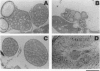
Abstract
Current theories of sexual differentiation maintain that ovarian estrogen prevents masculine development of the copulatory system in birds, whereas estrogen derived from testicular androgens promotes masculine sexual differentiation of neuroanatomy and sexual behavior in mammals. Paradoxically, some data suggest that the neural song system in zebra finches follows the mammalian pattern with estrogenic metabolites of testicular secretions causing masculine development. To test whether the removal of estrogen from males during early development would prevent the development of masculine song systems, zebra finches were treated embryonically with an inhibitor of estrogen synthesis. In addition, this treatment in genetic female zebra finches induced both functional ovarian and testicular tissue to develop, thus allowing the assessment of the direct effects of testicular secretions on song system development. In males, the inhibition of estrogen synthesis before hatching had a small but significant effect in demasculinizing one aspect of the neural song system. In treated females, the song systems remained morphologically feminine. These results suggest that masculinization of the song system is not determined solely by testicular androgens or their estrogenic metabolites.
Full text
PDF
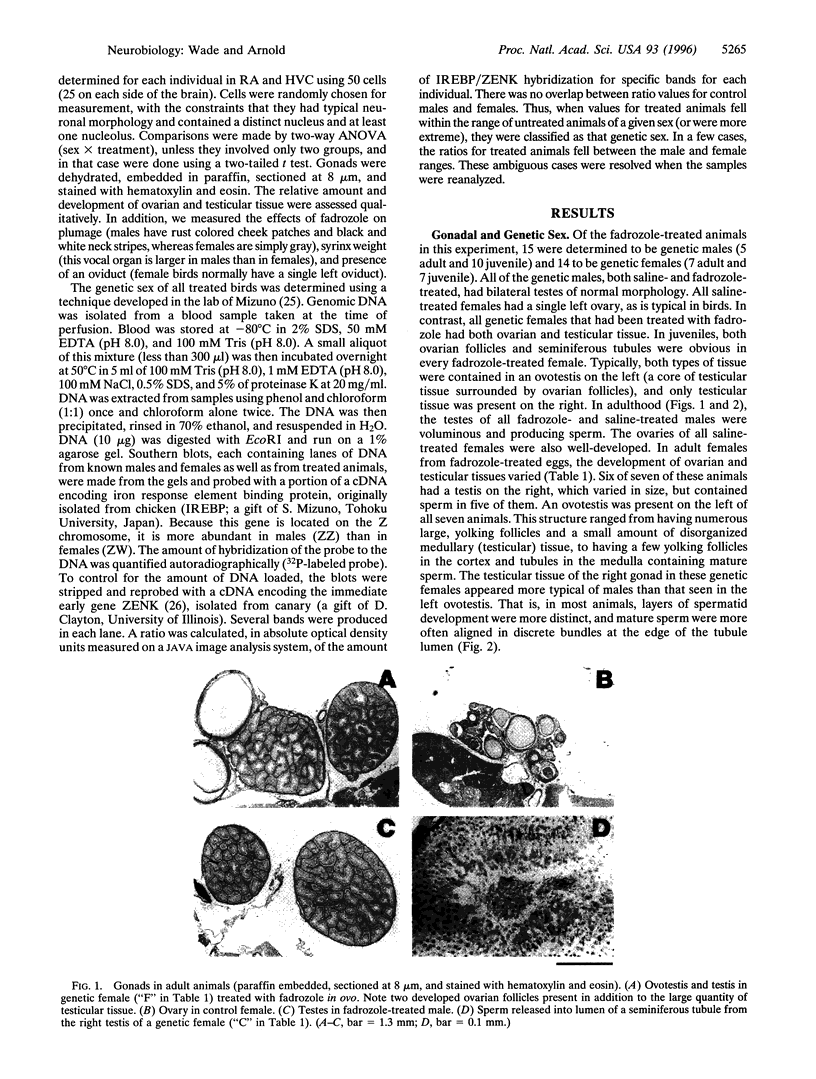
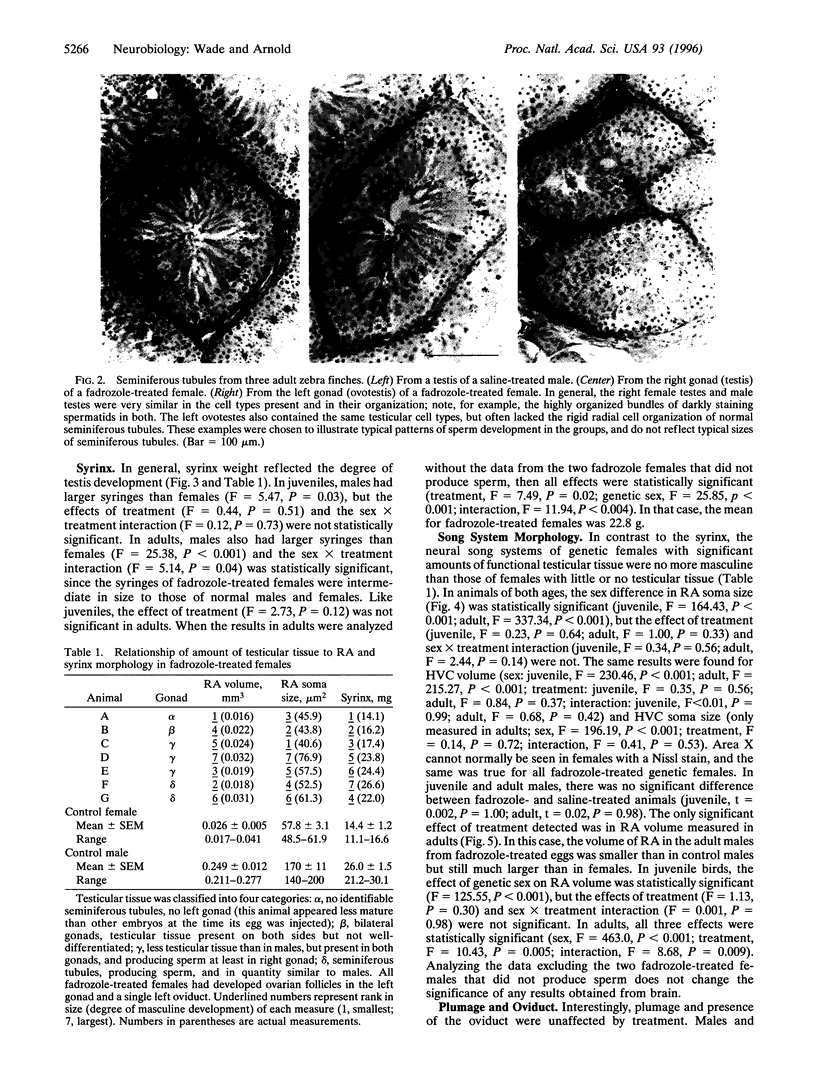
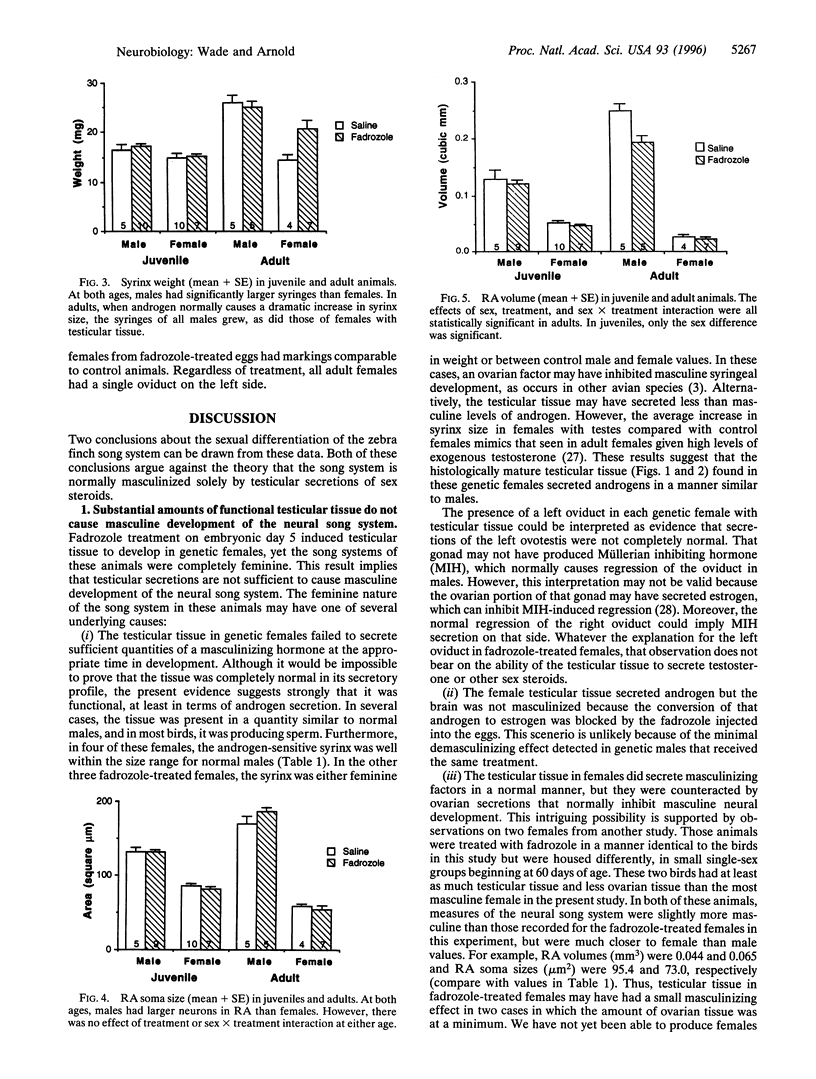

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins-Regan E., Abdelnabi M., Mobarak M., Ottinger M. A. Sex steroid levels in developing and adult male and female zebra finches (Poephila guttata). Gen Comp Endocrinol. 1990 Apr;78(1):93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E., Ascenzi M. Sexual differentiation of behavior in the zebra finch: effect of early gonadectomy or androgen treatment. Horm Behav. 1990 Mar;24(1):114–127. doi: 10.1016/0018-506x(90)90031-r. [DOI] [PubMed] [Google Scholar]
- Arnold A. P. The effects of castration on song development in zebra finches (Poephila guttata). J Exp Zool. 1975 Feb;191(2):261–278. doi: 10.1002/jez.1401910212. [DOI] [PubMed] [Google Scholar]
- Balthazart J., De Clerck A., Foidart A. Behavioral demasculinization of female quail is induced by estrogens: studies with the new aromatase inhibitor, R76713. Horm Behav. 1992 Jun;26(2):179–203. doi: 10.1016/0018-506x(92)90041-s. [DOI] [PubMed] [Google Scholar]
- Elbrecht A., Smith R. G. Aromatase enzyme activity and sex determination in chickens. Science. 1992 Jan 24;255(5043):467–470. doi: 10.1126/science.1734525. [DOI] [PubMed] [Google Scholar]
- Gurney M. E., Konishi M. Hormone-induced sexual differentiation of brain and behavior in zebra finches. Science. 1980 Jun 20;208(4450):1380–1383. doi: 10.1126/science.208.4450.1380. [DOI] [PubMed] [Google Scholar]
- Hutchison J. B., Wingfield J. C., Hutchison R. E. Sex differences in plasma concentrations of steroids during the sensitive period for brain differentiation in the zebra finch. J Endocrinol. 1984 Dec;103(3):363–369. doi: 10.1677/joe.0.1030363. [DOI] [PubMed] [Google Scholar]
- Hutson J. M., Donahoe P. K., MacLaughlin D. T. Steroid modulation of Mullerian duct regression in the chick embryo. Gen Comp Endocrinol. 1985 Jan;57(1):88–102. doi: 10.1016/0016-6480(85)90204-7. [DOI] [PubMed] [Google Scholar]
- Luine V., Nottebohm F., Harding C., McEwen B. S. Androgen affects cholinergic enzymes in syringeal motor neurons and muscle. Brain Res. 1980 Jun 16;192(1):89–107. doi: 10.1016/0006-8993(80)91011-2. [DOI] [PubMed] [Google Scholar]
- Mathews G. A., Arnold A. P. Antiestrogens fail to prevent the masculine ontogeny of the zebra finch song system. Gen Comp Endocrinol. 1990 Oct;80(1):48–58. doi: 10.1016/0016-6480(90)90147-e. [DOI] [PubMed] [Google Scholar]
- Mathews G. A., Arnold A. P. Tamoxifen's effects on the zebra finch song system are estrogenic, not antiestrogenic. J Neurobiol. 1991 Dec;22(9):957–969. doi: 10.1002/neu.480220907. [DOI] [PubMed] [Google Scholar]
- Mello C. V., Vicario D. S., Clayton D. F. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten M. D., Stocker-Buschina S. Fadrozole induces delayed effects on neurons in the zebra finch song system. Brain Res. 1995 Feb 13;671(2):317–320. doi: 10.1016/0006-8993(94)01370-w. [DOI] [PubMed] [Google Scholar]
- Nottebohm F., Arnold A. P. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976 Oct 8;194(4261):211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Saitoh Y., Ogawa A., Hori T., Kunita R., Mizuno S. Identification and localization of two genes on the chicken Z chromosome: implication of evolutionary conservation of the Z chromosome among avian species. Chromosome Res. 1993 Nov;1(4):239–251. doi: 10.1007/BF00710129. [DOI] [PubMed] [Google Scholar]
- Schlinger B. A., Arnold A. P. Brain is the major site of estrogen synthesis in a male songbird. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4191–4194. doi: 10.1073/pnas.88.10.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger B. A., Arnold A. P. Plasma sex steroids and tissue aromatization in hatchling zebra finches: implications for the sexual differentiation of singing behavior. Endocrinology. 1992 Jan;130(1):289–299. doi: 10.1210/endo.130.1.1727704. [DOI] [PubMed] [Google Scholar]
- Shen P., Schlinger B. A., Campagnoni A. T., Arnold A. P. An atlas of aromatase mRNA expression in the zebra finch brain. J Comp Neurol. 1995 Sep 11;360(1):172–184. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand C. D., Miranda R. C., Bentham W. D., Sohrabji F., Brown T. J., Hochberg R. B., MacLusky N. J. Estrogen receptors colocalize with low-affinity nerve growth factor receptors in cholinergic neurons of the basal forebrain. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4668–4672. doi: 10.1073/pnas.89.10.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vockel A., Pröve E., Balthazart J. Sex- and age-related differences in the activity of testosterone-metabolizing enzymes in microdissected nuclei of the zebra finch brain. Brain Res. 1990 Mar 19;511(2):291–302. doi: 10.1016/0006-8993(90)90174-a. [DOI] [PubMed] [Google Scholar]
- Wade J., Arnold A. P. Post-hatching inhibition of aromatase activity does not alter sexual differentiation of the zebra finch song system. Brain Res. 1994 Mar 14;639(2):347–350. doi: 10.1016/0006-8993(94)91752-3. [DOI] [PubMed] [Google Scholar]
- Wade J., Schlinger B. A., Arnold A. P. Aromatase and 5 beta-reductase activity in cultures of developing zebra finch brain: an investigation of sex and regional differences. J Neurobiol. 1995 Jun;27(2):240–251. doi: 10.1002/neu.480270210. [DOI] [PubMed] [Google Scholar]
- Wade J., Schlinger B. A., Hodges L., Arnold A. P. Fadrozole: a potent and specific inhibitor of aromatase in the zebra finch brain. Gen Comp Endocrinol. 1994 Apr;94(1):53–61. doi: 10.1006/gcen.1994.1059. [DOI] [PubMed] [Google Scholar]