Abstract
Objective
To evaluate long-term patient survival and causes of death after open (OR) or endovascular (ER) mesenteric revascularization for atherosclerotic chronic mesenteric ischemia using propensity score-matched comparison and clinical risk stratification.
Methods
The clinical data of 343 patients treated with mesenteric revascularization for chronic mesenteric ischemia between 1991 and 2010 were retrospectively reviewed. Clinical, anatomical, and procedure-related variables were analyzed using a multivariate model to identify independent predictors of any-cause early and late (>30 days) mortality. Cause of death was retrieved from review of the National Death Index. Patient survival was analyzed using Society for Vascular Surgery (SVS) comorbidity scores and propensity score-matched comparison based on independent predictors of any-cause mortality.
Results
There were 187 patients treated by OR and 156 patients treated by ER. Early procedure-related mortality was 2.6% (9/343), including five OR (2.7%) and four ER (2.6%) patients. Median follow-up was 96 ± 54 months (range, 1-168 months). There were 144 late deaths, most commonly from cardiac causes in 35% (51/144), followed by cancer in 15% (21/144), pulmonary complications in 13% (19/144), and mesenteric ischemia in 11% (16/144). A further 21 patients died from various identifiable causes, and 14 patients (10%) died of unknown causes. Overall, 25 patients (7.3%) died of mesenteric-related causes, including nine early and 16 late deaths (OR, 10/187; 8.0%, and ER, 6/156; 6.4%). Multivariate analysis identified age >80, diabetes, chronic kidney disease (CKD) stage IV or V, and home oxygen therapy as independent predictors (P < .05) of any cause of death. Diabetes and CKD stage IV or V were independently associated with mesenteric-related death (P < .05). Late patient survival at 5 years in the OR and ER groups was 75% ± 4% and 60% ± 9% for low SVS risk (<9), 52% ± 8% and 43% ± 9% for intermediate SVS risk (9-16), and 67% ± 15% and 30% ± 8% for high SVS risk (>16). Using propensity matched scores, 5-year survival was nearly identical for patients treated by OR (60%) or ER (57%; P = .7).
Conclusions
Long-term patient survival after mesenteric revascularization was not influenced by type of arterial reconstruction. Age >80 years, diabetes, CKD stage IV or V, and home oxygen were independent predictors of any-cause mortality. Diabetes and CKD stage IV or V were independently associated with mesenteric-related death.
Mesenteric artery stenting has surpassed open surgery as the most commonly used treatment for patients with atherosclerotic chronic mesenteric ischemia (CMI), relegating open surgical bypass to patients in whom mesenteric artery stenting fails or who have lesions unsuitable to stenting. Based on the best available evidence, endovascular revascularization (ER) has reduced early mortality and morbidity compared with open revascularization (OR), but it is associated with higher rates of restenosis and reintervention.1
Poor prognostic indicators for long-term patient survival after mesenteric revascularization include advanced age and presence of severe cardiac, pulmonary, or renal disease.2 The type of revascularization has not been shown to affect survival, but comparative analysis is limited by selection bias favoring OR for good-risk patients and ER for higher-risk patients. It is possible that survival may be affected by type of reconstruction, given that recurrent mesenteric ischemia and reinterventions are potentially associated with morbidity and mortality. The aim of this study was to evaluate long-term patient survival and causes of death in a large cohort of patients with CMI treated with OR or ER using standardized clinical scores and a propensity score-matched comparison.
METHODS
The study was approved by the institutional review board of the Mayo Clinic. The clinical, anatomical, and procedural data on all consecutive patients treated for atherosclerotic CMI with OR or ER between 1991 and 2010 were retrospectively reviewed. The diagnosis of CMI was made in patients with classic symptoms (abdominal pain, postprandial pain, weight loss, “food fear”) for >2 weeks and angiographic evidence of high-grade stenosis or occlusion of at least one mesenteric artery. Patients with acute or acute-on-chronic mesenteric ischemia, asymptomatic lesions, vasculitis, median arcuate ligament syndrome, or other nonatherosclerotic etiologies were excluded from the study.
Demographics, clinical characteristics, radiologic data, and operative data were obtained from the medical records. Operative risk was assessed using Society for Vascular Surgery (SVS) comorbidity scores.3 Early postprocedure period was defined as occurring within the first 30 days or within hospital stay if >30 days. Late follow-up period was defined as after 30 days or dismissal from the hospital until the last available clinical examination or correspondence. Follow-up consisted of clinical examination and duplex ultrasound every 6 months during the first year and annually thereafter. Late follow-up data were obtained from review of medical records, correspondence with patient or referral physician, phone interview, or questionnaire. The cause of death was retrieved by review of medical records or autopsy reports. The Minnesota and Olmsted County death certificate database and the National Death Index (Government account, Y11-0053) were consulted in all cases of deaths to retrieve the specific cause of death, providing an accurate account of the date and cause of death.
Statistical analysis and propensity score matching system
Data were analyzed using the SVS reporting standards.3 Patients were divided in three risk categories using the interquartile SVS sum score for age, cardiac, pulmonary, renal, and hypertension categories (score 0-30). Clinical risk stratification included low (score 0-8), intermediate (score 9-16), and high (score 17-30) risk. The primary end point was patient survival at 5 years. Patient survival was compared with the expected survival of age-matched and gender-matched controls from the general population of Olmstead County using the Olmstead Epidemiology Project database. The secondary end point was freedom from mesenteric-related death. A multivariate Cox proportional hazard model was used to identify independent predictors of any-cause early and late mortality. Patient survival after OR or ER was compared in both groups using propensity matched scores for all patients and according to SVS risk stratification. Pearson χ2 or Fisher exact test was used for analysis of categorical variables. Differences between means were tested with two-sided t-test, Wilcoxon rank-sum test, or Mann-Whitney test. All analyses were conducted by a statistician at the Department of Statistics using SAS software version 9.2 (SAS Institute Inc, Cary, NC). Two-sided P < .05 was used to determine statistical significance.
RESULTS
Patient population
During the study period, 343 patients with CMI were treated, including 256 women (75%) and 87 men (25%), with mean age of 68 ± 13 years. Patients treated with OR had higher rates of cigarette smoking (79% vs 57%) but lower rates of coronary artery disease (37% vs 64%), previous myocardial infarction (19% vs 36%), chronic obstructive pulmonary disease (19% vs 30%), chronic kidney disease stage (CKD) IV or V (14% vs 25%), and congestive heart failure (7% vs 19%) compared with patients treated with ER (Table I). There were more low-risk patients (67% vs 38%; P = .003) in the OR group and more intermediate-risk (28% vs 36%; P = .3) and high-risk (5% vs 26%; P < .001) patients in the ER group. Only nine OR patients (5%) met high-risk criteria using SVS scores. The use of statins (44% vs 31%), acetylsalicylic acid (80% vs 51%), clopidogrel (60% vs 12%), and β-blockers (43% vs 36%) was higher among ER patients compared with OR patients (P < .05).
Table I.
Demographics, clinical characteristics, and cardiovascular risk factors of 343 patients with chronic mesenteric ischemia treated with ORs or ERs
| All patients, N = 343, % |
OR
|
ER
|
P | |||
|---|---|---|---|---|---|---|
| n = 187 | % | n = 156 | % | |||
| Demographics | ||||||
| Age (mean ± SD) | 68 ± 13 | 65 ± 12 | 72 ± 12 | .01 | ||
| Female gender | 256 (75) | 143 | 77 | 113 | 72 | .7 |
| Clinical presentation | ||||||
| Abdominal pain | 324 (94) | 177 | 95 | 147 | 94 | .9 |
| Weight loss | 285 (83) | 159 | 85 | 126 | 80 | .7 |
| Food fear | 179 (52) | 97 | 52 | 82 | 52 | 1.0 |
| Nausea/vomiting | 91 (26) | 50 | 27 | 41 | 26 | .9 |
| Cardiovascular risk factors | ||||||
| Hypertension | 271 (79) | 137 | 73 | 134 | 85 | .3 |
| Smoking | 237 (69) | 148 | 79 | 89 | 57 | .05 |
| Hyperlipidemia | 195 (57) | 92 | 49 | 103 | 66 | .1 |
| Coronary artery disease | 170 (49) | 70 | 37 | 100 | 64 | .004 |
| Peripheral arterial disease | 140 (41) | 73 | 39 | 67 | 43 | .7 |
| Prior vascular surgery | 91 (26) | 46 | 25 | 45 | 29 | .9 |
| Prior myocardial infarction | 93 (27) | 37 | 19 | 56 | 36 | .01 |
| COPD | 82 (24) | 35 | 19 | 47 | 30 | .05 |
| Chronic renal insufficiency | 66 (19) | 27 | 14 | 39 | 25 | .04 |
| Diabetes | 66 (19) | 30 | 16 | 36 | 23 | .2 |
| Arrhythmia | 65 (19) | 30 | 16 | 35 | 22 | .2 |
| Valve disease | 51 (15) | 22 | 12 | 29 | 18 | .1 |
| Congestive heart failure | 43 (12) | 13 | 7 | 30 | 19 | .002 |
| Society for Vascular Surgery (mean ± SD)a | ||||||
| Age | 1.6 ± 0.9 | 1.2 ± 0.8 | 1.9 ± 0.9 | <.001 | ||
| Hypertension | 1.2 ± 0.9 | 1.0 ± 0.8 | 1.5 ± 0.9 | <.001 | ||
| Cardiac (×4) | 3.6 ± 4.0 | 2.7 ± 3.5 | 4.5 ± 4.5 | <.001 | ||
| Pulmonary (×2) | 0.8 ± 1.7 | 0.7 ± 1.4 | 1.0 ± 1.9 | .06 | ||
| Renal (×2) | 0.5 ± 1.1 | 0.4 ± 0.9 | 0.6 ± 1.3 | <.001 | ||
| Sum | 9.0 ± 7.0 | 6.5 ± 5.4 | 9.7 ± 6.0 | <.001 | ||
| Clinical risk categories | ||||||
| Low risk | 185 | 125 | 67 | 59 | 38 | .003 |
| Moderate risk | 109 | 53 | 28 | 56 | 36 | .3 |
| High risk | 50 | 9 | 5 | 41 | 26 | <.001 |
| Medical therapy | ||||||
| 1991-1999b | ||||||
| Acetylsalicylic acid | 66 (19) | 43 | 42 | 23 | 72 | 0.1 |
| Clopidogrel | 8 (2) | 4 | 4 | 4 | 12 | 0.2 |
| β-blockers | 27 (8) | 23 | 23 | 4 | 12 | 0.3 |
| Statins | 23 (7) | 21 | 21 | 2 | 6 | 0.2 |
| 2000-2010c | ||||||
| Acetylsalicylic acid | 155 (45) | 53 | 62 | 102 | 82 | 0.2 |
| Clopidogrel | 119 (35) | 18 | 21 | 101 | 81 | <0.001 |
| β-blockers | 114 (33) | 44 | 52 | 70 | 56 | 0.8 |
| Statins | 105 (31) | 38 | 45 | 67 | 54 | 0.5 |
COPD, Chronic obstructive pulmonary disease; ER, endovascular revascularization; OR, open revascularization; SD, standard deviation.
Clinical risk was estimated by using the Society for Vascular Surgery comorbidity score based on the presence of one or more comorbidities (ie, cardiac, pulmonary, and renal). This grading score has not been validated in a prospective study of patients with chronic mesenteric ischemia.
Based on the Society for Vascular Surgery comorbidity score risk statistic3
n = 102 OR patients; n = 32 ER patients.
n = 85 OR patients; n = 124 ER patients.
There was a shift from OR to ER after 2002 (Fig 1). An OR procedure was performed in 187 patients; 327 vessels were reconstructed (182 superior mesenteric artery, 135 celiac axis, and 10 inferior mesenteric artery) with antegrade bypasses in 156 patients (83%) and retrograde bypasses in 31 patients (17%) (Table II). In 156 patients and 173 vessels (116 superior mesenteric artery, 52 celiac axis, and five inferior mesenteric artery), ER was performed, including angioplasty and stenting in 128 patients and 138 vessels (80%) and primary angioplasty alone in 28 patients and 35 vessels (20%) (Table III).
Fig 1.
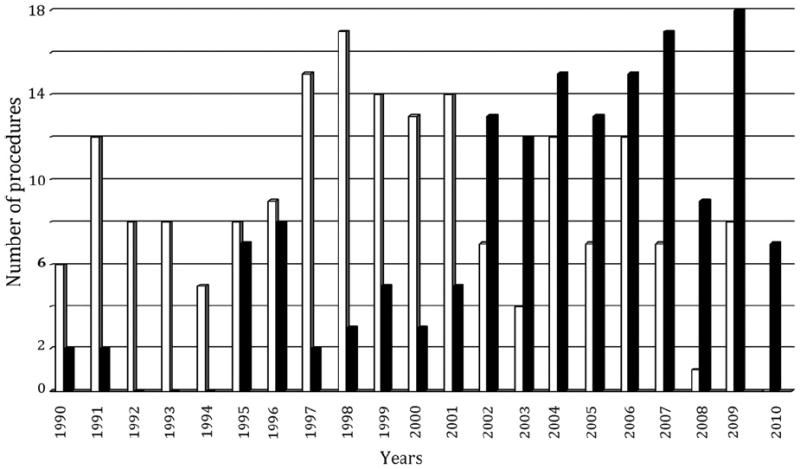
Distribution of patients treated with open surgical (open bars) or endovascular (solid bars) mesenteric revascularization between 1991 and 2010.
Table II.
Operative characteristics of 187 patients with chronic mesenteric ischemia treated with OR
| Operative characteristics | No. of patients/vessels (187/327) | % |
|---|---|---|
| Extension of disease | ||
| Single-vessel disease | 11 | 6 |
| Two-vessel disease | 103 | 55 |
| Three-vessel disease | 73 | 39 |
| Target vessela | ||
| Celiac artery | 135 | 41 |
| SMA | 182 | 56 |
| IMA | 10 | 3 |
| Single-vessel revascularization | 52 | 28 |
| Two-vessel revascularization | 133 | 71 |
| Three-vessel revascularization | 3 | 1 |
| Mean number of vessels | 1.75 ± 0.5 | |
| Technical data | ||
| Bypass | 177 | 94 |
| Bifurcated graft | 136 | 72 |
| Single graft | 41 | 22 |
| Endarterectomy/patch angioplasty | 9 | 5 |
| Endarterectomy/transposition | 1 | 1 |
| Configuration | ||
| Antegrade | 156 | 83 |
| Retrograde | 31 | 17 |
IMA, Inferior mesenteric artery; OR, open revascularization; SMA, superior mesenteric artery.
Denominator is the total number of vessels treated (174 for endovascular and 327 for open repairs).
Table III.
Operative characteristics of 156 patients with chronic mesenteric ischemia treated with ER
| Operative characteristics | No. of patients/vessels (156/173) | % |
|---|---|---|
| Target vessela | ||
| Length, mean | 15 ± 4.4 | |
| Diameter, mean | 5.8 ± 0.8 | |
| SMA | 116 | 67 |
| Celiac artery | 52 | 30 |
| IMA | 5 | 3 |
| One vessel treated | 125 | 72 |
| Two vessels treated | 24 | 28 |
| Mean number of vessels | 1.1 ± 0.6 | |
| Technical data | ||
| PTA/stent | 128/138 | 80 |
| PTA alone | 28/35 | 20 |
| Stent/balloon diameter, mm | 5.8 ± 0.6 | |
| Stent/balloon length, mm | 20.8 ± 8 | |
| Embolic protection device | 14 | 9 |
| Femoral approach | 106 | 68 |
| Brachial approach | 50 | 33 |
| Large profile (0.035-inch) | 81 | 52 |
| Small profile (≤0.018-inch) | 75 | 48 |
| Residual stenosis | ||
| Absent | 132 | 85 |
| <30% | 16 | 10 |
| >30% | 8 | 5 |
ER, Endovascular revascularization; IMA, inferior mesenteric artery; PTA, percutaneous transluminal angioplasty; SMA, superior mesenteric artery.
Denominator is the number of vessels (n = 173).
Mesenteric-related mortality
Nine (2.6%) early procedure-related deaths occurred, including five after OR (2.7%) and four (2.6%) after ER (P = 1.0). Causes of death after OR were myocardial infarction in two patients, mesenteric ischemia in two, and respiratory failure in one. In the ER group, two patients died of complications of distal embolization causing bowel ischemia and multi-system organ failure, and two patients died of myocardial infarction.
The median follow-up among 334 patients who survived the initial revascularization was 96 ± 54 months (range, 1-168 months). Follow-up was significantly longer after OR (131 ± 43 months; range, 1-168 months) compared with ER (64 ± 54 months; range, 1-105 months). Reinterventions for recurrent symptoms or a high-grade restenosis were needed in 24 patients (14%) treated with OR and in 30 patients (21%) who had ER (P = .6). Two patients died as a result of complications of reintervention, one in each group (OR, 1/24; 4.2%, and ER, 1/30; 3.3%; P = .87). Overall, 10 OR patients (5.8%) and six ER patients (4.1%) died of late mesenteric-related causes after 30 days or dismissal, including two patients who had complications of reintervention and 14 patients who died of acute mesenteric ischemia according to review of death certificates or autopsy reports. For the entire cohort, the combined rate of early and late mesenteric-related death was 8% for patients treated with OR (15/187) and 6.4% for patients treated with ER (10/156).
Any-cause mortality
There were 144 late deaths, 90 in the OR group and 54 in the ER group, including the 16 patients who died from late mesenteric-related causes. The cause of death was retrieved in 130 patients using medical records in 68 and review of death certificates in 62. The most common causes of late death (Table IV) in the OR and ER groups were cardiac events in 36 (21%) and 15 (10%) patients (P = .04), followed by cancer in 13 (7.5%) and eight (5.5%) patients (P = .5), respiratory complications in 12 (7%) and seven (5%) patients (P = .5), and mesenteric-related complications in 10 (5.8%) and six (4.1%) patients (P = .5). The cause of death remained unknown in 10 (6%) patients treated with OR and in four (3%) patients treated with ER (P = .2).
Table IV.
Early and late mortality in 343 patients with chronic mesenteric ischemia treated with OR or ER
| Outcomes |
OR
|
ER
|
P | ||
|---|---|---|---|---|---|
| n = 187 | % | n = 156 | % | ||
| Early | |||||
| Death | 5 | 2.7 | 4 | 2.6 | 1.0a |
| Cause of death | |||||
| Myocardial infarction | 2 | 1 | 2 | 1.3 | 1.0a |
| Mesenteric ischemia | 2 | 1 | 2 | 1.3 | 1.0a |
| Respiratory failure | 1 | 0.6 | 0 | 0 | 1.0a |
| Lateb | |||||
| Mean follow-up, months | 131 ± 43 | 64 ± 54 | |||
| Any cause of death | 90 | 52 | 54 | 37 | .09 |
| Causes of death | |||||
| Cardiac | 36 | 21 | 15 | 10 | .04 |
| Malignancy | 13 | 7.5 | 8 | 5.5 | .5 |
| Pulmonary | 12 | 7 | 7 | 5 | .5 |
| Mesenteric ischemia | 9 | 5 | 5 | 3.5 | .5 |
| Neurologic | 3 | 2 | 5 | 3.5 | .5a |
| Sepsis | 3 | 2 | 2 | 1.5 | 1.0a |
| Natural | 1 | 0.5 | 3 | 2 | .2a |
| Gastrointestinal bleeding | 0 | 0 | 3 | 2 | .1a |
| Renal | 1 | 0.5 | 1 | 0.5 | 1.0a |
| Reintervention-related death | 1 | 0.5 | 1 | 0.7 | 1.0a |
| Trauma | 1 | 0.5 | 0 | 0 | 1.0a |
| Unknown | 10 | 6 | 4 | 3 | .2 |
ER, Endovascular revascularization; OR, open revascularization.
Fisher exact test used.
Late surveillance included 172 patients in the OR group and 145 patients in the ER group.
Patient survival
The overall 5-year patient survival was 69% ± 4% in the OR group and 44% ± 5% in the ER group (P < .001) (Fig. 2). Patient survival was significantly less in both groups compared with the expected survival for age-matched and gender-matched control patients from the state of Minnesota. For the entire cohort, 5-year survival was 71% ± 4% for low-risk, 49% ± 6% for intermediate-risk, and 38% ± 7% for high-risk patients (P < .001) (Fig. 3). Among OR and ER groups, 5-year survival was 75% ± 4% and 60% ± 9% for low-risk patients (P = .02), 52% ± 8% and 43% ± 9% for intermediate-risk patients (P = .7), and 67% ± 15% and 30% ± 8% for high-risk patients (P = .01) (Fig 4). The rate of 5-year freedom from mesenteric-related death was 91% ± 2% after OR and 93% ± 4% after ER (P = 1.0) (Fig 5).
Fig 2.
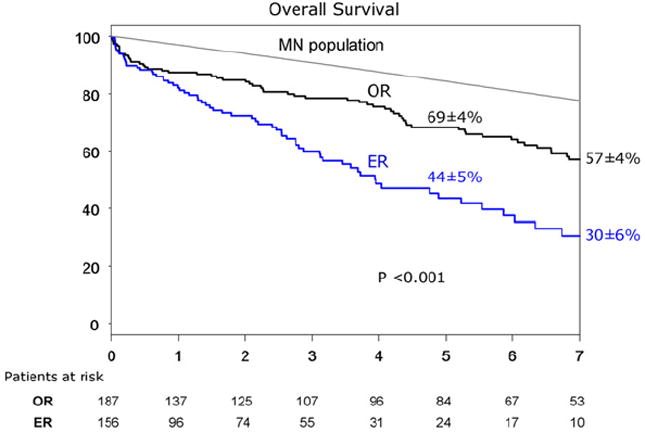
Kaplan-Meier 5-year survival curve of 343 patients with chronic mesenteric ischemia treated with open (OR) or endovascular (ER) revascularizations. MN, Minnesota.
Fig 3.
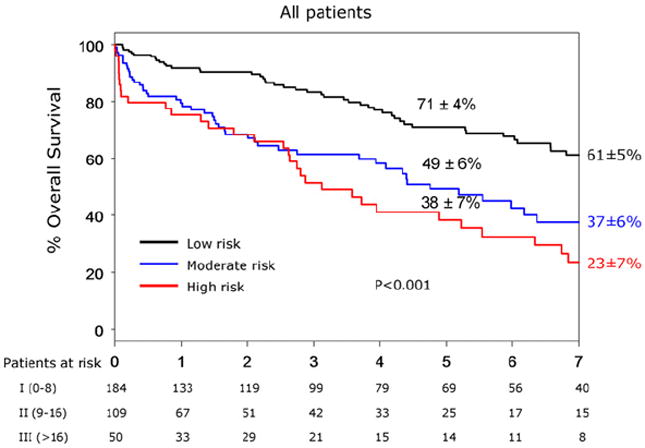
Kaplan-Meier 5-year survival curve of 343 patients with chronic mesenteric ischemia treated with open (OR) or endovascular (ER) revascularizations and divided into low, moderate, and high clinical risks.
Fig 4.
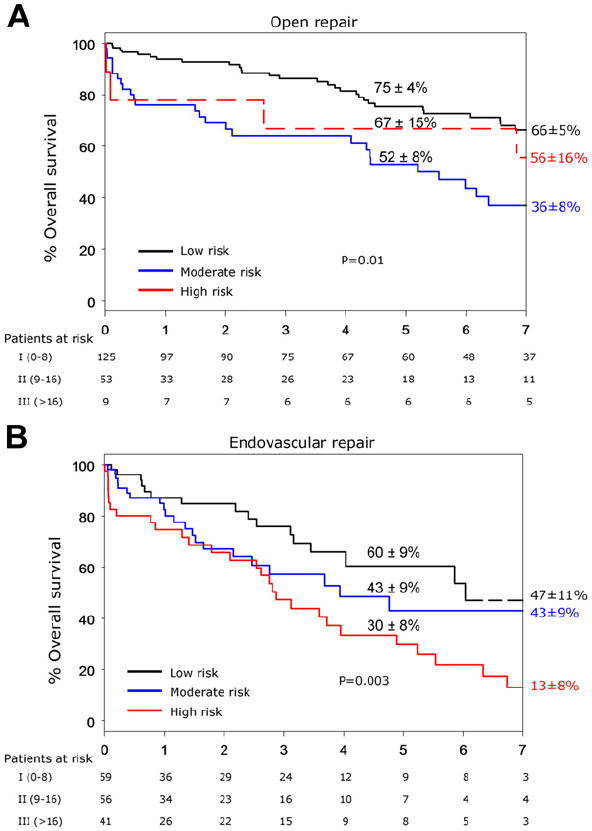
A, Kaplan-Meier 5-year survival curve of 187 patients with chronic mesenteric ischemia treated with open revascularizations (ORs) and divided into low, moderate, and high clinical risks. B, Kaplan-Meier 5-year survival curve of 156 patients with chronic mesenteric ischemia treated with endovascular revascularizations (ERs) and divided into low, moderate, and high clinical risks.
Fig 5.
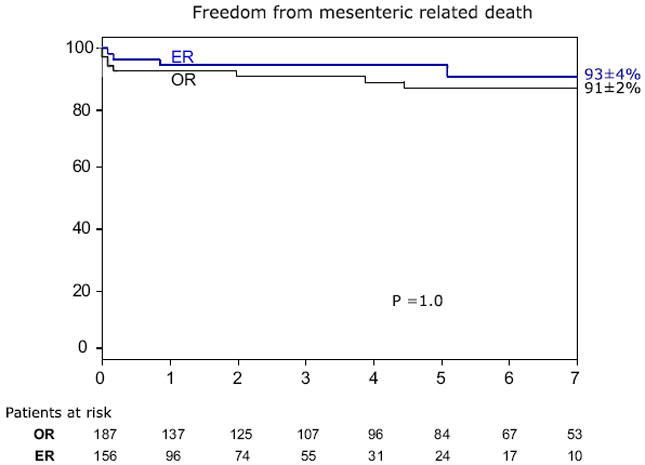
Kaplan-Meier curve depicting 5-year survival free from mesenteric-related deaths in patients with chronic mesenteric ischemia treated with open (OR) vs endovascular (ER) revascularizations.
Propensity score-matched comparison
The multivariate regression model identified age >80 years (odds ratio, 3.3; confidence interval [CI], 1.03-1.06; P ≤ .001), CKD stage IV or V (odds ratio, 5.5; CI, 1.4-16.6; P < .01), diabetes (odds ratio, 1.7; CI, 1.2-2.6; P < .01), and home oxygen therapy (odds ratio, 3.7; CI, 1.2-9.1; P < .001) as independent predictors of any-cause mortality (Table V). CKD stage IV or V (odds ratio, 3.4; CI, 3.3-345; P = .003) and diabetes (odds ratio, 4.2; CI, 1.7-10.5; P = .005) were independently associated with mesenteric-related death (Table VI). Using these independent predictors and baseline demographics, propensity score matching analysis was obtained in 254 patients (80%) from the entire cohort, including 127 patients treated with OR and 127 patients treated with ER. There were 63 patients (20%), including 45 in the OR group and 18 in the ER group, excluded from the propensity score analysis because of inability to find a paired match. The 5-year patient survival using propensity score-matched comparison was 60% for patients treated with OR and 57% for patients treated with ER (P = .7).
Table V.
Univariate and multivariate analysis for any cause of death in 343 patients with chronic mesenteric ischemia treated with OR or ER
| Any cause of death | HR | 95% CI | P |
|---|---|---|---|
| Univariate analysis | |||
| Age | 1.1 | 1.02-1.05 | <.001 |
| Diabetes | 1.7 | 1.1-2.5 | <.001 |
| Coronary artery disease | 2.4 | 1.6-3.7 | <.001 |
| Congestive heart failure | 2.6 | 1.6-4.0 | <.001 |
| Valve disease | 1.8 | 1.1-2.8 | .001 |
| Chronic renal insufficiency | 6.2 | 1.9-19.9 | <.001 |
| COPD | 2.1 | 1.4-3.1 | <.001 |
| Home oxygen therapy | 6.5 | 2.8-15 | <.001 |
| Prior myocardial infarction <30 days | 2.3 | 1.3-4.0 | <.01 |
| Prior CABG | 2.5 | 1.5-4.1 | <.001 |
| Celiac revascularization | 0.5 | 0.3-0.7 | <.001 |
| Multivariate analysis | |||
| Age >80 | 3.3 | 1.03-1.06 | <.001 |
| Chronic renal insufficiency | 5.5 | 1.4-16.6 | <.01 |
| Home oxygen therapy | 3.7 | 1.2-9.1 | <.001 |
| Diabetes | 1.7 | 1.2-2.6 | <.01 |
CABG, Coronary artery bypass grafting; CI, Confidence interval; COPD, chronic obstructive pulmonary disease; ER, endovascular revascularization; HR, hazard ratio; OR, open revascularization.
Table VI.
Univariate and multivariate analysis for mesenteric-related death in 343 patients with chronic mesenteric ischemia treated with OR or ER
| Mesenteric-related death | HR | 95% CI | P |
|---|---|---|---|
| Univariate analysis | |||
| Chronic renal insufficiency | 8.7 | 1.7-45 | .009 |
| Diabetes | 3.3 | 1.3-8.6 | .01 |
| SVS sum score >16 | 4.2 | 1.1-15.5 | .03 |
| Valve disease | 3.2 | 0.8-13 | .1 |
| Multivariate analysis | |||
| Chronic renal insufficiency | 3.4 | 3.3-345 | .003 |
| Diabetes | 4.2 | 1.7-10.5 | .005 |
CI, Confidence interval; ER, endovascular revascularization; HR, hazard ratio; OR, open revascularization; SVS, Society for Vascular Surgery.
DISCUSSION
Patients with CMI are often of advanced age and have a high prevalence of cardiovascular risk factors and atherosclerosis affecting other vascular beds. The choice of treatment is based on patient comorbidities, physician preference, and experience at the institution. A common criticism of prior reports addressing mesenteric revascularization is the lack of risk-stratified analysis; this introduces selection bias given that open repair is preferred in lower-risk patients and endovascular therapy is selected in higher-risk patients.4-11 Using propensity score-matched comparisons, this study showed that long-term patient survival was not affected by the type of reconstruction.
Despite reports of higher rates of reinterventions for symptom recurrence in patients treated with ER, this study showed similar rates of reinterventions (21% vs 14%) and of late mesenteric-related deaths (4.1% vs 5.8%) between patients treated with ER and patients treated with OR; overall, mesenteric-related death was the fourth most frequent cause of death, accounting for 10% of all late deaths after cardiac, cancer, and pulmonary causes. Late cardiac death was more common in the OR group, which is intriguing given that these patients had lower rates of cardiovascular risk factors. It is likely that improvements in medical therapy during the last decade (ie, statins, antiplatelet therapy, and β-blockers) accounted for lower rates of cardiovascular events in the ER group. Death from complications of reintervention occurred in two patients, one from each group; both patients presented with acute mesenteric ischemia because of graft or stent thrombosis. There were no deaths among patients who underwent reintervention for chronic symptoms. A further 14 patients died of acute mesenteric ischemia, a diagnosis that was obtained from review of death certificates or autopsy reports. A novel finding reported in this study is that acute mesenteric ischemia after mesenteric revascularizations accounts for 6% to 8% of all late deaths; therefore, continued patient surveillance is important to diagnose and treat graft or stent restenosis that could result in life-threatening acute mesenteric thrombosis.
Independent predictors of mesenteric-related death were advanced CKD (stage IV or V) and diabetes. In more recent studies,12,13 a decreased preoperative estimated glomerular filtration rate was found to be the best independent predictor of early and late death after other types of arterial reconstructions, including aortic and cardiac procedures. Many causes have been ascribed, including more advanced disease affecting smaller side branches and severe calcification, which potentially render the results of operation less durable and more prone to late recurrent ischemia from a scarce collateral network and poor mesenteric reserve.
Most late deaths were not the result of complications of mesenteric ischemia but instead were associated with other pre-existing clinical factors, more frequently cardiac and pulmonary diseases. Independent predictors of any-cause mortality were age >80 years, CKD stage IV or V, home oxygen therapy, and diabetes. The causes of late death have been poorly described in prior reports addressing CMI. In this study, all available death certificates were reviewed allowing an accurate description of the cause of death. The three most common causes of late death were cardiac, cancer and pulmonary in both groups, which is consistent with other reports of patients treated for vascular disease in other territories.14,15
The use of clinical risk stratification is important for analysis of outcomes after mesenteric revascularization. In our report, patient survival at 5 years was significantly higher in the OR group (69% vs 44%), which reflects selection bias with a greater number of higher risk patients in the ER group. To analyze patient survival according to clinical risk, we used the SVS comorbidity grading scores, but we acknowledge that this score system has not been validated in a prospective study of patients treated for CMI. Nevertheless, there was excellent correlation between survival and clinical risk: 71% ± 4% for low risk, 49% ± 6% for intermediate risk, and 38% ± 7% for high risk in both groups. In addition, survival was similar for each risk category when we compared patients treated with OR and ER, although there were not enough high-risk patients treated with OR to allow meaningful comparison.
Given that late patient survival is not influenced by type of reconstruction, treatment selection should be based on other factors that may affect durability of the procedure, such as anatomic characteristics of the lesion. Oderich et al16 reported that rates of restenosis are higher among patients treated for heavily calcified or long (>30 mm) lesions, particularly long occlusions. In these patients or in patients who fail endovascular therapy, open mesenteric reconstruction should be considered. Our current approach has been to offer ER to patients with anatomically suitable lesions, including patients with short ostial stenosis with mild-to-moderate calcifications.
This study is novel because it is the first to provide a comprehensive analysis of causes of death and late patient survival using propensity matched scores among patients treated for CMI. A careful review of death certificates and autopsy reports was also performed to improve accuracy when describing causes of late death. The large number of patients included in the cohort allowed us to use multivariate models to identify independent predictors of death with strong statistical power. However, there are several shortcomings that need to be discussed. Because of the retrospective design, we were unable to identify specific factors that may affect choice of therapy, specific technique, and device. Using the propensity score methodology, we were unable to identify a match for each patient; to allow a fair comparison between the open surgical and endovascular groups, a greater number of good-risk patients in the open surgical group was excluded to match the higher-risk endovascular patients. During the long study period, which spanned 2 decades, there has been significant progress in medical therapy, surgical techniques, and endovascular technology, which may have affected outcomes. Important improvements in medical therapy are difficult to analyze using a retrospective database and may have contributed to differences in cause of death between the two groups, including the higher rate of cardiac deaths in the OR group, predominantly treated in the 1990s. Finally, the follow-up period was longer among patients treated by open surgical repair, which may have affected rates of reinterventions and mesenteric-related deaths between patients treated by open and endovascular procedures.
CONCLUSIONS
Mesenteric-related mortality and any-cause mortality were not influenced by type of mesenteric revascularization. Long-term patient survival is nearly identical among patients treated by OR and ER techniques when matched for independent predictors of death using a propensity score-matched comparison.
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: GO, TT
Analysis and interpretation: GO, TT, PG, AD, MK, SM, SC, TB
Data collection: GO, TT
Writing the article: GO, TT, PG, AD, MK, SM, SC, TB
Critical revision of the article: GO, TT, PG, AD, MK, SM, SC, TB
Final approval of the article: GO, TT, PG, AD, MK, SM, SC, TB
Statistical analysis: GO, TT, SC
Obtained funding: GO
Overall responsibility: GO
Presented at the 2011 Vascular Annual Meeting of the Society for Vascular Surgery, Chicago, Ill, June 16-18, 2011.
Author conflict of interest: Dr Oderich is a consultant for Cook Medical, Inc.
References
- 1.Schermerhorn ML, Giles KA, Hamdan AD, Wyers MC, Pomposelli FB. Mesenteric revascularization: management and outcomes in the United States, 1988-2006. J Vasc Surg. 2009;50:341.e1–8.e1. doi: 10.1016/j.jvs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oderich GS, Bower TC, Sullivan TM, Bjarnason H, Cha S, Gloviczki P. Open versus endovascular revascularization for chronic mesenteric ischemia: risk-stratified outcomes. J Vasc Surg. 2009;49:1472.e3–9.e3. doi: 10.1016/j.jvs.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Chaikof EL, Fillinger MF, Matsumura JS, Rutherford RB, White GH, Blankensteijn JD, et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1061–6. doi: 10.1067/mva.2002.123991. [DOI] [PubMed] [Google Scholar]
- 4.van Petersen AS, Kolkman JJ, Beuk RJ, Huisman AB, Doelman CJ, Geelkerken RH. Open or percutaneous revascularization for chronic splanchnic syndrome. J Vasc Surg. 2010;51:1309–16. doi: 10.1016/j.jvs.2009.12.064. [DOI] [PubMed] [Google Scholar]
- 5.Oderich GS, Malgor RD, Ricotta JJ., 2nd Open and endovascular revascularization for chronic mesenteric ischemia: tabular review of the literature. Ann Vasc Surg. 2009;23:700–12. doi: 10.1016/j.avsg.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Dias NV, Acosta S, Resch T, Sonesson B, Alhadad A, Malina M, et al. Mid-term outcome of endovascular revascularization for chronic mesenteric ischaemia. Br J Surg. 2010;97:195–201. doi: 10.1002/bjs.6819. [DOI] [PubMed] [Google Scholar]
- 7.Atkins MD, Kwolek CJ, LaMuraglia GM, Brewster DC, Chung TK, Cambria RP. Surgical revascularization versus endovascular therapy for chronic mesenteric ischemia: a comparative experience. J Vasc Surg. 2007;45:1162–71. doi: 10.1016/j.jvs.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 8.Kasirajan K, O’Hara PJ, Gray BH, Hertzer NR, Clair DG, Greenberg RK, et al. Chronic mesenteric ischemia: open surgery versus percutaneous angioplasty and stenting. J Vasc Surg. 2001;33:63–71. doi: 10.1067/mva.2001.111808. [DOI] [PubMed] [Google Scholar]
- 9.Rawat N, Gibbons CP. Surgical or endovascular treatment for chronic mesenteric ischemia: a multicenter study. Ann Vasc Surg. 2010;24:935–45. doi: 10.1016/j.avsg.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Fioole B, van de Rest HJ, Meijer JR, van Leersum M, van Koeverden S, Moll FL, et al. Percutaneous transluminal angioplasty and stenting as first-choice treatment in patients with chronic mesenteric ischemia. J Vasc Surg. 2010;51:386–91. doi: 10.1016/j.jvs.2009.08.055. [DOI] [PubMed] [Google Scholar]
- 11.Brown DJ, Schermerhorn ML, Powell RJ, Fillinger MF, Rzucidlo EM, Walsh DB, et al. Mesenteric stenting for chronic mesenteric ischemia. J Vasc Surg. 2005;42:268–74. doi: 10.1016/j.jvs.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 12.Nathan DP, Brinster CJ, Woo EY, Carpenter JP, Fairman RM, Jackson BM. Predictors of early and late mortality following open extent IV thoracoabdominal aortic aneurysm repair in a large contemporary single-center experience. J Vasc Surg. 2011;53:299–306. doi: 10.1016/j.jvs.2010.08.085. [DOI] [PubMed] [Google Scholar]
- 13.van Straten AH, Soliman Hamad MA, Koene BM, Martens EJ, Tan ME. Which method of estimating renal function is the best predictor of mortality after coronary artery bypass grafting? Neth Heart J. 2011;19:464–9. doi: 10.1007/s12471-011-0184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce JD, Edwards MS, Stafford JM, et al. Open repair of aortic aneurysms involving the renal vessels. Ann Vasc Surg. 2007;21:676–86. doi: 10.1016/j.avsg.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Chong T, Nguyen L, Owens CD, Conte MS, Belkin M. Suprarenal aortic cross-clamp position: A reappraisal of its effects on outcomes for open abdominal aortic aneurysm repair. J Vasc Surg. 2009;49:873–80. doi: 10.1016/j.jvs.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 16.Oderich GS, Macedo TA, Malgor RD, Bower TC, Vritiska T, Duncan AA, et al. Natural history and predictors of mesenteric artery in-stent restenosis in patients with mesenteric ischemia. J Vasc Surg. 2009;49(Suppl 1):A1–22. S1–58, e1–2. [Google Scholar]


