Summary
Glycoproteins are an important class of biomolecules involved in a number of biological recognition processes. However, natural and recombinant glycoproteins are usually produced as mixtures of glycoforms that differ in the structures of the pendent glycans, which are difficult to separate in pure glycoforms. As a result, synthetic homogeneous glycopeptides and glycoproteins have become indispensable probes for detailed structural and functional studies. A number of elegant chemical and biological strategies have been developed for synthetic construction of tailor-made, full-size glycoproteins to address specific biological problems. In this review, we highlight recent advances in chemical and chemoenzymatic synthesis of homogeneous glycoproteins. Selected examples are given to demonstrate the applications of tailor-made, glycan-defined glycoproteins for deciphering glycosylation functions.
Keywords: glycoprotein, glycopeptide, native chemical ligation, enzymatic ligation, chemical synthesis, chemoenzymatic synthesis, transglycosylation
Introduction
It is well documented that protein glycosylation, as one of the most prevalent posttranslational modifications (PTMs), can profoundly affect a protein’s intrinsic properties such as folding, stability, and intracellular trafficking (Helenius and Aebi, 2001; Petrescu et al., 2006). Moreover, the glycans attached can directly participate in a wide variety of biological recognition processes: cell adhesion, signaling, development, host-pathogen interaction and immune response, to name a few (Dube and Bertozzi, 2005; Dwek, 1996; Haltiwanger and Lowe, 2004; Hart and Copeland, 2010; Jefferis, 2009; Nimmerjahn and Ravetch, 2008b; Varki, 1993). In contrast to other posttranslational modifications that involve only a simple functional group transfer such as protein phosphorylation and methylation, protein glycosylation in general is much more diverse and complex. More than 40 different types of sugar-amino acid linkages have been characterized, involving at least 13 different proximal monosaccharides and 8 different amino acid residues (Spiro, 2002). In fact, each single linkage type can represent a unique posttranslational modification (PTM) with defined functional consequence. As a result, protein glycosylation should not be collectively perceived simply as “another” PTM given the huge diversity in both the types of modification and functions.
There are two major types of glycoproteins, namely N-linked and O-linked glycoproteins. In N-glycoproteins, the glycan is attached to the amide side chain of an asparagine residue in a consensus (Asn-Xaa-Ser/Thr) sequence; in a common O-glycoprotein, the sugar is linked to the hydroxy group of a Ser or Thr residue. While common N- and O-glycans frequently appear and function at cell surface, a special O-glycosylation, the attachment of a simple monosaccharide (N-acetylglucosamine, GlcNAc) appears as a common PTM of nuclear and cytoplasmic proteins, which plays important roles in a number of physiological processes and disease states (Hart et al., 2011; Zachara and Hart, 2002). Except for O-GlcNAc glycosylation that involves the transfer of only a monosaccharide, the biosynthesis of typical N- and O-glycoproteins often involves multiple steps under the actions of a large panel of enzymes (glycosyltransferases, glycosidases, and other carbohydrate modifying enzymes) (Figure 1). For example, N-glycoprotein biosynthesis starts with the assembly of a dolichol-linked oligosaccharide precursor, Glc3Man9GlcNAc2 in the ER, and its subsequent transfer by a multi-subunit oligosaccharyltransferase (OST) to the amide side chain of an asparagine (Asn) in a consensus sequence Asn-X-Ser/Thr of a nascent polypeptide. The oligosaccharide precursor is then processed to the mono-glucosylated glycoform (Glc1Man9GlcNAc2), which is the key intermediate for protein folding in protein quality control mediated by the calnexin/calreticulin chaperone. Once correctly folded, the precursor is trimmed further to Man8GlcNAc2-protein, which is then translocated to Golgi apparatus for further trimming and processing to give different high-mannose and complex type glycoforms (Figure 1a). In contrast to N-glycosylation that begins with the assembly of a large precursor oligosaccharide, the common O-glycosylation of proteins occurs in a stepwise fashion in the Golgi apparatus, starting with the attachment of the first sugar, N-acetylgalactosamine (GalNAc) to the hydroxy group of Ser or Thr residue in an α-glycosidic linkage under the catalysis of a polypeptide:GalNAc transferase (ppGalNAcT). Further extension of the sugar chain is achieved by additions of sugar residues one-by-one under respective glycosyltransferase to form various O-glycan core structures (Figure 1b). These unique biosynthetic pathways result in the generation of a large number of diverse and complex glycoprotein glycoforms, which share the same polypeptide backbone but differ in the structures of the pendent glycans. The huge structural diversity lays the molecular basis for diverse biological roles and functions. However, the structural heterogeneity in glycosylation also poses tremendous challenges in elucidating the biological functions of glycoproteins. Unfortunately controlling glycosylation to a well-defined glycoform during protein expression is still a formidable task to achieve, and current chromatographic techniques are unable to isolate different glycoprotein glycoforms on a practical scale. The urgent need of structurally well-defined homogeneous glycoproteins for functional studies and biomedical applications has stimulated a great interest in developing chemical and biochemical methods for making full-size homogeneous glycoproteins. Many elegant synthetic strategies have been explored and have been discussed in a number of excellent reviews, which include total chemical synthesis (Buskas et al., 2006; Gaidzik et al., 2013; Gamblin et al., 2009; Grogan et al., 2002; Guo and Shao, 2005; Pratt and Bertozzi, 2005; Unverzagt and Kajihara, 2013; Yuan et al., 2010), chemoselective ligation (Chalker et al., 2011; Hang and Bertozzi, 2001), chemoenzymatic synthesis (Rich and Withers, 2009; Schmaltz et al., 2011; Wang, 2008; Wang and Lomino, 2012), and glycosylation engineering of glycan biosynthetic pathways (Castilho and Steinkellner, 2012; Hamilton and Gerngross, 2007; Jacobs et al., 2009; Stanley, 1992; Wildt and Gerngross, 2005). Indeed, concurrent with the sophistication of synthetic methodology, chemists are now ready to take the challenges of making those extremely large and most complex biomolecules like full-size glycoproteins in flask (Lowary, 2013; Wang and Davis, 2013). The present review intends to focus on recent advances in chemical and chemoenzymatic synthesis of homogeneous glycoproteins. Examples are selected to showcase the applications of tailor-made, glycan-defined glycoproteins for deciphering the roles and functions of protein glycosylation.
Figure 1. Biosynthetic pathways for glycoproteins.
(a) the biosynthesis of typical N-linked glycoproteins; (b) the biosynthesis of mucin type O-linked glycoproteins
Major approaches for glycoprotein synthesis
Glycoproteins are the conjugates of sugars and proteins. From the chemistry point of view, glycoproteins are a class of natural products that belong to the most challenging synthetic targets due to the very large size (typically over 15 KDa), the complex branching structures of the oligosaccharide components, the heterogeneity of glycosylation, and the multi-functionality from both the oligosaccharide and polypeptide portions. The conventional solid-phase peptide synthesis (SPPS) protocol has been frequently applied for making small to middle-size N- and O-glycopeptides (Hojo and Nakahara, 2007; Seitz, 2000). The oligosaccharide moiety is introduced either by using glycosylated amino acids as building blocks during SPPS or by convergent coupling between a glycosylamine and a free aspartic acid residue in a protected polypeptide. However, extension of this approach to larger glycopeptides has been problematic, due to the low efficiency of couplings, poor solubility of protected polypeptides, and the susceptibility of the O-glycosidic bonds to acidic hydrolysis under strong acidic conditions (e.g., TFA or HF treatment) required for final global peptide de-protections. To address these issues, several important synthetic strategies have been explored. These strategies, including native chemical ligation, chemoenzymatic transformation, “tag and modify” method, and direct enzymatic glycosylation (Figure 2), have now made it possible to assemble free polypeptide/glycopeptide and oligosaccharide fragments together in a well-controlled manner to provide full-size homogeneous glycoproteins. It should be noted that, as an essential component of glycoproteins, the synthesis of the N- and O-glycans alone can be very tedious, complex, and challenging by itself, which often requires extensive protecting group manipulations and careful selections of glycosylation methods in order to achieve regio- and stereo-selectivity in each step of glycosidic bond formation. Fortunately, exciting progresses have been made in this area, including the development of convergent chemical and chemoenzymatic methods for synthesizing highly branched N-glycans (Eller et al., 2007; Koizumi et al., 2013; Shivatare et al., 2013; Walczak and Danishefsky, 2012; Wang et al., 2013b), the development of one-pot multiple enzyme systems for constructing complex oligosaccharides (Muthana et al., 2009), and the invention of the pre-activation one-pot tandem glycosylation strategy (Huang et al., 2004; Sun et al., 2008). In addition, some complex N-glycans can be prepared from natural sources in pure forms by isolation and purification. For example, the bi-antennary complex type N-glycan can be readily isolated from chicken egg yolks (Kajihara et al., 2004; Seko et al., 1997) and high-mannose N-glycans including Man9GlcNAc2 can be prepared from soybean flour by fractional precipitation, enzymatic digestion of the crude soybean agglutinin glycoprotein fraction, and chromatographic purification of the released glycans (Wang et al., 2004). It was also demonstrated that Man9GlcNAc2 glycan could be also obtained from chicken egg yolks by pronase digestion and chromatographic separation (Makimura et al., 2012). The accessibility to these well-defined glycoprotein glycans has lifted a major roadblock toward in vitro synthetic construction of homogeneous glycoproteins.
Figure 2.
Major strategies for synthesis of homogeneous glycoproteins
Total chemical synthesis of glycoproteins via native chemical ligation
The invention of native chemical ligation (NCL) for total chemical synthesis of proteins has revolutionized protein chemistry (Dawson and Kent, 2000; Dawson et al., 1994). Similarly, the application of NCL and its modified versions for ligation of peptides and glycopeptides has enabled the synthesis of large homogeneous glycoproteins for deciphering functions (Figure 2a). The original version of NCL relies on chemoselective reaction between two partners, a peptide acid thioester and an N-terminal cysteine residue of another peptide fragment, to form a native peptide amide linkage. This ligation involves a reversible transthioesterification between the thioester and the N-terminal cysteine residue to generate a transient thioester intermediate, which then undergoes a rapid, irreversible intramolecular S → N acyl transfer to form a native peptide bond at the junction without the need of side-chain protections on other amino acids including those internal Cys residues. Bertozzi and co-workers reported the first NCL-based total chemical synthesis of an 82-amino acid glycoprotein carrying two O-GalNAc residues, a glycosylated form of the antimicrobial protein diptericin (Shin et al., 1999). Since the primary sequence of diptericin did not contain Cys residues, a G25C mutation was introduced to enable a retrosynthetic disconnection at the site. Notably the authors devised an alkanesulfonamide “safety-catch” linker that enables the synthesis of glycopeptide thioester via the Fmoc-based SPPS protocol. Unverzagt and co-workers reported the first synthesis of a glycopeptide thioester carrying a complex type N-glycan using the “safety-catch” linker strategy and applied it for NCL to form larger N-glycopeptides (Mezzato et al., 2005). The first total chemical synthesis of a full-size glycoprotein carrying a complex type N-glycan, a glycoform of the 76-amino acid chemokine monocyte chemotactic protein-3 (MCP-3), was achieved by Kajihara and co-workers, using two consecutive native chemical ligations of three peptide/glycopeptide fragments followed by folding to provide the native glycoprotein (Yamamoto et al., 2008).
To circumvent the limitations of reliance on Cys residues for ligation – many primary sequences may not have Cys residues at strategically useful positions for NCL, various auxiliary-based strategies have been developed to mimic the cysteine-based NCL (Payne and Wong, 2010). Notably, an array of thiolated amino acids as latent residues for Ala, Gln, Leu, Lys, Phe, Pro, Val and Thr has been devised and tested. After ligation, the thiol group is selectively removed by radical desulfurization to restore the respective native amino acid residues in the sequence. This development has provided the flexibility to retrosynthetically disconnect the glycoprotein sequence at appropriate junctions and significantly expanded the scope of NCL for protein and glycoprotein synthesis (Payne and Wong, 2010; Unverzagt and Kajihara, 2013). The recent success in the chemical synthesis of glycoprotein hormone α- and β-subunits (Aussedat et al., 2012; Nagorny et al., 2012), glycosylated human interferon-β (Sakamoto et al., 2012), and full-size erythropoietin (Murakami et al., 2012; Wang et al., 2012) showcases the power of the ligation methods for total glycoprotein synthesis. Moreover, expressed protein ligation (EPL) has been successfully explored for glycoprotein synthesis, in which a large intact protein thiosester or an N-terminal Cys-containing protein domain is recombinantly expressed and used as ligation partners (Muir, 2003; Muir et al., 1998; Payne and Wong, 2010; Schwarzer and Cole, 2005). A very impressive early example, reported by Bertozzi and co-workers, is the synthesis of GlyCAM-1, a heavily glycosylated glycoprotein involved in leukocyte homing (Macmillan and Bertozzi, 2004). The authors designed an elegant three-piece ligation scheme, in which the heavily glycosylated N- and C-terminal glycopeptide domains were prepared by chemical synthesis and the internal non-glycosylated protein domain was expressed in E. coli. Two consecutive ligations provided the full-length glycoprotein carrying multiple GalNAc residues. More recently, EPL has been successfully applied for the synthesis of full-length ribonuclease C (Piontek et al., 2009a; Piontek et al., 2009b) and a glycoform of full-length erythropoietin carrying two complex type N-glycans (Hirano et al., 2009). The combination of EPL and NCL has further overcome the size limitation in traditional NCL.
Chemoenzymatic glycosylation remodeling of glycoproteins
Glycosylation remodeling of heterogeneous glycoproteins, either from natural or from recombinant source, is emerging as a very promising approach toward homogeneous glycoprotein glycoforms (Wang and Lomino, 2012). This approach has been particularly useful for N-glycoproteins and it involves two key manipulations: enzymatic trimming to remove the heterogeneous N-glycans leaving only the innermost GlcNAc residue at the glycosylation site and sugar chain elongation by glycosyltransferases or endoglycosidases (Figure 2b). As a classic example, Wong and co-workers successfully converted ribonuclease B (RNase B), a glycoprotein carrying heterogeneous high-mannose N-glycans at Asn-34, to a single homogeneous glycoform carrying a sialyl Lewis X tetrasaccharide moiety (Witte et al., 1997). After deglycosylation of RNase B by Endo-H, the sialyl Lewis X moiety was built up at the Asn-linked GlcNAc in the protein by stepwise additions of galactose, sialic acid, and fucose using the respective glycosyltransferases. In contrast to the stepwise addition of monosaccharides by glycosyltrasferases, a more appealing convergent remodeling approach is the single-step transfer of a pre-assembled intact N-glycan en bloc to the GlcNAc-protein via the endoglycoosidase-catalyzed transglycosylation (Wang, 2008, 2011). This was enabled by two major developments: the exploitation of synthetic sugar oxazolines (the transition-state mimics) as donor substrates for transglycosylation (Fujita et al., 2001; Li et al., 2005a; Rising et al., 2006) and the discovery of glycosynthases (novel glycosidase mutants) that are devoid of product hydrolysis activity but are capable of taking the highly active sugar oxazolines as substrates for transglycosylation (Huang et al., 2009b; Umekawa et al., 2008). Several bacterial and fungus endoglycosidases, including Endo-A, Endo-M, Endo-D, Endo-S, and Endo-F3, which possess distinct glycan and acceptor substrate specificity, have been converted into glycosynthases for synthetic applications (Wang and Lomino, 2012). These enzymes have been used for the synthesis of large and complex glycopeptides including HIV-1 glycopeptide antigens (Amin et al., 2013; Huang et al., 2009a; Huang et al., 2010b; Li et al., 2005a; Li et al., 2005b); for glycosylation remodeling to produce homogeneous glycoproteins carrying natural and selectively modified (e.g., azide-tagged or fluorinated) N-glycans (Amin et al., 2011; Huang et al., 2009b; Huang et al., 2010a; Li et al., 2006; Ochiai et al., 2008; Orwenyo et al., 2013; Schwarz et al., 2010; Umekawa et al., 2010); and for glycoengineering of intact IgG antibodies (Fan et al., 2012; Huang et al., 2012; Wei et al., 2008; Zou et al., 2011). This chemoenzymatic method was also successfully combined with NCL for synthesizing full-size glycosylated proteins (Asahina et al., 2013; Hojo et al., 2012). A notable feature of this chemoenzymatic approach is its convergence and its ability to reconstitute the N-glycans maintaining the conserved native N-glycan core, which opens an exciting new avenue to well-defined glycopeptides and glycoproteins for deciphering biological functions (vide infra).
Chemoselective ligation between tagged protein and glycan
Chemoselective glycosylation of recombinant proteins via the so-called “tag and modify” strategy (Chalker et al., 2011) provides another convergent approach to homogeneous glycoproteins. In this approach, specific tags are introduced at pre-determined glycosylation sites by site-directed mutagenesis. After expression, the tagged protein is conjugated with a functionalized glycan at the tagged site via bio-orthogonal chemo-selective ligation (Figure 2c). Among the natural amino acid residues, cysteine (Cys) is particularly useful – it can be readily introduced into a protein by site-directed mutagenesis and the free Cys residue can be selectively reacted with a range of cysteine-reactive functional groups for site-specific modification including glycosylation (Chalker et al., 2011). Early examples using the Cys-tag method include site-specific glycosylation of EPO (Hirano et al., 2009; Macmillan et al., 2001), site-specific introduction of a glycan at the conserved Fc glycosylation site to probe the glycosylation effects on antibody’s effector functions (Watt et al., 2003), and synthesis of novel anti-bacterial glycodendriproteins (Rendle et al., 2004). In addition to Cys residue, a series of functionalized unnatural amino acid residues such as those containing bioorthogonally reactive tags (azide-, aldehyde-, ketone, alkyne, alkene, etc.) can be now introduced into proteins via novel genetic manipulations of the expression system. For example, azido-homoalanine (Aha) and homopropargylglycine (Hpg) can be incorporated into proteins by employing a Met (−) auxotrophic E. coli strain to express the target protein using the corresponding unnatural amino acids to replace methionine (Fernández-González et al., 2010; Wang et al., 2008; Wiltschi et al., 2008); aldehyde tag can be selectively introduced by inserting a CXPXR motif at the site followed by in situ oxidation of the Cys residue in this sequence to a formylglycine group by a co-expressed formylglycine generating enzyme (Carrico et al., 2007; Wu et al., 2009). This approach was successfully applied to site-specific glycosylation of a human growth hormone via oxime formation with an aminooxy-functionalized glycan (Hudak et al., 2011). As a remarkable example showing the usefulness of the “tag and modify” technology, Davis and co-workers reported the design and synthesis of a synthetic glycoprotein to functionally mimic the P-selectin glycoprotein ligand-1 (PSGL-1) (van Kasteren et al., 2007). Based on the fact that two posttranslational modifications, a sulfate group at Tyr-48 and a sialylated glycan at the Ser-57 of PSGL-1, are essential for the binding of PSGL-1 to P-selectin in the inflammatory response, the authors sought to use the LacZ-type reporter enzyme (a β-galactosidase) as the scaffold protein and to introduce a sulfotyrosine at position 439 and a sialyl Lewis X oligosaccharide at position 43 to mimic the global presentation of the two functional groups in PSGL-1. This was achieved by introduction of two distinct and orthogonal tags (azide and cysteine) in the scaffold followed by two chemoselective ligations to incorporate the respective functional groups. It was further demonstrated that the synthetic glycoprotein, with the sulfotyrosine and the sialyl Lewis X moiety being installed at the right sites, could efficiently bind to P-selectin while the scaffold protein still maintained the enzymatic activity. This property was successfully used to detect in vivo inflammatory brain lesions by its specific recognition of P-selectin and subsequent enzymatic reactions for X-Gal tissue staining. In general, the “tag and modify” technology is quite flexible and allows a quick access to various homogeneously glycosylated proteins for functional studies. The only major drawback of this approach is the introduction of unnatural sugar-amino acid linkage in the glycoprotein, which might not perfectly mimic the natural counterparts. In addition, the unnatural linkages might be immunogenic if used in humans.
Direct enzymatic glycosylation of polypeptides and proteins
In N-glycoprotein biosynthesis, a key step is the enzymatic transfer of a large oligosaccharide from a dolichol-phosphate glycolipid to the Asn in a consensus NXS/T sequon of the nascent protein by the oligosaccharyl transferases (OST) (Helenius and Aebi, 2004). Naturally, it would be highly valuable if OST can be employed for in vitro protein glycosylation (Figure 2d). While it has been shown that OST does glycosylate polypeptides (Dempski and Imperiali, 2002; Imperiali and Hendrickson, 1995; Xu and Coward, 1997), a practical application of the OST for in vitro glycoprotein synthesis remains to be fulfilled. Major hurdles include the instability of the enzyme, the huge complexity of the membrane-bound enzyme that involves up to nine protein subunits as a complex for activity, and the inability of the enzyme to glycosylate folded proteins. In contrast, PglB, a single-subunit oligosaccharyl transferase that was responsible for protein N-glycosylation in Campylobacter Jejuni was shown to be able to glycosylate polypeptide (Chen et al., 2007; Glover et al., 2005; Kowarik et al., 2006; Li et al., 2010; Weerapana and Imperiali, 2006). In addition, PglB has very relaxed substrate specificity on the extended oligosaccharide structures and is capable of transferring even large bacterial polysaccharides to a protein to form linkage-defined polysaccharide-protein conjugates, which provides a very promising approach to making bacterial glycoconjugate vaccines (Feldman et al., 2005; Terra et al., 2012). Nevertheless, PglB requires an extended N-glycosylation sequence (D/E-X-N-X-S/T) for recognition and it can transfer oligosaccharides to the extended sequence only when it is located in a flexible region, as the enzyme has no or only marginal activity on folded protein (Kowarik et al., 2006). In addition, PglB cannot efficiently transfer mammalian N-glycan from the corresponding glycolipid (Chen et al., 2007), and demonstrates only a marginal activity to recognize the mammalian N-glycan core for glycosylation (<1% yield) in an engineered E. coli system (Valderrama-Rincon et al., 2012). Rational protein engineering on the basis of the crystal structure (Lizak et al., 2011) and directed evolution may help broaden the substrate specificity of PglB. Recently, a special cytoplasmic N-glycosyltransferase (NGT) from a pathogenic bacterium was characterized (Schwarz et al., 2011). This NGT is able to transfer a glucose monosaccharide at the NXS/T consensus sequence in a polypeptide, but uses nucleotide-activated monosaccharide (UDP-Glc) instead of glycolipids as the donor substrate. Wang and co-workers successfully combined the NGT glycosylation with endoglycosidase-catalyzed transglycosylation to directly glycosylate polypeptides with complex glycans (Lomino et al., 2013). On the other hand, the polypeptide:N-acetylgalactosamine transferases (ppGalNAcTs) has been efficiently used to glycosylate polypeptides to make O-glycopeptides. A notable example is the efficient chemoenzymatic synthesis of the MUC1 tandem glycopeptide repeats for cancer vaccines (Sorensen et al., 2006) (vide infra). Direct enzymatic glycosylation of recombinant proteins in vitro could become a very attractive and promising approach to making homogeneous glycoproteins when more efficient enzymes with relaxed substrate specificity are available.
Total chemical synthesis of erythropoietin (EPO)
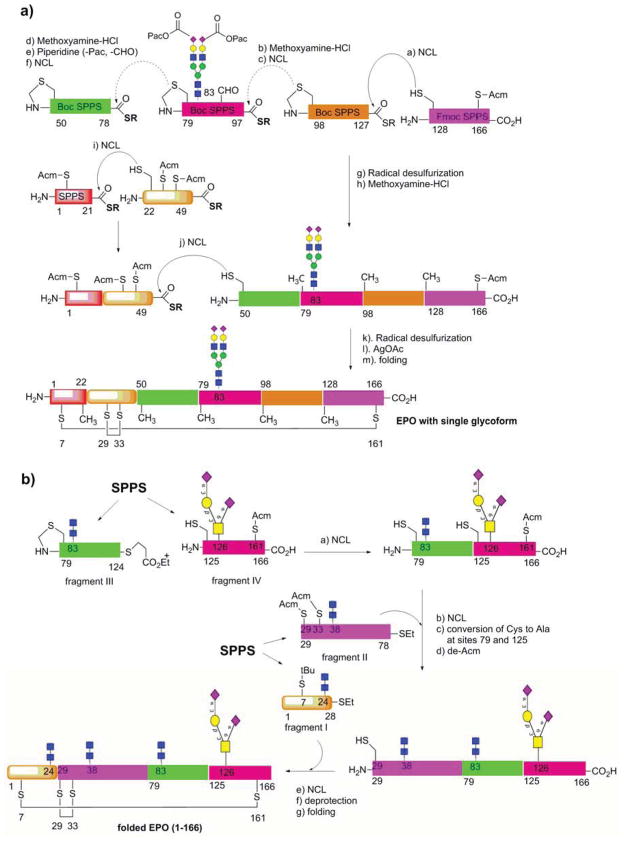
Erythropoietin (EPO) is a therapeutic glycoprotein that boosts the production of red blood cells and is widely used for the treatment of anemia after cancer chemotherapy. Natural EPO is a glycoprotein that consists of 166 amino acid residues carrying a conserved O-glycan at Ser-126 and three N-glycans at the Asn-24, Asn-38, and Asn-83 conserved N-glycosylation sites, respectively. It is well established that appropriate glycosylation, particularly the terminal sialylation of the N-glycans, is critically important for the serum’s half-life and thus in vivo biological activity of EPO. The relatively large size and complex glycosylation pattern of EPO make it an attractive and challenging synthetic target for chemists to test new synthetic strategies (Wilson et al., 2013). Kajihara and co-workers recently reported a total chemical synthesis of a full-length EPO glycoform that carries a sialylated bi-antennary complex N-glycan at the Asn-83 site (Murakami et al., 2012). Instead of using the existing 4 Cys residues for NCL, which are unfavorably positioned close to the N or C-terminus of EPO, the authors decided to disconnect the EPO sequence at 5 appropriate Ala sites to provide 5 peptide fragments and 1 glycopeptide (aa 79–97) fragment, which can be readily synthesized by SPPS (Figure 3a). In this strategy, the identified N-terminal Ala residues were replaced with free Cys or thiazolidine-protected Cys in the fragments to facilitate NCL, and the 4 native Cys residues were “permanently” masked via Acm-protection during ligations. To make the glycopeptide fragment (aa 79–97) via Boc-chemistry, the sialic acid residues were protected as phenacyl esters, which is stable during acid treatment and thiolysis required to release the corresponding glycopeptide thioester. The full-length polypeptide was constituted through tandem ligations of the fragments, and the Cys residues generated after NCL were readily converted into the native Ala residues via radical desulfurification. Finally deprotection of the Acm-Cys and oxidative refolding provided a synthetic version of EPO that contains two mutations (E21A and Q78A to facilitate ligation) and carries a sialylated bi-antennary N-glycan at Asn-83 (Figure 3a). Because the site, number, and sialylation of glycans attached are closely correlated to EPO’s in vivo hematopoietic activity (Higuchi et al., 1992), this synthetic approach should be quite flexible for producing selectively glycosylated EPO glycoforms to study the effects of individual N-glycans or their combinations on the in vivo biological activity of EPO.
Figure 3. Total chemical synthesis of glycoprotein erythropoietin (EPO).
(a) chemical synthesis of an EPO glycoform carrying a single N-glycan; (b) chemical synthesis of a full-length native EPO glycoform carrying glycans at all the four conserved glycosylation sites.
In another study, Danishefsky and co-workers described a total chemical synthesis of a full-length EPO with completely native amino acid sequence that carries an O-glycan at Ser-126 and three truncated N-glycans (the chitobiose cores) at the Asn-24, Asn-38, and Asn-83 conserved N-glycosylation sites, respectively (Wang et al., 2012). This remarkable total synthesis is on top of a series of previous painstaking studies from the Danishefsky laboratory, which have addressed a number of challenging problems on the road toward the total synthesis (Wilson et al., 2013). In the retrosynthetic design, the authors disconnected EPO into four glycopeptide fragments (I–IV), which allows iterative alanine- and cysteine-based ligations to reconstitute the full-length EPO (Figure 3b). In this strategy, the Ala-79 in fragment III and the Ala-125 in fragment IV were temporarily replaced with Cys residue to facilitate NCL. The N-glycopeptides were synthesized by a convergent coupling between a chitobiosyl amine and the respective protected polypeptide carrying a free Asp at the glycosylation sites. The sialylated O-glycopeptide (IV) was prepared using a glycosylated Ser as the building block in SPPS. Native ligation between III and IV gave the polypeptide (79–166), which was then ligated with fragment II. The resulting ligation product was subjected to global metal free radical de-sulfurization to convert the free Cys residues to the native Ala residues to provide polypeptide (29–166) after deprotection of the Acm-protected native Cys residues. Finally, native chemical ligation between the polypeptide (29–166) and glycopeptide thioester (I), with in situ deprotection of the Acm-protected Cys residues, gave a full-length, glycosylated EPO after correct folding. More recently, an ultimate total chemical synthesis of full-length native EPO carrying full-size natural glycans at all the four conserved glycosylation sites (three sialylated complex type N-glycans and one O-glycan) was accomplished by Danishefsky and co-workers (Wang et al., 2013a). To achieve this remarkable synthesis, the authors had to redesign the synthetic route by moving the retrosynthetic junction sites away from the conserved N-glycan sites, as the presence of the bulky complex type N-glycans adjacent to the ligation sites prevents efficient native chemical ligation. The synthetic version of glycosylated EPO showed comparable erythropoietic activity as the recombinant EPO in both a cell proliferation assay and in mice, while the non-glycosylated version of EPO turned to aggregate and showed much lower erythropoietic activity (Wang et al., 2012; Wang et al., 2013a). These results clearly demonstrate the importance of appropriate glycosylation for the stability and biological activity of erythropoietin. The total chemical synthesis of full-length native EPO carrying all full-size glycans represents a milestone achievement in chemical synthesis of complex natural products.
Synthesis of glycan-defined glycoprotein probes for deciphering the molecular mechanism of lectin-mediated protein quality control
It is well known that N-glycosylation plays an important role in the lectin-mediated protein quality control (Aebi et al., 2010; Helenius and Aebi, 2001; Spiro, 2000). In this process, the lectin-like molecular chaperones, calnexin (CNX) and calreticulin (CRT), recognize the monoglucosylated N-glycan in a protein and recruit other molecular chaperones such as protein disulfide isomerase-like protein ERp57 to form a complex for appropriate folding. Once the last Glc residue is removed by α-glucosidase II (G-II), the resulting Man9GlcNAc2-protein will be released from the CNX/CRT folding cycle. At this point, an ER-residing UDP-glucose:glycoprotein glucosyltransferase (UGGT) can serve as a “folding sensor” to detect and re-glucosylate the mis-folded protein to generate the mono-glucosylated glycoform, which enters into the CNX/CRT cycle again for refolding (Figure 2). Despite tremendous efforts in this field, the molecular mechanism of the protein quality control process is still not fully understood. A major obstacle is the difficulty to obtain homogeneous, structurally well-defined glucosylated N-glycoproteins for detailed mechanistic and functional studies (Zapun et al., 1997). To address the issues, Ito and co-workers have synthesized a number of monoglucosylated N-glycans and their derivatives, and used them to probe molecular recognitions mediated by the lectin-like chaperones and UGGT (Ito et al., 2005; Iwamoto et al., 2013; Takeda et al., 2009). Despite these impressive contributions, homogeneous natural glycoproteins with defined glycosylation and folding/misfolding states are still urgently required for deciphering the detailed molecular mechanism of the protein quality control system. Two recent synthetic studies have shed lights in this direction (Figure 4). In one study, Wang and co-workers applied a chemoenzymatic method for the synthesis of selectively glucosylated Man9GlcNAc2 glycoprotein glycoforms, using RNase B as the model glycoprotein (Amin et al., 2011). The synthetic construction of the target glycoproteins includes a total chemical synthesis of a galactose-masked Glc1Man9GlcNAc glycan oxazoline and its enzymatic transfer to the deglycosylated RNase B (Figure 4a). Selective removal of the terminal galactose by a β-galactosidase gave the Glc1Man9GlcNAc2-RNase glycoform in excellent yield. In addition, a Man9GlcNAc2 glycoform was synthesized separately by transglycosylation with the Man9GlcNAc oxazoline. CD and RNA-hydrolyzing analysis revealed that these synthetic RNase glycoforms maintained essentially the same global conformations and were fully active as the natural bovine RNase B. SPR binding studies revealed that the Glc1Man9GlcNAc2-RNase had high affinity to lectin CRT, while the synthetic Man9GlcNAc2-RNase glycoform and natural RNase B that lack the glucose moiety did not show any CRT-binding activity. Interestingly, partially denatured Glc1Man9GlcNAc2-RNase could eb efficiently recognized by lectin CRT. These results confirmed the essential role of the glucose moiety in the molecular recognition by the lectin-like chaperone of misfolded proteins.
Figure 4. Synthesis of glycoprotein probes for deciphering the molecular mechanism of lectin-mediated protein quality control.
(a) a chemoenzymatic synthesis of several homogeneous glycoforms of ribonuclease B; (b) a chemical synthesis of intentionally misfolded homogeneous glycoforms of interleukin-8.
In another study, Kajihara and co-workers carried out a chemical synthesis of intentionally misfolded homogeneous glycopropteins and used them to study the recognition by enzyme UGGT, the protein folding sensor (Izumi et al., 2012). Intereleukin-8 was chosen as the model glycoprotein and a Man9GlcNAc2 was artificially installed at Asn-36 in the sequence. The synthesis started with the native chemical ligation between a peptide fragment (aa1–33) and a glycopeptide fragment (aa34–72), and the free full-length polypeptide was subject to oxidative folding to provide correctly folded glycoprotein and several misfolded intermediates with disulfide shuffling (Figure 4b). Notably, the authors were able to isolate the folded and misfolded glycoproteins by RP-HPLC and fully characterized their folding status. Enzymatic assays indicated that UGGT could efficiently recognize the misfolded monomer and dimer to introduce a glucose tag in the glycan of the misfolded proteins, but it showed only very low activity on the correctly folded glycoprotein. This remarkable study provides unambiguous evidence on the role of enzyme UGGT as the folding sensor to recruit and tag the misfolded glycoproteins. The resulting monoglucosylated mis-folded glycoproteins are then recruited by the CNX/CRT chaperones to enter the refolding cycle that also involves other chaperone proteins. Taken together, these synthetic homogeneous glycoprotein probes should be highly valuable for a detailed mechanistic study on how those molecular chaperones work in concert to distinguish between mis-folded and folded glycoproteins in the protein quality control system.
Glycoengineering of IgG antibodies for deciphering functions
Monoclonal antibodies (mAbs) are an important class of therapeutic proteins that are used for treatment of cancer, autoimmune, and infectious diseases (Adams and Weiner, 2005; Aggarwal, 2011; Jefferis, 2009). It has been demonstrated that different structures of the N-glycans attached at the conserved N-glycosylation site (Asn297) of the Fc domain can profoundly impact the effector functions of antibodies, including the antibody-dependent cellular cytotoxicity (ADCC) (Jefferis, 2009; Nimmerjahn and Ravetch, 2008a). For example, the lack of the core fucose, as well as the attachment of a bisecting GlcNAc moiety, significantly enhances the affinity of antibody for the FcγIIIa receptor (FcγRIIIa), which is responsible for ADCC (Ferrara et al., 2011; Shields et al., 2002). On the other hand, the terminal α-2,6-sialylated Fc glycoform, a minor species in the intravenous immunoglobulin (IVIG), was recently identified as the active species for the anti-inflammatory activity of IVIG, as evaluated in a mouse model of rheumatoid arthritis (RA) (Anthony et al., 2008a; Anthony et al., 2008b; Kaneko et al., 2006). The importance of Fc glycosylation on the biological functions and therapeutic outcome of IgG antibodies has stimulated tremendous interest in developing methods to control antibody’s Fc glycosylation. Among several biochemical and cell-based methods, Wang and co-workers have developed a chemoenzymatic glycoengineering approach that is particularly useful for site-specific Fc glycosylation remodeling to provide various pure antibody glycoforms (Fan et al., 2012; Huang et al., 2012; Wei et al., 2008; Zou et al., 2011). Following the initial success in glycosylation remodeling of recombinant Fc domains obtained from yeast and CHO cell expression (Fan et al., 2012; Wei et al., 2008; Zou et al., 2011), the authors have successfully extended the approach to glycoengineering of intact IgG monoclonal antibodies (Huang et al., 2012). This endeavor was enabled by the remarkable Fc deglycosylation activity of EndoS, an endo-β-N-acetylglucosaminidase (ENGase) from Streptococcus pyogenes (Allhorn et al., 2008; Collin and Olsen, 2001; Goodfellow et al., 2012), and the highly efficient transglycosylation by the novel EndoS-based glycosynthases (EndoS-D233A and EndoS-D233Q) that are devoid of product hydrolysis activity (Huang et al., 2012). This approach was demonstrated by glycoengineering of rituximab, a therapeutic monoclonal antibody (Figure 5). Deglycosylation by EndoS efficiently removed the heterogeneous Fc N-glycans (mainly the G0F, G2F, and G2F glycoforms, where G0–2 indicates the number of terminal galactose in the glycan and F means core fucosylated). The resulting homogenous GlcNAc-IgG was then used as the acceptor for the glycosynthase-catalyzed transglycosylation using respective glycan oxazolines as the donor substrates. Both natural and selectively modified N-glycans could be attached to form homogeneous IgG glycoforms. The non-fucosylated G2 glycoform was achieved by deglycosylation with both EndoS and an α-fucosidase, followed by transglycosylation with a desialylated complex type glycan oxazoline. It should be noted that all the enzymatic transformations were carried out under mild conditions (aqueous buffer, pH 7.0–7.4, ambient temperature) without the need of denaturing the proteins. The transglycosylation was driven to completion by using an excess molar equivalent of glycan oxazolines, which were recovered as free glycan form after reaction and could be readily converted back to oxazoline in a single step. SPR binding analysis with FcγRIIIa-F158 showed that the affinity of the homogeneous G2 glycoform for the FcγIIIa receptor was 20-fold higher than that of the commercial rituximab. On the other hand, the fully sialylated Fc glycoform (S2G2 form) may gain anti-inflammatory activity (Anthony et al., 2008a). This chemoenzymatic approach enables a quick access to various well-defined IgG glycoforms that are hitherto difficult to obtain by other methods for functional studies. In addition, the method also holds promise for development of more effective antibody-based therapeutics.
Figure 5.

a chemoenzymatic approach to glycoengineering of IgG antibodies.
Synthesis of mucin glycopeptides as anti-cancer vaccines
Aberrant glycosylation in glycolipids and glycoproteins is often associated with cancer progression and metastasis. The tumor-associated carbohydrate antigens are thus emerging as novel biomarkers for diagnosis and as attractive targets for cancer vaccines (Gaidzik et al., 2013; Wilson and Danishefsky, 2013). For example, MUC1, the type 1 mucin glycoproteins are present on most epithelial cells, which are often overexpressed in cancer cells and appended with truncated O-linked glycans (with only GalNAc, Galβ1, 3GalNAc, or sialylated GalNAc) instead the more extended large O-glycans found in normal cells. The extracellular domain of MUC1 contains tandem repeats of the 20 amino acid sequence, HGVTSAPDTRPAPGSTAPPA, which has up to five potential O-glycosylation sites ((Thr-4, Ser-5, Thr-9, Ser-15, and Thr-16). Tremendous studies have been carried out to develop vaccines targeting these unique antigenic structures. Synthesis has played an essential role in understanding how the glycosylation patterns affect the antigenicity and immunogenicity of MUC1 glycopeptides. A common approach to the synthesis of MUC1 glycopeptides is to use pre-assembled glycosylated amino acids as building blocks in SPPS, which permits the installation of different truncated O-glycans at pre-determined sites. As a typical example, Li, Kunz, and co-workers performed microwave-supported solid-phase synthesis of the MUC1 glycopeptides containing the STn or 2,6-ST antigen at Ser-15, and the Tn or T antigen at the Thr-9 site (Cai et al., 2012) (Figure 6a). A triethylene glycol spacer with a free terminal amino group was introduced at the N-terminus to facilitate its coupling with BSA via the squaric acid ester method to provide the MUC1 glycopeptide-BSA conjugate. It should be noted that, in O-glycopeptide synthesis, particular care should be taken to avoid the base-catalyzed β-elimination of glycans during sugar deprotection. Immunization in Balb/c mice showed that the glycoconjugate was able to induce strong IgG type antibody responses and the antibodies demonstrated high-affinity binding to breast cancer cells of the MCF-7 cell line. The present study, together with previous investigations, has revealed that attachment of the Tn and STn antigens at the immuno-dominant Thr-9 and Ser-15 regions is important for stimulating strong antibody responses. In another study, Clausen and co-workers reported a remarkable chemoenzymatic synthesis of large MUC1 glycopeptides carrying multimeric Tn, T, and STn motifs (Sorensen et al., 2006). In this approach, a MUC1 60-mer polypeptide consisting of 3 copies of the tandem 20-mer repeat was synthesized by SPPS. It was then enzymatically glycosylated using the site-selective recombinant polypeptide GalNAc transferases (GalNAc-T2, -T4, and -T11) to introduce different numbers of Tn antigens (2, 3, and 5 Tn per tandem repeat), taking advantage of the substrate specificity of the GalNAc transferases. Extension of the sugar chains via glycosylations with the recombinant core 1 β3Gal transferase (C1Gal-T1) and the recombinant murine sialyltransferase (ST6GalNAc-I) resulted in the incorporation of T and STn antigens, respectively (Figure 6b). Notably MUC1 glycopeptides fully glycosylated at all the five potential glycosylation sites in each 20-mer repeat were achieved by a combined use of these glycosyltransferases, and the products were characterized by MALDI-TOF MS analysis and antibody binding study. The synthetic MUC1 glycopeptides with different glycosylation patterns were conjugated to KLH and the resulting glycoconjugates were used as immunogens to evaluate the effects of different Tn/T/STn glycosylation patterns and density on the immunogenicity. It was shown that MUC1 glycopeptides with complete glycan occupancy (five sites per repeat) elicited the strongest antibody response against MUC1 expressed in breast cancer cell lines in both Balb/c and MUC1-trangenic mice. The elicited antibodies showed high specificity for cancer cells. This study suggests that a higher dense presentation of Tn and STn antigens in the context of the MUC1 polypeptide may provide a more efficient anticancer vaccine candidate.
Figure 6. Synthesis of mucin glycopeptides as anti-cancer vaccines.
(a) a chemical synthesis of MUC1 glycopeptides carrying T and ST glycans at defined glycosylation sites; (b) a chemoenzymatic synthesis of large MUC1 glycopeptides carrying multiple Tn, T, and STn glycans.
Synthetic HIV-1 glycopeptides for characterizing the glycan specificity of HIV-neutralizing antibodies
Recent discovery of a new class of glycan-dependent broadly neutralizing antibodies (bnAbs), represented by PG9, PG16, PGT121, PGT128 and PGT135, has provided new templates for HIV vaccine design (Bonsignori et al., 2011; Doores and Burton, 2010; Walker et al., 2011; Walker et al., 2009; Walker et al., 2010). These bnAbs appear to target conserved glycopeptide epitopes located at the variable (V1/V2 or V3) regions of HIV-1 gp120. Remarkable studies on the crystal structures of PG9, PG16, PGT128, PGT121, and PGT135 and their complexes with gp120 outer domains have revealed novel modes of glycopeptide antigen recognition, which provides important templates for designing glycopeptide immunogens (Burton et al., 2012; Wang, 2013). However, further characterization of the glycan specificity of these neutralizing antibodies was complicated by the heterogeneity in glycosylation of gp120 and recombinant scaffolded gp120 domains, because of the difficulties in controlling the structures of individual N-glycans at distinct glycosylation sites. To further characterize the glycan specificity with a goal of reconstituting the minimal glycopeptide epitopes of PG9 and PG16 for vaccine design, Wang and co-workers (Amin et al., 2013) designed cyclic V1V2 glycopeptides derived from two HIV-1 strains (CAP45 and ZM109), in which a disulfide bond was engineered to constrain the β-sheet conformations as observed in the crystal structure of the PG9 Fab in complex with the scaffolded V1V2 domain (McLellan et al., 2011). The synthesis was achieved by a chemoenzymatic approach, as demonstrated for the preparation of three typical ZM109 V1V2 glycopeptides (Figure 7a). Installation of two Man5GlcNAc2 glycans at the Asn160 and Asn173 sites were efficiently achieved by the simultaneous transfer of two Man5GlcNAc moieties from the corresponding glycan oxazoline substrate to the GlcNAc moieties of the cyclic peptide precursor. To install different glycans at the Asn160 and Asn173 sites, the first transglycosylation with Man5GlcNAc-oxazoline was controlled to give two mono-glycosylated intermediates, which were separated by HPLC. The isolated isomers were then separately glycosylated with a sialylated N-glycan at the remaining GlcNAc moiety under the catalysis of EndoM-N175A mutant to provide glycopeptides carrying two distinct N-glycans. By this synthetic approach, more than 25 homogeneous cyclic V1V2 glycopeptides from HIV-1 CAP45 and ZM109 strains were constructed and the overall synthetic yields for most of the glycopeptides were excellent. SPR and ELISA binding analysis indicated that both the nature and the location of the N-glycans in the context of the V1V2 sequence are important for antibody recognition, as indicated by the fact that free N-glycans and peptide alone as well as the glycopeptides with mis-placed N-glycans showed no or only marginal binding activities under the same conditions. These studies confirmed the essence of a Man5GlcNAc2 glycan at Asn160 for recognition by antibody PG9 and PG16, and further revealed a critical role of a sialylated N-glycan at the secondary glycosylation site (Asn156 or Asn173) for high affinity binding, which was not revealed by the original PG9 structural study (McLellan et al., 2011). The best glycopeptides showed a μM affinity for the PG9 Fab in SPR analysis. But they were able to detect PG9 and PG16 antibodies at 50 pM to 1.5 nM concentrations in an ELISA format (due to the high avidity). In another study, Danishefsky and co-workers synthesized several V1V2 glycopeptides derived from HIV-1 strain A244 by a convergent chemical assembly approach (Aussedat et al., 2013) (Figure 7b). Notably, the native chemical ligation between two fragments carrying bulky N-glycans proximal to the ligation junction gave an impressive 55% yield. Initial binding with PG9 whole IgG antibody indicated that the glycopeptide carrying two Man5GlcNAc2 glycans (at Asn156 and Asn160) exhibited high affinity (KD = 300 nM) for PG9. Surprisingly, the glycopeptide carrying two truncated Man3GlcNAc2 glycans also showed comparable affinity for PG9 in the SPR analysis. Later analysis suggested that the high-affinity binding was attributed to the dimerization of the synthetic glycopeptides that contain a free cysteine residue under the assay conditions (Alam et al., 2013). This result is consistent with the finding that PG9 preferably recognizes gp120 trimer and an additional Man5GlcNAc2 glycan at Asn160 of a neighboring gp120 molecule also contributes to binding (Julien et al., 2013). Taken together, these studies suggest that an oligomeric form of the V1V2 glycopeptides may be better mimics for the neutralizing epitopes of PG9 and PG16.
Figure 7. Synthetic HIV V1V2 glycopeptides for characterizing the glycan specificity of HIV-neutralizing antibodies.
(a) a chemoenzymatic synthesis of V1V2 glycopeptide antigens carrying two distinct N-glycans at the conserved glycosylation sites; (b) a chemical synthesis of V1V2 glycopeptide antigens carrying the same N-glycans at the two conserved N-glycosylation sites.
Conclusions
Synthesis of full-length natural glycoproteins, given their extremely large size and enormously complex structures from the glycan portions, was once viewed as only a remote dream for synthetic chemists. The concerted efforts from different research laboratories worldwide and, in particular, the development of those sophisticated chemical and biochemical ligation methods have now made it possible to assemble various natural and tailor-made glycoproteins involving diverse glycosylation patterns. Despite these tremendous progresses, it should be pointed out that the synthetic technologies for constructing glycoproteins are still far from maturation. Theoretically, the application of iterative native chemical ligation and other auxiliary-based ligations provides an opportunity to assemble glycoproteins of unlimited size and to install distinct glycans at different sites in the sequence. In reality, however, each target glycoprotein may pose special challenges that require a careful choice of ligation strategy and an innovative design of ligation components. Novel enzymes are required to permit site-specific glycosylation of polypeptides and folded recombinant proteins. Novel concepts remain to be explored that would allow site-specific alteration of glycans at pre-determined sites in a multiply glycosylated glycoprotein, similar to site-directed mutagenesis of amino acids in recombinant DNA technology. With continuous efforts in improving existing methods and in exploring new synthetic concepts, it is expected that a number of homogeneous glycoproteins with increasing complexity will be made to address important structural and biological problems that would be otherwise difficult to tackle in the lack of appropriate molecular probes. Moreover, the ability to construct well-defined tailor-made glycoproteins will certainly facilitate the development of more effective glycoprotein-based therapeutics in the future.
Acknowledgments
This work was financially supported by the National Institutes of Health (NIH grants R01 GM080374 and R01 GM096973).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- Aebi M, Bernasconi R, Clerc S, Molinari M. N-glycan structures: recognition and processing in the ER. Trends Biochem Sci. 2010;35:74–82. doi: 10.1016/j.tibs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Aggarwal S. What’s fueling the biotech engine--2010 to 2011. Nat Biotechnol. 2011;29:1083–1089. doi: 10.1038/nbt.2060. [DOI] [PubMed] [Google Scholar]
- Alam SM, Dennison SM, Aussedat B, Vohra Y, Park PK, Fernandez-Tejada A, Stewart S, Jaeger FH, Anasti K, Blinn JH, et al. Recognition of synthetic glycopeptides by HIV-1 broadly neutralizing antibodies and their unmutated ancestors. Proc Natl Acad Sci USA. 2013;110:18214–18219. doi: 10.1073/pnas.1317855110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allhorn M, Olsen A, Collin M. EndoS from Streptococcus pyogenes is hydrolyzed by the cysteine proteinase SpeB and requires glutamic acid 235 and tryptophans for IgG glycan-hydrolyzing activity. BMC Microbiol. 2008;8:3. doi: 10.1186/1471-2180-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin MN, Huang W, Mizanur RM, Wang LX. Convergent Synthesis of Homogeneous Glc(1)Man(9)GlcNAc(2)-Protein and Derivatives as Ligands of Molecular Chaperones in Protein Quality Control. J Am Chem Soc. 2011;133:14404–14417. doi: 10.1021/ja204831z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin MN, McLellan JS, Huang W, Orwenyo J, Burton DR, Koff WC, Kwong PD, Wang LX. Synthetic glycopeptides reveal the glycan specificity of HIV-neutralizing antibodies. Nat Chem Biol. 2013;9:521–526. doi: 10.1038/nchembio.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008a;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008b;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina Y, Kamitori S, Takao T, Nishi N, Hojo H. Chemoenzymatic synthesis of the immunoglobulin domain of Tim-3 carrying a complex-type N-glycan by using a one-pot ligation. Angew Chem Int Ed. 2013;52:9733–9737. doi: 10.1002/anie.201303073. [DOI] [PubMed] [Google Scholar]
- Aussedat B, Fasching B, Johnston E, Sane N, Nagorny P, Danishefsky SJ. Total synthesis of the alpha-subunit of human glycoprotein hormones: toward fully synthetic homogeneous human follicle-stimulating hormone. J Am Chem Soc. 2012;134:3532–3541. doi: 10.1021/ja2111459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aussedat B, Vohra Y, Park PK, Fernandez-Tejada A, Alam SM, Dennison SM, Jaeger FH, Anasti K, Stewart S, Blinn JH, et al. Chemical Synthesis of Highly Congested gp120 V1V2 N-Glycopeptide Antigens for Potential HIV-1-Directed Vaccines. J Am Chem Soc. 2013;135:13113–13120. doi: 10.1021/ja405990z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011;85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, et al. A blueprint for HIV vaccine discovery. Cell host & microbe. 2012;12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskas T, Ingale S, Boons GJ. Glycopeptides as Versatile Tools for Glycobiology. Glycobiology. 2006;16:113R–136R. doi: 10.1093/glycob/cwj125. [DOI] [PubMed] [Google Scholar]
- Cai H, Huang ZH, Shi L, Sun ZY, Zhao YF, Kunz H, Li YM. Variation of the glycosylation pattern in MUC1 glycopeptide BSA vaccines and its influence on the immune response. Angew Chem Int Ed. 2012;51:1719–1723. doi: 10.1002/anie.201106396. [DOI] [PubMed] [Google Scholar]
- Carrico IS, Carlson BL, Bertozzi CR. Introducing genetically encoded aldehydes into proteins. Nat Chem Biol. 2007;3:321–322. doi: 10.1038/nchembio878. [DOI] [PubMed] [Google Scholar]
- Castilho A, Steinkellner H. Glyco-engineering in plants to produce human-like N-glycan structures. Biotechnology journal. 2012;7:1088–1098. doi: 10.1002/biot.201200032. [DOI] [PubMed] [Google Scholar]
- Chalker JM, Bernardes GJ, Davis BG. A “Tag-and-Modify” Approach to Site-Selective Protein Modification. Acc Chem Res. 2011;44:730–741. doi: 10.1021/ar200056q. [DOI] [PubMed] [Google Scholar]
- Chen MM, Glover KJ, Imperiali B. From peptide to protein: comparative analysis of the substrate specificity of N-linked glycosylation in C. jejuni. Biochemistry. 2007;46:5579–5585. doi: 10.1021/bi602633n. [DOI] [PubMed] [Google Scholar]
- Collin M, Olsen A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001;20:3046–3055. doi: 10.1093/emboj/20.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PE, Kent SB. Synthesis of native proteins by chemical ligation. Annu Rev Biochem. 2000;69:923–960. doi: 10.1146/annurev.biochem.69.1.923. [DOI] [PubMed] [Google Scholar]
- Dawson PE, Muir TW, Clark-Lewis I, Kent SB. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- Dempski RE, Jr, Imperiali B. Oligosaccharyl transferase: gatekeeper to the secretory pathway. Curr Opin Chem Biol. 2002;6:844–850. doi: 10.1016/s1367-5931(02)00390-3. [DOI] [PubMed] [Google Scholar]
- Doores KJ, Burton DR. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J Virol. 2010;84:10510–10521. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube DH, Bertozzi CR. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- Dwek RA. Glycobiology: Toward understanding the function of sugars. Chem Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- Eller S, Schuberth R, Gundel G, Seifert J, Unverzagt C. Synthesis of pentaantennary N-glycans with bisecting GlcNAc and core fucose. Angew Chem Int Ed. 2007;46:4173–4175. doi: 10.1002/anie.200604788. [DOI] [PubMed] [Google Scholar]
- Fan SQ, Huang W, Wang LX. Remarkable transglycosylation activity of glycosynthase mutants of Endo-D, an endo-beta-N-acetylglucosaminidase from Streptococcus pneumoniae. J Biol Chem. 2012;287:11272–11281. doi: 10.1074/jbc.M112.340497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman MF, Wacker M, Hernandez M, Hitchen PG, Marolda CL, Kowarik M, Morris HR, Dell A, Valvano MA, Aebi M. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc Natl Acad Sci USA. 2005;102:3016–3021. doi: 10.1073/pnas.0500044102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-González M, Boutureira O, Bernardes GJ, Chalker JM, Young MA, Errey JC, Davis BG. Site-selective chemoenzymatic construction of synthetic glycoproteins using endoglycosidases. Chem Sci. 2010;1:709–715. [Google Scholar]
- Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between Fc{gamma}RIII and antibodies lacking core fucose. Proc Natl Acad Sci USA. 2011;108:12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Shoda S, Haneda K, Inazu T, Takegawa K, Yamamoto K. A novel disaccharide substrate having 1,2-oxazoline moiety for detection of transglycosylating activity of endoglycosidases. Biochim Biophys Acta. 2001;1528:9–14. doi: 10.1016/s0304-4165(01)00164-7. [DOI] [PubMed] [Google Scholar]
- Gaidzik N, Westerlind U, Kunz H. The development of synthetic antitumour vaccines from mucin glycopeptide antigens. Chem Soc Rev. 2013;42:4421–4442. doi: 10.1039/c3cs35470a. [DOI] [PubMed] [Google Scholar]
- Gamblin DP, Scanlan EM, Davis BG. Glycoprotein synthesis: an update. Chem Rev. 2009;109:131–163. doi: 10.1021/cr078291i. [DOI] [PubMed] [Google Scholar]
- Glover KJ, Weerapana E, Numao S, Imperiali B. Chemoenzymatic synthesis of glycopeptides with PglB, a bacterial oligosaccharyl transferase from Campylobacter jejuni. Chem Biol. 2005;12:1311–1315. doi: 10.1016/j.chembiol.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow JJ, Baruah K, Yamamoto K, Bonomelli C, Krishna B, Harvey DJ, Crispin M, Scanlan CN, Davis BG. An endoglycosidase with alternative glycan specificity allows broadened glycoprotein remodelling. J Am Chem Soc. 2012;134:8030–8033. doi: 10.1021/ja301334b. [DOI] [PubMed] [Google Scholar]
- Grogan MJ, Pratt MR, Marcaurelle LA, Bertozzi CR. Homogeneous glycopeptides and glycoproteins for biological investigation. Annu Rev Biochem. 2002;71:593–634. doi: 10.1146/annurev.biochem.71.110601.135334. [DOI] [PubMed] [Google Scholar]
- Guo Z, Shao N. Glycopeptide and glycoprotein synthesis involving unprotected carbohydrate building blocks. Med Res Rev. 2005;25:655–678. doi: 10.1002/med.20033. [DOI] [PubMed] [Google Scholar]
- Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annu Rev Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- Hamilton SR, Gerngross TU. Glycosylation engineering in yeast: the advent of fully humanized yeast. Curr Opin Biotechnol. 2007;18:387–392. doi: 10.1016/j.copbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Hang HC, Bertozzi CR. Chemoselective approaches to glycoprotein assembly. Acc Chem Res. 2001;34:727–736. doi: 10.1021/ar9901570. [DOI] [PubMed] [Google Scholar]
- Hart GW, Copeland RJ. Glycomics hits the big time. Cell. 2010;143:672–676. doi: 10.1016/j.cell.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Oh-eda M, Kuboniwa H, Tomonoh K, Shimonaka Y, Ochi N. Role of sugar chains in the expression of the biological activity of human erythropoietin. J Biol Chem. 1992;267:7703–7709. [PubMed] [Google Scholar]
- Hirano K, Macmillan D, Tezuka K, Tsuji T, Kajihara Y. Design and synthesis of a homogeneous erythropoietin analogue with two human complex-type sialyloligosaccharides: combined use of chemical and bacterial protein expression methods. Angew Chem Int Ed. 2009;48:9557–9560. doi: 10.1002/anie.200904376. [DOI] [PubMed] [Google Scholar]
- Hojo H, Nakahara Y. Recent progress in the field of glycopeptide synthesis. Biopolymers. 2007;88:308–324. doi: 10.1002/bip.20699. [DOI] [PubMed] [Google Scholar]
- Hojo H, Tanaka H, Hagiwara M, Asahina Y, Ueki A, Katayama H, Nakahara Y, Yoneshige A, Matsuda J, Ito Y, et al. Chemoenzymatic synthesis of hydrophobic glycoprotein: synthesis of saposin C carrying complex-type carbohydrate. J Org Chem. 2012;77:9437–9446. doi: 10.1021/jo3010155. [DOI] [PubMed] [Google Scholar]
- Huang W, Giddens J, Fan SQ, Toonstra C, Wang LX. Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions. J Am Chem Soc. 2012;134:12308–12318. doi: 10.1021/ja3051266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Groothuys S, Heredia A, Kuijpers BH, Rutjes FP, van Delft FL, Wang LX. Enzymatic glycosylation of triazole-linked GlcNAc/Glc-peptides: synthesis, stability and anti-HIV activity of triazole-linked HIV-1 gp41 glycopeptide C34 analogues. ChemBioChem. 2009a;10:1234–1242. doi: 10.1002/cbic.200800741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Li C, Li B, Umekawa M, Yamamoto K, Zhang X, Wang LX. Glycosynthases enable a highly efficient chemoenzymatic synthesis of N-glycoproteins carrying intact natural N-glycans. J Am Chem Soc. 2009b;131:2214–2223. doi: 10.1021/ja8074677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Yang Q, Umekawa M, Yamamoto K, Wang LX. Arthrobacter endo-beta-N-acetylglucosaminidase shows transglycosylation activity on complex-type N-glycan oxazolines: one-pot conversion of ribonuclease B to sialylated ribonuclease C. ChemBioChem. 2010a;11:1350–1355. doi: 10.1002/cbic.201000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhang X, Ju T, Cummings RD, Wang LX. Expeditious chemoenzymatic synthesis of CD52 glycopeptide antigens. Org Biomol Chem. 2010b;8:5224–5233. doi: 10.1039/c0ob00341g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Huang L, Wang H, Ye XS. Iterative one-pot synthesis of oligosaccharides. Angew Chem Int Ed. 2004;43:5221–5224. doi: 10.1002/anie.200460176. [DOI] [PubMed] [Google Scholar]
- Hudak JE, Yu HH, Bertozzi CR. Protein glycoengineering enabled by the versatile synthesis of aminooxy glycans and the genetically encoded aldehyde tag. J Am Chem Soc. 2011;133:16127–16135. doi: 10.1021/ja206023e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiali B, Hendrickson TL. Asparagine-linked glycosylation: specificity and function of oligosaccharyl transferase. Bioorg Med Chem. 1995;3:1565–1578. doi: 10.1016/0968-0896(95)00142-5. [DOI] [PubMed] [Google Scholar]
- Ito Y, Hagihara S, Matsuo I, Totani K. Structural approaches to the study of oligosaccharides in glycoprotein quality control. Curr Opin Struct Biol. 2005;15:481–489. doi: 10.1016/j.sbi.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Iwamoto S, Isoyama M, Hirano M, Yamaya K, Ito Y, Matsuo I, Totani K. Reconstructed glycan profile for evaluation of operating status of the endoplasmic reticulum glycoprotein quality control. Glycobiology. 2013;23:121–131. doi: 10.1093/glycob/cws130. [DOI] [PubMed] [Google Scholar]
- Izumi M, Makimura Y, Dedola S, Seko A, Kanamori A, Sakono M, Ito Y, Kajihara Y. Chemical synthesis of intentionally misfolded homogeneous glycoprotein: a unique approach for the study of glycoprotein quality control. J Am Chem Soc. 2012;134:7238–7241. doi: 10.1021/ja3013177. [DOI] [PubMed] [Google Scholar]
- Jacobs PP, Geysens S, Vervecken W, Contreras R, Callewaert N. Engineering complex-type N-glycosylation in Pichia pastoris using GlycoSwitch technology. Nat Protoc. 2009;4:58–70. doi: 10.1038/nprot.2008.213. [DOI] [PubMed] [Google Scholar]
- Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8:226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- Julien JP, Lee JH, Cupo A, Murin CD, Derking R, Hoffenberg S, Caulfield MJ, King CR, Marozsan AJ, Klasse PJ, et al. Asymmetric recognition of the HIV-1 trimer by broadly neutralizing antibody PG9. Proc Natl Acad Sci USA. 2013;110:4351–4356. doi: 10.1073/pnas.1217537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajihara Y, Suzuki Y, Yamamoto N, Sasaki K, Sakakibara T, Juneja LR. Prompt chemoenzymatic synthesis of diverse complex-type oligosaccharides and its application to the solid-phase synthesis of a glycopeptide with Asn-linked sialyl-undeca- and asialo-nonasaccharides. Chem Eur J. 2004;10:971–985. doi: 10.1002/chem.200305115. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- Koizumi A, Matsuo I, Takatani M, Seko A, Hachisu M, Takeda Y, Ito Y. Top-down chemoenzymatic approach to a high-mannose-type glycan library: synthesis of a common precursor and its enzymatic trimming. Angew Chem Int Ed. 2013;52:7426–7431. doi: 10.1002/anie.201301613. [DOI] [PubMed] [Google Scholar]
- Kowarik M, Numao S, Feldman MF, Schulz BL, Callewaert N, Kiermaier E, Catrein I, Aebi M. N-linked glycosylation of folded proteins by the bacterial oligosaccharyltransferase. Science. 2006;314:1148–1150. doi: 10.1126/science.1134351. [DOI] [PubMed] [Google Scholar]
- Li B, Song H, Hauser S, Wang LX. A highly efficient chemoenzymatic approach toward glycoprotein synthesis. Org Lett. 2006;8:3081–3084. doi: 10.1021/ol061056m. [DOI] [PubMed] [Google Scholar]
- Li B, Zeng Y, Hauser S, Song H, Wang LX. Highly efficient endoglycosidase-catalyzed synthesis of glycopeptides using oligosaccharide oxazolines as donor substrates. J Am Chem Soc. 2005a;127:9692–9693. doi: 10.1021/ja051715a. [DOI] [PubMed] [Google Scholar]
- Li H, Li B, Song H, Breydo L, Baskakov IV, Wang LX. Chemoenzymatic synthesis of HIV-1 V3 glycopeptides carrying two N-glycans and effects of glycosylation on the peptide domain. J Org Chem. 2005b;70:9990–9996. doi: 10.1021/jo051729z. [DOI] [PubMed] [Google Scholar]
- Li L, Woodward R, Ding Y, Liu XW, Yi W, Bhatt VS, Chen M, Zhang LW, Wang PG. Overexpression and topology of bacterial oligosaccharyltransferase PglB. Biochem Biophys Res Commun. 2010;394:1069–1074. doi: 10.1016/j.bbrc.2010.03.126. [DOI] [PubMed] [Google Scholar]
- Lizak C, Gerber S, Numao S, Aebi M, Locher KP. X-ray structure of a bacterial oligosaccharyltransferase. Nature. 2011;474:350–355. doi: 10.1038/nature10151. [DOI] [PubMed] [Google Scholar]
- Lomino JV, Naegeli A, Orwenyo J, Amin MN, Aebi M, Wang LX. A two-step enzymatic glycosylation of polypeptides with complex N-glycans. Bioorg Med Chem. 2013;21:2262–2270. doi: 10.1016/j.bmc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowary T. Context and complexity: The next big thing in synthetic glycobiology. Curr Opin Chem Biol. 2013;17:990–996. doi: 10.1016/j.cbpa.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Macmillan D, Bertozzi CR. Modular assembly of glycoproteins: towards the synthesis of GlyCAM-1 by using expressed protein ligation. Angew Chem Int Ed. 2004;43:1355–1359. doi: 10.1002/anie.200352673. [DOI] [PubMed] [Google Scholar]
- Macmillan D, Bill RM, Sage KA, Fern D, Flitsch SL. Selective in vitro glycosylation of recombinant proteins: semi- synthesis of novel homogeneous glycoforms of human erythropoietin. Chem Biol. 2001;8:133–145. doi: 10.1016/s1074-5521(00)90065-6. [DOI] [PubMed] [Google Scholar]
- Makimura Y, Kiuchi T, Izumi M, Dedola S, Ito Y, Kajihara Y. Efficient synthesis of glycopeptide-alpha-thioesters with a high-mannose type oligosaccharide by means of tert-Boc-solid phase peptide synthesis. Carbohydr Res. 2012;364:41–48. doi: 10.1016/j.carres.2012.10.011. [DOI] [PubMed] [Google Scholar]
- McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzato S, Schaffrath M, Unverzagt C. An orthogonal double-linker resin facilitates the efficient solid-phase synthesis of complex-type N-glycopeptide thioesters suitable for native chemical ligation. Angew Chem Int Ed. 2005;44:1650–1654. doi: 10.1002/anie.200461125. [DOI] [PubMed] [Google Scholar]
- Muir TW. Semisynthesis of proteins by expressed protein ligation. Annu Rev Biochem. 2003;72:249–289. doi: 10.1146/annurev.biochem.72.121801.161900. [DOI] [PubMed] [Google Scholar]
- Muir TW, Sondhi D, Cole PA. Expressed protein ligation: a general method for protein engineering. Proc Natl Acad Sci USA. 1998;95:6705–6710. doi: 10.1073/pnas.95.12.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Okamoto R, Izumi M, Kajihara Y. Chemical Synthesis of an Erythropoietin Glycoform Containing a Complex-type Disialyloligosaccharide. Angew Chem Int Ed. 2012 doi: 10.1002/anie.201109034. [DOI] [PubMed] [Google Scholar]
- Muthana S, Cao H, Chen X. Recent progress in chemical and chemoenzymatic synthesis of carbohydrates. Curr Opin Chem Biol. 2009;13:573–581. doi: 10.1016/j.cbpa.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagorny P, Sane N, Fasching B, Aussedat B, Danishefsky SJ. Probing the frontiers of glycoprotein synthesis: the fully elaborated beta-subunit of the human follicle-stimulating hormone. Angew Chem Int Ed. 2012;51:975–979. doi: 10.1002/anie.201107482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Anti-Inflammatory Actions of Intravenous Immunoglobulin. Annu Rev Immunol. 2008a;26:513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008b;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- Ochiai H, Huang W, Wang LX. Expeditious chemoenzymatic synthesis of homogeneous N-glycoproteins carrying defined oligosaccharide ligands. J Am Chem Soc. 2008;130:13790–13803. doi: 10.1021/ja805044x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwenyo J, Huang W, Wang LX. Chemoenzymatic synthesis and lectin recognition of a selectively fluorinated glycoprotein. Bioorg Med Chem. 2013;21:4768–4777. doi: 10.1016/j.bmc.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RJ, Wong CH. Advances in chemical ligation strategies for the synthesis of glycopeptides and glycoproteins. Chem Commun (Camb) 2010;46:21–43. doi: 10.1039/b913845e. [DOI] [PubMed] [Google Scholar]
- Petrescu AJ, Wormald MR, Dwek RA. Structural aspects of glycomes with a focus on N-glycosylation and glycoprotein folding. Curr Opin Struct Biol. 2006;16:600–607. doi: 10.1016/j.sbi.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Piontek C, Ring P, Harjes O, Heinlein C, Mezzato S, Lombana N, Pohner C, Puttner M, Varon Silva D, Martin A, et al. Semisynthesis of a homogeneous glycoprotein enzyme: ribonuclease C: part 1. Angew Chem Int Ed. 2009a;48:1936–1940. doi: 10.1002/anie.200804734. [DOI] [PubMed] [Google Scholar]
- Piontek C, Varon Silva D, Heinlein C, Pohner C, Mezzato S, Ring P, Martin A, Schmid FX, Unverzagt C. Semisynthesis of a homogeneous glycoprotein enzyme: ribonuclease C: part 2. Angew Chem Int Ed. 2009b;48:1941–1945. doi: 10.1002/anie.200804735. [DOI] [PubMed] [Google Scholar]
- Pratt MR, Bertozzi CR. Synthetic glycopeptides and glycoproteins as tools for biology. Chem Soc Rev. 2005;34:58–68. doi: 10.1039/b400593g. [DOI] [PubMed] [Google Scholar]
- Rendle PM, Seger A, Rodrigues J, Oldham NJ, Bott RR, Jones JB, Cowan MM, Davis BG. Glycodendriproteins: a synthetic glycoprotein mimic enzyme with branched sugar-display potently inhibits bacterial aggregation. J Am Chem Soc. 2004;126:4750–4751. doi: 10.1021/ja031698u. [DOI] [PubMed] [Google Scholar]
- Rich JR, Withers SG. Emerging methods for the production of homogeneous human glycoproteins. Nat Chem Biol. 2009;5:206–215. doi: 10.1038/nchembio.148. [DOI] [PubMed] [Google Scholar]
- Rising TW, Claridge TD, Moir JW, Fairbanks AJ. Endohexosaminidase M: exploring and exploiting enzyme substrate specificity. ChemBioChem. 2006;7:1177–1180. doi: 10.1002/cbic.200600183. [DOI] [PubMed] [Google Scholar]
- Sakamoto I, Tezuka K, Fukae K, Ishii K, Taduru K, Maeda M, Ouchi M, Yoshida K, Nambu Y, Igarashi J, et al. Chemical synthesis of homogeneous human glycosyl-interferon-beta that exhibits potent antitumor activity in vivo. J Am Chem Soc. 2012;134:5428–5431. doi: 10.1021/ja2109079. [DOI] [PubMed] [Google Scholar]
- Schmaltz RM, Hanson SR, Wong CH. Enzymes in the synthesis of glycoconjugates. Chem Rev. 2011;111:4259–4307. doi: 10.1021/cr200113w. [DOI] [PubMed] [Google Scholar]
- Schwarz F, Fan YY, Schubert M, Aebi M. Cytoplasmic N-glycosyltransferase of Actinobacillus pleuropneumoniae is an inverting enzyme and recognizes the NX(S/T) consensus sequence. J Biol Chem. 2011;286:35267–35274. doi: 10.1074/jbc.M111.277160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz F, Huang W, Li C, Schulz BL, Lizak C, Palumbo A, Numao S, Neri D, Aebi M, Wang LX. A combined method for producing homogeneous glycoproteins with eukaryotic N-glycosylation. Nat Chem Biol. 2010;6:264–266. doi: 10.1038/nchembio.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer D, Cole PA. Protein semisynthesis and expressed protein ligation: chasing a protein’s tail. Curr Opin Chem Biol. 2005;9:561–569. doi: 10.1016/j.cbpa.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Seitz O. Glycopeptide synthesis and the effects of glycosylation on protein structure and activity. ChemBioChem. 2000;1:214–246. doi: 10.1002/1439-7633(20001117)1:4<214::AID-CBIC214>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Seko A, Koketsu M, Nishizono M, Enoki Y, Ibrahim HR, Juneja LR, Kim M, Yamamoto T. Occurence of a sialylglycopeptide and free sialylglycans in hen’s egg yolk. Biochim Biophys Acta. 1997;1335:23–32. doi: 10.1016/s0304-4165(96)00118-3. [DOI] [PubMed] [Google Scholar]
- Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- Shin Y, Winans KA, Backes BJ, Kent SBH, Ellman JA, Bertozzi CB. Fmoc-Based Synthesis of Peptide-α Thioesters: Application to the Total Chemical Synthesis of a Glycoprotein by Native Chemical Ligation. J Am Chem Soc. 1999;121:11684–11689. [Google Scholar]
- Shivatare SS, Chang SH, Tsai TI, Ren CT, Chuang HY, Hsu L, Lin CW, Li ST, Wu CY, Wong CH. Efficient convergent synthesis of bi-, tri-, and tetra-antennary complex type N-glycans and their HIV-1 antigenicity. J Am Chem Soc. 2013;135:15382–15391. doi: 10.1021/ja409097c. [DOI] [PubMed] [Google Scholar]
- Sorensen AL, Reis CA, Tarp MA, Mandel U, Ramachandran K, Sankaranarayanan V, Schwientek T, Graham R, Taylor-Papadimitriou J, Hollingsworth MA, et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16:96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
- Spiro RG. Glucose residues as key determinants in the biosynthesis and quality control of glycoproteins with N-linked oligosaccharides. J Biol Chem. 2000;275:35657–35660. doi: 10.1074/jbc.R000022200. [DOI] [PubMed] [Google Scholar]
- Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- Stanley P. Glycosylation engineering. Glycobiology. 1992;2:99–107. doi: 10.1093/glycob/2.2.99. [DOI] [PubMed] [Google Scholar]
- Sun B, Srinivasan B, Huang X. Pre-activation-based one-pot synthesis of an alpha-(2,3)-sialylated core-fucosylated complex type bi-antennary N-glycan dodecasaccharide. Chem Eur J. 2008;14:7072–7081. doi: 10.1002/chem.200800757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Totani K, Matsuo I, Ito Y. Chemical approaches toward understanding glycan-mediated protein quality control. Curr Opin Chem Biol. 2009;13:582–591. doi: 10.1016/j.cbpa.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Terra VS, Mills DC, Yates LE, Abouelhadid S, Cuccui J, Wren BW. Recent developments in bacterial protein glycan coupling technology and glycoconjugate vaccine design. Journal of medical microbiology. 2012;61:919–926. doi: 10.1099/jmm.0.039438-0. [DOI] [PubMed] [Google Scholar]
- Umekawa M, Huang W, Li B, Fujita K, Ashida H, Wang LX, Yamamoto K. Mutants of Mucor hiemalis endo-beta-N-acetylglucosaminidase show enhanced transglycosylation and glycosynthase-like activities. J Biol Chem. 2008;283:4469–4479. doi: 10.1074/jbc.M707137200. [DOI] [PubMed] [Google Scholar]
- Umekawa M, Li C, Higashiyama T, Huang W, Ashida H, Yamamoto K, Wang LX. Efficient glycosynthase mutant derived from Mucor hiemalis endo-beta-N-acetylglucosaminidase capable of transferring oligosaccharide from both sugar oxazoline and natural N-glycan. J Biol Chem. 2010;285:511–521. doi: 10.1074/jbc.M109.059832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverzagt C, Kajihara Y. Chemical assembly of N-glycoproteins: a refined toolbox to address a ubiquitous posttranslational modification. Chem Soc Rev. 2013;42:4408–4420. doi: 10.1039/c3cs35485g. [DOI] [PubMed] [Google Scholar]
- Valderrama-Rincon JD, Fisher AC, Merritt JH, Fan YY, Reading CA, Chhiba K, Heiss C, Azadi P, Aebi M, DeLisa MP. An engineered eukaryotic protein glycosylation pathway in Escherichia coli. Nat Chem Biol. 2012;8:434–436. doi: 10.1038/nchembio.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kasteren SI, Kramer HB, Jensen HH, Campbell SJ, Kirkpatrick J, Oldham NJ, Anthony DC, Davis BG. Expanding the diversity of chemical protein modification allows post-translational mimicry. Nature. 2007;446:1105–1109. doi: 10.1038/nature05757. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak MA, Danishefsky SJ. Solving the convergence problem in the synthesis of triantennary N-glycan relevant to prostate-specific membrane antigen (PSMA) J Am Chem Soc. 2012;134:16430–16433. doi: 10.1021/ja307628w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, Phogat SK, Poignard P, Burton DR. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 2010;6:e1001028. doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Winblade Nairn N, Johnson RS, Tirrell DA, Grabstein K. Processing of N-terminal unnatural amino acids in recombinant human interferon-beta in Escherichia coli. ChemBioChem. 2008;9:324–330. doi: 10.1002/cbic.200700379. [DOI] [PubMed] [Google Scholar]
- Wang LX. Chemoenzymatic synthesis of glycopeptides and glycoproteins through endoglycosidase-catalyzed transglycosylation. Carbohydr Res. 2008;343:1509–1522. doi: 10.1016/j.carres.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LX. The amazing transglycosylation activity of endo-beta-N-acetylglucosaminidases. Trends Glycosci Glycotechnol. 2011;23:33–52. doi: 10.4052/tigg.23.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LX. Synthetic carbohydrate antigens for HIV vaccine design. Curr Opin Chem Biol. 2013;17:997–1005. doi: 10.1016/j.cbpa.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LX, Davis BG. Realizing the Promise of Chemical Glycobiology. Chem Sci. 2013;4:3381–3394. doi: 10.1039/C3SC50877C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LX, Lomino JV. Emerging technologies for making glycan-defined glycoproteins. ACS Chem Biol. 2012;7:110–122. doi: 10.1021/cb200429n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LX, Ni J, Singh S, Li H. Binding of high-mannose-type oligosaccharides and synthetic oligomannose clusters to human antibody 2G12: implications for HIV-1 vaccine design. Chem Biol. 2004;11:127–134. doi: 10.1016/j.chembiol.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Wang P, Dong S, Brailsford JA, Iyer K, Townsend SD, Zhang Q, Hendrickson RC, Shieh J, Moore MA, Danishefsky SJ. At last: erythropoietin as a single glycoform. Angew Chem Int Ed. 2012;51:11576–11584. doi: 10.1002/anie.201206090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Dong S, Shieh JH, Peguero E, Hendrickson R, Moore MA, Danishefsky SJ. Erythropoietin derived by chemical synthesis. Science. 2013a;342:1357–1360. doi: 10.1126/science.1245095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Chinoy ZS, Ambre SG, Peng W, McBride R, de Vries RP, Glushka J, Paulson JC, Boons GJ. A general strategy for the chemoenzymatic synthesis of asymmetrically branched N-glycans. Science. 2013b;341:379–383. doi: 10.1126/science.1236231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt GM, Lund J, Levens M, Kolli VS, Jefferis R, Boons GJ. Site-specific glycosylation of an aglycosylated human IgG1-Fc antibody protein generates neoglycoproteins with enhanced function. Chem Biol. 2003;10:807–814. doi: 10.1016/j.chembiol.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Weerapana E, Imperiali B. Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology. 2006;16:91R–101R. doi: 10.1093/glycob/cwj099. [DOI] [PubMed] [Google Scholar]
- Wei Y, Li C, Huang W, Li B, Strome S, Wang LX. Glycoengineering of human IgG1-Fc through combined yeast expression and in vitro chemoenzymatic glycosylation. Biochemistry. 2008;47:10294–10304. doi: 10.1021/bi800874y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt S, Gerngross TU. The humanization of N-glycosylation pathways in yeast. Nat Rev Microbiol. 2005;3:119–128. doi: 10.1038/nrmicro1087. [DOI] [PubMed] [Google Scholar]
- Wilson RM, Danishefsky SJ. A vision for vaccines built from fully synthetic tumor-associated antigens: from the laboratory to the clinic. J Am Chem Soc. 2013;135:14462–14472. doi: 10.1021/ja405932r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RM, Dong S, Wang P, Danishefsky SJ. The winding pathway to erythropoietin along the chemistry-biology frontier: a success at last. Angew Chem Int Ed. 2013;52:7646–7665. doi: 10.1002/anie.201301666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschi B, Wenger W, Nehring S, Budisa N. Expanding the genetic code of Saccharomyces cerevisiae with methionine analogues. Yeast. 2008;25:775–786. doi: 10.1002/yea.1632. [DOI] [PubMed] [Google Scholar]
- Witte K, Sears P, Martin R, Wong CH. Enzymatic glycoprotein synthesis: Preparation of ribonuclease glycoforms via enzymatic glycopeptide condensation and glycosylation. J Am Chem Soc. 1997;119:2114–2118. [Google Scholar]
- Wu P, Shui W, Carlson BL, Hu N, Rabuka D, Lee J, Bertozzi CR. Site-specific chemical modification of recombinant proteins produced in mammalian cells by using the genetically encoded aldehyde tag. Proc Natl Acad Sci USA. 2009;106:3000–3005. doi: 10.1073/pnas.0807820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Coward JK. 13C- and 15N-labeled peptide substrates as mechanistic probes of oligosaccharyltransferase. Biochemistry. 1997;36:14683–14689. doi: 10.1021/bi9719511. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Tanabe Y, Okamoto R, Dawson PE, Kajihara Y. Chemical synthesis of a glycoprotein having an intact human complex-type sialyloligosaccharide under the Boc and Fmoc synthetic strategies. J Am Chem Soc. 2008;130:501–510. doi: 10.1021/ja072543f. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Chen J, Wan Q, Wilson RM, Danishefsky SJ. Toward fully synthetic, homogeneous glycoproteins: advances in chemical ligation. Biopolymers. 2010;94:373–384. doi: 10.1002/bip.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachara NE, Hart GW. The emerging significance of O-GlcNAc in cellular regulation. Chem Rev. 2002;102:431–438. doi: 10.1021/cr000406u. [DOI] [PubMed] [Google Scholar]
- Zapun A, Petrescu SM, Rudd PM, Dwek RA, Thomas DY, Bergeron JJ. Conformation-independent binding of monoglucosylated ribonuclease B to calnexin. Cell. 1997;88:29–38. doi: 10.1016/s0092-8674(00)81855-3. [DOI] [PubMed] [Google Scholar]
- Zou G, Ochiai H, Huang W, Yang Q, Li C, Wang LX. Chemoenzymatic synthesis and Fcgamma receptor binding of homogeneous glycoforms of antibody Fc domain. Presence of a bisecting sugar moiety enhances the affinity of Fc to FcgammaIIIa receptor. J Am Chem Soc. 2011;133:18975–18991. doi: 10.1021/ja208390n. [DOI] [PMC free article] [PubMed] [Google Scholar]