Abstract
DNAX-activating protein of 12 kD (DAP12) is an immunoreceptor tyrosine-based activation motif (ITAM)–containing adaptor protein found in myeloid cells and natural killer cells, and it couples to various receptors that mediate either cellular activation or inhibition. DAP12 inhibits Toll-like receptor (TLR) signaling, such as that of TLR4 in response to its ligand lipopolysaccharide (LPS), as well as cytokine responses by coupling to TREM2 (triggering receptor expressed on myeloid cells 2) at the plasma membrane. Understanding the mechanisms that inhibit inflammatory responses in macrophages is important for the development of therapies to treat inflammatory diseases. We show that inhibition of LPS responses by DAP12 is mediated by the adaptor protein DOK3 (downstream of kinase 3). DOK3 physically associated with the ITAM of DAP12 through its phosphotyrosine-binding domain. In response to LPS, DOK3 was phosphorylated in a DAP12- and Src-dependent manner, which led to translocation of phosphorylated DOK3 to the plasma membrane. DOK3-deficient cells exhibited increased production of proinflammatory cytokines and activation of extracellular signal–regulated kinase (ERK). Compared to wild-type mice, DOK3-deficient mice had increased susceptibility to challenge with a sublethal dose of LPS and produced increased serum concentrations of the inflammatory cytokine tumor necrosis factor–α (TNF-α). Together, these data suggest the mechanism by which DAP12 and TREM2 inhibit LPS signaling in macrophages to prevent inflammation.
INTRODUCTION
The presence of trace amounts of the bacterial product lipopolysaccharide (LPS; also known as endotoxin) in the circulation accompanies some inflammatory conditions, including obesity and aging (1, 2). Studies demonstrated that such low-grade endotoxemia (LPS concentration ≤1 ng/ml, referred to as “low-dose” LPS) might play a role in the initiation of chronic conditions, including diabetes, atherosclerosis, and Parkinson’s disease (3–5). Although low-dose LPS fails to activate the transcription factor nuclear factor κB (NF-κB), it stimulates the production of proinflammatory cytokines that may lead to progressive compromise of cellular and tissue function (6). Therefore, understanding the mechanism by which low-dose LPS signals are regulated is necessary to prevent excessive inflammation and cellular dysfunction.
The adaptor protein DNAX-activating protein of 12 kD (DAP12; also known as TYROBP and KARAP) (7–10) is among the known mediators of LPS responses. DAP12 is an immunoreceptor tyrosine-based activation motif (ITAM)–containing protein that couples to a host of immunoreceptors expressed on the surfaces of macrophages, dendritic cells, osteoclasts, microglia, and natural killer (NK) cells (11). Upon association with these receptors, DAP12 mediates cellular processes, such as migration, phagocytosis, and NK-mediated cell killing, by activating signaling pathways, such as those mediated by the tyrosine kinase Syk, phosphatidylinositol 3-kinase (PI3K), phospholipase Cγ, the guanine nucleotide exchange factor (GEF) Vav, the mitogen-activated protein kinase (MAPK) extracellular signal–regulated kinase (ERK), and Ca2+ flux. DAP12-deficient [DAP12 knockout (KO)] mice are more susceptible to low-grade endotoxemia than are wild-type mice, and they exhibit enhanced cytokine production and ERK activation (12). Additional studies demonstrated that the inhibitory effects of DAP12 on Toll-like receptor (TLR) responses in macrophages are mediated by the transmembrane receptor TREM2 (triggering receptor expressed on myeloid cells 2) (13, 14).
The molecular mechanism by which DAP12 mediates inhibition of TLR signaling is not well understood. Early studies suggested that DAP12 mediates inhibition of TLR responses specifically by inhibiting the ERK pathway downstream of TLR4, the receptor activated by LPS. Compared to their wild-type counterparts, DAP12 KO bone marrow–derived macro-phages (BMDMs) show an increase in the magnitude of phosphorylation of ERK in response to LPS. In contrast, the magnitudes of activation of the MAPKs p38 and c-Jun N-terminal kinase (JNK) and of the transcription factor NF-κB are similar between wild-type and DAP12 KO macrophages (12).
The adaptor proteins downstream of kinase 1 (DOK1) to DOK3 also inhibit LPS responses (15, 16). DOK proteins have an N-terminal pleckstrin homology (PH) domain, a phosphotyrosine-binding (PTB) domain, and four tyrosine-containing motifs that can bind to Src homology 2 (SH2) domains when phosphorylated (17). DOK1 to DOK3 are mainly found in immune cells, and they are closely related, with some functional redundancy, although each DOK protein has distinct properties and functions. Whereas DOK3 cannot bind to the guanosine triphosphatase–activating protein (GAP) RasGAP, DOK1 and DOK2 limit ERK activation by binding to p120 RasGAP in response to the constitutively active viral oncoproteins v-Abl and v-Src and to LPS, but not to other TLR ligands (16–18). Mice deficient in DOK1 to DOK3 develop lung cancer and histiocytic sarcomas (19, 20), revealing the functional importance of DOK proteins in regulating cellular responses in vivo.
Previous studies have shown that low-dose LPS stimulates increased cytokine production and enhanced ERK phosphorylation in the absence of the signaling adaptor protein DAP12 (12). Additional studies revealed that TREM2, a DAP12-associated receptor, mediates the inhibitory effects of DAP12 in macrophages (13, 14); however, the mechanism by which TREM2 and DAP12 inhibit low-dose LPS signaling remains unknown. We hypothesized that DOK proteins might participate in DAP12 ITAM–mediated inhibition of TLR responses by inhibiting the ERK pathway in response to LPS. Our data suggest a previously uncharacterized mechanism underlying the DAP12- and TREM2-mediated inhibition of LPS responses in macrophages that may help our understanding of the enhanced inflammatory responses that are deleterious to the body.
RESULTS
LPS stimulates the association of DOK3 with the ITAM motif of DAP12
We speculated that low-dose LPS might stimulate the formation of a signaling complex that could inhibit TLR4 responses. To test this hypothesis, we first identified the proteins that were stimulated to associate with DAP12 after stimulation of cells with low-dose LPS. We stimulated BMDMs from wild-type mice with LPS (1 ng/ml), lysed the cells, and then subjected them to immunoprecipitation with an anti-DAP12 antibody. One of the LPS-dependent, DAP12-associated proteins that we identified was DOK3. In response to LPS, DOK3 coimmunoprecipitated with DAP12 40 min after stimulation (Fig. 1A).
Fig. 1. LPS stimulates an association between DOK3 and the ITAM of DAP12.
(A) BMDMs from wild-type (WT) mice were treated with LPS (1 ng/ml) for the indicated times, and then cell lysates were immunoprecipitated (IP) with anti-DAP12 antibody and analyzed by Western blotting with antibodies specific for DOK3 and DAP12. (B) Left: BMDMs were treated with LPS (1 ng/ml or 1 mg/ml) for 40 min, lysed, and then incubated with the indicated GST proteins to pull down DOK3 PTB–associated proteins. Samples were then analyzed by Western blotting with antibodies against GST or DAP12. Right: BMDMs were treated with LPS (1 ng/ml) for 40 min, lysed, and then incubated with the indicated GST proteins to pull down Syk SH2 domain–associated proteins. Samples were then analyzed by Western blotting with antibodies against GST or DAP12. (C) List of biotinylated DAP12 ITAM peptides. The specific phosphorylated tyrosine (Y) is noted by a lowercase, bolded “p.” (D) The purified GST-DOK3-PTB fusion protein was incubated with the indicated ITAM peptides in lysis buffer (top) or with ITAM peptides in BMDM lysates (bottom) overnight at 4°C. Protein complexes were precipitated with NeutrAvidin beads, extensively washed, and analyzed by Western blotting with antibodies specific for GST (top) or Syk (bottom). Western blots in (A), (B), and (D) are representative of three independent experiments.
We next characterized the association of DOK3 with DAP12. DOK family members, including DOK3, contain PTB domains that mediate many interactions with phosphoproteins. Unlike the PTBs of DOK1 and DOK2, which preferentially interact with a short peptide sequence encompassing a NPXpY motif within SH2 domain–containing inositol phosphatase (SHIP1), the T cell receptor (TCR) ζ chain (TCRζ), and CD3ε (17, 21), the PTB of DOK3 binds to phosphoproteins, such as Abl, by alternative means (22). Because DAP12 does not contain an NPXpY motif, we speculated that the PTB domain of DOK3 might bind to the ITAM of DAP12. To test this hypothesis, we generated a glutathione S-transferase (GST)–DOK3–PTB fusion protein for pulldown assays. GST-DOK3-PTB pulled down DAP12 from lysates of macrophages stimulated with LPS (1 ng/ml), whereas the GST control protein did not (Fig. 1B). The GST-DOK3-PTB fusion protein failed to pull down DAP12 from the lysates of cells stimulated with a higher concentration of LPS (“high-dose” LPS, 1 mg/ml). A GST fusion protein containing the SH2 domain of the tyrosine kinase Syk (GST-Syk-SH2), which binds to the phosphorylated ITAM of DAP12 (23), did not associate with DAP12 in cells treated with low-dose LPS. Consistent with these data, LPS did not stimulate changes in the phosphorylation state of DAP12 (fig. S1).
Although these data suggested that DAP12 interacted with the PTB domain of DOK3, we could not exclude the possibility of the indirect binding of DOK3 to an SH2 domain–containing protein that was bound to DAP12. To confirm that DOK3 directly bound to DAP12, we made five peptides corresponding to the DAP12 ITAM (SPYQELQGQRPEVYSDL): an unphosphorylated wild-type peptide, peptides containing the individual phosphorylated tyrosines Tyr65 (Y65) or Tyr76 (Y76), a doubly tyrosine-phosphorylated (YY) peptide, and a peptide in which both tyrosines were mutated to phenylalanines (FF) (Fig. 1C). Biotinylation of these peptides enabled the pulldown of peptide-associated proteins with NeutrAvidin beads. To detect direct interactions between the PTB of DOK3 and the ITAM of DAP12, we purified the GST-DOK3-PTB fusion protein and incubated it with the biotinylated ITAM peptides in lysis buffer. NeutrAvidin-precipitated DAP12 ITAM–associated protein complexes were analyzed by Western blotting with anti-GST antibody to detect GST-PTB interactions with DAP12. We found that the PTB domain of DOK3 bound to the DAP12 ITAM peptides (Fig. 1D). The FF mutant ITAM peptide failed to pull down the GST-DOK3-PTB fusion protein, indicating that the tyrosines within the ITAM were critical for the association between the DAP12 peptide and the DOK3 PTB domain. To confirm that the ITAM peptides were capable of interacting with endogenous proteins, we used the same biotinylated peptides to precipitate the tyrosine kinase Syk from lysates of macrophages (Fig. 1D). Together, these data reveal a previously uncharacterized association between DOK3 and DAP12 that is dependent on the PTB domain of DOK3 and the ITAM of DAP12.
Loss of DOK3 leads to enhanced cytokine responses to low-dose LPS
Given the LPS-dependent association between DOK3 and DAP12, we hypothesized that DAP12 might function through DOK3 to inhibit macrophage responses to low-dose LPS. We stimulated BMDMs from wild-type and DOK3-deficient (DOK3 KO) mice with increasing concentrations of LPS. In response to low concentrations of LPS (0.5 or 1 ng/ml), DOK3 KO BMDMs secreted increased concentrations of the proinflammatory cytokines tumor necrosis factor–α (TNF-α), interleukin-6 (IL-6), and IL-12 compared to wild-type BMDMs (Fig. 2). At higher concentrations of LPS, the amounts of cytokines secreted by the wild-type and DOK3 KO BMDMs were similar (Fig. 2) and were consistent with the responses of DAP12 KO BMDMs (12). To determine whether DOK3 participated in responses to other TLR ligands, we stimulated DOK3 KO and wild-type BMDMs with the TLR9 ligand CpG oligonucleotide (0.04 to 1 mM) or the TLR3 ligand polyinosinic-polycytidylic acid [poly(I:C)] (1 to 50 mg/ml). Stimulation with CpG induced production of similar amounts of TNF-α and IL-6 by wild-type and DOK3 KO BMDMs. Poly(I:C) stimulated production of similar amounts of TNF-α by both cell types; however, DOK3 KO BMDMs produced substantially less IL-6 in response to poly(I:C) than did wild-type BMDMs. These data suggest that DOK3 might function downstream of DAP12 to inhibit responses of macrophages to low-dose LPS, whereas it enhances TLR3-dependent IL-6 production.
Fig. 2. Loss of DOK3 leads to enhanced cytokine production in response to low-dose LPS.
(A to C) BMDMs from WT (black bars) or DOK3-deficient (DOK3 KO) (white bars) mice were stimulated with increasing concentrations of (A) LPS, (B) CpG, or (C) poly(I:C) for 16 hours. Supernatants were collected, and the concentrations of TNF-α, IL-6, and IL-12p40 were measured by enzyme-linked immunosorbent assay (ELISA). Data are means ± SD from two independent experiments. *P < 0.05; **P < 0.005; ns, not significant.
DOK3-deficient macrophages exhibit enhanced ERK phosphorylation in response to low-dose LPS
To determine the molecular mechanism by which DOK3 inhibited responses to low-dose LPS, we examined the kinetics of activation of three MAPKs and the NF-κB pathway. We found that ERK signaling was enhanced in DOK3 KO macrophages in response to low-dose LPS compared to that in wild-type macrophages (Fig. 3A). Although ERK activation was enhanced in DOK3 KO macrophages, it was not inappropriately sustained because the amounts of phosphorylated ERK (pERK) were reduced to those of unstimulated cells 60 min after exposure of both wild-type and DOK3 KO macrophages to LPS. The extents of activation of p38 and JNK, as determined by detection of their phosphorylation by Western blotting analysis, were similar in DOK3 KO and wild-type macrophages (Fig. 3A). Although the abundance of pJNK in stimulated DOK3 KO macrophages was slightly increased in the representative experiment shown, this was not reproducible over multiple experiments. We also determined the role of DOK3 in the LPS-dependent activation of the NF-κB pathway by examining the degradation of the NF-κB inhibitor IκBa, which retains NF-κB in the cytoplasm, thereby preventing its transcriptional activity. After we exposed the cells to low-dose LPS, we found that the abundances of IκBa were similar in wild-type and DOK3 KO macrophages (Fig. 3B). Together, these data suggest that the principal difference in LPS-stimulated signaling between wild-type and DOK3 KO macrophages is ERK phosphorylation.
Fig. 3. DOK3 KO macrophages exhibit enhanced ERK phosphorylation in response to low-dose LPS.
(A and B) WT and DOK3 KO BMDMs were stimulated with LPS (1 ng/ml) for the indicated times. (A) Left: Cell lysates were analyzed by Western blotting for total and phosphorylated forms of ERK, JNK, and p38. β-Actin was used as a loading control. Right: Bar graph shows the densitometric analysis of Western blots from three independent experiments showing the mean fold change ± SD in the abundance of pERK relative to that of total ERK normalized to time zero. Statistical analysis was by two-way analysis of variance (ANOVA). (B) Western blotting analysis of IκBα abundance in lysates of WT and DOK3 KO BMDMs after stimulation with LPS (1 ng/ml) for the indicated times. β-Actin served as a loading control. Data are representative of three independent experiments.
LPS-dependent phosphorylation of DOK3 is DAP12-dependent
Because the phosphorylation of DOK3 downstream of other immunoreceptors is critical to mediate their signaling (18, 24–28), we tested whether low-dose LPS induced the phosphorylation of DOK3. LPS stimulated the phosphorylation of DOK3 in wild-type BMDMs as determined by Western blotting analysis of DOK3 immunoprecipitates with an anti-phosphotyrosine (pTyr) antibody (Fig. 4A). Further, to determine whether DAP12 was required for LPS-induced DOK3 phosphorylation, we performed similar experiments with DAP12-deficient (DAP12 KO) BMDMs. We found that LPS-dependent DOK3 phosphorylation was substantially reduced in DAP12 KO BMDMs compared to that in wild-type BMDMs (Fig. 4A). Increased concentrations of LPS also stimulated DOK3 phosphorylation in wild-type BMDMs; however, at a high concentration of LPS (100 ng/ml), the amounts of total and phosphorylated DOK3 protein were decreased compared to those in cells stimulated with a lower concentration of LPS (Fig. 4B), which was likely a result of the degradation of DOK3 protein, as we previously reported (15). Together, these data suggest that LPS stimulates DOK3 phosphorylation in a DAP12-dependent manner.
Fig. 4. LPS-stimulated phosphorylation of DOK3 is dependent on SFKs and DAP12.
(A) WT or DAP12 KO BMDMs were treated with LPS (1 ng/ml) for 40 min. Cell lysates were subjected to immunoprecipitation with an anti-DOK3 antibody and analyzed by Western blotting with antibodies specific for phosphotyrosine residues (pTyr) or DOK3. (B) WT BMDMs were stimulated with LPS (10 or 100 ng/ml) for 40 min. Cell lysates were subjected to immunoprecipitation with anti-DOK3 antibody and analyzed by Western blotting for pTyr and DOK3. (C) WT BMDMs were stimulated with LPS (1 ng/ml) in the presence of solvent control (DMSO), the Src inhibitor PP2, or the Syk inhibitor Bay for 40 min. Cell lysates were analyzed by Western blotting with antibodies against pTyr and pDOK3. (D and E) WT BMDMs (D) and RAW264.7 cells transfected with plasmids encoding FLAG-DOK3 or FLAG-DOK3ΔPTB (E) were treated with LPS (1 ng/ml) for 40 min, and then cytosolic and membrane protein fractions were extracted. Cytosolic fractions were verified by Western blotting analysis for the presence of GAPDH, whereas membrane fractions were verified with a pancadherin antibody. (D) Cellular fractions were subjected to immunoprecipitation with an anti-DOK3 antibody and analyzed by Western blotting for pTyr and DOK3. (E) Membrane protein samples were subjected to immunoprecipitation with anti-FLAG antibody and analyzed by Western blotting with antibodies against pTyr and FLAG. Data are representative of three independent experiments.
LPS stimulates DOK3 phosphorylation by Src-family kinases
In macrophages, LPS-dependent signaling leads to the activation of Srcfamily kinases (SFKs) and Syk-family kinases (29). Because LPS led to DOK3 phosphorylation, we hypothesized that LPS-dependent activation of Src, Syk, or both might be required for DOK3 phosphorylation. To test this, we treated wild-type BMDMs with an Src inhibitor (PP2), a Syk inhibitor (Bay), or vehicle control [dimethyl sulfoxide (DMSO)] before exposing them to low-dose LPS. Cell lysates were immunoprecipitated with anti-DOK3 antibody, and samples were analyzed by Western blotting with an anti-pTyr antibody. The Src inhibitor PP2 substantially reduced basal as well as LPS-dependent DOK3 phosphorylation, whereas the Syk inhibitor had no effect (Fig. 4C). Thus, low-dose LPS stimulated the tyrosine phosphorylation of DOK3 in an SFK-dependent manner.
LPS stimulates the localization of phosphorylated DOK3 to the plasma membrane
Activation of DAP12 by SFKs causes the recruitment and activation of PI3K at the plasma membrane and the generation of phosphatidylinositol 3,4,5-trisphosphate (PIP3), which results in the recruitment of PH domain–containing proteins to the plasma membrane. DOK3 translocates to the plasma membrane from the cytosol in a PH domain–dependent manner after engagement of the B cell receptor (BCR) (26). Thus, we investigated whether LPS stimulated similar changes in the cellular localization of DOK3 in macrophages. We analyzed the cytosolic and membrane fractions of cells before and after stimulation with LPS. We stimulated RAW264.7 cells (a mouse macrophage cell line) in suspension with low-dose LPS over time and then prepared cytosolic and membrane fractions. We confirmed the effectiveness of cell fractionation by Western blotting analysis for cadherins in the membrane fractions and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the cytosolic fractions (Fig. 4D). We immuno-precipitated DOK3 from the cytosolic and membrane fractions and then analyzed the samples by Western blotting with antibodies specific for pTyr and total DOK3. Most of the DOK3 protein was found in the cytosolic fractions of cells before and after stimulation with LPS (Fig. 4D). A small amount of DOK3 was associated with the membrane fraction of cells before stimulation with LPS; however, 40 min after stimulation, pDOK3 was predominantly detected in the membrane fractions. These data suggest that LPS stimulates the translocation of pDOK3 from the cytosol to the plasma membrane.
Although DOK3 could become membrane-associated through its PH domain, we speculated that the interaction between DAP12 and the PTB domain of DOK3 might be necessary for DOK3 to translocate to the membrane. Thus, we generated a FLAG-tagged DOK3 mutant protein in which the PTB domain was deleted (FLAG-DOK3ΔPTB) (28). In studies of RAW264.7 cells transfected with plasmid encoding FLAG-DOK3ΔPTB, we detected the mutant DOK3 only in the cytosol either before or after stimulation with LPS (Fig. 4E). Moreover, we could not detect pDOK3 in either the cytosol or membrane fractions in cells transfected with plasmid encoding FLAG-DOK3DPTB. These data suggest that the PTB domain of DOK3 is required for both its phosphorylation and membrane translocation in response to LPS.
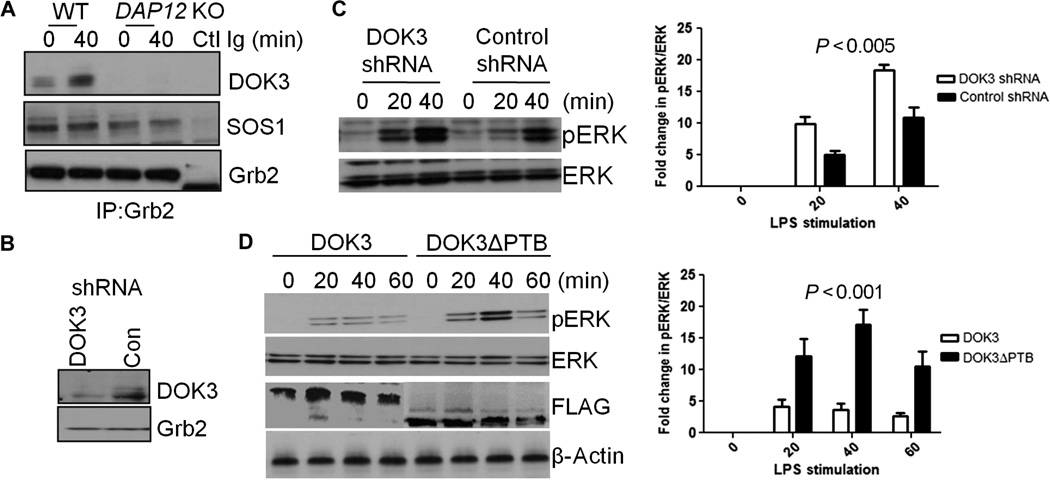
LPS stimulates the association of DOK3 with Grb2 in a DAP12-dependent manner
Previous reports showed that pDOK3 associates with the cytosolic adaptor protein Grb2 (growth factor receptor–bound protein 2) and the Ras GEF Sos1 (son of sevenless 1) to prevent activation of the Ras-ERK pathway downstream of protein-tyrosine kinases in transfected human embryonic kidney (HEK) 293T cells (18). Thus, we wanted to determine whether a similar mechanism occurred after stimulation of macrophages with low-dose LPS. To answer this, we analyzed Grb2-associated proteins in macrophages before and after stimulation with LPS. We immunoprecipitated Grb2 from wild-type or DAP12 KO BMDMs and then analyzed the samples by Western blotting with antibodies specific for Grb2, Sos1, and DOK3 (Fig. 5A). DOK3 was associated with Sos1 and Grb2 in unstimulated wild-type macrophages, but the extent of this association was considerably increased in response to low-dose LPS. DOK3 failed to associate with Grb2 or Sos1 in DAP12 KO BMDMs either before or after stimulation with LPS. These data suggest that the DAP12-dependent tyrosine phosphorylation of DOK3 is required for the association of DOK3 with Grb2 and Sos1. Thus, DAP12 might limit LPS-dependent ERK activation by promoting the association of DOK3 with Grb2 and Sos1.
Fig. 5. LPS stimulates an association between DOK3 and Grb2 in a DAP12-dependent manner.
(A) WT and DAP12 KO BMDMs were treated with LPS (1 ng/ml) for 40 min. Cell lysates were subjected to immunoprecipitation with an anti-Grb2 antibody or with an isotype control immunoglobulin (Ctl Ig) and analyzed by Western blotting with antibodies specific for DOK3, Sos1, and Grb2. (B) RAW264.7 cells transduced with lentivirus expressing DOK3-specific shRNA or with control lentivirus expressing luciferase were lysed and analyzed by Western blotting with an antibody specific for DOK3. (C) Left: DOK3 KD RAW264.7 cells were stimulated with LPS (1 ng/ml) for the indicated times, and cell lysates were analyzed by Western blotting with antibodies specific for pERK and total ERK. Right: Bar graph shows the densitometric analysis of Western blots from three independent experiments showing the mean fold change ± SD in the abundance of pERK relative to that of total ERK normalized to time zero. Statistical analysis was by two-way ANOVA. (D) Left: DOK3 KD RAW264.7 cells transfected with plasmids encoding FLAG-DOK3 or FLAG-DOK3ΔPTB were stimulated with LPS (1 ng/ml) for the indicated times, and cell lysates were analyzed by Western blotting for pERK, total ERK, FLAG, and β-actin as a loading control. Right: Bar graph shows the densitometric analysis of Western blots from three independent experiments showing the mean fold change ± SD in the abundance of pERK relative to that of total ERK normalized to time zero. Statistical analysis was by two-way ANOVA. Data are representative of three independent experiments. Statistical analysis of the data in the bar graphs in (C) and (D) was by two-way ANOVA.
To further investigate this hypothesis, we generated a stable DOK3 knockdown (KD) RAW264.7 cell line with DOK3-specific short hairpin RNA (shRNA) (Fig. 5B). These DOK3 KD cells had an enhanced response to low-dose LPS, with increased ERK activation similar to that observed in DOK3 KO BMDMs (Fig. 5C). To further test the role of DAP12-dependent DOK3 function in ERK activation, we transfected DOK3 KD cells with plasmids encoding FLAG-DOK3 or FLAG-DOK3ΔPTB, a DOK3 mutant protein that is unable to bind to DAP12. The presence of FLAG-tagged proteins was confirmed by Western blotting analysis of cell lysates with an anti-FLAG antibody (Fig. 5D). We analyzed cell lysates for ERK activation in untreated cells and at 20, 40, and 60 min after treatment with low-dose LPS. As compared to cells expressing wild-type FLAG-DOK3, cells expressing FLAG-DOK3ΔPTB had enhanced ERK activation, similar to that observed in DOK3 KO cells (Fig. 5D). Thus, the involvement of DAP12 in inhibiting LPS-dependent ERK activation appears to be mediated by the ability of DOK3 to associate with DAP12 and to bind to Grb2 and Sos1
DOK3-deficient mice have increased mortality and serum TNF-α in response to a sublethal dose of LPS
Having shown that DOK3 was necessary to limit LPS-dependent cytokine responses in a DAP12-dependent manner, we next determined whether DOK3 was required to limit host responses to LPS in vivo. We treated wild-type and DOK3 KO mice with a sublethal dose of LPS (10 mg/kg body weight), and their survival was monitored over 6 days. Significantly more DOK3 KO mice died compared to wild-type mice (Fig. 6A). Additional cohorts of mice were treated with a sublethal dose of LPS, and serum was collected after 40 min. We found that TNF-α concentrations were increased in the serum from DOK3 KO mice compared to that from wild-type mice; however, this difference was not statistically significant (P = 0.1102). We tried to capture the in vivo association of DAP12 with DOK3 by analyzing peritoneal macrophages isolated at 40 min after LPS stimulation of mice. Immunoprecipitation of DOK3 or DAP12 and Western blotting analysis for DAP12 and DOK3 failed to show that DAP12 coimmunoprecipitated with DOK3 or vice versa (fig. S2). However, on the basis of the increased sensitivity to LPS challenge, we conclude that DOK3 is required to limit LPS responses in vivo.
Fig. 6. DOK3 KO mice exhibit increased TNF-α production and mortality in response to challenge with a sublethal dose of LPS.
(A) DOK3 KO and control S129 mice were treated with a sublethal dose of LPS by intraperitoneal injection and were monitored for survival over the next 6 days. DOK3 KO mice had statistically significantly increased mortality [P < 0.005, log-rank (Mantel-Cox) test] compared to S129 mice. Data are from three experiments with a total of 24 S129 mice and 22 DOK3 KO mice. (B) Sera were collected from control and DOK3 KO mice 40 min after challenge with LPS (10 mg/kg). TNF-α concentrations were measured by ELISA (P = 0.1102, unpaired t test). Data are from two experiments with a total of nine WT and eight DOK3 KO mice.
DOK3 associates with SHIP1 and DAP12
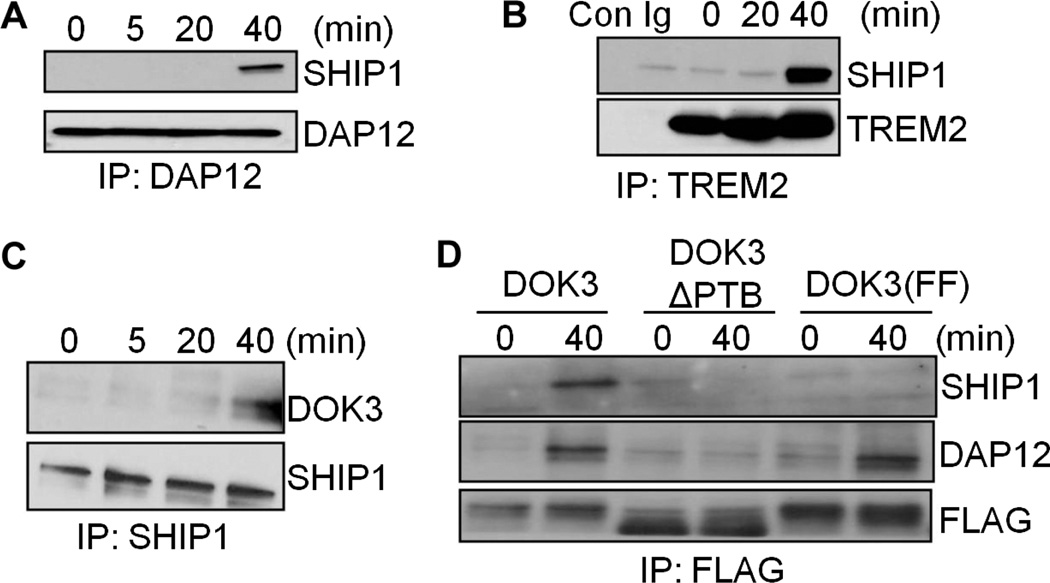
Previously, we showed that the recruitment of the phosphatase SHIP1 to DAP12 inhibits ERK activation in response to cross-linking of the receptor TREM2 (30). To determine whether SHIP1 participated in the DAP12-mediated inhibition of LPS-dependent ERK activation, we examined associations among DAP12, DOK3, and SHIP1 in macrophages in response to LPS. Protein complexes were immunoprecipitated with anti-DAP12 (Fig. 7A), anti-TREM2 (Fig. 7B), or anti-SHIP1 (Fig. 7C) antibodies, and samples were analyzed by Western blotting with antibodies specific for DOK3, SHIP1, DAP12, or TREM2. We found that LPS stimulated associations between SHIP1 and DAP12 (Fig. 7A) and between SHIP1 and TREM2 (Fig. 7B) 40 min after treatment. Similarly, DOK3 and SHIP1 were coimmunoprecipitated from lysates of cells treated for 40 min with LPS (Fig. 7C). Thus, LPS stimulated the association of DOK3 with SHIP1 and DAP12 in macrophages.
Fig. 7. DOK3 acts as a linker between SHIP1 with DAP12.
(A to C) BMDMs from WT mice were treated with LPS (1 ng/ml) for the indicated times, and cell lysates were subjected to immunoprecipitation (IP) with (A) anti-DAP12 antibody, (B) anti-TREM2 antibody, or (C) anti-SHIP1 antibody and analyzed by Western blotting with antibodies specific for SHIP1, DAP12, TREM2, and DOK3. The experiments in (B) also included samples that were subjected to immunoprecipitation with control immunoglobulin (Con Ig). (D) RAW264.7 cells transfected with plasmids encoding FLAG-DOK3, FLAG-DOK3ΔPTB, or FLAG-DOK3(FF) were treated with LPS (1 ng/ml) for 40 min, and cell lysates were subjected to immunoprecipitation with anti-FLAG antibody and were analyzed by Western blotting with antibodies specific for DAP12, SHIP1, or FLAG. Data are representative of three independent experiments.
We showed that both SHIP1 and DOK3 bound to the DAP12 ITAM; therefore, it was possible that DOK3 and SHIP1 might compete for binding to this motif. Alternatively, because SHIP1 associates with DOK3 through two of four tyrosines found within the C-terminal domain of DOK3 (27), it was possible that DOK3 might recruit SHIP1 to DAP12 or vice versa. To test this hypothesis, we transfected RAW264.7 cells with plasmids encoding FLAG-DOK3, FLAG-DOK3ΔPTB, or FLAG-DOK3(FF) (27), the latter of which lacks the SHIP1-binding domain of DOK3. We used an anti-FLAG antibody to immunoprecipitate DOK3-associated proteins, and we confirmed the presence of each FLAG-tagged protein by Western blotting (Fig. 7D). FLAG-DOK3 coimmunoprecipitated with both DAP12 and SHIP1 from cells treated with LPS for 40 min, whereas FLAG-DOK3ΔPTB failed to coimmunoprecipitate with DAP12 or SHIP1. In addition, FLAG-DOK3(FF) associated with DAP12 in response to LPS, but it failed to associate with SHIP1. These data suggest that LPS stimulates the association of DOK3 with DAP12, which is then followed by the recruitment of SHIP1 to DOK3.
SHIP1 is dispensable for the LPS-dependent phosphorylation of DOK3, its association with Grb2, and its membrane localization
SHIP1 interacts with many proteins, including Src and c-Abl (31); therefore, it is possible that SHIP1 could influence DOK3 phosphorylation indirectly by bringing kinases in proximity to DOK3. To determine whether SHIP1 was required for the phosphorylation of DOK3 in response to LPS, we stimulated wild-type and SHIP1-deficient (SHIP1 KO) BMDMs with LPS, immunoprecipitated DOK3, and analyzed the samples by Western blotting with antibodies specific for DOK3 or pTyr (Fig. 8A). LPS stimulated the phosphorylation of DOK3 in wild-type and SHIP1 KOBMDMs. To confirm that SHIP1 was not required for DAP12-mediated phosphorylation of DOK3 or for the membrane localization of DOK3, we transfected RAW264.7 cells with plasmid encoding FLAG-DOK3(FF), stimulated the cells with low-dose LPS, isolated cytosolic and membrane fractions from the cells, and performed immunoprecipitations with anti-FLAG antibody (Fig. 8B). We found that FLAG-DOK3(FF) was predominantly localized in the cytosol, with a small amount found in the membrane fractions, similar to that of endogenous DOK3 (Fig. 4C). Phosphorylated FLAG-DOK3(FF) was appropriately localized to the membrane fraction after stimulation of the cells with LPS (Fig. 8B). These data suggest that SHIP1 is dispensable for both the phosphorylation and membrane localization of DOK3 in response to low-dose LPS.
Fig. 8. SHIP1 is dispensable for the LPS-dependent phosphorylation of DOK3, its association with Grb2, and its membrane localization.
(A) WT and SHIP1 KO BMDMs were treated with LPS (1 ng/ml) for 40 min. Cell lysates were subjected to immunoprecipitation with anti-DOK3 antibody and then analyzed by Western blotting with antibody for pTyr. (B) RAW264.7 cells transfected with plasmid encoding FLAG-DOK3(FF) were treated with LPS (1 ng/ml) for 40 min, and cytosolic and membrane protein fractions were extracted. Fractions were subjected to immunoprecipitation with anti-FLAG antibody and then analyzed by Western blotting with antibodies specific for pTyr or FLAG. Cell fractions were analyzed by Western blotting with a pan-cadherin antibody (membrane) or an anti-GAPDH antibody (cytosol) to determine the efficiency of the extraction procedure. (C and D) WT and SHIP1 KO BMDMs were treated with LPS (1 ng/ml) for 40 min, and cells were lysed. (C) Cell lysates were subjected to immunoprecipitation with an anti-Grb2 antibody, and samples were analyzed by Western blotting with antibodies specific for DOK3, Sos1, and Grb2. (D) Whole-cell lysates were analyzed by Western blotting with antibodies specific for pERK and total ERK. Data are representative of three independent experiments.
We then asked whether SHIP1 was required for the LPS-dependent association between DOK3, Grb2, and Sos1. We stimulated wild-type and SHIP1 KO BMDMs with low-dose LPS and immunoprecipitated Grb2-associated proteins with anti-Grb2 antibody. We found that DOK3, Grb2, and Sos1 coimmunoprecipitated from LPS-stimulated SHIP1 KO cells, just as they did in wild-type BMDMs (Fig. 7C). Thus, SHIP1 was not required for the interactions among these proteins. Additionally, we found that the extent of ERK activation in SHIP1 KO macrophages in response to low-dose LPS was similar to that in LPS-treated wild-type cells (Fig. 7D). These data suggest that SHIP1, although recruited to DAP12 and DOK3 in response to LPS, does not participate in DAP12-dependent inhibition of LPS-induced ERK activation.
DISCUSSION
We identified a previously uncharacterized mechanism by which DAP12 limits LPS responses in macrophages. As shown in our model (fig. S3), our results suggest that low-dose LPS leads to the DAP12-dependent phosphorylation of DOK3 and the recruitment of pDOK3 to the plasma membrane. There, pDOK3 inhibits LPS-dependent ERK activation by binding to Sos1 and Grb2 downstream of DAP12, thus preventing the activation of Ras and ERK, as well as the subsequent production of inflammatory cytokines. The functional importance of DOK3 for the inhibition of LPS responses was seen in the increased mortality of DOK3 KO mice in response to challenge with low-dose LPS. These data suggest a critical role for DOK3 during the DAP12-mediated inhibition of LPS signaling.
Compared to wild-type macrophages, DOK3 KO macrophages exhibited increased production of inflammatory cytokines and ERK activation in response to LPS in a manner similar to BMDMs from DAP12 KO and TREM2 KO mice. Unlike DAP12 and TREM2, DOK3 did not inhibit TLR9-or TLR3-dependent cytokine responses. Indeed, deficiency in DOK3 was associated with decreased IL-6 production in response to the TLR3 ligand poly(I:C). These data suggest that DOK3 may serve different functions, depending on the nature of the TLR ligand. Supporting this hypothesis, whereas DOK1 inhibits TLR2-dependent IL-6 production in astrocytes, it stimulates TLR2-dependent IL-6 production in microglia (32). Given the redundancy among DOK1, DOK2, and DOK3, it is remarkable that deficiency in individual members of the DOK family exerts physiological effects in vitro and in vivo.
Supporting a role for DOK3 as an indispensable mediator of TLR4 signaling, we previously showed that DOK3 plays a role in regulating endotoxin tolerance (15). Whereas our current studies showed that the association of DOK3 with Sos1 and Grb2 in response to low-dose LPS was required to inhibit ERK activation, at higher concentrations of TLR ligands, including LPS at >100 ng/ml and CpG at 0.5 µM, DOK3 becomes ubiquitinated and degraded (15). The ubiquitination of DOK3 by the E3 ubiquitin ligase Cbl-b and its subsequent degradation occurs 30 min after TLR stimulation and is associated with maximal TLR-dependent ERK activation (15). Degradation of DOK3 leads to the proteasomal degradation of Sos1, suggesting that DOK3 may serve to further limit ERK activation by regulating Sos1 abundance. Unlike our current studies that showed that the phosphorylation status of DOK3 was critical to regulate low-dose LPS responses, the phosphorylation status of DOK3 did not affect its degradation in response to high-dose LPS. Additional studies are needed to determine whether the degradation of DOK3 in response to high-dose LPS occurs in a DAP12-dependent manner.
The phosphorylation of DOK3 seems to play a critical role in its localization and function. Tyrosine-phosphorylated DOK3 sequesters Grb2 and inhibits the Ras-ERK pathway downstream of constitutively active v-SRC in transfected HEK 293T cells (18). Here, we found that low-dose LPS stimulated the phosphorylation of DOK3 in an Src-dependent manner, which led to formation of a Sos1- and Grb2-containing complex that was required to inhibit ERK activation. In the absence of DAP12, LPS failed to stimulate DOK3 phosphorylation, thereby preventing formation of the complex of DOK3, Grb2, and Sos1, and resulting in increased ERK activation. Thus, the phosphorylation status of DOK3 appears to be required for specific protein-protein interactions and to control its intracellular localization.
We found that unstimulated, adherent wild-type BMDMs contained basal amounts of tyrosine-phosphorylated DOK3, and that this protein migrated faster when resolved by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) than did DOK3 phosphorylated in response to LPS (Figs. 4, A and B, and 8A). In adherent macrophages, basal tyrosine phosphorylation of DOK3 may be a result of integrin-mediated activation of DAP12 (33). Consistent with this hypothesis, nonadherent macrophages stimulated in suspension had minimal amounts of pDOK3 in either the cytosolic or membrane fractions (Fig. 4, C and D). Thus, it is possible that DOK3 might regulate basal integrin-dependent ITAM signaling in macrophages; however, additional studies are needed to confirm this and to further define the role of DOK3 in integrin-mediated signaling in macrophages.
We also explored the role of upstream Src- and Syk-family kinases in the regulation of DOK3 phosphorylation. Src-independent DOK3 phosphorylation is implicated in fibrinogen-induced platelet spreading downstream of the integrin aIIb3 (25). However, in that study, stimulation of the collagen receptor glycoprotein VI, which associates with the ITAM adaptor protein FcRγ (γ chain of the Fc receptor), was Src-dependent (25). Although other studies have shown that Syk is critical for DAP12-dependent inhibition of TLR responses (12), here we found that inhibition of SFKs, but not of Syk, prevented both basal and LPS-dependent phosphorylation of DOK3. Thus, one or more of the SFKs may play an integral role in mediating the phosphorylation status of DOK3 as a result of tonic DAP12 ITAM signaling through integrins, as well as TLR-dependent signals.
The binding of SHIP1 to DOK proteins, including DOK3, is involved in the inhibition of integrin signaling in platelets and of BCR and TCR signaling (17). In B cells, stimulation of the BCR induces the tyrosine phosphorylation of DOK3, which recruits SHIP1 and inhibits JNK signaling (27). We previously showed that SHIP1 inhibits DAP12 signaling downstream of the engagement of TREM2 or the stimulation of colony-stimulating factor 1 receptor (c-fms) by macrophage colony-stimulating factor (M-CSF) in osteoclasts (30). Here, we showed that LPS stimulated the association of DOK3 with SHIP1; however, SHIP1 was not required for DAP12-mediated inhibition of ERK signaling in response to low-dose LPS. There are conflicting views about the role of SHIP1 in regulating LPS signaling; some studies show that SHIP1 enhances LPS-stimulated cytokine production, whereas others suggest that SHIP1 is inhibitory (34, 35). Differences in the role of SHIP1 in regulating TLR4 responses may be, in part, a result of its binding association with DAP12 and DOK3. In response to low-level LPS, DOK3 inhibited LPS signaling independently of SHIP1; however, in response to higher concentrations of LPS, during which DOK3 is degraded, SHIP1 may play a more important inhibitory role.
We showed by multiple methods that DOK3 and DAP12 associated with each other either directly or within a complex in vitro; however, a limitation of our study is that we were unable to show an association between DOK3 and DAP12 in vivo. We tried to capture this association in peritoneal macrophages isolated at 40 min after stimulation of mice with LPS (fig. S2). Although we did not detect an association between DOK3 and DAP12 in vivo after challenge with LPS, we recognize the limitation of this study as being that we used only one dose of LPS and one period. The in vivo kinetics of the association between DAP12 and DOK3 may be very different from those seen in vitro in a purified population of macrophages; however, on the basis of our sublethal LPS challenge experiments, we still conclude that DOK3 is necessary to inhibit LPS responses in vivo, which supports our in vitro findings.
Together, our data led us to identify DOK3 as a critical mediator of the DAP12-dependent inhibition of LPS signaling in macrophages. These studies provide insight into the mechanisms underlying the inhibition of TLR signaling by TREM2 and DAP12. In addition, our findings reveal a previously uncharacterized association between DOK3 and DAP12 that may have farreaching implications for myeloid cells, osteoclasts, and other immune cells. We suggest that lackofregulationoftonic ITAM signalingbyDOK3 may play a role in chronic inflammatory diseases or in the progression of inflammation-induced malignant transformation.
MATERIALS AND METHODS
Mice
129/Sv and C57/Bl6 mice were obtained from commercial vendors and housed at the Oklahoma Medical Research Foundation (OMRF) or the Jilin University Laboratory Animal Center. DOK3-deficient mice (on a 129/Sv background) were provided by P. P. Pandolfi (Departments of Medicine and Pathology, Harvard Medical School) (19). SHIP1-deficient mice (on a C57BL/6 background) were provided by G. Krystal by way of K. M. Coggeshall (OMRF). DAP12-deficient mice were provided by R. McEver (OMRF). Animals were maintained according to the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care at OMRF, and animal experiment protocols were approved by the review committees from the OMRF, the University of Oklahoma Health Sciences Center (OUHSC), and the Jilin University Laboratory Animal Center and were in compliance with all guidelines.
Reagents
Antibodies against the following proteins of interest were used in this study: DOK3, β-actin, IκBα, SHIP1, and Sos1 (from Santa Cruz Biotechnology); GST, ERK, pERK, JNK, pJNK, p38, pp38, Grb2, pan-cadherin, and pTyr (from Cell Signaling Technology); GAPDH (Abcam); and FLAG M2 (Upstate Biotechnology). Fluorescently conjugated secondary antibodies were obtained from Invitrogen, and anti-DAP12 antibody was a gift from T. Takai (Tohoku University, Japan). Antibody against TREM2 (clone 78) was previously described (36) and was provided by M. C. Nakamura and W. E. Seaman (University of California, San Francisco). Escherichia coli O55:B5 LPS, Salmonella typhosa LPS, polymyxin B, and poly(I:C) were purchased from Sigma-Aldrich. CpG oligonucleotides were purchased from InvivoGen.
Macrophage cell culture
The RAW264.7 macrophage cell line was purchased from the American Type Culture Collection and maintained in α-minimum essential medium (αMEM). For primary macrophage cultures, BMDMs were derived from flushed bone marrow from mouse long bones as previously described (36). After 2 days, nonadherent BMDMs were collected and transferred at a density of 5 × 106 cells and were cultured for an additional 2 to 5 days. BMDMs were maintained in αMEM supplemented with 10% endotoxin-free fetal bovine serum, 5% CMG (conditioned medium containing M-CSF), M-CSF, penicillin, streptomycin, and glutamine.
Transfection and transduction
shRNA lentiviral particles (Santa Cruz Biotechnology) were used according to the manufacturer’s instructions. Briefly, RAW264.7 cells were cultured to 50% confluence, and the medium was removed and replaced with 1 ml of polybrene (5 µg/ml)/medium mixture per well (for a 12-well plate). The cells were infected by adding 6 µl of DOK3 shRNA lentiviral particles (1 × 106 infectious units of virus), and stable clones expressing the DOK3-specific shRNA were selected with puromycin (5 µg/ml). Transfection of RAW264.7 cells with the plasmids pCMV-FLAG-DOK3, pCMV-FLAG-DOK3ΔPTB (the PTB domain deletion mutant of DOK3), and pCMV-FLAG-DOK3FF (in which Tyr325 and Tyr343 were substituted with phenylalanines) was performed by nucleofection following the appropriate Amaxa protocol.
Construction of complementary DNA plasmids
The plasmid pCMV-FLAG-DOK3 was constructed by subcloning complementary DNA (cDNA) encoding mouse DOK3 (IMAGE no. 40126241) into pCMV-FLAG (Sigma). The plasmid pCMV-FLAG-DOK3FF was generated with the QuikChange Site-Directed Mutagenesis Kit (Stratagene). The GST-DOK3-PTB construct was generated by subcloning the cDNA encoding the PTB domain of DOK3 (corresponding to amino acid residues 114 to 255) into the GST fusion protein expression vector pGEX4T3 by polymerase chain reaction (PCR) with DreamTaq Green PCR Master Mix (Open Biosystems). The plasmid pCMV-FLAG-DOK3ΔPTB was generated by overlap extension PCR. Briefly, pCMV-FLAG-DOK3 was used as a template to construct the PTB-deleted mutant. To amplify the cDNA fragment encoding amino acid residues 1 to 113 of DOK3, the forward primer was from the DNA sequence of the FLAG tag, and the reverse primer contained a linker of DNA sequence encoding amino acid residues 256 to 262 of DOK3. The cDNA encoding amino acid residues 256 to 444 of DOK3 was amplified by PCR from DOK3 (IMAGE no. 40126241) as a template. Finally, overlap extension PCR was performed to generate the pCMV-FLAG-DOK3ΔPTB plasmid. The generated constructs were confirmed by DNA sequencing.
In vitro peptide pulldown assays
The in vitro peptide assays were based on methods previously described (30, 37, 38). Biotinylated wild-type, phosphorylated, and mutant DAP12 ITAM peptides with the core sequence SPYQELQGQRPEVYSDL (Fig. 1C) were synthesized by Quality Controlled Biochemicals. Whole-cell lysates were incubated with biotinylated peptides overnight at 4°C with rotation. Peptides and associated proteins were collected with NeutrAvidin-Sepharose (Pierce), washed with radioimmunoprecipitation assay (RIPA) buffer, and then subjected to SDS-PAGE and Western blotting analysis. In other assays, biotinylated peptides were added to purified, recombinant GST-DOK3-PTB in RIPA buffer for 4 hours at 4°C. Peptide-associated proteins were collected and processed as described earlier. Western blot membranes were incubated with antibody against GST to detect GST-fusion proteins that were pulled down with the peptides.
Stimulation of cells with LPS stimulation and measurement of cytokines
BMDMs or RAW264.7 cells were stimulated with LPS for the times indicated in the figure legends. Conditioned media and cell lysates were collected. To measure secreted cytokines by ELISA, day 5 BMDMs were seeded into 48- or 24-well tissue plates at 1 × 105 or 1 × 106 cells per well overnight in the presence of LPS, CpG, or poly(I:C). CpG and poly(I:C) were first mixed in the presence of polymyxin B (10 mg/ml) to remove contaminating LPS. The amounts of TNF-α, IL-6, and IL-12p40 in duplicate supernatant samples were measured by ELISA (eBioscience).
Immunoprecipitations and extraction of membrane proteins
Cell lysates were prepared with ice-cold RIPA buffer as previously described (39). Immunoprecipitations were performed by incubating the precleared cell lysates with primary antibody on ice for 1 hour and then adding protein A or protein G beads at 4°C for 1 hour with rotation. The beads were then washed, and the immunoprecipitation complexes were dissolved in SDS-PAGE sample buffer before being resolved by SDS-PAGE. The proteins were then transferred onto polyvinylidene difluoride membranes, incubated with the appropriate primary antibodies, and visualized with the enhanced chemiluminescence system (Pierce). For membrane protein extraction, macrophages were removed from tissue culture plates, resuspended in medium, and stimulated with LPS (1 ng/ml) for the times indicated in the figure legends. Cytosolic and membrane proteins were extracted with a ProteoExtract Transmembrane protein extraction kit (Novagen). Cells were resuspended in Extraction Buffer 1 to extract cytosolic proteins, and Extraction Buffer 2A was used subsequently to extract the membrane protein fraction.
Expression and purification of GST fusion proteins and pulldown assays
GST alone or the GST-DOK3-PTB fusion protein was expressed in E. coli (JM109) cells and purified by affinity glutathione-agarose beads, as described previously (30). The protein-bead complexes (15 to 20 mg of protein) were incubated with cell lysates (~1 mg) at 4°C for 2 hours with rotation. The beads were washed three times with RIPA buffer and dissolved in SDS-PAGE sample buffer for analysis by Western blotting.
In vivo LPS challenge
Three cohorts of age- and sex-matched wild-type mice (n = 24 mice) and DOK3 KO mice (n = 22 mice), both male and female of 2 to 6 months of age, were injected with S. typhosa LPS in phosphate-buffered saline intraperitoneally at 60 to 75 mg/kg. Mice were monitored twice daily for 7 days. To determine the association between DAP12 and DOK3 in cells in vivo, mice were injected with S. typhosa LPS intraperitoneally at 10 mg/kg. After 40 min, peritoneal macrophages and BMDMs were collected, lysed, and subjected to immunoprecipitation as described earlier. Serum was collected by terminal cardiac puncture and analyzed for TNF-α by ELISA.
Statistical analysis
Statistical analysis was performed by Student’s t test, unless otherwise specified, and was performed with GraphPad Prism Plus software.
Supplementary Material
Acknowledgments
We thank T. Johnson (OUHSC) for help with figure production.
Funding: This work was funded by NIH grant DE019398 and a VA Career Development Award to M.B.H. This work was also funded by Jilin University grants 430504001043, 450060445662, and 430505010272 to Q.P.
Author contributions: Q.P. performed the research, analyzed the data, and wrote the manuscript; C.L.L. performed the research, analyzed the data, and edited the manuscript; S.M. performed and analyzed the research and edited the manuscript; M.B.H. designed the research, analyzed the data, and wrote the manuscript.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencesignaling.org/cgi/content/full/6/289/ra72/DC1
Fig. S1. The abundance of pDAP12 is not increased in response to LPS.
Fig. S2. Stimulation of peritoneal macrophages with LPS in vivo fails to induce a stable complex containing DOK3 and pDAP12.
Fig. S3. DAP12 and DOK3 mediate inhibition of LPS-dependent cytokine production.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 2.Goto T, Edén S, Nordenstam G, Sundh V, Svanborg-Edén C, Mattsby-Baltzer I. Endotoxin levels in sera of elderly individuals. Clin. Diagn. Lab. Immunol. 1994;1:684–688. doi: 10.1128/cdli.1.6.684-688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno-Navarrete JM, Manco M, Ibáñez J, García-Fuentes E, Ortega F, Gorostiaga E, Vendrell J, Izquierdo M, Martínez C, Nolfe G, Ricart W, Mingrone G, Tinahones F, Fernández-Real JM. Metabolic endotoxemia and saturated fat contribute to circulating NGAL concentrations in subjects with insulin resistance. Int. J. Obes. 2010;34:240–249. doi: 10.1038/ijo.2009.242. [DOI] [PubMed] [Google Scholar]
- 4.Wiesner P, Choi SH, Almazan F, Benner C, Huang W, Diehl CJ, Gonen A, Butler S, Witztum JL, Glass CK, Miller YI. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor κB and activator protein-1: Possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ. Res. 2010;107:56–65. doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maitra U, Gan L, Chang S, Li L. Low-dose endotoxin induces inflammation by selectively removing nuclear receptors and activating CCAAT/enhancer-binding protein d. J. Immunol. 2011;186:4467–4473. doi: 10.4049/jimmunol.1003300. [DOI] [PubMed] [Google Scholar]
- 7.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 8.Olcese L, Cambiaggi A, Semenzato G, Bottino C, Moretta A, Vivier E. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J. Immunol. 1997;158:5083–5086. [PubMed] [Google Scholar]
- 9.Tomasello E, Olcese L, Vély F, Geourgeon C, Bléry M, Moqrich A, Gautheret D, Djabali M, Mattei MG, Vivier E. Gene structure, expression pattern, and biological activity of mouse killer cell activating receptor-associated protein (KARAP)/DAP-12. J. Biol. Chem. 1998;273:34115–34119. doi: 10.1074/jbc.273.51.34115. [DOI] [PubMed] [Google Scholar]
- 10.Campbell KS, Cella M, Carretero M, López-Botet M, Colonna M. Signaling through human killer cell activating receptors triggers tyrosine phosphorylation of an associated protein complex. Eur. J. Immunol. 1998;28:599–609. doi: 10.1002/(SICI)1521-4141(199802)28:02<599::AID-IMMU599>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Hamerman JA, Ni M, Killebrew JR, Chu CL, Lowell CA. The expanding roles of ITAM adapters FcRγ and DAP12 in myeloid cells. Immunol. Rev. 2009;232:42–58. doi: 10.1111/j.1600-065X.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat. Immunol. 2005;6:579–586. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: Inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J. Immunol. 2006;177:2051–2055. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 14.Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, Colonna M. Cutting edge: TREM-2 attenuates macrophage activation. J. Immunol. 2006;177:3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 15.Peng Q, O’Loughlin JL, Humphrey MB. DOK3 negatively regulates LPS responses and endotoxin tolerance. PLoS One. 2012;7:e39967. doi: 10.1371/journal.pone.0039967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinohara H, Inoue A, Toyama-Sorimachi N, Nagai Y, Yasuda T, Suzuki H, Horai R, Iwakura Y, Yamamoto T, Karasuyama H, Miyake K, Yamanashi Y. Dok-1 and Dok-2 are negative regulators of lipopolysaccharide-induced signaling. J. Exp. Med. 2005;201:333–339. doi: 10.1084/jem.20041817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mashima R, Hishida Y, Tezuka T, Yamanashi Y. The roles of Dok family adapters in immunoreceptor signaling. Immunol. Rev. 2009;232:273–285. doi: 10.1111/j.1600-065X.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- 18.Honma M, Higuchi O, Shirakata M, Yasuda T, Shibuya H, Iemura S, Natsume T, Yamanashi Y. Dok-3 sequesters Grb2 and inhibits the Ras-Erk pathway downstream of protein-tyrosine kinases. Genes Cells. 2006;11:143–151. doi: 10.1111/j.1365-2443.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- 19.Berger AH, Niki M, Morotti A, Taylor BS, Socci ND, Viale A, Brennan C, Szoke J, Motoi N, Rothman PB, Teruya-Feldstein J, Gerald WL, Ladanyi M, Pandolfi PP. Identification of DOK genes as lung tumor suppressors. Nat. Genet. 2010;42:216–223. doi: 10.1038/ng.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mashima R, Honda K, Yang Y, Morita Y, Inoue A, Arimura S, Nishina H, Ema H, Nakauchi H, Seed B, Oda H, Yamanashi Y. Mice lacking Dok-1, Dok-2, and Dok-3 succumb to aggressive histiocytic sarcoma. Lab. Invest. 2010;90:1357–1364. doi: 10.1038/labinvest.2010.121. [DOI] [PubMed] [Google Scholar]
- 21.Dong S, Corre B, Foulon E, Dufour E, Veillette A, Acuto O, Michel F. T cell receptor for antigen induces linker for activation of T cell-dependent activation of a negative signaling complex involving Dok-2, SHIP-1, and Grb-2. J. Exp. Med. 2006;203:2509–2518. doi: 10.1084/jem.20060650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cong F, Yuan B, Goff SP. Characterization of a novel member of the DOK family that binds and modulates Abl signaling. Mol. Cell. Biol. 1999;19:8314–8325. doi: 10.1128/mcb.19.12.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McVicar DW, Taylor LS, Gosselin P, Willette-Brown J, Mikhael AI, Geahlen RL, Nakamura MC, Linnemeyer P, Seaman WE, Anderson SK, Ortaldo JR, Mason LH. DAP12-mediated signal transduction in natural killer cells. A dominant role for the Syk protein-tyrosine kinase. J. Biol. Chem. 1998;273:32934–32942. doi: 10.1074/jbc.273.49.32934. [DOI] [PubMed] [Google Scholar]
- 24.Neumann K, Oellerich T, Heine I, Urlaub H, Engelke M. Fc gamma receptor IIb modulates the molecular Grb2 interaction network in activated B cells. Cell. Signal. 2011;23:893–900. doi: 10.1016/j.cellsig.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Senis YA, Antrobus R, Severin S, Parguiña AF, Rosa I, Zitzmann N, Watson SP, García A. Proteomic analysis of integrin αIIbβ3 outside-in signaling reveals Src-kinase-independent phosphorylation of Dok-1 and Dok-3 leading to SHIP-1 interactions. J. Thromb. Haemost. 2009;7:1718–1726. doi: 10.1111/j.1538-7836.2009.03565.x. [DOI] [PubMed] [Google Scholar]
- 26.Stork B, Neumann K, Goldbeck I, Alers S, Kähne T, Naumann M, Engelke M, Wienands J. Subcellular localization of Grb2 by the adaptor protein Dok-3 restricts the intensity of Ca2+ signaling in B cells. EMBO J. 2007;26:1140–1149. doi: 10.1038/sj.emboj.7601557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robson JD, Davidson D, Veillette A, Inhibition of the Jun N-terminal protein kinase pathway by SHIP-1. a lipid phosphatase that interacts with the adaptor molecule Dok-3. Mol. Cell. Biol. 2004;24:2332–2343. doi: 10.1128/MCB.24.6.2332-2343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemay S, Davidson D, Latour S, Veillette A. Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol. Cell. Biol. 2000;20:2743–2754. doi: 10.1128/mcb.20.8.2743-2754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat. Immunol. 2010;11:734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- 30.Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, Humphrey MB. TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci. Signal. 2010;3 doi: 10.1126/scisignal.2000500. ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.March ME, Ravichandran K. Regulation of the immune response by SHIP. Semin. Immunol. 2002;14:37–47. doi: 10.1006/smim.2001.0340. [DOI] [PubMed] [Google Scholar]
- 32.Downer EJ, Johnston DG, Lynch MA. Differential role of Dok1 and Dok2 in TLR2-induced inflammatory signaling in glia. Mol. Cell. Neurosci. 2013;56C:148–158. doi: 10.1016/j.mcn.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Mócsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat. Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An H, Xu H, Zhang M, Zhou J, Feng T, Qian C, Qi R, Cao X. Src homology 2 domain-containing inositol-5-phosphatase 1 (SHIP1) negatively regulates TLR4-mediated LPS response primarily through a phosphatase activity- and PI-3K–independent mechanism. Blood. 2005;105:4685–4692. doi: 10.1182/blood-2005-01-0191. [DOI] [PubMed] [Google Scholar]
- 35.Fang H, Pengal RA, Cao X, Ganesan LP, Wewers MD, Marsh CB, Tridandapani S. Lipopolysaccharide-induced macrophage inflammatory response is regulated by SHIP. J. Immunol. 2004;173:360–366. doi: 10.4049/jimmunol.173.1.360. [DOI] [PubMed] [Google Scholar]
- 36.Humphrey MB, Daws MR, Spusta SC, Niemi EC, Torchia JA, Lanier LL, Seaman WE, Nakamura MC. TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function. J. Bone Miner. Res. 2006;21:237–245. doi: 10.1359/JBMR.051016. [DOI] [PubMed] [Google Scholar]
- 37.Tridandapani S, Kelley T, Pradhan M, Cooney D, Justement LB, Coggeshall KM. Recruitment and phosphorylation of SH2-containing inositol phosphatase and Shc to the B-cell Fcγ immunoreceptor tyrosine-based inhibition motif peptide motif. Mol. Cell. Biol. 1997;17:4305–4311. doi: 10.1128/mcb.17.8.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tridandapani S, Pradhan M, LaDine JR, Garber S, Anderson CL, Coggeshall KM. Protein interactions of Src homology 2 (SH2) domain-containing inositol phosphatase (SHIP): Association with Shc displaces SHIP from FcγRIIb in B cells. J. Immunol. 1999;162:1408–1414. [PubMed] [Google Scholar]
- 39.Mócsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA, Nakamura MC. The immunomodulatory adapter proteins DAP12 and Fc receptor γ-chain (FcRγ) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.