Abstract
Drosophila seminal fluid proteins elicit physiological and behavioral changes in the female after mating. For example, the seminal protein sex peptide (SP) causes females to lay more eggs, reduce receptivity to re-mating, consume more food and produce more concentrated excreta upon mating. It has been reported that SP indirectly increases food consumption as a result of its stimulation of egg production, but its role in producing more concentrated excreta in the mated female was reported to be independent of egg production. Additionally, it has been shown that SP's effect on food consumption persists for several days after mating, while it is unknown whether this is true for its effect on excretion.
SP can have both transient and long-term effects on mated females; the latter occur because of the peptide's binding to, and slow release from, sperm in the female. Here we used timed measures of excretion by female flies that had mated to males mutant in SP or in its regulators, to test the duration of SP's effect on excretion. We found that SP's effect on excretion persists for at least ~1 week after mating, and that this persistence requires that SP bind to and be released from sperm. Although these binding/release requirements of SP are similar to those for increased egg production (and consequent increased food intake) following mating, we find that the long-term change in excretion phenotype is only partially dependent on the presence of eggs in the female. Our data indicate that a change in intestinal transit is part of the long-term post-mating response elicited by the gradual release of sperm-bound SP in the female after mating, even though it is not fully dependent of other long-term responses elicited by SP.
Keywords: Drosophila melanogaster, feeding, excretion, post-mating response, sperm, sex peptide
1. Introduction
In Drosophila melanogaster, and many other insects, seminal fluid proteins transferred during mating elicit numerous changes in female behavior and physiology (reviewed in Avila et al., 2011 and Wolfner, 2009). Since nutrition plays a key role in reproductive success (Chapman and Partridge, 1996), it is not surprising that one post-mating change in the Drosophila female involves an increase in food intake (Carvalho et al., 2006). A recent study found that while food intake increases after mating, intestinal transit is significantly decreased (Cognigni et al., 2011). This slower intestinal transit results in more concentrated fecal deposits, referred to as reproductive oblong deposits (RODs), and is suggested to be an advantageous mechanism to increase nutrient absorption at a energetically demanding time for the female (Cognigni et al, 2011). In addition to increased food intake and slower intestinal transit, mating elicits a change in food preference by female Drosophila: virgin females prefer carbohydrates, while mated females prefer protein-rich food (Ribeiro and Dickson, 2010; Vargas et al., 2010). These responses are biologically important to the female since mating increases oogenesis and egg laying, which are energetically costly. Previous studies show that the male accessory gland protein, sex peptide (SP), is involved in each of these responses (Barnes et al., 2008; Carvalho et al., 2006; Cognigni et al., 2011; Ribeiro and Dickson, 2010; Vargas et al., 2010).
SP has multiple roles in the post-mating response, such as increasing egg laying and reducing receptivity to re-mating, both short-term (within the first 24 hours after mating) and long-term (up to 2 weeks after mating). Within the first 24 hours after mating, SP stimulates oocyte progression and accumulation of yolk proteins in the oocyte leading to increased egg production (Moshitzky et al., 1996; Soller et al., 1997). Sex peptide also plays major roles in the long-term response (LTR). As part of the LTR, SP decreases receptivity to re-mating and increases egg laying for up to 10 days after mating (Chapman et al., 2003; Liu and Kubli, 2003). SP produces these long-term effects by binding to sperm in the female reproductive tract and then being gradually released, by tryptic cleavage, from stored sperm over several days (Peng et al., 2005). Therefore, sperm are essential for the long-term persistence of SP's effects (Manning, 1962, 1967; Peng et al., 2005). For egg production and receptivity changes, SP exerts its effects on the female through the sex peptide receptor (SPR) found on neurons that express fruitless (fru) and pickpocket (ppk) in the female reproductive tract (Häsemeyer et al., 2009; Yang et al., 2009).
Previously, it was shown that increased feeding after mating is a long-term response that is dependent upon egg production (Barnes et al., 2008). Therefore, it is likely that SP increases feeding indirectly by increasing egg production. However, Cognigni et al. (2011) found SP was also responsible for producing more concentrated excreta, independent of egg production. These data suggest that increased food consumption and slower intestinal transit are separable processes.
Cognigni et al. (2011) measured fly excreta production over a single 72 hour window, so it was not possible to determine whether the effect of SP that they observed was short-term, long-term or both. Therefore, to address this question, we measured ROD production in females at different times post-mating. By using crosses with males that produce normal or modified SP, and with males that do not transfer sperm, we investigated whether the change in intestinal transit is a long-term response that requires the release of sperm-bound SP. We also modified Cognigni et al.'s assay to indirectly measure food intake by quantifying the mean total fecal output per fly. We found that ROD production is a long-term response that requires the release of sperm-bound SP to persist. However, unlike increased feeding behavior, the change in intestinal transit, as reflected by ROD production, is only partially dependent upon egg production.
2. Materials and Methods
2.1. Flies
Sex peptide null males (SP0/Δ130) were generated by crossing the SP null line (SP0/TM3, Sb ry) and the SP deficiency line (Δ130/TM3, Sb Ser) (Liu and Kubli, 2003). SP cleavage mutant males (w/Y; SP-TGQQ/+; SP0/Δ130) were obtained by crossing the SP null line (SP0/TM3, Sb ry) to the SP-TGQQ line (y w/Y; SP-TGQQ/SP-TGQQ; Δ130/TM3, Sb Ser) (Peng et al., 2005). The SP null mutant line, the SP deficiency line and the SPTGQQ lines were all kind gifts of Eric Kubli (University of Zurich, Zurich, Switzerland). Spermless and eggless flies (tud1 bw sp/+) were the progeny of a cross of tud1 bw sp females to Canton S (CS) males. tud1 is a recessive maternal effect mutation that results in offspring that are unable to form a germline (Boswell and Mahowald, 1985). Sperm-and egg-producing controls for this experiment were also tud1 bw sp/+, but were generated by crossing tud1 bw sp/CyO females to CS males. CG1656 and CG1652 knockdown males were obtained by crossing a transgenic line carrying the RNAi construct for CG1656 or CG1652 (Ravi Ram and Wolfner, 2007) to a ubiquitous driver line (tubulin-Gal4/TM3, Sb). CG1656 and CG1652 are gene duplicates and off-targets of one-another (Ravi Ram and Wolfner, 2007). Males that were UAS-RNAi/TM3, Sb were used as controls. Flies were reared on yeast-glucose media at room temperature (as in Ravi Ram and Wolfner, 2009), collected as virgins after eclosing, and aged in single-sex groups for 3–5 days prior to use in experiments.
2.2. Excreta assay
Each mating was carried out by placing a single 5-day old virgin female with a single 5-day old male in a glass vial containing a square piece of moistened filter paper. Matings were observed, and pairs with unusually short matings (<15 minutes) were discarded so that we only studied pairs that had adequate time to transfer ejaculate. Upon completion of mating, four females were placed into a 35mm petri dish containing a wedge of standard yeast-glucose food supplemented with 0.5% Bromophenol blue (B5525, Sigma) (Cognigni et al., 2011). Females were left undisturbed for 24 hours, after which they were aspirated to a new 35mm petri dish containing a new wedge of food. Digital images of the petri dish were obtained using an Epson Perfection 2400 Photo scanner. This was repeated every 24 hours to obtain scans at 24, 48, and 72 hours post-mating. The assay was performed in multiple sets of 3 to 4 flies for each genotype and condition tested. For longer-term tests, flies were transferred to fresh plates on days 1, 3, 5 and 7 after mating, and the plates were scanned as above to quantify excreta from days 1, 2+3, 4+5 and 6+7, respectively.
2.3. Quantitative analysis of excreta
The percentage of reproductive oblong deposits (RODs) (Cognigni et al., 2011) was calculated by determining the number of RODs relative to the total number of fecal spots. In order to quantify the total amount of excreta on each plate, Metamorph® imaging software was used. Briefly, the pixel area, optical density, and integrated optical density (IOD; pixel area multiplied by the optical density) were obtained for each fecal spot. Parameters for shape and size were set at 0.2–1.0 shape factor and 50–700 square pixels, respectively, to eliminate background noise. In order to quantify the amount of excreta per fly, the IOD for all excreta spots on one petri dish were added and the sum was divided by the number of flies in the set. This also allowed us to adjust for flies that were lost or died throughout the 72 hour or one week experiment. For statistical analysis, pair-wise comparisons of IOD of excreta per fly for the sets were performed using ANOVA in JMP to determine the statistical differences between genotype, days post-mating and mated versus virgin flies.
3. Results
3.1. The post-mating change in ROD production is a long-term response that persists beyond the first day after mating
Cognigni et al. (2011) reported that mated females produce significantly more reproductive oblong deposits (RODs) than virgin females during a 72 hour window after mating. To determine whether the response occurred within the first 24 hours (short-term) or over the entire 72 hours (short-term and long-term) we subdivided this window. We mated 4 CS females to CS males and quantified the excreta in each of the first three days following mating (see Materials and Methods). We found that CS females that had mated to CS males produced significantly more RODs compared to CS virgin females at 24 hours (p=0.01), 48 hours (p<0.0001) and 72 hours (p<0.0001) after the start of mating (ASM) (Fig. 1A). In order to quantify the total amount of excreta at these time points, we calculated the integrated optical density (IOD) (see Materials and Methods). Consistent with a previous report that increased feeding is dependent on increased egg laying (Barnes et al., 2008), itself a long-term post-mating response (Chapman et al., 2003; Liu and Kubli, 2003), we found that the mean IOD of excreta per mated female was significantly higher than the mean IOD of excreta per virgin female at 24 hours (p<0.0001), 48 hours (p<0.0001) and 72 hours (p=0.0424) ASM (Fig. 1B). We conclude that in addition to increased feeding, ROD production, a measure of intestinal transit, is a long-term post-mating response that persists for at least 72 hours after mating.
Figure 1.
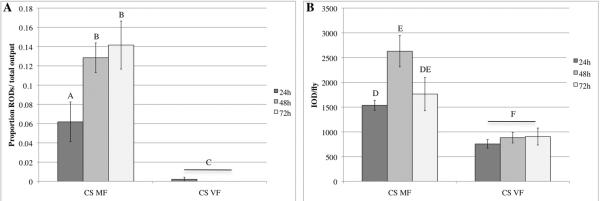
The effect of mating on excreta produced by Drosophila females at 24 hours, 48 hours and 72 hours post-mating. Bars with the same letter are statistically similar; MF = mated females, VF = virgin females. (A) The proportion of reproductive oblong deposits (RODs) was calculated at each time point. Females mated to CS males (CS MF) produced more RODs than CS virgin females (CS VF) at 24 hours (p=0.01), 48 hours (p<0.0001) and 72 hours (p<0.0001). (B) The integrated optical density (IOD) of excreta despoits per fly was calculated by totaling the IOD (pixel area x optical density) for the entire petri dish and dividing by the number of flies. The IOD of excreta per fly for CS MF was significantly higher than CS VF at 24 hours (p<0.0001), 48 hours (p<0.0001) and 72 hours (p=0.0424). Total numbers of flies used are as follows: for 24 hours, nCSMF = 39 and nCSVF = 40, for 48 hours, nCSMF = 36 and nCSVF = 36, for 72 hours, nCSMF = 35 and nCSVF = 29.
3.2. The role of sex peptide in the post-mating increase in ROD production
3.2.1. Sex peptide regulates ROD production for at least ~1 week post-mating
The seminal protein, sex peptide (SP), is necessary for the post-mating change in intestinal transit (Cognigni et al., 2011). To test whether SP affects ROD production and total IOD of excreta per fly, long-term as well as short-term, we first mated females to SP-deficient (or control) males and assessed ROD production on each of the first three days post-mating (Fig. 2A). At 24, 48 and 72 hours ASM, CS females mated to control males (that transferred SP) produced significantly more RODs than CS virgin females (p=0.0016, p=0.0014 and p=0.0014, respectively). In addition, at 48 hours and 72 hours ASM, control-mated females produced significantly more RODs than females mated to SP null males (p=0.001 and p=0.0014). This indicates that SP is responsible for eliciting long-term ROD production. However, in this experiment, ROD production at 24 hours was not significantly different between females mated to control males and females mated to SP null males, and in both cases was significantly greater than that of unmated females. Although this could suggest that another seminal fluid protein, besides SP, is able to elicit ROD production within the first 24 hours, we did not observe this effect in later experiments using SP null males with other wild type females (Fig. 4C), suggesting that the 24 hour response we observed here was specific to the CS line we used. In order to determine whether SP's effect on ROD production could persist long-term, we next mated CS females to either control males or SP null males and quantified ROD production on days 1, 2+3, 4+5 and 6+7 (fig. 2C). Control-mated females produced more RODs than virgins or than SP null-mated females at each time point (p=0.0061, p<0.0001, p<0.0001 and p<0.0001, respectively). Further, there were no differences between SP null-mated females and virgin females at all time points, indicating that SP is required for the increase in ROD production for at least ~1 week after mating. [Since the long-term response is already well established by 72 hours post-mating, for convenience we used a 72-hour timepoint for most of our remaining experiments.]
Figure 2.
Sex peptide elicits long-term post-mating excretion change in females. (A) Females mated to control males (SP+ MF) produced significantly more RODs than virgin females (CS VF) at 24 hours (p=0.0016), 48 hours (p=0.0014) and 72 hours (p=0.0014) and than CS females that had mated to SP null males (SP0 MF) at 48 hours (p=0.001) and 72 hours (p=0.0014). SP null-mated females produced significantly more RODs than virgin females at 24 hours (p=0.0235). (B) The mean IOD of excreta per fly for SP+ mated females was significantly higher than SP0 mated females at 24 hours (p=0.017), 48 hours (p=0.0306) and 72 hours (p=0.001) and virgin females at each time point (p=0.0012, p=0.0299 and p=0.0042, respectively), while there was no difference between SP0 mated females and virgin females at any time point. In panels A and B, bars with the same letter are statistically similar. Total numbers of flies used are as follows: for 24 hours, nSP0MF = 40, nSP+MF = 39 and nCSVF = 40, for 48 hours, nSP0MF = 38, nSP+MF = 39 and nCSVF = 38, for 72 hours, nSP0MF = 38, nSP+MF = 39 and nCSVF = 37. (C) Females mated to control males (SP+ MF) produced significantly more RODs than virgin females (CS VF) 1 day (p=0.0224), 2+3 days (p<0.0001), 4+5 days (p<0.0001) and 6+7 days (p<0.0001) ASM and more RODs than females mated to SP null males (SP0 MF) at each time point (p=0.0061, p<0.0001, p<0.0001, and p<0.0001, respectively). (D) The mean IOD of excreta per fly for SP+ mated females was significantly higher than SP0 mated females at 1 day (p=0.036), 2+3 days (p=0.0002), 4+5 days (p<0.0001) and 6+7 days (p<0.0001) ASM and higher than virgin females at each time point (p=0.0488, p=0.0002, p=0.0003 and p<0.0001, respectively). In panels C and D, measurements were taken at 1 day ASM, 3 days ASM, 5 days ASM and 7 days ASM. p<0.05, p**<0.01, p***<0.0001. Total numbers of flies used in C and D are as follows: for day 1, nSP0MF = 36, nSP+MF = 39 and nCSVF = 44, for days 2+3, nSP0MF = 35, nSP+MF = 39 and nCSVF = 39, for days 4+5, nSP0MF = 31, nSP+MF = 38 and nCSVF = 38, for days 6+7, nSP0MF = 31, nSP+MF = 38 and nCSVF = 38.
Figure 4.
SP elicits long-term ROD production partially independent of egg production. (A) Mated control females (M Control F) produced significantly more RODs than virgin control females (V Control F) and mated eggless females (M Eggless F) at 24 hours (p=0.0026 and p=0.0054), 48 hours (p<0.0001 and p=0.0003) and 72 hours ASM (p=0.0001 and p=0.0008), while mated eggless females produced more RODs than virgin eggless females (V Eggless F) only at 72 hours after mating (p=0.0156). (B) Mated control females had a significantly higher IOD of excreta per fly than virgin control females and mated eggless females at 24 hours (p=0.0004 and p=0.002), 48 hours (p=0.0005 and p=0.0003) and 72 hours (p<0.0001 and p<0.0001). There was no significant difference between eggless mated females and eggless virgin females. (C) Eggless females mated to control males (SP+ M) produced more RODs than eggless females mated to SP0 males (SP0 M) and eggless virgin females at 48 hours (p=0.014 and p=0.0167) and 72 hours (p=0.0045 and p=0.0095) ASM. Control females mated to SP+ males produced significantly more RODs than eggless females mated to SP+ males at all three time points ASM (p=0.01, p=0.0034, and p=0.0002, respectively). (D) There was no significant difference in IOD of excreta per fly between eggless females mated to SP0 males, eggless females mated to SP+ males and eggless virgin females at 24 hours, 48 hours and 72 hours ASM. Control females mated to SP+ males at a higher IOD of excreta per fly than control females mated to SP0 males at 48 hours (p=0.0006) and 72 hours (p=0.0007) and control virgin females at all three time points ASM (p=0.0052, p=0.0003 and p<0.0001, respectively). Bars with the same letter are statistically similar. Total numbers of flies used for A and B are as follows: for 24 hours, nMEF = 51, nVEF = 52, nMCF = 52 and nVCF = 52, for 48 hours, nMEF = 49, nVEF = 50, nMCF = 49 and nVCF = 50, for 72 hours, nMEF = 44, nVEF = 48, nMCF = 47 and nVCF = 45. Sample sizes for B and C are as follows: for eggless females at 24 hours, nSP0M = 30, nSP+M = 33, nV = 28, for 48 hours, nSP0M = 29, nSP+M = 33, nV = 27, for 72 hours, nSP0M = 28, nSP+M = 33, nV = 22. For control females at 24 hours, nSP0M = 27, nSP+M = 41, nV = 42, for 48 hours nSP0M = 27, nSP+M = 40, nV = 41, for 72 hours, nSP0M = 25, nSP+M = 39, nV = 38.
As expected from the known SP-dependent increase in feeding post-mating (Carvalho et al., 2006) and the coupling of increased feeding to increased egg production (Barnes et al., 2008), the IOD measurements showed that females mated to control males had a significantly higher IOD of excreta per fly than both CS virgins (p=0.0012, p=0.0299, p=0.0042) and females mated to SP null males (p=0.017, p=0.0306, p=0.001) at each time point, while there was no significant difference between CS virgins and females mated to SP null males at any time point (Fig. 2B). Furthermore, the SP-dependent increase in IOD persisted for at least 6–7 days after mating (Fig. 2D).
3.2.2. SP binding to and release from sperm is required for the long-term increase in ROD production
In order to determine whether the gradual cleavage of sperm-bound SP is responsible for eliciting the post-mating change in ROD production, we mated females to three different types of males: male progeny of tudor females (spermless males), males that produce a form of SP that cannot be cleaved from sperm (Peng et al., 2005), and males that were knocked down for seminal fluid proteins CG1656 and CG1652, which are lectins that have been implicated in binding SP to sperm (Ravi Ram and Wolfner, 2009).
At all three time points that we examined (24, 48 and 72 hours ASM), females mated to spermless males produced significantly fewer RODs than females mated to control males (p=0.0018, p=0.0019 and p<0.0001, respectively; Fig. 3A). At 48 hours ASM, however, females mated to spermless males produced significantly more RODs than virgin females, presumably due to unbound SP transferred in the seminal fluid. This was also true for 24 hours ASM, however it was not statistically different due to an unexpected number of RODs produced by virgin females at 24 hours ASM in this experiment. Importantly, by 72 hours ASM, females mated to spermless males did not produced more RODs than virgin females, suggesting that stored sperm is required in the female for ROD production to persist long-term. Since the transfer of sperm is necessary for the long-term increase in egg production after mating, it would be expected that this would also be true for increased feeding. As expected, at 48 and 72 hours post-mating, females mated to spermless males had a significantly lower IOD of excreta per fly than females mated to control males (p=0.0023 and p<0.0001, respectively). At 24 hours there was no significant difference in IOD between spermless- and control-mated females (p=0.1164), again likely due the effect of unbound SP that was transferred in the seminal fluid (Fig. 3B). Together, these data suggest that stored sperm is required in the female for both increased feeding and ROD production to persist long-term.
Figure 3.
The gradual release of sperm-bound sex peptide is responsible for the long-term increase in ROD production after mating. (A) Females mated to control males (Control MF) produced significantly more RODs than females mated to spermless males (Spermless MF) and virgin females (CS VF) at 24 hours (p=0.0018 and p=0.0005), 48 hours (p=0.0019 and p=0.0002) and 72 hours after mating (p<0.0001 and p<0.0001). Spermless mated-females produced significantly more RODs than virgin females only at 48 hours (p=0.0243). (B) Mean IOD of excreta per fly for control-mated females was higher than virgin females at 24 hours (p=0.0008), 48 hours (p<0.0001) and 72 hours (p<0.0001) and higher than spermless-mated females at 48 hours (p=0.0023) and 72 hours (p<0.0001). (C) Females mated to control males produced more RODs than virgin females at all 3 time points ASM (p=0.0026, p<0.0001 and p<0.0001) and more RODs than females mated to males with non-cleavable SP (SP-TGQQ MF) at 48 and 72 hours (p=0.0179 and p<0.0001). SP-TGQQ -mated females also produced more RODs than virgin females at all 3 time points (p=0.0004, p<0.0001, p=0.0065). (D) Mean IOD of excreta per fly for control mated females was significantly higher than SP-TGQQ -mated females at 48 and 72 hours ASM (p=0.0101 and p=0.0216). (E) Control-mated females produced more RODs than virgin females at each time point after mating (p=0.0197, p<0.0001 and p<0.0001) and more RODs than females mated to CG1656 knockdown males (1656 KD MF) at 48 hours (p=0.0002) and 72 hours (p=0.0003). (F) Females mated to control males and females mated to CG1656 knockdown males had a higher IOD of excreta per fly than virgin females at 24 hours (p=0.007 and p=0.0034), 48 hours (p=0.0154 and p=0.0185) and 72 hours (p=0.0007 and p=0.0005) while there was no significant difference between females mated to control or knockdown males. Bars with the same letter are statistically similar. Total numbers of flies used are as follows: for 24 hours, nSPERMLESS = 64, nCONTROL = 63, nCSVF = 64, nSP-TGQQ = 52, nCONTROL = 67, nCSVF = 59, n1656KD = 35, nCONTROL = 36, nCSVF = 24, for 48 hours, nSPERMLESS = 59, nCONTROL = 61, nCSVF = 63, nSP-TGQQ = 52, nCONTROL = 67, nCSVF = 58, n1656KD = 31, nCONTROL = 35, nCSVF = 24, for 72 hours, nSPERMLESS = 56, nCONTROL = 53, nCSVF = 61, nSP-TGQQ = 51, nCONTROL = 64, nCSVF = 58, n1656KD = 27, nCONTROL = 34, nCSVF = 24.
In order to investigate whether the gradual release of SP from sperm is necessary for ROD production to persist, females were mated to males that produce a form of SP that cannot be released from sperm (SP-TGQQ males) (Peng et al., 2005). As an independent test of whether SP binding to sperm is needed for long-term ROD production, we also examined females that had mated to males that were knocked down for CG1656 and CG1652, seminal fluid proteins necessary to bind SP to sperm in the female (Ravi Ram and Wolfner, 2007, 2009). If the gradual release of sperm-bound SP is required for the post-mating increase in ROD production to persist, females that had mated to SP-TGQQ males or CG1656/1652 knockdown males should lack this long-term response. Indeed, we found that at 48 hours and 72 hours ASM, females mated to control males produced significantly more RODs (p=0.0179 and p<0.0001) and had a higher IOD of excreta per fly (p=0.0101 and p=0.0216) than females mated to SP-TGQQ males (Fig. 3C and D). Also, females mated to CG1656 knockdown males produced significantly fewer RODs at 48 and 72 hours post-mating than females mated to control males (p=0.0002 and p=0.0003) (Fig. 3E). Given that CG1656 and CG1652 are off-targets of one another (Ravie Ram and Wolfner, 2007), similar results were obtained with CG1652 knockdown males (data not shown). Interestingly, there was no difference in IOD of excreta between control- and knockdown-mated females at any time point ASM (Fig. 3F). This was unexpected given the observation that the cleavage of sperm-bound SP is necessary for the increase in feeding long-term (Fig. 3D). One possibility is that since RNAi does not completely knockdown the expression of CG1656, its residual expression was enough to allow sufficient SP persistence to increase the amount of excreta. However, given our results, we conclude that the binding of SP to sperm and its gradual release from sperm is essential for the long-term increase in ROD production after mating.
3.3. The post-mating increase in ROD production is only partially dependent upon egg production
Peng et al. (2005) found that females mated to transgenic males with SP that either could not be bound to sperm or could not be cleaved from sperm only showed only a short-term increase in post-mating egg laying; the long-term response was abolished. Additionally, previous data suggests that the post-mating increase in feeding is dependent upon egg production (Barnes et al., 2008). Therefore, it is not surprising that we observed that the increase in feeding as measured by IOD of excreta depends on SP binding to sperm. SP's effect on feeding may occur indirectly through egg production.
However, Cognigni et al. (2011) found that increased ROD production occurs after mating even in females whose oogenesis was arrested by an ovoD1 mutation (Oliver et al., 1987). To investigate if long-term ROD production occurs in females that cannot produce any eggs, we mated germline-less females (see Materials and Methods) to CS males and quantified their ROD production and IOD of excreta per fly at 24, 48 and 72 hours ASM. At 72 hours ASM, mated eggless females produced significantly more RODs than virgin eggless females (p=0.0156) (Fig. 4A). They also produced more RODs at 48 hours, though this was not statistically significant. Therefore, these data suggest that long-term ROD production after mating can occur even without egg production. However, we found that at each time point ASM, mated eggless females produced significantly fewer RODs than mated control females (p=0.0054, p=0.0003 and p=0.0008, respectively) (Fig. 4A). This may suggest that ROD production is not completely uncoupled from egg production. Additionally, as expected, our data show that the IOD of excreta per fly is completely dependent upon egg production since mated eggless females had a significantly lower IOD of excreta per fly than mated control females at each time point (p=0.002, p=0.0003, p<0.0001), while mated eggless females showed no significant difference in IOD when compared to virgin eggless females (p=0.3707, p=0.2186, p=0.0627) (Fig. 4B). These data suggest that total excreta upon mating (and, indirectly, food consumption) is dependent upon egg production, while long-term ROD production may be uncoupled from egg production to some extent. Our data show that ROD production is not completely independent, however, since there were still significant differences between mated control females and mated eggless females.
To further investigate the finding from Cognigni et al. (2011) that the requirement for SP in ROD production may be uncoupled from egg production, we sought to determine if SP was responsible for eliciting the long-term ROD production in eggless females. Cognigni et al. (2011) had found that sterile ovoD1 females produced RODs when mated to wild type males but not when mated to SP null males. Here, we show that eggless females mated to wild type males produce significantly more RODs than eggless females mated to SP null males at 48 and 72 hours ASM (p=0.014 and p=0.0045, respectively) (Fig. 4C), indicating that SP elicits long-term ROD production independently of egg production. We found, however, that the proportion of RODs produced by eggless females mated to wild type males was significantly lower than control females mated to wild type males at all three time points ASM (p=0.01, p=0.0034, and p=0.0002). This furthermore suggests that SP elicits long-term ROD production independently of egg production, but the two are not entirely separable responses.
4. Discussion
We used a previously described assay (Cognigni et al., 2011) to investigate whether the post-mating change in the processing of food in the Drosophila female intestine elicited by SP is part of the long-term post-mating response. Cognigni et al. (2011) previously reported that over a single 72-hour window, mated females produced more RODs than virgin females and this effect required SP from the males. It was unclear, however, whether the RODs they observed derived from short-term effects of SP or also reflected its long-term effects, which are sperm-dependent. When we examined ROD production at time points within and beyond the 72 hour window, we found that ROD production was elevated not only at 24 hours, but also at 48 and 72 hours ASM as well as for at least ~1 week after mating. This indicates that SP regulates ROD production as part of both the short-term and the long-term response to mating.
While it was also reported that SP elicits ROD production independent of egg production (Cognigni et al., 2011), this was only partially the case in our experiments (Fig. 4A). The difference may be explained by the type of eggless females used in the two studies: the tudor progeny used in this study completely lack a germline while Cognigni et al. (2011) used ovoD1 females which have a germline, but whose oocytes arrested in development (Oliver et al., 1987). We conclude that ROD production is likely dependent upon two factors: egg production and SP. The long-term increase in feeding behavior, however, is dependent on an increase in egg production that results from the gradual cleavage of sperm-bound SP in the female reproductive tract over several days (Barnes et al., 2008; this study).
Our data and those from previous studies on post-mating feeding behavior in Drosophila females indicate multiple, and possibly separable, roles for SP in eliciting these changes in the female that ultimately maximize the female's nutritional intake at an energetically demanding time. First, SP increases food consumption after mating (Carvalho et al., 2006) and this is dependent on SP increasing egg production (Barnes et al., 2008). The gradual cleavage of sperm-bound SP is responsible for the long-term increase in egg production post-mating (Liu and Kubli, 2003; Peng et al., 2005), and our data show that the cleavage of SP is also necessary for increased food consumption, consistent with SP's role in food intake being an indirect result of its role in increasing egg production (Barnes et al., 2008). Second, SP causes a change in intestinal transit post-mating, partially independent of egg production (Cognigni et al., 2011; this study). Cognigni et al. (2011) showed that the intestinal contents of mated females are more concentrated than those of virgin females; mating-dependent production of RODs, as highly concentrated excreta, further supports this conclusion (Cognigni et al., 2011). We showed here that ROD production is a long-term response that requires the gradual cleavage of sperm-bound SP to persist, and thus is likely mediated by the C terminus of SP (Peng et al., 2005). Future studies, including investigation of changes in the timing of ROD production after post-mating feeding, are needed to define the precise nature of SP's role in modifying intestinal transit and excreta characteristics. Finally, previous studies have shown that SP is involved in the switch in preference from carbohydrate-rich food to protein-rich food after mating, also independent of egg production (Ribeiro and Dickson, 2010). Therefore, it appears that SP is responsible for coordinating several behaviors that lead to the optimization of nutrient intake and processing the female upon mating, providing a reproductive advantage for maximal fertility.
5. Conclusions
In summary, we investigated the role of the seminal protein SP in changing the rate and characteristics of excretion by Drosophila females, during the first post-mating week. We quantified both total fecal output (as a measure of food consumption) and the proportion of highly concentrated RODs (which reflect slower intestinal transit (Cognigni et al., 2013)). We found that increased food intake/excretion and slower intestinal transit are long-term post-mating responses that require the binding of SP to sperm in females, followed by SP's gradual release by cleavage from the sperm. The region (and sperm-binding) of SP required for long-term ROD production are similar to those that regulate long-term egg production, but the long-term increase in ROD production is partially independent of egg production (in contrast to the increase in total food consumption).
Acknowledgements
This work was supported by NIH grant R01-HD038921 (to MFW). For part of these studies, JA was also supported on NIH Training Grant # T32 GM07617. We thank Irene Miguel-Aliaga (University of Cambridge) and Carol Bayles (Cornell University) for technical advice, the Cornell University Microscopy and Imaging Facility for Metamorph® use, and Clement Chow, Geoff Findlay, Frank Avila, and an anonymous reviewer for their comments on this manuscript.
References
- Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annual Review of Entomology. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AI, Wigby S, Boone JM, Partridge L, Chapman T. Feeding, fecundity and lifespan in female Drosophila melanogaster. Proceedings of the Royal Society B Biological Sciences. 2008;275(1643):1675–1683. doi: 10.1098/rspb.2008.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell RE, Mahowald AP. Tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell. 1985;43:97–104. doi: 10.1016/0092-8674(85)90015-7. [DOI] [PubMed] [Google Scholar]
- Carvalho GB, Kapahi P, Anderson DJ, Benzer S. Allocrine Modulation of Feeding Behavior by the Sex Peptide of Drosophila. Current Biology. 2006;16:692–696. doi: 10.1016/j.cub.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Partridge L. Female fitness in Drosophila melanogaster: An interaction between the effect of nutrition and of encounter rate with mates. Proceedings: Biological Sciences. 1996;22:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proceedings of the National Academy of Sciences USA. 2003;100(17):9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric Neurons and Systemic Signals Couple Nutritional and Reproductive Status with Intestinal Homeostasis. Cell Metabolism. 2011;13:92–104. doi: 10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häsemeyer M, Yapici N, Heberlein U, Dickinson BJ. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 2009;61(4):511–518. doi: 10.1016/j.neuron.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proceedings of the National Academy of Sciences USA. 2003;100(17):9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning A. A Sperm Factor Affecting the Receptivity of Drosophila melanogaster Females. Nature. 1962;194:252–253. [Google Scholar]
- Manning A. The control of sexual receptivity in Drosophila. Animal Behaviour. 1967;15(2–3):239–250. doi: 10.1016/0003-3472(67)90006-1. [DOI] [PubMed] [Google Scholar]
- Moshitzky P, Fleischmann I, Chaimov N, Suadan P, Klauser S, Kubli E, Applebaum SW. Sex-peptide activates juvenile hormone biosynthesis in the Drosophila melanogaster corpus allatum. Archives of Insect Biochemistry and Physiology. 1996;32(3–4):363–374. doi: 10.1002/(SICI)1520-6327(1996)32:3/4<363::AID-ARCH9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Oliver B, Perrimon N, Mahowald AP. The ovo locus is required for sex-specific germ line maintenance in Drosophila. Genes & Development. 1987;1:913–923. doi: 10.1101/gad.1.9.913. [DOI] [PubMed] [Google Scholar]
- Peng J, Chen S, Büsser S, Liu H, Honegger T, Kubli E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Current Biology. 2005;15:207–213. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Ravi Ram K, Wolfner MF. Sustained Post-Mating Response in Drosophila melanogaster Requires Multiple Seminal Fluid Proteins. PLoS Genetics. 2007;3(12):e238. doi: 10.1371/journal.pgen.0030238. doi:10.1371/journal.pgen.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram K, Wolfner MF. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proceedings of the National Academy of Sciences USA. 2009;106(36):15384–15389. doi: 10.1073/pnas.0902923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Current Biology. 2010;20:1000–1005. doi: 10.1016/j.cub.2010.03.061. [DOI] [PubMed] [Google Scholar]
- Soller M, Bownes M, Kubli E. Mating and Sex Peptide Stimulate the Accumulation of Yolk in Oocytes of Drosophila melanogaster. European Journal of Biochemistry. 1997;243(3):732–738. doi: 10.1111/j.1432-1033.1997.00732.x. [DOI] [PubMed] [Google Scholar]
- Vargas MA, Luo N, Yamaguchi A, Kapahi P. A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Current Biology. 2010;20:1006–1011. doi: 10.1016/j.cub.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner MF. Battle and ballet: molecular interactions between the sexes in Drosophila. Journal of Heredity. 2009;100(4):399–410. doi: 10.1093/jhered/esp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, Jan YN. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron. 2009;61(4):519–526. doi: 10.1016/j.neuron.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]





