Abstract
A total of 6257 helminths of 19 taxa were recovered from the digestive tract and lungs of 67 bobcats in Illinois. Infections caused by Alaria mustelae, Diphyllobothrium latum, and Macracanthorhynchus ingens are reported for the first time in bobcats. From all the taxa recovered, only three species occurred in high prevalence and caused intense infections: Taenia rileyi, Alaria marcianae, and Toxocara cati, with prevalence and mean intensity of 70% and 6; 42% and 193, and 25% and 14 individuals, respectively. Prevalence lower than 15% of 14 helminth species suggests bobcats are not continuously exposed to infective stages of a single parasite, and may be exposed to a large variety of generalists during their lifespan. No significant difference in parasite species according to host sex or age was detected, except for Diphyllobothrium spp., which were found more frequently in females and in trapped bobcats, and the hookworm, Ancylostoma caninum, which infected juveniles more frequently. Average species richness per infracommunity was 2.4 (±1.2), and the parasite component community showed low qualitative similarity with neighbor communities. The taxa A. caninum, Alaria spp., Diphyllobothrium spp., Paragonimus kellicotti, and T. cati are etiological agents of epizootic and zoonotic diseases.
Keywords: Epizootic, Zoonotic helminths, Carnivore, Lynx rufus, Bobcat, Diphyllobothrium latum
Abstract
Un total de 6257 helminthes de 19 taxa ont été collectés de l’appareil digestif et des poumons de 67 lynx roux dans l’Illinois. Les infections causées par Alaria mustelae, Diphyllobothrium latum et Macracanthorhynchus ingens sont rapportées pour la première fois chez les lynx roux. De tous les taxa collectés, seules trois espèces présentaient une prévalence importante et causaient des infections intenses : Taenia rileyi, Alaria marcianae et Toxocara cati, avec des prévalences et intensités moyennes de respectivement 70 % et 6 individus, 42 % et 193 et 25 % et 14. Les prévalences inférieures à 15 % de 14 espèces d’helminthes suggèrent que les lynx roux ne sont pas exposés en permanence aux stades infectieux d’un parasite unique, et peuvent être exposés à une grande variété de généralistes au cours de leur vie. Aucune différence significative dans les espèces de parasites selon sexe et l’âge n’a été détectée, sauf pour Diphyllobothrium spp., qui a été trouvé plus fréquemment chez les femelles et chez les lynx piégés, et l’ankylostome Ancylostoma caninum, qui infectait plus souvent les juvéniles. La richesse en espèces moyenne par infracommunauté était de 2.4 (±1.2), et la communauté des parasites a montré une faible similarité qualitative avec les communautés voisines. Les taxa A. caninum, Alaria spp., Diphyllobothrium spp., Paragonimus kellicotti et T. cati sont des agents étiologiques de maladies épizootiques et de zoonoses.
Introduction
In the United States, the top five neglected parasitic infections include four zoonotic diseases, namely Chagas disease, cysticercosis, toxocariasis, and toxoplasmosis [4]. Excluding cysticercosis, the etiological agents of these diseases include parasites that infect domestic and wild felids [7, 10, 21, 65]. Bobcats (Lynx rufus) are the most abundant and widely distributed wild felid species in North America. They range from southern Canada to the northern half of Mexico and are nearly ubiquitous throughout the US, including areas of high human density [23, 24]. Highly agricultural areas of the Midwest including locations in Illinois, Indiana, and Ohio provide relatively poor habitat for bobcats [42, 71]. However, hunting and trapping regulations have allowed populations to rebound from overharvesting; bobcats have been protected from legal harvest in Illinois since 1971 and were listed as threatened in the state until 1999 when they were down-listed to non-game species status [41, 43]. Bobcat population densities were estimated to be 0.27/km2 in the late 1990s and have increased at a rate >6% since then [41]. The increase in bobcat populations is considered a success in wildlife management [53], yet it also raises concerns about the proportional increase in their role as reservoirs of epizootic and zoonotic parasites [5, 51, 64]. Currently, bobcat abundance is stable or increasing in every state except Florida and Delaware, with a population estimate of about 2.3 million individuals in the US [53].
Parasites of bobcats have been surveyed in bordering regions of Canada and Mexico, as well as in 14 states in the US, including characterization of infections in populations from Arkansas, Georgia, Massachusetts, Nebraska, North and South Carolina, Texas, Virginia, and West Virginia [26, 50, 55, 57, 58, 61, 67, 69, 73]. Each population appears to harbor a unique community of parasites that appear to be a function of the habitat, prey availability, and climate of the region [67, 69, 73]. The differences among communities appear to be congruent with the expectation of an inverse correlation between the similarity of helminth communities and geographic distance in populations of vertebrates [31, 45, 47].
Among the parasites that infect bobcats, three taxa of protozoans and five of helminths are epizootic, and therefore are shared with other carnivores including coyotes, cougars, and domestic cats and dogs; these include Cytauxzoon felis, Diphyllobothrium mansonoides, Echinococcus oligarthus, Mesocestoides variabilis, P. kellicotti, Sarcocystis spp., Taenia spp., and Toxoplasma gondii. Six known species of parasites reported in bobcats are known to be agents of zoonotic disease including P. kellicotti, E. oligarthus, D. mansonoides, Dirofilaria striata, Toxocara cati, and Toxoplasma gondii [10, 72]. The prevalence of these and other etiological agents of zoonotic disease are higher in areas where bobcats, cougars, and domestic cats occur in sympatry [5].
Given the potential for cougars to recolonize the Midwest [34], the high density of bobcats [53, 74] and domestic cats [37], and the continued expansion of human populations [12], it is necessary to document and archive the parasites infecting bobcats. The objective of the present study is to characterize the helminth component community in an area of the Southeast Temperate Plains near an agricultural zone and compare it against helminth component communities from the same ecological region (Georgia, North and South Carolina, Virginia, and West Virginia), the Southeast Coastal Plains (New England), the Ozark/Ouachita Forest (Arkansas), the Semiarid Plains (Nebraska and Texas), and Mixed Woods (Minnesota). To complete this goal, we also characterize infections in bobcats, compare the effect of age and sex on infections, and establish the qualitative similarities of bobcat helminth communities across their distributional range.
Materials and methods
Study area
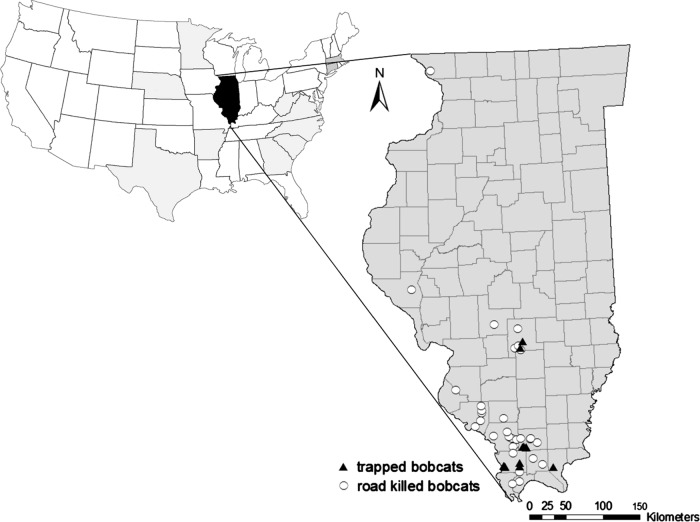
This survey was completed in the 39 southernmost counties in Illinois (46,436 km2) and only 1 bobcat was obtained from the northern part of the state (Figure 1). The population of bobcats in southern Illinois resides at the edge of areas considered as highly suitable habitat (forested regions) and maize monoculture, generally considered poor habitat for these felines. The southern region of Illinois is human-dominated (ca. 21.5 persons/km2) and comprises the southern till plain, Wabash border, Shawnee hills, Ozark, lower Mississippi river bottomlands, and coastal plain natural divisions [40, 62]. Streams and roads are abundant in the landscape. Land cover consists primarily of closed-canopy mixed hardwood forests (44%; Quercus and Carya spp.), grasslands (21%), and cropland (19%), [38]. Wetlands (8%), open water (6%), and urban (2%) cover types comprise the remainder of the study area [2].
Figure 1.
Collection locations for trapped (13) or road killed (32) individuals used to characterize infections suffered by bobcats (Lynx rufus) in Illinois during 2003–2012. States where other surveys have been completed are shaded gray.
Parasitological examination
Sixty-seven bobcats (52 adults, 15 juveniles) were collected between July 2003 and August 2012. Most of the individuals (96%) were collected in the period between 2010 and 2012. The total included 34 males (27 adults and 7 juveniles) and 33 females (25 adults and 8 juveniles). All animals were in fair to good condition with adults weighing from 5.0 to 17.3 kg (males) and 5.5–10.4 kg (females). The sample included 46 road killed bobcats and 21 individuals that were trapped for the project. From the latter set, 13 individuals were captured by licensed trappers seeking other species.
Specimens were weighed, sexed, and either immediately processed or frozen at −20 °C until necropsy. All methods were supported by the Institutional Animal Care and Use Committee of Southern Illinois University (Assurance Number A-3078-01). Age was determined using the presence of annuli in canine cementum layers of a lower canine tooth [15]. Bobcat ages were grouped into three distinct categories: young of year (YOY) (<1 year), juvenile (1–2 years), and adult (>3 years). These age categories are commonly used in the characterization and comparison of infections in carnivores [25, 30].
The helminthological examination included inspection of the body cavity and internal organs. All organs were prospected for helminths including the heart, lung, liver, stomach, and lower intestinal tract. All macroscopically evident parasites were separated into Petri dishes containing saline solution or tap water and were fixed and preserved immediately. Organ contents were sieved through 350- and 500-μm mesh. The filtered materials were processed by sedimentation and the sediment was examined to recuperate all parasites [20].
Parasites were preserved in a solution of 70% ethanol or formaldehyde; live worms were relaxed by inducing osmotic shock or submerged in glacial acetic acid. Subsequently, worms were fixed as described above. Platyhelminthes were stained in Semichon’s acetic carmine prior to mounting in Canada balsam. Identification of the parasites was completed using specialized literature [11, 17, 36, 52, 68]. Voucher specimens were deposited in the United States National Parasite Collection (USNPC, Bethesda, Maryland).
Data analysis
Ecological definitions of abundance, intensity, prevalence, and communities follow Bush et al. [9]. Simpson’s diversity index was calculated to measure the concentration of dominance of helminth species [29]. Values of intensity and species richness were first tested for normality (α = 0.05 throughout) and then transformed using the logarithm of the original value plus 1. To test for significant differences among parasite communities in roadkills and trapped animals, normalized data of intensity of each parasite species and species richness were tested using a Student t-test. The same test was used to compare species intensity and species richness depending on sex. ANOVA was used for test differences among the three age groups. Data that could not be transformed were analyzed using non-parametric Kruskal-Wallis tests for age and sex. The Pearson Chi-square test with a correction for continuity for samples sizes <200 was used to compare expected with observed values of frequency of occurrence for pairs of helminth species [66]. The Chi-square test was also used to determine significant associations between species richness and intensity of species with either host age or sex.
Gastrointestinal helminth component communities were compared against similar bobcat parasite surveys with sample sizes >30 individuals, with the exception of communities from Arkansas, which was based on 10 individuals [26]. The expectation was to find clustering of these communities depending on five ecoregions including: the Southeast Temperate Plains (Illinois, Georgia, North and South Carolina, Virginia, and West Virginia), the Southeast Coastal Plains (New England), the Ozark/Ouachita Forest (Arkansas), the Semiarid Plains (Nebraska and Texas), and Mixed Woods (Minnesota) [39, 49, 55, 67, 69, 73]. These ecoregions follow the definitions provided by the Environmental Protection Agency [3]. Qualitative similarity of these parasite communities was estimated by means of Jaccard’s similarity index using SAS [59].
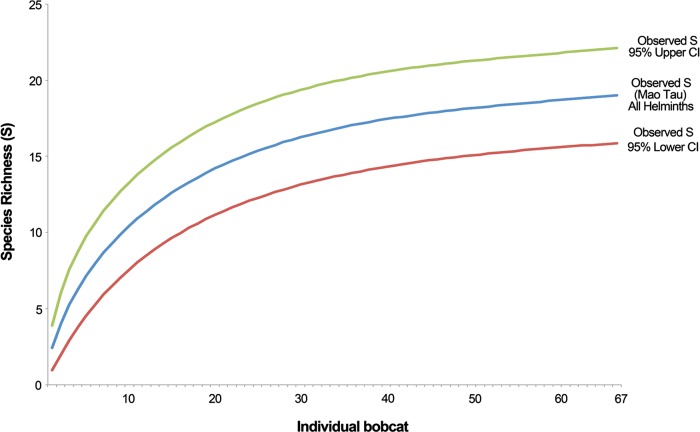
As a generalist mesopredator the bobcat is expected to accumulate a large number of helminth species. To visualize this trend a rarefaction curve was constructed based on a sample-based approach, using the program EstimateS 9.1.0 [13]. This curve was constructed based on the expected richness in the pooled sample of infracommunities based on the empirical dataset. Curves for the lower and upper 95% confidence intervals were also constructed. All estimates were made at 67 nodes, replicating the original number of observations, and are based on 100 iterations, with sampling at each node and replacement of individuals between replicates.
Results
A total of 6257 helminths of 18 species, including 6 cestodes, 4 digeneans, 7 nematodes, and one acanthocephalan were recovered (Table 1). Internal organs positive for infection included the stomach, small intestine, and lungs. From that number, a single species of nematode was recovered from the stomach, whereas 14 species were found in the small intestine (three digeneans, six tapeworms, four nematodes, and one acanthocephalan). Three species were recovered from the lungs, including one digenean and two nematodes. Only one bobcat was free of helminth infection. Mean species richness in infracommunities was 2.4 (±1.2 SD) with a range from 0 to 6. Simpson’s index was relatively low (x = 0.13). Both lower and upper 95% confidence intervals of the estimated species richness (S) do not converge with the expected S. The latter curve does not reach a plateau (Figure 2). No significant differences were found with respect to collection method and as a result, all roadkills and trapped bobcats were pooled. There were no differences in species intensity between males and females, and only Diphyllobothrium spp. were found more commonly in females (χ 2 1,67 = 4.45, p = 0.04) and trapped animals (χ 2 1,67 = 6.12, p = 0.01). The dog hookworm, A. caninum, was common in young animals and never found in adults (χ 2 2,67 = 6.24, p = 0.04). Concomitantly, A. caninum was present at higher intensities in YOY and juvenile bobcats when compared with adults (F 1,5 = 8.20, p = 0.04). No significant differences were found with respect to parasite intensity for sex or age class. There were no significant differences of prevalence according to sex or age class.
Table 1.
Characterization of helminth infections from bobcats, Lynx rufus, in Illinois, based on 67 individuals. Collection numbers from the United States National Parasite Collection are included.
| Prevalence (%) | Mean abundance, ±SD | Intensity |
USNPC No. | ||
|---|---|---|---|---|---|
| Range | Mean, ±SD | ||||
| Trematoda | |||||
| Alaria americana (I) | 5 | 0(±3) | 1–22 | 8(±12) | 106449 |
| Alaria marcianae (I) | 42 | 81(±385) | 1–2872 | 193(±547) | 106448 |
| Alaria mustelae (I) | 5 | 0(±1) | 2–4 | 3(±1) | 106710 |
| Paragonimus kellicotti (L) | 6 | 0(±2) | 1–16 | 5(±7) | 106450 |
| Cestoda | |||||
| Diphyllobothrium mansonoides (I) | 9 | 1(±5) | 1–33 | 11(±13) | 106708 |
| Diphyllobothrium latum (I) | 6 | 0(±1) | 1–3 | 2(±1) | 106707 |
| Mesocestoides sp. (I) | 2 | 0(±4) | 31 | 31 | 106706 |
| Taenia pseudolaticollis (I) | 5 | 1(±4) | 1–31 | 11(±17) | 106445, 106709 |
| Taenia pisiformis (I) | 5 | 0(±2) | 1–10 | 7(±5) | 106444 |
| Taenia rileyi (I) | 70 | 4(±4) | 1–18 | 6(±4) | 106446, 106447 |
| Nematoda | |||||
| Anafilaroides rostratus (L) | 8 | 0(±1) | 1–7 | 3(±2) | 106716 |
| Ancylostoma caninum (I) | 9 | 1(±3) | 1–18 | 6(±7) | 106713 |
| Molineus barbatus (I) | 14 | 1(±4) | 1–26 | 8(±10) | 106712 |
| Physaloptera sp. (S) | 2 | 0(±0) | 1 | 1 | 106714 |
| Toxascaris leonina (I) | 6 | 0(±1) | 1–6 | 3(±2) | 106452 |
| Toxocara cati (I) | 25 | 3(±11) | 1–60 | 14(±18) | 106451 |
| Vogeloides felis (L) | 6 | 0(±0) | 1–2 | 2(±1) | 106715 |
| Ascarid juveniles (I) | 20 | 1(±3) | 1–16 | 4(±5) | |
| Acanthocephala | |||||
| Macracanthorhynchus ingens (I) | 2 | 0(±0) | 1 | 1 | 106711 |
(I) Small intestine, (L) Lung, (S) Stomach.
Figure 2.
Rarefaction curves of parasite species recovery per infracommunity in bobcats collected during 2003–2012. The Mau τ index was employed to calculate the observed species and the confidence intervals per node (infracommunity).
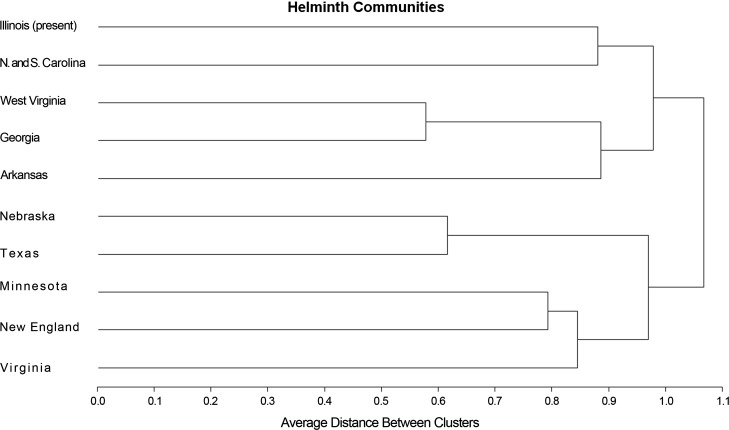
The comparison of parasite communities (Table 2) resulted in low similarity among all regions (Figure 3). The distance between clusters was >0.5 for all locations. The most similar parasite communities include pairs in Georgia and West Virginia (0.56), and Nebraska and Texas (0.53). The communities with the greatest similarity with southern Illinois were those in North and South Carolina (0.33). The most dissimilar were Arkansas to Texas (0.05) and New England to Arkansas (0.08). The area accumulation curve shows non-convergence between the estimated number of species and their confidence intervals.
Table 2.
Gastrointestinal helminths recorded in the bobcat, Lynx rufus, in the United States. The presence or absence of the parasites is denoted in a binary mode, with one (1) indicating presence. This table was used to reconstruct the Jaccard’s similarity among component communities in 10 different surveys of parasites of bobcats.
| Locality | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arkansas (n = 10) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Georgia (n = 10) | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Minnesota (n = 50) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N. & S. Carolina (n = 16) | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nebraska (n = 75) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| New England (n = 100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Illinois (n = 67) | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Virginia (n = 70) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| West Virginia (n = 143) | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Texas (n = 66) | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
1, Alaria marcianae; 2, A. americana; 3, A. mustelae; 4, Diphyllobothrium mansonoides; 5, D. latum; 6, Mesocestoides corti; 7, M. variabilis; 8, Taenia taeniaeformis; 9, T. pisiformis; 10, T. hydatigena; 11, T. laticollis; 12, T. ovis; 13, T. macrocystis; 14, T. pseudolaticollis; 15, T. rileyi; 16, Capillaria plica; 17, C. putorii; 18, Trichuris felis; 19, Toxascaris leonine; 20, Toxocara cati; 21, T. canis; 22, Ancylostoma tubaeforme; 23, A. braziliense; 24, A. caninum; 25, Molineus barbatus; 26, Physaloptera praeputialis; 27, P. rara; 28, Cyathospirura chevreuxi; 29, C. felineus; 30, Didelphonema longispiculatum; 31, Pterygodermatites cahirensis; 32, Oncicola canis; 33, Gongylonema pulchrum; 34, Trichostrongylus affinis; 35, T. axei; 36, Macracanthorhynchus ingens.
Figure 3.
Dendogram representing Jaccard’s similarity index among parasite communities of 46 species of bobcat parasites across 10 different localities. Only species of parasites present in the digestive tract have been included in the analysis. Average distance was used to reconstruct the distance between clusters.
Discussion
Helminths infecting bobcats include a large number of generalist species; with the exception of D. latum, A. mustelae, and M. ingens, all observed species have been previously reported from bobcats elsewhere in their range. All 19 taxa varied in prevalence, intensity, and abundance, although 15 occurred with prevalence lower than 10%. Parasites of relatively high prevalence included T. rileyi (70%), A. marcianae (42%), and T. cati (25%); these species have been found in other bobcat-dwelling helminth surveys and are considered either bobcat-specific, such as T. rileyi, or feline-specific, such as T. cati [26, 39, 49, 55, 67, 69, 73]. Four taxa contain known agents of zoonotic disease, including Alaria spp., Diphyllobothrium spp., T. rileyi, and Toxocara cati [16, 18, 28, 44, 63]. The majority of parasite species found are epizootic, in that they may use other species including coyotes, red and grey fox (Vulpes vulpes, Urocyon cinereoargenteus), and domestic dogs and cats (C. lupus familiaris, Felis catus) as alternate definitive hosts [54]. Their presence in bobcats may be the result of the generalist nature of these parasites and the opportunistic diet of bobcats [46]; this diet includes lagomorphs, rodents, white-tailed deer, and birds as common prey species [35, 70, 74]. Since the vast majority of the species have heteroxenous patterns of transmission, low values of prevalence also suggest that individual bobcats do not encounter infective stages of these parasites very frequently. Species considered in this category include A. americana, A. mustelae, A. caninum, D. latum, D. mansonoides, M. barbatus, M. ingens, P. kellicotti, T. pseudolaticollis, T. pisiformis, and T. leonina. All of these species are epizootic and most appeared with relatively low prevalence, which is considered a characteristic of generalist parasites that are able to infect a large number of individuals of different species [19]. Several of these parasites have a specific requirement to complete their life cycles and transmission to the definitive host [1, 8]. As a consequence, infective stages of parasites using intermediate and paratenic hosts would be consumed sporadically, resulting in infrequent reinfections that result in parasites showing low prevalence. For example, species of Alaria and Diphyllobothrium must have a free-living life stage as well as aquatic intermediate hosts, which include planorbid snails and copepods, respectively.
No significant correlation was found between infection and intensity of any given parasite with age or sex. The only exception was infections caused by species of Diphyllobothrium, which were more common in females than in males. This was opposite to the expected typical pattern of carnivores, with a sex bias toward males. Males generally have a higher parasite load than females in experimental situations and a slightly significant male bias has been documented in field studies [60]. We do not have a satisfactory explanation for this trend, and further testing would enable researchers to test if these patterns persist, and if temporal dietary fluctuations of females may explain this pattern. Furthermore, A. caninum was found more commonly in juveniles than in adults. This is consistent with previous findings, and it is a direct consequence of the host feline developing a stronger acquired immune system after initial infections [22, 25, 30]. Species of Diphyllobothrium were found more commonly in trapped animals than in roadkills. This difference may be a factor of the restricted trapping area and small-scale habitat differences than as a result of the collection method; previous studies in southern Illinois found bobcat habitat was commonly intersected with roads [42, 43].
Rarefaction curves show that although a larger accumulation of helminth species is still expected, those species that may be considered common or specialists to bobcats were recovered as a result of our efforts. Bobcat-dwelling infracommunities tend to accumulate a large number of generalist species; however, there are five species that appear to be common to the bobcat, including A. marcianae, T. rileyi, and T. cati. The high variability and low concentration of dominant helminth species, indicated by the low Simpson’s index, is consistent with previous studies and indicative of a parasite community dominated by a few species (Taenia rileyi and A. marcianae). Simpson’s diversity index in bobcats from Illinois (0.13) is considerably similar to the value obtained for communities in Texas (0.1), even though the average species richness was lower for communities in Illinois relative to those in Texas, (2.4 vs. 7.4, respectively). Stone and Pence (1978) suggested that the differences in helminth communities likely reflect basic differences in specific collection localities, as well as regional differences. While different geographic areas may display different communities, it is localized home ranges that influence these infections.
The parasite community of bobcats in Illinois is relatively rich, with at least 18 species present. The comparison of bobcat parasite communities resulted in relatively low taxonomic similarity among component communities according to the low values of Jaccard’s index. This most likely can be attributed to relatively few shared parasites between the groups, since most of the parasite species occur in low prevalence in a single locality. Geographic distance has been shown to be an important determinant of the likelihood of presence of parasite species [48]. Bobcats, especially given their adaptability, reflect this variation. However, there is likely variation between localities that reflect the diverse habitats and prey species utilized by bobcats. Given the nidality of transmission of infectious diseases, Stone and Pence [67] suggested that differences among the composition of communities infecting individual bobcats were likely the result of the localized levels prevailing on the home range of an individual bobcat, rather than a regional effect. This may result in moderate or low similarity at the level of the component community detected in the dendogram (Figure 3).
The dendogram appears to show two clusters that reflect a putative meaningful regional similarity. This is seen in the clustering of communities from Minnesota and Nebraska (Semiarid Plains), and the Southeastern Temperate Plains (Georgia, Illinois, North and South Carolina, and West Virginia). The pattern appears to be challenged by the clustering of communities from Minnesota, Virginia, and New England that belong to three different geographic regions [3]. In this respect, a revision of the specimens used to make the identification appears to be necessary; however, at the present time it was not possible to locate the specimens from these three studies to complete the reexamination.
Areas in the US are experiencing a change in mammalian carnivore dynamics. Some areas: the Southeastern Temperate Plains, the Southeast Coastal Plains, and the Ozark/Ouachita/Appalachian Forest, have been highlighted as optimal for the potential recolonization of cougars (Puma concolor), which is congruent with the increase in cougar confirmed records in the region [32–34]. If cougars do indeed recolonize the Midwest, cougars will serve as an additional compatible definitive host for a large number of parasites, including the feline specialist T. cati. As cougars move east, they will be exposed to the parasites already present in the area, and will also have the potential of dispersing species from their native ranges [6, 27].
Although our study offers a baseline on the helminths present in bobcats in Illinois, more precision is necessary relative to the role of the season in the variations in helminth fauna, if any. This could be influenced by temperature changes, ability for larval development in the environment, and potential seasonal dietary shifts and how that may impact transmission from intermediate hosts [56]. These same factors could be used spatially as environmental variables to compare parasite presence based on specific locality. Correlations between species presence and environmental factors such as precipitation, land cover, or human density could provide further insight into localized infection areas. Geographic Information System (GIS) and related technologies are increasingly used to analyze the geography of disease, specifically the relationships between pathological factors and their geographical environments [14].
Very few of the helminths can be considered specific to felines, including T. rileyi and To. cati. Most of the species are generalists able to infect other carnivores; therefore, bobcats serve as competent reservoirs of agents of epizootic disease that may affect domestic carnivores. In the present study, three of the five species of helminths able to induce zoonotic disease were recorded; these species include P. kellicotti, D. mansonoides, and Toxocara cati. In addition, bobcats in Illinois are infected with another agent of zoonotic disease, D. latum.
Acknowledgments
Funding for this project was provided by the Cooperative Wildlife Research Laboratory and Department of Zoology at Southern Illinois University. The Illinois Department of Transportation (IDOT), Illinois Department of Natural Resources (IDNR) and Dan Woolard assisted in the collection of road killed and trapped bobcats. A special thanks to Sara Ressing and Marie Tosa who also helped capture bobcats.
Cite this article as: Hiestand SJ, Nielsen CK, Jiménez FA: Epizootic and zoonotic helminths of the bobcat (Lynx rufus) in Illinois and a comparison of its helminth component communities across the American Midwest. Parasite, 2014, 21, 4.
References
- 1.Altizer S, Nunn CL, Thrall PH, Gittleman JL, Antonovics J, Cunningham AA, Dobson AP, Ezenwa V, Jones KE, Pedersen AB, Poss M, Pulliam JRC. 2003. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annual Review of Ecology Evolution and Systematics, 34, 517–547 [Google Scholar]
- 2.Anonymous 1996. Digital Data Set of Illinois, CD-ROM, vol. 1, Illinois Department of Natural Resources: Springfield, Illinois [Google Scholar]
- 3.Anonymous 2013. Ecoregions of North America. Environmental Protection Agency 2013, 09/16/2013 [cited 2013 December 20, 2013]. Available from: http://www.epa.gov/wed/pages/ecoregions/na_eco.htm – Downloads [Google Scholar]
- 4.Anonymous 2013. Neglected Parasitic Infections in the United States. Parasites [cited 2013 December 18, 2013; November 22, 2013]. Available from: http://www.cdc.gov/parasites/npi.html
- 5.Bevins SN, Carver S, Boydston EE, Lyren LM, Alldredge M, Logan KA, Riley SP, Fisher RN, Vickers TW, Boyce W, Salman M, Lappin MR, Crooks KR, VandeWoude S. 2012. Three pathogens in sympatric populations of pumas, bobcats, and domestic cats: implications for infectious disease transmission. Plos One, 7(2), e31403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bordes F, Morand S, Kelt DA, Van Vuren DH. 2009. Home range and parasite diversity in mammals. American Naturalist, 173(4), 467–474 [DOI] [PubMed] [Google Scholar]
- 7.Brown EL, Roellig DM, Gompper ME, Monello RJ, Wenning KM, Gabriel MW, Yabsley MJ. 2010. Seroprevalence of Trypanosoma cruzi among eleven potential reservoir species from six states across the southern United States. Vector Borne and Zoonotic Diseases, 10(8), 757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunner JL, Schock DM, Davidson EW, Collins JP. 2004. Intraspecific reservoirs: complex life history and the persistence of a lethal ranavirus. Ecology, 85(2), 560–566 [Google Scholar]
- 9.Bush AO, Lafferty KD, Lotz JM, Shostak AW. 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology, 83(4), 575–583 [PubMed] [Google Scholar]
- 10.Carver S, Scorza AV, Bevins SN, Riley SP, Crooks KR, Vandewoude S, Lappin MR. 2012. Zoonotic parasites of bobcats around human landscapes. Journal of Clinical Microbiology, 50(9), 3080–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandler AC. 1954. New strigeids from Minnesota birds and mammals. American Midland Naturalist, 52, 133–141 [Google Scholar]
- 12.Cohen JE. 2003. Human population: the next half century. Science, 302(5648), 1172–1175 [DOI] [PubMed] [Google Scholar]
- 13.Colwell RK. 2013. EstimateS: Statistical estimation of species richness and shared species from samples. Available from: http://purl.oclc.org/estimates [Google Scholar]
- 14.Cromley EK. 2003. GIS and disease. Annual Review of Public Health, 24, 7–24 [DOI] [PubMed] [Google Scholar]
- 15.Crowe DM. 1972. The presence of annuli in bobcat tooth cementum layers. Journal of Wildlife Management, 36, 1330–1332 [Google Scholar]
- 16.Eberhard ML, Alfano E. 1998. Adult Toxocara cati infections in U.S. children: report of four cases. American Journal of Tropical Medicine and Hygiene, 59(3), 404–406 [DOI] [PubMed] [Google Scholar]
- 17.Erickson AB. 1944. Helminths of Minnesota Canidae in relation to food habits, and a host list and key to the species reported in North America. American Midland Naturalist, 32, 358–372 [Google Scholar]
- 18.Fisher M. 2003. Toxocara cati: an underestimated zoonotic agent. Trends in Parasitology, 19(4), 167–170 [DOI] [PubMed] [Google Scholar]
- 19.Futuyma DJ, Moreno G. 1988. The evolution of ecological specialization. Annual Review of Ecology and Systematics, 19, 207–233 [Google Scholar]
- 20.Gardner SL. 1996. Essential techniques for collection of parasites during surveys of mammals, in Measuring and monitoring biological diversity. Standard methods for mammals, Wilson D, Cole R, Nichols JD, Rudran R, Foster M, Eds Smithsonian Institution Press: Washington DC: p. 291–298 [Google Scholar]
- 21.Gerhold RW, Jessup DA. 2013. Zoonotic diseases associated with free-roaming cats. Zoonoses and Public Health, 60(3), 189–195 [DOI] [PubMed] [Google Scholar]
- 22.Gholami I, Daryani A, Sharif M, Amouei A, Mobedi I. 2011. Seroepidemiological survey of helminthic parasites of stray dogs in Sari City, northern Iran. Pakistan Journal of Biological Sciences, 14(2), 133–137 [DOI] [PubMed] [Google Scholar]
- 23.Hansen K. 2006. Bobcat: Master of Survival. Oxford University Press: New York, NY [Google Scholar]
- 24.Harrison RL. 1998. Bobcats in residential areas: distribution and homeowner attitudes. Southwestern Naturalist, 43(4), 469–475 [Google Scholar]
- 25.Haukisalmi V, Henttonen H, Tenora F. 1987. Parasitism by helminths in the gray-sided vole (Clethrionomys rufocanus) in northern Finland – influence of density, habitat and sex of the host. Journal of Wildlife Diseases, 23(2), 233–241 [DOI] [PubMed] [Google Scholar]
- 26.Heidt GA, Rucker RA, Kennedy ML, Baeyens ME. 1988. Hematology, intestinal parasites, and selected disease antibodies from a population of bobcats (Felis rufus) in Central Arkansas. Journal of Wildlife Diseases, 24(1), 180–183 [DOI] [PubMed] [Google Scholar]
- 27.Hénaux V, Powell LA, Hobson KA, Nielsen CK, LaRue MA. 2011. Tracking large carnivore dispersal using isotopic clues in claws: an application to cougars across the Great Plains. Methods in Ecology and Evolution, 2(5), 489–499 [Google Scholar]
- 28.Hoberg EP. 2002. Taenia tapeworms: their biology, evolution and socioeconomic significance. Microbes and Infection, 4(8), 859–866 [DOI] [PubMed] [Google Scholar]
- 29.Holmes JC, Podesta R. 1968. The helminths of wolves and coyotes from the forested regions of Alberta. Canadian Journal of Zoology-Revue Canadienne De Zoologie, 46(6), 1193–1204 [Google Scholar]
- 30.Kisielewska K. 1970. Ecological organization of helminth groupings in Clethrionomys glareolus (Schreb.) (Rodentia). Structure and seasonal dynamics of helminth groupings in a host population in the Bialowieza National Park. Acta Parasitologica Polonica, 18, 121–147 [Google Scholar]
- 31.Krasnov BR, Poulin R, Shenbrot GI, Mouillot D, Khokhlova IS. 2005. Host specificity and geographic range in haematophagous ectoparasites. Oikos, 108(3), 449–456 [Google Scholar]
- 32.LaRue MA, Nielsen CK. 2008. Modelling potential dispersal corridors for cougars in midwestern North America using least-cost path methods. Ecological Modelling, 212(3–4), 372–381 [Google Scholar]
- 33.LaRue MA, Nielsen CK. 2011. Modelling potential habitat for cougars in midwestern North America. Ecological Modelling, 222(3), 897–900 [Google Scholar]
- 34.LaRue MA, Nielsen CK, Dowling M, Miller K, Wilson B, Shaw H, Anderson CR. 2012. Cougars are recolonizing the midwest: analysis of cougar confirmations during 1990–2008. Journal of Wildlife Management, 76(7), 1364–1369 [Google Scholar]
- 35.Litvaitis JA, Stevens CL, Mautz WW. 1984. Age, sex, and weight of bobcats in relation to winter Diet. Journal of Wildlife Management, 48(2), 632–635 [Google Scholar]
- 36.Loos-Frank B. 2000. An up-date of Verster’s (1969) “Taxonomic revision of the genus Taenia Linnaeus” (Cestoda) in table format. Systematic Parasitology, 45(1), 155–183 [DOI] [PubMed] [Google Scholar]
- 37.Loss SR, Will T, Marra PP. 2013. The impact of free-ranging domestic cats on wildlife of the United States. Nature Communications, 4, 1396. [DOI] [PubMed] [Google Scholar]
- 38.Luman DM, Joselyn M, Suloway L. 1996. Critical trends assessment project: landcover database, in Illinois Natural History Survey, Illinois Natural History Survey C, Illinois, Ed.Illinois Natural History Survey: Champaign, Illinois [Google Scholar]
- 39.Miller GC, Harkema R. 1968. Helminths of some wild mammals in the southeastern United States. Proceedings of the Helminthological Society of Washington, 35(2), 118–125 [Google Scholar]
- 40.Neely RD, Heister CG. 1987. The natural resources of Illinois: introductions and guide, in State of Illinois, Illinois Natural History Survey. Doea NR, Ed.Illinois Natural History Survey: Champaign, Illinois: p. 224 [Google Scholar]
- 41.Nielsen CK, Woolf A. 2001. Spatial organization of bobcats (Lynx rufus) in southern Illinois. American Midland Naturalist, 146(1), 43–52 [Google Scholar]
- 42.Nielsen CK, Woolf A. 2002. Habitat-relative abundance relationship for bobcats in southern Illinois. Wildlife Society Bulletin, 30(1), 222–230 [Google Scholar]
- 43.Nielsen CK, Woolf A. 2002. Survival of unexploited bobcats in southern Illinois. Journal of Wildlife Management, 66(3), 833–838 [Google Scholar]
- 44.Otranto D, Eberhard ML. 2011. Zoonotic helminths affecting the human eye. Parasites & Vectors, 4, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pence DB. 1990. Helminth community of mammalian hosts: concepts at the infracommunity, component community and compound community levels, in Parasite Communities: Patterns and Processes. Esch GW, Busch AO, Aho JM, Eds.Chapman and Hall: New York, NY, p. 233–260 [Google Scholar]
- 46.Pence DB, Eason S. 1980. Comparison of the helminth faunas of two sympatric top carnivores from the rolling plains of Texas. Journal of Parasitology, 66(1), 115–120 [PubMed] [Google Scholar]
- 47.Poulin R. 2003. The decay of similarity with geographical distance in parasite communities of vertebrate hosts. Journal of Biogeography, 30(10), 1609–1615 [Google Scholar]
- 48.Poulin R, Morand S. 1999. Geographical distances and the similarity among parasite communities of conspecific host populations. Parasitology, 119, 369–374 [DOI] [PubMed] [Google Scholar]
- 49.Progulske DR. 1952. The Bobcat and Its Relations to Prey Species in Virginia. Virginia Polytechnich Institute: Virginia, USA [Google Scholar]
- 50.Reichard MV, Caudell DL, Kocan AA. 2004. Survey of helminth lung parasites of bobcats (Lynx rufus) from Alabama, Kansas, New Mexico, Oklahoma, and Virginia, USA. Comparative Parasitology, 71(1), 88–90 [Google Scholar]
- 51.Riley SPD, Foley J, Chomel B. 2004. Exposure to feline and canine pathogens in bobcats and gray foxes in urban and rural zones of a National Park in California. Journal of Wildlife Diseases, 40(1), 11–22 [DOI] [PubMed] [Google Scholar]
- 52.Riser NW. 1956. The hooks of Taenoid cestodes from North American felids. American Midland Naturalist, 56, 133–137 [Google Scholar]
- 53.Roberts NM, Crimmins SM. 2010. Bobcat population status and management in North America: evidence of large-scale population increase. Journal of Fish and Wildlife Management, 1(2), 169–174 [Google Scholar]
- 54.Robertson ID, Irwin PJ, Lymbery AJ, Thompson RCA. 2000. The role of companion animals in the emergence of parasitic zoonoses. International Journal for Parasitology, 30(12–13), 1369–1377 [DOI] [PubMed] [Google Scholar]
- 55.Rollings CT. 1945. Habitats, food and parasites of the bobcat in Minnesota. Journal of Wildlife Management, 9, 131–145 [Google Scholar]
- 56.Saeed I, Maddox-Hyttel C, Monrad J, Kapel CMO. 2006. Helminths of red foxes (Vulpes vulpes) in Denmark. Veterinary Parasitology, 139(1–3), 168–179 [DOI] [PubMed] [Google Scholar]
- 57.Salinas-López N, Jiménez-Guzmán F, Cruz-Reyes A. 1996. Presence of Echinococcus oligarthus (Diesing, 1863) Lühe, 1910 in Lynx rufus texensis Allen, 1895 from San Fernando, Tamaulipas State, in north-east Mexico. International Journal for Parasitology, 26(7), 793–796 [DOI] [PubMed] [Google Scholar]
- 58.Sarmiento L, Stough BD. 1956. Troglostrongylus wilsoni (Stough, 1953) n. comb. (Nematoda: Metastrongylidae) from the lungs of the bobcat, Lynx rufus rufus. Journal of Parasitology, 42(1), 45–48 [PubMed] [Google Scholar]
- 59.SAS Institute Inc 2009. The data analysis for this paper was generated using SAS software. Copyright, SAS Institute Inc. [Google Scholar]
- 60.Schalk G, Forbes MR. 1997. Male biases in parasitism of mammals: effects of study type, host age, and parasite taxon. Oikos, 78(1), 67–74 [Google Scholar]
- 61.Schitoskey EC. 1981. Helminths of South Dakota bobcats. Proceedings from South Dakota Academy of Science, 60, 135–141 [Google Scholar]
- 62.Schwegman JE. 1972. Comprehensive plan for the Illinois Nature Preserves System, Part II, the natural divisions of Illinois in Illinois Nature Preserves Commission, Conservation INPCIFACIASIDo, Ed.Illinois Nature Preserves Commission: Rockford, Illinois, p. 32 [Google Scholar]
- 63.Shabbir MZ, Rabbani M, Yaqub T, Ahmad A, Zia-ur-Rehman, Umair S. 2010. Comparative clinical epidemiology of toxocariosis in dogs and cats. Pakistan Journal of Zoology, 42(2), 129–133 [Google Scholar]
- 64.Shock BC, Murphy SM, Patton LL, Shock PM, Olfenbuttel C, Beringer J, Prange S, Grove DM, Peek M, Butfiloski JW, Hughes DW, Lockhart JM, Bevins SN, VandeWoude S, Crooks KR, Nettles VF, Brown HM, Peterson DS, Yabsley MJ. 2011. Distribution and prevalence of Cytauxzoon felis in bobcats (Lynx rufus), the natural reservoir, and other wild felids in thirteen states. Veterinary Parasitology, 175(3–4), 325–330 [DOI] [PubMed] [Google Scholar]
- 65.Smith KE, Fischer JR, Dubey JP. 1995. Toxoplasmosis in a Bobcat (Felis rufus). Journal of Wildlife Diseases, 31(4), 555–557 [DOI] [PubMed] [Google Scholar]
- 66.Sokal RR, Rohlf FJ. 1995. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd edn W. H. Freeman: New York, NY, p. 887 [Google Scholar]
- 67.Stone JE, Pence DB. 1978. Ecology of helminth parasitism in bobcat from west Texas. Journal of Parasitology, 64(2), 295–302 [PubMed] [Google Scholar]
- 68.Tiekotter KL. 1981. A rapid technique for identification of Taenoid cestodes using unstained scolices. Transactions of the Nebraska Academy of Sciences and Affiliated Societies, 9, 55–56 [PMC free article] [PubMed] [Google Scholar]
- 69.Tiekotter KL. 1985. Helminth species diversity and biology in the bobcat, Lynx rufus (Schreber), from Nebraska. Journal of Parasitology, 71(2), 227–234 [PubMed] [Google Scholar]
- 70.Toweill DE, Anthony RG. 1988. Annual diet of bobcats in Oregon’s cascade range. Northwest Science, 62(3), 99–103 [Google Scholar]
- 71.Tucker SA, Clark WR, Gosselink TE. 2008. Space use and habitat selection by bobcats in the fragmented landscape of south-central Iowa. Journal of Wildlife Management, 72(5), 1114–1124 [Google Scholar]
- 72.van Keulen H, Macechko PT, Wade S, Schaaf S, Wallis PM, Erlandsen SL. 2002. Presence of human Giardia in domestic, farm and wild animals, and environmental samples suggests a zoonotic potential for giardiasis. Veterinary Parasitology, 108(2), 97–107 [DOI] [PubMed] [Google Scholar]
- 73.Watson TG, Nettles VF, Davidson WR. 1981. Endoparasites and selected infectious agents in bobcats (Felis rufus) from West Virginia and Georgia. Journal of Wildlife Diseases, 17(4), 547–554 [DOI] [PubMed] [Google Scholar]
- 74.Woolf A, Nielsen CK, Weber T, Gibbs-Kieninger TJ. 2002. Statewide modeling of bobcat, Lynx rufus, habitat in Illinois, USA. Biological Conservation, 104(2), 191–198 [Google Scholar]