (Figures 3A and 3B; Table S1), similar to the results from immunostaining described above, and very few in the ML. At this early time point and 7 dpi (data not shown), none of the recombined cells were colabeled with TBR2 (Figure S1B; Table S1) or DCX (Table S1), showing that the starting population does not comprise the progeny of aNSCs, as previously indicated by other approaches (Bonaguidi et al., 2011; Lagace et al., 2007). It also reveals that the labeled aNSCs were largely quiescent, as their progeny 7 days after labeling had not yet passed to the early steps of the neuronal lineage, such as IPs and early NBs.
Figure 3.

Split-Cre-Based Fate Mapping of Prominin1+/hGFAP-eGFP+ Cells in the DG of Adult GFP Reporter Mice
(A) Examples of recombined cells at 3 dpi, including GFAP+ radial astrocytes (RG), horizontal astrocytes (HA), and hilus astrocytes (arrow).
(B) Schematic illustrating recombined cells according to their location and morphology. The pie chart depicts their relative proportions based on immunohistochemical markers and morphological analyses at 3 dpi.
(C) DCX+ recombined cells at 1 mpi.
(D) Example of a recombined putative clone consisting of a GFAP+ RG (arrow), an IP, and two GFAP− NBs at 1 mpi.
(E) Overview of recombined cells in the DG at 3 mpi.
(F) Magnified image of the boxed area in (E) showing several recombined DCX+ NBs.
(G) Single-stack image of a recombined NEUN+ mature granule neuron at 3 mpi.
(H) Same as in (B) at 3 mpi.
Scale bars, 100 μm (A and E) and 20 μm (C, D, F, and G). GZ, granular zone; ML, molecular layer. See also Figure S1 and Table S1.
By 1 mpi, GFP+/DCX+ NBs with the typical immature granule neuron morphology had emerged from the GFP-expressing population (Figure 3C), demonstrating that some cells that were initially labeled by the split-Cre approach had generated immature neurons by this time (see also Figures 4A and 4C). Notably, even at 1 mpi, GFAP+ radial and horizontal astrocytes were still the majority of GFP+ cells, further supporting their quiescent nature. Interestingly, in some instances of low-density infection, we observed GFP+ cell clusters, possibly clonally derived cells, consisting of a GFAP+ radial astrocyte in close proximity to a GFAP− cell and several NBs (Figure 3D).
Figure 4.
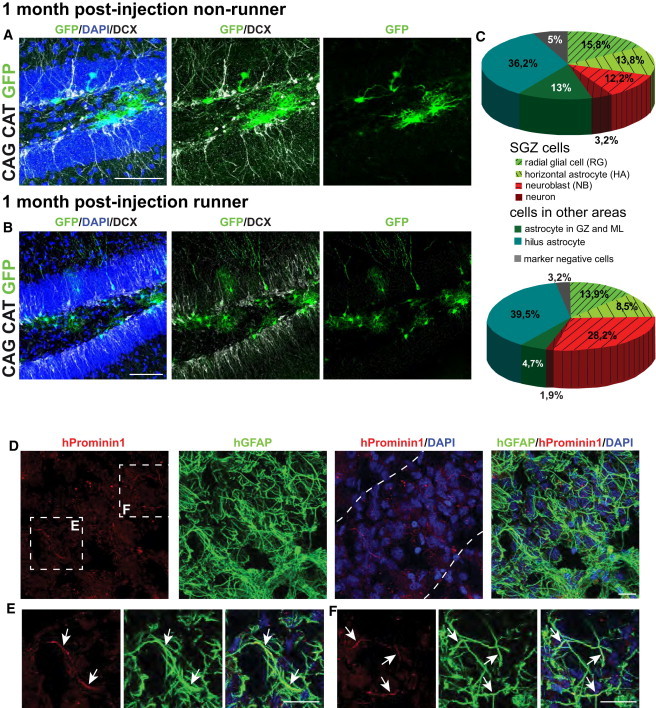
Split-Cre Injections in Running versus Nonrunning Mice, and hProminin/hGFAP Expression in the Human DG
(A and B) Overview of split-Cre injections in the DG of CAG CAT GFP nonrunning (A) and running (B) mice.
(C) Pie charts illustrate the relative proportion of recombined cells.
(D) Expression of hProminin1 (red) in processes of hGFAP+ astrocytes (green) in the human DG (outlined by dotted lines; for further characteristics of the subject, see Figure S2C); magnification of boxed areas in (E and F).
(E) Colocalization of hProminin1 and hGFAP (arrows) in radially oriented glial cells in the DG as well as in other glial processes (F).
Scale bars, 100 μm (A and B) and 20 μm (D–F). See also Figure S1 and Table S1.
At 3 mpi, GFP+/GFAP+ cells with radial and horizontal morphology reminiscent of the initial population (Figure 3H; Table S1) persisted in the SGZ, indicating some degree of self-renewal. We could still detect GFP+ NBs expressing DCX, suggesting that they were recently generated and had not yet fully matured (11%; n = 1680 GFP+ cells, 3 animals; Figures 3E and 3F; Table S1). Most importantly, many mature GFP+/NEUN+ neurons were observed, accounting for 47% of the GFP+ cells (Figures 3G and 3H; Table S1), consistent with the further maturation of the NBs generated at earlier stages. Notably, glial cells did not increase in the SGZ and GZ populations, implying that the labeled cells were largely neurogenic even in 5-month-old mice. These results strongly suggest that glial cells coexpressing Prominin1 and hGFAP in the SGZ of the adult DG are long-term neurogenic and self-renewing aNSCs.
It has been shown that physical activity increases neurogenesis by effects on various stages of the lineage (Brandt et al., 2010; Lugert et al., 2010; Steiner et al., 2008). To test whether the Prominin1+/hGFAP+ lineage also responds to a running stimulus, mice were housed with free access to running wheels directly after the injection of split-Cre viruses and analyzed at 1 mpi. In agreement with previous findings, the number of newly generated GFP+/DCX+ NBs was more than doubled in the running mice in comparison to the nonrunners (Figures 4A–4C; Table S1). Thus, SGZ NBs expanded at the expense of non-SGZ glia, whereas the SGZ glia showed no obvious difference. These data demonstrate that the Prominin1+/hGFAP+ lineage reacts to voluntary exercise by increasing the generation of new neurons as previously described for quiescent aNSCs (Lugert et al., 2010).
Human Prominin1+/GFAP+ Cells in Human DG
Because the combination of Prominin1 and GFAP labels NSCs in all neurogenic regions of the mouse brain, we investigated the expression of both markers in the postmortem human brain. hGFAP is expressed by many astrocytes of the human hippocampus. Interestingly, we found a very selective localization of human Prominin1 (hProminin1) along radial and nonradial GFAP+ processes (Figures 4D–4F) in the human DG. This coexpression was consistently observed irrespective of the age of the subject (between 51 and 87 years; Figures 4D–4F and S2A–S2C). Thus, the adult human DG also harbored cells coexpressing GFAP and Prominin1, similar to neurogenic regions in other mammalian and nonmammalian vertebrates.
Discussion
During brain development, aNSCs originate from their region-specific embryonic counterparts, suggesting common hallmarks between embryonic and adult radial glial cells. We previously showed that Prominin1 in combination with hGFAP is a highly selective marker for NSCs in the embryonic forebrain and the adult SEZ (Beckervordersandforth et al., 2010; Pinto et al., 2008). Here, we extended this labeling technique to the DG and demonstrated that Prominin1+/hGFAP+ cells are long-term, self-renewing aNSCs that increase the generation of new neurons upon physical activity. These data, together with our expression analysis in human DG samples and the widespread expression of Prominin1 in neurogenic zones of other vertebrates (Jászai et al., 2013), strongly suggest that the combinatorial expression of hGFAP and Prominin1 is a widespread marker for NSCs in many species and at different developmental stages.
In comparison with our approach, previous analyses of aNSCs in the DG by different single-marker-labeling strategies had the disadvantage of targeting other cell types in addition to aNSCs (e.g., astrocytes in the brain parenchyma with GLASTCreERT2, as shown in Ninkovic et al., 2007; GFAP-CreERT2, as shown in Hirrlinger et al., 2006 or GFAPtk, as shown in Morshead et al., 2003; or ependymal cells and IPs with Nestin-CreERT2, as shown in Bonaguidi et al., 2011 and Lagace et al., 2007). Likewise, injection of viral vectors (MLV-based Cre retroviruses) largely labels fast-proliferating progenitor cells in the SGZ (Jagasia et al., 2009; Zhao et al., 2006). Targeting Prominin1/hGFAP-coexpressing cells has the advantage that it avoids initial labeling of IPs and NBs. The ability to follow the aNSC lineage directly from its origin, including radial and nonradial aNSCs while excluding NSC progeny, is an important improvement that allows us to analyze stem cell characteristics and lineage progression under specific conditions (e.g., age, disease, running, and learning).
Interestingly, Prominin1 is found in other adult stem cell populations, such as those in the hematopoietic system and small intestine (Snippert et al., 2009; Zhu et al., 2009) and is a marker of tumor-initiating cells in several cancers (Neuzil et al., 2007). It was originally discovered in humans as an antibody against the AC133 antigen (Shmelkov et al., 2005), a glycosylation-dependent epitope of CD133. Prominin1+ cells isolated from the adult human kidney were shown to be capable of self-renewal and multilineage differentiation, and to contribute to renal tissue regeneration (Bussolati et al., 2005). Our finding that Prominin1 is expressed in both major sets of adult murine NSCs further supports its widespread presence in adult stem cell populations. Prominin1 has been implicated at the functional level in self-renewal of stem cells (Ettinger et al., 2011). Its association with cholesterol-based membrane microdomains and protrusion-specific localization led to the hypothesis that Prominin1 plays a role in the biogenesis and functional maintenance of plasma membrane protrusions, and there is evidence that the release of Prominin1-containing membrane particles may contribute to cell differentiation (Corbeil et al., 2010). Although the function of Prominin1 in embryonic and adult NSCs needs further investigation, here we substantiate the wide applicability of Prominin1 in combination with an additional tissue-specific antigen as a valuable tool for identifying stem cells.
Experimental Procedures
Animals and Stereotactic Injections
hGFAP-eGFP (Nolte et al., 2001) and CAG CAT GFP reporter (Nakamura et al., 2006) mice were kept under standard housing conditions. For the voluntary exercise paradigm, running wheels were put in the cages. Experimental procedures were performed in accordance with German and European Union guidelines and were approved by the institutional animal care committee and the Government of Upper Bavaria under license number 55.2-1-54-2531-144/07. Stereotactic injections were performed as previously described (Jagasia et al., 2009) with 1 μl of virus per DG at the following coordinates: −2.0 anterior/posterior, ±1.6 medial/lateral, and −1.9 to −2.1 dorsal/ventral relative to bregma (in millimeters). Split-Cre viral constructs were as described in Beckervordersandforth et al. (2010).
Human Tissue
Tissues were procured from patients who were admitted to the Department of Neuropathology, University of Debrecen, Debrecen, Hungary, and died between January and April 2011. The institutional review board and local ethics committee approved the study, and informed consent was obtained from the relatives of all patients analyzed.
Tissue collection (occipital cortex and hippocampus) was performed in subjects without neurological or psychiatric disease who died from nonneurological causes. Brain tissue was frozen and stored at −80°C until further analysis.
Immunohistochemistry
Animal handling, sectioning of brains, and immunohistochemistry are described in detail in the Supplemental Experimental Procedures.
The following primary antibodies were used: rat anti-CD133 (1:100; eBioscience), chicken anti-GFP (1:2000; Aves), mouse anti-GFAP (1:500; Sigma), rabbit anti-GFAP (1:500; DAKO), rabbit anti-DCX (1:500; abcam), guinea pig anti-DCX (1:500; Millipore), mouse anti-Nestin (1:200; Millipore), rabbit anti-SOX2 (1:500; Millipore), mouse anti-S100β (1:1000; Sigma), rat anti-BrdU (1:200; Serotec), mouse anti-BrdU (1:200; Becton Dickinson), mouse anti-KI67 (1:200; DAKO), mouse anti-NEUN (1:100; Millipore), rabbit anti-TBR2 (1:500; abcam), rabbit anti-human GFAP (1:1,000; DAKO), and mouse anti-human PROMININ1 (80B258; Karbanova et al., 2008).
Primary antibodies were visualized with Alexa-conjugated secondary antibodies (all 1:400; Invitrogen). Biotinylated secondary antibodies (1:400; Vector Laboratories) were used in combination with Alexa-conjugated streptavidin (Invitrogen) to enhance CD133 and GFP signals. As a negative control, staining was done using secondary antibody only.
Acknowledgments
We thank Andrea Steiner-Mezzadri for technical help, Dennis Corbeil for providing the hProminin1 antibody, and Daniel Coutu for help with confocal microscopy. This work was supported by grants from the DFG (German Research Foundation) to M.G. (SFB 870, GO640/8.1, 9.1) and R.B. (BE 5136/1-1).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information
References
- Beckervordersandforth R., Tripathi P., Ninkovic J., Bayam E., Lepier A., Stempfhuber B., Kirchhoff F., Hirrlinger J., Haslinger A., Lie D.C. In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell. 2010;7:744–758. doi: 10.1016/j.stem.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Bonaguidi M.A., Wheeler M.A., Shapiro J.S., Stadel R.P., Sun G.J., Ming G.L., Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt M.D., Maass A., Kempermann G., Storch A. Physical exercise increases Notch activity, proliferation and cell cycle exit of type-3 progenitor cells in adult hippocampal neurogenesis. Eur. J. Neurosci. 2010;32:1256–1264. doi: 10.1111/j.1460-9568.2010.07410.x. [DOI] [PubMed] [Google Scholar]
- Bussolati B., Bruno S., Grange C., Buttiglieri S., Deregibus M.C., Cantino D., Camussi G. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil D., Marzesco A.M., Wilsch-Bräuninger M., Huttner W.B. The intriguing links between prominin-1 (CD133), cholesterol-based membrane microdomains, remodeling of apical plasma membrane protrusions, extracellular membrane particles, and (neuro)epithelial cell differentiation. FEBS Lett. 2010;584:1659–1664. doi: 10.1016/j.febslet.2010.01.050. [DOI] [PubMed] [Google Scholar]
- Coskun V., Wu H., Blanchi B., Tsao S., Kim K., Zhao J., Biancotti J.C., Hutnick L., Krueger R.C., Jr., Fan G. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc. Natl. Acad. Sci. USA. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas J.M., Michurina T.V., Peunova N., Park J.H., Tordo J., Peterson D.A., Fishell G., Koulakov A., Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger A.W., Wilsch-Brauninger M., Marzesco A.M., Bickle M., Lohmann A., Maliga Z., Karbanova J., Corbeil D., Hyman A.A., Huttner W.B. Proliferating versus differentiating stem and cancer cells exhibit distinct midbody-release behaviour. Nature communications. 2011;2:503. doi: 10.1038/ncomms1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M., Huttner W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Hirrlinger P.G., Scheller A., Braun C., Hirrlinger J., Kirchhoff F. Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen-inducible DNA recombinase variant CreERT2. Glia. 2006;54:11–20. doi: 10.1002/glia.20342. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J., Scheller A., Hirrlinger P.G., Kellert B., Tang W., Wehr M.C., Goebbels S., Reichenbach A., Sprengel R., Rossner M.J., Kirchhoff F. Split-cre complementation indicates coincident activity of different genes in vivo. PLoS ONE. 2009;4:e4286. doi: 10.1371/journal.pone.0004286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagasia R., Steib K., Englberger E., Herold S., Faus-Kessler T., Saxe M., Gage F.H., Song H., Lie D.C. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J. Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jászai J., Graupner S., Tanaka E.M., Funk R.H., Huttner W.B., Brand M., Corbeil D. Spatial distribution of prominin-1 (CD133)-positive cells within germinative zones of the vertebrate brain. PLoS ONE. 2013;8:e63457. doi: 10.1371/journal.pone.0063457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbanova J., Missol-Kolka E., Fonseca A.V., Lorra C., Janich P., Hollerova H., Jaszai J., Ehrmann J., Kolar Z., Liebers C. The stem cell marker CD133 (Prominin-1) is expressed in various human glandular epithelia. J. Histochem. Cytochem. 2008;56:977–993. doi: 10.1369/jhc.2008.951897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Chesler E.J., Lu L., Williams R.W., Gage F.H. Natural variation and genetic covariance in adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA. 2006;103:780–785. doi: 10.1073/pnas.0510291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A., Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace D.C., Whitman M.C., Noonan M.A., Ables J.L., DeCarolis N.A., Arguello A.A., Donovan M.H., Fischer S.J., Farnbauch L.A., Beech R.D. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J. Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U., Zimmerman L.B., McKay R.D. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Lugert S., Basak O., Knuckles P., Haussler U., Fabel K., Götz M., Haas C.A., Kempermann G., Taylor V., Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Morshead C.M., Garcia A.D., Sofroniew M.V., van Der Kooy D. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur. J. Neurosci. 2003;18:76–84. doi: 10.1046/j.1460-9568.2003.02727.x. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Colbert M.C., Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ. Res. 2006;98:1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- Neuzil J., Stantic M., Zobalova R., Chladova J., Wang X., Prochazka L., Dong L., Andera L., Ralph S.J. Tumour-initiating cells vs. cancer ‘stem’ cells and CD133: what’s in the name? Biochem. Biophys. Res. Commun. 2007;355:855–859. doi: 10.1016/j.bbrc.2007.01.159. [DOI] [PubMed] [Google Scholar]
- Ninkovic J., Mori T., Götz M. Distinct modes of neuron addition in adult mouse neurogenesis. J. Neurosci. 2007;27:10906–10911. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte C., Matyash M., Pivneva T., Schipke C.G., Ohlemeyer C., Hanisch U.K., Kirchhoff F., Kettenmann H. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia. 2001;33:72–86. [PubMed] [Google Scholar]
- Pinto L., Mader M.T., Irmler M., Gentilini M., Santoni F., Drechsel D., Blum R., Stahl R., Bulfone A., Malatesta P. Prospective isolation of functionally distinct radial glial subtypes—lineage and transcriptome analysis. Mol. Cell. Neurosci. 2008;38:15–42. doi: 10.1016/j.mcn.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Raponi E., Agenes F., Delphin C., Assard N., Baudier J., Legraverend C., Deloulme J.C. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia. 2007;55:165–177. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N., Nguyen T., Ihrie R.A., Mirzadeh Z., Tsai H.H., Wong M., Gupta N., Berger M.S., Huang E., Garcia-Verdugo J.M. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmelkov S.V., St Clair R., Lyden D., Rafii S. AC133/CD133/Prominin-1. Int. J. Biochem. Cell Biol. 2005;37:715–719. doi: 10.1016/j.biocel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Snippert H.J., van Es J.H., van den Born M., Begthel H., Stange D.E., Barker N., Clevers H. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology. 2009;136:2187–2194.e1. doi: 10.1053/j.gastro.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Spalding K.L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H.B., Boström E., Westerlund I., Vial C., Buchholz B.A. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B., Zurborg S., Hörster H., Fabel K., Kempermann G. Differential 24 h responsiveness of Prox1-expressing precursor cells in adult hippocampal neurogenesis to physical activity, environmental enrichment, and kainic acid-induced seizures. Neuroscience. 2008;154:521–529. doi: 10.1016/j.neuroscience.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Suh H., Consiglio A., Ray J., Sawai T., D’Amour K.A., Gage F.H. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T.L., Wierick A., Sykes A.M., Waldau B., Corbeil D., Carmeliet P., Kempermann G. Prominin-1 allows prospective isolation of neural stem cells from the adult murine hippocampus. J. Neurosci. 2013;33:3010–3024. doi: 10.1523/JNEUROSCI.3363-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann A., Corbeil D., Hellwig A., Huttner W.B. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc. Natl. Acad. Sci. USA. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Teng E.M., Summers R.G., Jr., Ming G.L., Gage F.H. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Gibson P., Currle D.S., Tong Y., Richardson R.J., Bayazitov I.T., Poppleton H., Zakharenko S., Ellison D.W., Gilbertson R.J. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


