Abstract
Proteins in the seminal fluid of animals with internal fertilization effect numerous responses in mated females that impact both male and female fertility. Among these proteins is the highly represented class of proteolysis regulators (proteases and their inhibitors). Though proteolysis regulators have now been identified in the seminal fluid of all animals in which proteomic studies of the seminal fluid have been conducted (as well as several other species in which they have not), a unified understanding of the importance of proteolysis to male fertilization success and other reproductive processes has not yet been achieved. In this review, we provide an overview of the identification of proteolysis regulators in the seminal fluid of humans and Drosophila melanogaster, the two species with the most comprehensively known seminal fluid proteomes. We also highlight reports demonstrating the functional significance of specific proteolysis regulators in reproductive and post-mating processes. Finally, we make broad suggestions for the direction of future research into the roles of both active seminal fluid proteolysis regulators and their inactive homologs, another significant class of seminal fluid proteins. We hope that this review aids researchers in pursuing a coordinated study of the functional significance of proteolysis regulators in semen.
Keywords: proteolysis, seminal fluid, mass spectrometry, fertility
INTRODUCTION
Seminal fluid—the non-sperm components of the male ejaculate—contains hundreds of proteins as well as non-protein components that, together, are vital for male fertility (Avila et al. 2011; Poiani 2006; Rodríguez-Martínez et al. 2011). The functions of seminal fluid proteins (Sfps) in insects and mammals have been recently reviewed (Avila et al. 2011; Rodríguez-Martínez et al. 2011). In aggregate, Sfps are vital to male fertility, playing important roles in sperm activation and storage (Bloch Qazi and Wolfner 2003; Kanwar et al. 1979; King et al. 2011; Lu et al. 2011; Neubaum and Wolfner 1999; Osanai and Chen 1993; Ou et al. 2012; Smith and Stanfield 2011; Wong et al. 2008a; Zhao et al. 2012); pregnancy establishment in mammals (Daimon and Wada 2005; Doyle et al. 2012; Guerin et al. 2011; Kanwar et al. 1979; Moldenhauer et al. 2009; Ziecik et al. 2011); female immune function (Domanitskaya et al. 2007; Guerin et al. 2011; Moldenhauer et al. 2009); ovulation (in stimulated ovulators) (Heifetz et al. 2000; Marshall et al. 2009; Ratto et al. 2010; Xu and Wang 2011); and female post-mating behaviors in insects (Gillott 2003; Sirot et al. 2009). Individually, however, only a small proportion of Sfps have been assigned functions, and the molecular mechanisms for their functions are even less well understood.
Sfp homologs are difficult to identify between distantly related organisms as a result of the rapid evolution of many male reproductive proteins (Clark et al. 2006; Clark and Swanson 2005; Haerty et al. 2007; Swanson et al. 2001; Swanson and Vacquier 2002), and also likely due to large differences in mating systems between species. On the other hand, the major protein classes that comprise Sfps are conserved between taxa as distant as insects and mammals (Mueller et al. 2004). Proteases and their inhibitors are among these major conserved classes. The identities and functions of specific male-derived proteases and protease inhibitors in the seminal fluid will be the focus of this review.
Due to the complexity of the seminal fluid, identifying functions for individual Sfps has been very challenging. One way to tackle this complexity is to focus on proteins that are likely to coordinate the functions of multiple other Sfps. Proteolysis regulators (proteases and their inhibitors) are one such protein class in the seminal fluid because they have the ability to regulate functions of multiple proteins through degradation, removal of inhibitory peptides, or through processing of prohormones. A focus on the substrates and fertility consequences of proteolysis regulators in the seminal fluid may lead to a more integrated, pathway-centric understanding of Sfp function.
Proteolysis is central to many vital biological processes, such as immunity, blood clotting, cell cycle regulation, and tissue morphogenesis (Heutinck et al. 2010; Udvardy 1996). An external signal can be rapidly amplified through the activation of a single protease that regulates multiple downstream pathways (Behrendt 2012; Di Cera 2011). For the purposes of this review, we will refer to all enzymes that hydrolyze peptide bonds of either whole proteins or peptides as `proteases'. Serine proteases in particular can also be rapidly, and irreversibly, inactivated through the action of protease inhibitors (Behrendt 2012; Di Cera 2011; Farady and Craik 2010; Silverman et al. 2010). Both activation and inhibition of proteases can be achieved without new protein synthesis, making them ideal for regulating extracellular processes such as interactions between male and female proteins following ejaculation in internally fertilizing animals. Despite this, the roles of seminal fluid proteases in fertility remain poorly understood.
Proteolysis regulators are common constituents of seminal fluid
Proteomic and transcriptomic studies of the seminal fluid of many animals, including humans, have identified large numbers of Sfp proteolysis regulators (Tables 1–3). The most extensive proteomic studies are those from human and Drosophila seminal fluid, so we will focus on results from these species in the identification section of this review. Later sections will include examples from many other organisms as well. Sfp proteases in humans and insects fall into several protease classes, including metalloproteases, cysteine proteases, and aspartic proteases, though the majority are trypsin- or chymotrypsin-like serine proteases (Tables 1 and 3).
Table 1.
Human seminal fluid proteases.
| Protease class | Protein name | GI | References |
|---|---|---|---|
| ADAM metalloprotease | |||
| ADAMTS-1 | 1264483 | (Pilch and Mann 2006) | |
| ADAM 7 | 296439449 | (Pilch and Mann 2006) | |
|
| |||
| Aminopeptidase | |||
| LAP3 | 48146373 | (Pilch and Mann 2006) | |
| Aminoacylproline aminopeptidase | 68566146 | (Pilch and Mann 2006) | |
| Aminopeptidase N | 143811362 | (Pilch and Mann 2006; Utleg et al. 2003) | |
|
| |||
| Asparaginyl peptidase | |||
| Legumain | 56682964 | (Pilch and Mann 2006) | |
|
| |||
| Aspartate | |||
| Gastricin | 129796 | (Fung et al. 2004; Utleg et al. 2003) | |
|
| |||
| Carboxypeptidase | |||
| Carboxypeptidase C | 11055992 | (Pilch and Mann 2006) | |
| Carboxypeptidase D | 25089854 | (Pilch and Mann 2006) | |
| Carboxypeptidase E | 4503009 | (Pilch and Mann 2006) | |
| Carboxypeptidase M | 53832021 | (Pilch and Mann 2006) | |
| Carboxypeptidase O | 27436871 | (Pilch and Mann 2006) | |
| Carboxypeptidase Z | 62388875 | (Pilch and Mann 2006) | |
| Glutamate carboxypeptidase-like protein 1 | 23396498 | (Pilch and Mann 2006) | |
| Pancreatic carboxypeptidase B1 | 54607080 | (Pilch and Mann 2006) | |
| Probable serine carboxypeptidase CPVL precursor | 67476930 | (Pilch and Mann 2006) | |
| Prolylcarboxypeptidase | 4826940 | (Pilch and Mann 2006) | |
| Glutamate carboxyeptidase (CPGL) | 15620780 | (Utleg et al. 2003) | |
|
| |||
| Caspase | |||
| Caspase 14 | 6912286 | (Pilch and Mann 2006) | |
| Paracaspase | 20455075 | (Pilch and Mann 2006) | |
|
| |||
| Papain-like cysteine | |||
| Cathepsin B | 22538437 | (Pilch and Mann 2006) | |
| Cathepsin D | 4503143 | (Pilch and Mann 2006) | |
| Cathepsin F | 6042196 | (Pilch and Mann 2006) | |
| Cathepsin G | 4503149 | (Pilch and Mann 2006) | |
| Cathepsin H | 21264388 | (Pilch and Mann 2006) | |
| Cathepsin O | 4557501 | (Pilch and Mann 2006) | |
| Cathepsin L1 | 22202619 | (Pilch and Mann 2006) | |
| Cathepsin Z | 22538442 | (Pilch and Mann 2006) | |
| PPGB | 56001072 | (Pilch and Mann 2006) | |
|
| |||
| Other cysteine | |||
| Calpain 1 | 12408656 | (Pilch and Mann 2006) | |
|
| |||
| Matrix metalloprotease | |||
| MMP2 | 11342666 | (Pilch and Mann 2006) | |
| MMP7 | 4505219 | (Pilch and Mann 2006) | |
| MMP14 | 317373419 | (Pilch and Mann 2006) | |
|
| |||
| Other Peptidase | |||
| Dipeptidase 3 | 193211608 | (Pilch and Mann 2006) | |
| Dipeptidyl-peptidase II | 21617867 | (Pilch and Mann 2006) | |
| Dipeptidyl-peptidase III | 20532389 | (Pilch and Mann 2006) | |
| Tripeptidyl-peptidase I | 62896635 | (Pilch and Mann 2006) | |
| Tripeptidyl peptidase II | 55661754 | (Pilch and Mann 2006) | |
| Peptidase A | 23396498 | (Pilch and Mann 2006) | |
| Puromycin-sensitive aminopeptidase | 51704228 | (Pilch and Mann 2006) | |
| Insulin protease | 215274252 | (Pilch and Mann 2006) | |
| Dipeptidyl peptidase IV | 1352311 | (Pilch and Mann 2006; Utleg et al. 2003) | |
| Neprilysin | 128062 | (Utleg et al. 2003) | |
|
| |||
| Serine | |||
| KLK12 | 37182171 | (Yousef and Diamandis 2001) | |
| KLK14 | 156257445 | (Yousef and Diamandis 2001) | |
| KLK15 | 9957760 | (Yousef and Diamandis 2001) | |
| KLK4 | 64653115 | (Yousef and Diamandis 2001) | |
| KLK5 | 37183138 | (Yousef and Diamandis 2001) | |
| KLK8 | 37183190 | (Yousef and Diamandis 2001) | |
| KLK11 | 5803199 | (Pilch and Mann 2006; Yousef and Diamandis 2001) | |
| KLK2 | 5031829 | (Pilch and Mann 2006; Yousef and Diamandis 2001) | |
| Acrosin light chain | 115502349 | (Pilch and Mann 2006) | |
| Brain-specific serine protease 4 | 11545839 | (Pilch and Mann 2006) | |
| C1r-like serine protease analog protein | 182705204 | (Pilch and Mann 2006) | |
| complement component 1 | 62897163 | (Pilch and Mann 2006) | |
| complement component 2 | 74755562 | (Pilch and Mann 2006) | |
| complement factor B | 67782358 | (Pilch and Mann 2006) | |
| complement factor I | 317373341 | (Pilch and Mann 2006) | |
| matriptase | 13124575 | (Pilch and Mann 2006) | |
| prostasin | 4506153 | (Pilch and Mann 2006) | |
| serine protease 1 | 4506145 | (Pilch and Mann 2006) | |
| TMPRSS2 | 115502469 | (Pilch and Mann 2006; Utleg et al. 2003) | |
| PSA (KLK3) | 4502173 | (Fung et al. 2004; Pilch and Mann 2006; Utleg et al. 2003) | |
|
| |||
| Thermolysin-like | |||
| Angiotensin I converting enzyme | 4503273 | (Pilch and Mann 2006) | |
| Angiotensin-converting enzyme 2 | 48525714 | (Pilch and Mann 2006) | |
|
| |||
| Ubiquitin processing | |||
| Deubiquitinating enzyme 14 | 1729927 | (Pilch and Mann 2006) | |
Proteases identified in human seminal plasma or prostasomes, listed by protease class. Classes are based either on experimental data or computational prediction. GI: NCBI identifier.
Table 3.
Proteases, inhibitors, and protease homologs in Drosophila melanogaster seminal fluid.
Proteases, protease inhibitors, and their homologs in Drosophila melanogaster seminal fluid, listed by protein class. Most protein functions listed are based on predictions made by detection of conserved protease or protease inhibitor domains. “Non-AG expression?” denotes whether gene expression is detected outside of the male accessory glands, based on data in FlyAtlas (Chintapalli et al. 2007. Dashed lines indicate that the protein is absent from the database.
Highest gene expression level is outside the male accessory gland.
Annotation as protease inhibitor is based on sequence similarity to known serine protease inhibitor genes, rather than on direct detection of conserved domains.
Proteases are classified based on their hydrolysis mechanism (Polgár 1989): serine proteases have a conserved catalytic triad consisting of a His, Ser, and Asp that coordinates a water molecule. The serine residue acts as a nucleophile to attack the carbonyl carbon of the substrate's scissile peptide bond (Polgár 1989). Serine proteases are the most common protease class in the proteomes of both insects and mammals (Heutinck et al. 2010; Page and Di Cera 2008; Ross et al. 2003; Shah et al. 2008). There are 816 known and predicted proteases in the human genome (and 438 non-protease homologs) (Rawlings et al. 2012). Of these, 346 are serine proteases or their homologs. The largest single family is the S1 (chymotrypsin-like) serine proteases, of which there are 144 in humans (Rawlings et al. 2012). In Drosophila melanogaster, expansion of the serine protease family has led to a similar number of these genes as seen in humans, despite the lower overall gene content in Drosophila (Ross et al. 2003). There are a predicted 501 proteases and 268 homologues of proteases in the D. melanogaster proteome, and 379 are serine proteases (Rawlings et al. 2012). 268 of these belong to the S1 family of serine proteases, a significant proportion of them being non-protease homologues (32%).
Metalloproteases are so named because they use a metal ion (such as zinc) to polarize a water molecule within the active site; the water molecule is then used to hydrolyze the scissile peptide bond of the substrate (Polgár 1989). The extracellular matrix metalloproteases (MMPs) (Zitka et al. 2010) and the astacin metalloproteases (Bond and Beynon 1995) are important members of this class. Cysteine proteases use a nucleophilic cysteine for hydrolysis (Polgár 1989). Cysteine proteases are most common in plants (Domsalla and Melzig 2008), but they are also very important in human physiology, acting as lysosomal enzymes and showing tissue-specific expression that has been tied to processes such as bone growth and lung function (Chapman et al. 1997). Many cathepsins (Turk et al. 2012), which are present in seminal fluid, are cysteine proteases. Finally, aspartic proteases use an aspartate as their catalytic residue (Polgár 1989). The digestive proteases pepsin and gastricin (which is also a constituent of the seminal fluid (Fung et al. 2004; Utleg et al. 2003)) are examples of aspartic proteases (Szecsi 1992).
Proteolysis must be tightly regulated to prevent premature activation of pathways or tissue damage that may result from overactive proteases. Protease inhibitors play an important part in regulation of proteolytic activity. Just as serine proteases are the most common class of protease in seminal fluid, serine protease inhibitors (including serpins and the Kazal- and Kunitz-type inhibitors) are also the most prevalent class of protease inhibitors, though cysteine protease inhibitors are also common. The prevalence of serine proteases and their inhibitors in the seminal fluid is expected, given the large proportion of these classes in the proteome (Page et al. 2007): of 187 known or predicted protease inhibitors (and their homologues) in D. melanogaster, approximately 110 are inhibitors of serine proteases (Rawlings et al. 2012), of which 29 are serpins (Gubb et al. 2010). There are far more protease inhibitors in humans: 1845 inhibitors and homologs in total, with at least 180 serine protease inhibitors (including 39 serpins), though it is not yet known which protease classes are inhibited by the majority of human predicted protease inhibitors (Rawlings et al. 2012). In addition to catalytically active proteases and protease inhibitors, two related classes of proteins are represented in Drosophila seminal fluid: catalytically inactive protease homologs and non-inhibitory serpins (discussed later).
IDENTIFICATION OF PROTEOLYSIS REGULATORS IN SEMINAL FLUID
Proteolysis regulators in human seminal fluid
A large number of proteases (Table 1) and protease inhibitors (Table 2) have been identified in human seminal plasma. The majority of these proteins are known from empirical studies to act as either proteases or protease inhibitors, though a small number are classified as proteolysis regulators based on computational prediction. Ejaculate samples can be easily obtained from many mammalian species, including humans, to allow direct proteomic analysis of the seminal fluid, with or without the presence of sperm (reviewed in Duncan and Thompson 2007). Fung et al. (Fung et al. 2004) used 1D and 2D PAGE followed by mass spectrometry to identify over 100 proteins in normal human seminal fluid, including the serine protease prostate-specific antigen (PSA) and its major substrates, Semenogelins I and II. Many of these proteins, PSA included, were present in multiple modified forms, suggesting a role for post-translational modification in their regulation. A later study (Pilch and Mann 2006) took advantage of recent advances in proteomics to create a high-confidence list of 923 human seminal plasma proteins. Based on current protein annotations, this study identified 58 non-proteasomal proteases and 38 protease inhibitors. A complementary proteomic analysis of human prostasomes (tiny membranous vesicles secreted by the prostate into the seminal fluid) identified a total of 139 proteins, which included 1 protease not identified in the seminal fluid studies, the glutamate carboxypeptidase CPGL (Utleg et al. 2003); the remaining proteases identified in this study overlapped with the other proteomic studies of the seminal plasma (Fung et al. 2004; Pilch and Mann 2006).
Table 2.
Human seminal fluid protease inhibitors.
| Protease inhibitor class | Protein name | GI | Reference |
|---|---|---|---|
| Cysteine | |||
| Cystatin A | 48145945 | (Pilch and Mann 2006) | |
| Cystatin SN | 118188 | (Pilch and Mann 2006) | |
| CST6 | 74724686 | (Pilch and Mann 2006) | |
| Cystatin C | 4503107 | (Fung et al. 2004) | |
| Cystatin S | 4503109 | (Fung et al. 2004) | |
|
| |||
| Metalloprotease inhibitor | |||
| Tissue inhibitor of metalloproteinase 1 | 4507509 | (Pilch and Mann 2006) | |
| Tissue inhibitor of metalloproteinase 2 | 4507511 | (Pilch and Mann 2006) | |
| Tissue inhibitor of metalloproteinase 3 | 4507513 | (Pilch and Mann 2006) | |
|
| |||
| Peptidase inhibitor | |||
| Peptidase inhibitor 15 | 7705676 | (Pilch and Mann 2006) | |
|
| |||
| Serine | |||
| Alpha-1-antitrypsin | 1703025 | (Fung et al. 2004; Pilch and Mann 2006) | |
| Plasma serine protease inhibitor | 194018472 | (Fung et al. 2004) | |
| Acrosin-trypsin inhibitor | 123985 | (Pilch and Mann 2006) | |
| Alpha-1-antichymotrypsin | 112874 | (Pilch and Mann 2006) | |
| Alpha-2-antiplasmin | 112907 | (Pilch and Mann 2006) | |
| Amyloid-beta A4 protein | 112927 | (Pilch and Mann 2006) | |
| Amyloid-like protein 2 | 1703344 | (Pilch and Mann 2006) | |
| Angiotensinogen | 113880 | (Pilch and Mann 2006) | |
| Anosmin-1 | 134048661 | (Pilch and Mann 2006) | |
| Antileukoproteinase I | 113636 | (Pilch and Mann 2006) | |
| Antithrombin III | 113936 | (Pilch and Mann 2006) | |
| CD109 (TGF-beta-1-binding protein) | 117949389 | (Pilch and Mann 2006) | |
| Epididymal secretory protein E4 | 20141958 | (Pilch and Mann 2006) | |
| Inter-alpha-trypsin inhibitor heavy chain H5 | 187609608 | (Pilch and Mann 2006) | |
| Kallistatin | 68067608 | (Pilch and Mann 2006) | |
| Kunitz-type protease inhibitor 1 | 61252335 | (Pilch and Mann 2006) | |
| Kunitz-type protease inhibitor 3 | 152031689 | (Pilch and Mann 2006) | |
| Kunitz-type protease inhibitor 4 | 74749362 | (Pilch and Mann 2006) | |
| Maspin (serpin B5) | 229462757 | (Pilch and Mann 2006) | |
| Neuroserpin (serpin I1) | 3183087 | (Pilch and Mann 2006) | |
| PEBP-1 | 1352726 | (Pilch and Mann 2006) | |
| PEDF (serpin F1) | 313104314 | (Pilch and Mann 2006) | |
| Peptidase inhibitor 15 (SugarCrisp) | 74735410 | (Pilch and Mann 2006) | |
| Placental thrombin inhibitor (serpin B6) | 161784343 | (Pilch and Mann 2006) | |
| Plasma protease C1 inhibitor | 124096 | (Pilch and Mann 2006) | |
| Protein AMBP | 122801 | (Pilch and Mann 2006) | |
| Protein C inhibitor (serpin A5) | 322510122 | (Pilch and Mann 2006) | |
| Putative protease inhibitor WAP8 | 126302615 | (Pilch and Mann 2006) | |
| Squamous cell carcinoma antigen 1 (serpin B3) | 20141712 | (Pilch and Mann 2006) | |
| Thyroxine-binding globulin (serpin A7) | 1351236 | (Pilch and Mann 2006) | |
| Tissue factor pathway inhibitor 2 | 1351226 | (Pilch and Mann 2006) | |
| Transcortin (serpin A6) | 115851 | (Pilch and Mann 2006) | |
| SPINT3 | 189571689 | (Clauss et al. 2011) | |
| SPINT4 | 110626171 | (Clauss et al. 2011) | |
| SPINT5 (sequence only available from mouse) | 53794664 | (Clauss et al. 2011) | |
Protease inhibitors identified in human seminal plasma, listed by protease inhibitor class. Protease classes are based either on experimental data or computational prediction. GI: NCBI identifier.
Together, these proteomic studies of human seminal fluid (Fung et al. 2004; Pilch and Mann 2006) and purified prostasomes (Utleg et al. 2003) identified over 100 proteases or protease inhibitors (Tables 1 and 2). Of the proteolysis regulators identified in these three studies, there were 14 serine proteases and 32 serine protease inhibitors (these numbers do not exactly match those given in the original papers, as protein annotations have been updated; the updated information is used in Tables 1 and 2). It is likely that more proteases remain to be identified, as even the most comprehensive of these studies failed to find more than three kallikrein-like serine proteases (KLKs 2, 3, and 11) (Pilch and Mann 2006), though at least nine are secreted into the seminal fluid (Yousef and Diamandis 2001). Additional proteins may not be detected using these proteomic methods if they are products of novel, unannotated genes. Recently, three novel Sfp genes with Kunitz-family protease inhibitor domains found to be expressed in human epididymis, and are likely secreted into the seminal plasma (Clauss et al. 2011). These genes have evolved recently and are much smaller than other protease inhibitor genes, making their identification by standard gene model prediction methods difficult (Clauss et al. 2011).
Proteolysis regulators in insect seminal fluid
Proteolysis regulators are also highly represented in the seminal fluid of Drosophila melanogaster, as evidenced by several proteomic and transcriptomic studies (Table 3) (Ayroles et al. 2011; Findlay et al. 2009; Findlay et al. 2008; Mueller et al. 2005; Swanson et al. 2001; Takemori and Yamamoto 2009; Walker et al. 2006). Recent proteomic studies of the D. melanogaster male reproductive tract have found that proteolysis regulators are expressed in all tissues of the male reproductive tract: accessory glands, testes, seminal vesicles, ejaculatory duct, and ejaculatory bulb (Takemori and Yamamoto 2009; Yamamoto and Takemori 2010). In contrast with the classification of human seminal fluid proteolysis regulators, most of the proteolysis regulators in Drosophila seminal fluid are classified based on computational gene predictions. However, given the high level of sequence conservation among these protein classes (proteases and serpins, in particular) and their extensive curation in the Drosophila genomes (Garrett et al. 2009; Ross et al. 2003), these predictions are likely to accurately reflect the biochemical function of these proteins.
Findlay et al. (Findlay et al. 2008) used heavy isotope labeling of Drosophila melanogaster female proteins to identify Sfps that were transferred to the female reproductive tract, while excluding female proteins from the analysis. Using this method, combined with shotgun proteomics, they found that over 20% of Sfps that they identified were proteolysis regulators (15 proteases and 14 protease inhibitors predicted among 138 high-confidence proteins) (Findlay et al. 2008). Further analysis of these data identified additional Sfps, including protease inhibitors (Findlay et al. 2009), that were not previously predicted based on the D. melanogaster genome assembly, for similar reasons as discussed earlier for newly annotated human Sfps (Clauss et al. 2011).
To date, these and other studies (Mueller et al. 2005; Swanson et al. 2001) have identified 21 proteases, 17 protease inhibitors, 10 predicted inactive serine protease homologs, and 5 predicted non-inhibitory serpin homologs in the seminal fluid of D. melanogaster (Table 3). Future work may uncover new Sfp proteolysis regulators in Drosophila, as several predicted proteolysis regulators were identified in a genome-wide expression study that uncovered over 100 genes with expression patterns correlated to those of known Sfps (Ayroles et al. 2011). This gene list likely contains previously unknown Sfp genes, including proteolysis regulators and their homologs, as well as genes important for Sfp gene regulation.
Proteomic analysis of male accessory glands (the main site of Sfp synthesis in Drosophila) from a distant relative of D. melanogaster, D. mojavensis, also identified many proteases and protease inhibitors that are likely components of the seminal fluid (Kelleher et al. 2009). Six predicted proteases and 1 predicted protease inhibitor had an Sfp homolog in D. melanogaster (Findlay et al. 2008). An additional 15 predicted proteases and 8 predicted protease inhibitors were specific to the seminal fluid in D. mojavensis, though their homologs exist in D. melanogaster as non-seminal proteins (Kelleher et al. 2009). These included one protease (GI18354, a predicted trypsin-like serine protease) and one protease inhibitor (GI10660, a predicted cysteine protease inhibitor) that arose from D. mojavensis-specific gene duplications (Kelleher et al. 2009). These findings indicate that proteolysis regulators may have species-specific roles in seminal fluid. This is consistent with data demonstrating that Sfps in D. melanogaster and D. mojavensis are, as a class, more likely to be evolving under positive selection than are other protein classes (Haerty et al. 2007; Swanson et al. 2001; Wagstaff and Begun 2005).
While Drosophila certainly has the most fully annotated Sfp proteome, recent work in other arthropod species has identified proteases and/or protease inhibitors in the seminal fluid of mosquitoes (Dottorini et al. 2007; Sirot et al. 2011a), sandflies (Azevedo et al. 2012), medflies (Davies and Chapman 2006), honeybees (Baer et al. 2009), butterflies and moths (Nagaoka et al. 2012; Walters and Harrison 2008; Walters and Harrison 2010), Tribolium (South et al. 2011), crickets (Andrés et al. 2006), and ticks (Weiss et al. 2002). Vertebrate species other than humans in which proteolysis regulators have been identified in the seminal fluid include mouse (Claydon et al. 2012; Dean et al. 2011), bull (Kelly et al. 2006; Moura et al. 2010), stallion (Novak et al. 2010), fish (Mommens et al. 2008; Wojtczak et al. 2005), and birds (Kotłowska et al. 2005; Thurston et al. 1993).
FUNCTIONS OF SEMINAL FLUID PROTEASES AND THEIR INHIBITORS
Given the recent explosion in Sfp identification across diverse taxa, we can hope to uncover common themes for Sfp function as well as species-specific Sfp roles. Studies of Sfp proteolysis regulators, especially in model organisms, will aid in understanding of pathways mediated by seminal fluid. Below, we highlight functional studies of proteolysis regulators in the seminal fluid.
Sfp-mediated proteolysis and its regulation in insect ovulation and egg-laying
In most insects, mating—specifically receipt of Sfps—induces high levels of oogenesis, ovulation, and egg-laying (Avila et al. 2011). Serine protease Sfps have been implicated in several species in this mating-induced increase in egg production. A trypsin-like serine protease in the Allonemobius socius complex of crickets is necessary for full induction of egg-laying after mating (Marshall et al. 2009). This protease, ejac-sp, is one of the most abundant proteins in the cricket spermatophore (a discrete package of seminal fluid and sperm that is deposited by the male for female insemination). The abundance of ejac-sp has been shown to decline with male age, along with the male's ability to induce egg-laying in females (Marshall et al. 2009). RNAi knockdown of the ejac-sp protein in male crickets led to reduced levels of the protein in the ejaculate, which correlated with reduced numbers of eggs laid by females mated to these males. Ejac-sp is also a candidate speciation gene, as it shows a high level of divergence due to positive selection between two related cricket species (Marshall et al. 2011; Marshall et al. 2009).
In Drosophila melanogaster, a predicted trypsin-like serine protease in the seminal fluid, seminase (CG10586), is also required for normal induction of egg-laying in mated females (LaFlamme et al. 2012). Females mated to seminase RNAi knockdown males lay eggs in similar numbers to controls during the first 24 hours after mating, but fail to maintain a high level of egg-laying in the following days, resulting in a large decrease in the total number of eggs laid over a 10 day experiment. This reduction appears to be due to a defect in sperm release from the seminal receptacle, the major sperm storage organ in D. melanogaster females. Seminase interacts genetically (and likely physically) with several other Sfps in a network that ultimately leads to binding of the peptide hormone sex peptide (another Sfp) to sperm (LaFlamme et al. 2012; Ravi Ram and Wolfner 2007; Ravi Ram and Wolfner 2009) as illustrated in Figure 1. Sex peptide is required for the maintenance of post-mating responses in D. melanogaster, including elevated levels of egg-laying and decreased sexual receptivity (Chapman et al. 2003; Peng et al. 2005). Sex peptide is also required for the efficient release of sperm from storage over time (Avila et al. 2010). Sex peptide achieves this long-term effect by being gradually released from sperm by an as yet unidentified trypsin protease (Peng et al. 2005) and signaling through its receptor in the female (Yapici et al. 2008). Seminase is required to localize sex peptide and other Sfps in the network to the female sperm storage organs and is thus indirectly responsible for sex peptide's long-term effects (LaFlamme et al. 2012).
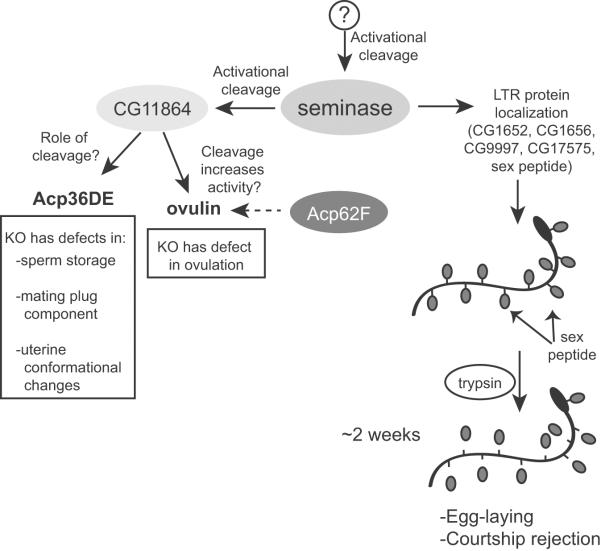
Figure 1. The Drosophila seminal fluid protease `seminase' regulates long- and short-term post-mating responses.
The trypsin-like protease seminase is cleaved while still in the male reproductive tract during mating, leading to the processing of the astacin metalloprotease CG11864. Both cleavages are predicted to active the respective proteases. Seminase is required for localization of other Sfps to the female sperm storage organs, which in turn is required for the post-mating processes regulated by sex peptide (right half of figure). As depicted, sex peptide is cleaved only from the sperm tail and not the head (Peng et al. 2005). In the short-term response pathway, seminase—through activation of CG11864—regulates processing of two Sfps: ovulin and Acp36DE (left half of figure). The trypsin protease inhibitor Acp62F may also interact with this pathway, as it affects ovulin processing. See text for references.
Seminase also has a seemingly unrelated role in initiating a protease cascade that leads to proteolytic cleavage of Sfps involved in earlier post-mating processes (LaFlamme et al. 2012) (Figure 1). Seminase itself is cleaved during mating, while still in the male reproductive tract, suggestive of activational pro-peptide removal, and may be responsible for quickly activating multiple post-mating processes mediated by Sfps. Seminase is required for the predicted activational processing of an astacin-family zinc metalloprotease in the seminal fluid, CG11864. The cleavage of CG11864 is also initiated in the male reproductive tract as well, in transit to the female (Ravi Ram et al. 2006). After deposition into the female tract, CG11864 is needed for processing of downstream Sfps: the sperm storage protein Acp36DE and the ovulation-inducing prohormone ovulin (Ravi Ram et al. 2006). Ovulin is cleaved in three sequential steps from its N-terminal end to give four final cleavage products (Park and Wolfner 1995). The trypsin protease inhibitor Acp62F (another Sfp) also affects ovulin processing; knock-out of Acp62F results in slower ovulin processing (Mueller et al. 2008). Despite the presence of active CG11864 in the male tract during mating, where ovulin is also present, cleavage of ovulin only occurs after deposition into the female reproductive tract, suggesting an important role for the female in this protease cascade. It is also possible that females may play a larger role in regulation of proteolytic pathways after mating, given the enrichment of proteolysis-related proteins upregulated in the female after mating (see discussion later in this review). The involvement of both male and female factors in the CG11864 proteolytic pathway suggests that precise regulation of ovulin processing is important for its physiological function.
Ovulin processing may lead to an increase in ovulin's activity, as ectopic expression of full-length ovulin as well as two C-terminal cleavage products can induce ovulation in virgin females (Heifetz et al. 2005). However, no functional role for ovulin's cleavage products has been tested in the context of mating. Despite the unclear role of CG11864 in male fertility, the seminase/CG11864 pathway is a useful in vivo model for studying the regulation of protease pathways in seminal fluid.
Protease pathways in semen coagulatio
Upon ejaculation in humans, proteins secreted by the seminal vesicles, Semenogelins I and II (SgI and SgII) and fibronectin, are mixed with other components of the semen to cause coagulation of the ejaculate (Lilja et al. 1987). Semenogelin (Sg) proteins create a matrix that immobilizes a portion of the ejaculated sperm in a structure called the seminal clot (Lilja et al. 1989) (illustrated in Figure 2). This gel-like structure is degraded within approximately 20–30 minutes post-ejaculation by the action of kallikrein-like proteases (KLKs) in the prostatic fluid in a process known as semen liquefaction (Lilja et al. 1989). KLKs are trypsin- or chymotrypsin-like serine proteases encoded by 15 genes at the chromosomal locus 19q13.4 (Borgoño and Diamandis 2004). KLKs are required both for semen liquefaction and skin desquamation in humans (reviewed in Pampalakis and Sotiropoulou 2007). Mutations in KLKs and defects in their regulation have been linked to various cancers, including prostate cancer (Borgoño and Diamandis 2004).
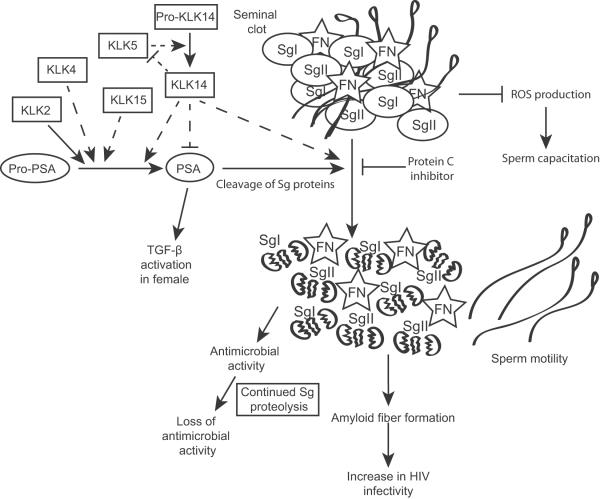
Figure 2. A protease cascade regulates semen liquefaction in humans.
The chymotrypsin protease KLK3/PSA is activated from its pro-form after ejaculation. Pro-PSA can be activated to PSA in vitro, by several KLK proteases, including KLK4, KLK5, KLK14, and KLK15 (dashed lines represent in vitro evidence). KLK2 has been shown in vivo to be required for PSA activation in a mouse model. KLK14 may also inactivate PSA and has the ability to directly cleave Semenogelin proteins (as does KLK 5—not shown). Seminal clot formation inhibits reactive oxygen species (ROS) production, which is required for sperm capacitation. Degradation of the seminal clot leads to several downstream consequences, including sperm motility for previously trapped sperm, antimicrobial activity of the semen, and may increase HIV infectivity. See text for references.
Semen liquefaction is the end result of a seminal fluid KLK protease cascade. KLKs 2, 3, 4, 5, 8, 11, 12, 14, and 15 are secreted by the prostate (Yousef and Diamandis 2001) and act in a protease cascade initiated by the reduction in Zn2+ concentration that follows mixing of the prostatic fluids with other seminal fluids (Pampalakis and Sotiropoulou 2007). Seminal liquefaction is required to release spermatozoa trapped by the seminal clot, and defects in liquefaction may lead to fertility problems (Lwaleed et al. 2004).
Pro-KLK3 (the inactive form of KLK3, also known as prostate-specific antigen, PSA) is ultimately activated by the KLK cascade and directly cleaves the Sg proteins. Activated PSA is subsequently inactivated, in part, by the serine protease inhibitor, protein C inhibitor (PCI) (Suzuki et al. 2007). PCI also forms tertiary structures with the Sg proteins, further contributing to the inactivation of PSA.
Which KLKs are directly responsible for activating PSA is not entirely clear. Williams et al. (Williams et al. 2010) created transgenic mouse lines expressing human PSA and KLK2 in the prostate; these are the only two KLKs without homologs in the mouse (Lundwall and Brattsand 2008). They found that PSA was only active in the mouse prostate when co-expressed with KLK2, suggesting that KLK2 may be important for regulation of PSA activity.
KLK14 has been identified as a potential activator and subsequent inhibitor of PSA (Emami and Diamandis 2008). Addition of active KLK14 to ejaculate samples rapidly and transiently increased chymotrypsin-like activity against a synthetic peptide (which could be attributed to PSA), compared to samples treated with a KLK14-specific inhibitor. Longer incubations showed a reversal of this effect, with greater chymotrypsin-like activity in samples treated with the KLK14 inhibitor relative to those treated with active KLK14 (Emami and Diamandis 2008). Samples treated with active KLK14 also showed greater fragmentation of PSA, suggesting an inhibitory mechanism by internal cleavage. KLK14 can also directly degrade purified SgI and SgII in vitro, complementing the function of PSA. Consistent with this, Emami and Diamandis (2008) found that patients with clinically delayed seminal clot liquefaction also had significantly lower KLK14 expression than normal patients.
Other seminal fluid serine proteases may also be involved in the KLK cascade. For example, the serine protease plasmin (the activated form of plasminogen) is able to activate KLK11 in vitro (Luo et al. 2006). In addition to proteins mentioned above, many of which are also expressed as blood plasma proteins, classic hemostatic factors may also contribute to seminal clotting and liquefaction (Lwaleed et al. 2007; Lwaleed et al. 2004; Lwaleed et al. 2005), including a major role for tissue factor (TF; thromboplastin) (Fernández and Heeb 2007; Lwaleed et al. 2006). Consistent with an important role of TF in seminal fluid, levels of this protein are much higher in semen (average of 21 ng/mL (Fernández and Heeb 2007)) than in blood (average of 85 pg/mL (Fernández et al. 1997)).
The extent to which the human seminal clotting/liquefaction pathways can be extrapolated to other species is unclear, as even primate species differ in regards to whether they form a seminal clot (as in humans) or a solid structure known as a copulatory or mating plug (as in chimpanzees). Unlike the human seminal clot, which traps sperm prior to liquefaction, mating plugs form more slowly and do not trap large numbers of sperm in a matrix. However, Sg proteins are used in the formation of both structures, and both may be acted upon by proteases (either male or female). Among primates, the presence and strength of selection on Sg genes appears to correlate with inferred levels of sperm competition (i.e.: the level of polyandry) (Carnahan and Jensen-Seaman 2008; Clark and Swanson 2005; Dorus et al. 2004). The semen coagulum or the copulatory plug may help prevent fertilization by subsequent mates within a short time period after the first mating, it may prevent loss of sperm, or may promote fertilization by some as yet unknown mechanism, all of which could contribute to an advantage for one male over his rivals in sperm competition. In chimpanzees, which experience high levels of sperm competition, the SgI protein coding sequence has become greatly expanded due to the duplication of several tandem repeats (Jensen-Seaman and Li 2003), providing potentially more sites for cross-linking or non-covalent interactions during copulatory plug formation.
Sg genes show signatures of strong positive selection in chimpanzee (Dorus et al. 2004; Kingan et al. 2003), as does transglutaminase 4 (TGM4), which functions in cross-linking Sg proteins in many species (Clark and Swanson 2005). TGM4 also shows signatures of positive selection in bonobos (Carnahan and Jensen-Seaman 2008; Clark and Swanson 2005; Jensen-Seaman and Li 2003). In contrast, SgI, SgII, and TGM4 are all pseudogenes in gorilla, which experiences low, if any, sperm competition (Carnahan and Jensen-Seaman 2008). However, pseudogenization of Sg genes is not a universal trend in species with low sperm competition. In humans, which experience low sperm competition, SgII and TGM4 appear to be evolving nearly neutrally, though they are clearly functional, as opposed to the non-functional, SgI, SgII, and TGM4 genes in gorilla (Carnahan and Jensen-Seaman 2008). Gibbons also have low (if any) sperm competition and do not show widespread loss of the Sg proteins or TGM4 (though SgI appears to be a pseudogene, containing a premature stop codon, in at least one gibbon species (Jensen-Seaman and Li 2003) and TGM4 is similarly truncated in another species (Clark and Swanson 2005)).
While TGM4 is not itself a protease, its function may be important in regulating proteolysis in the semen coagulum or in mating plugs of various species, including chimpanzees and mice (mating plugs are similar, though not equivalent, structures to the human semen coagulum). For example, the mouse mating plug is formed by the crosslinking action of transglutaminase in the seminal fluid. Cross-linking of mating plug proteins makes the plug resistant to proteolysis, presumably by female-expressed proteases (Tseng et al. 2012). The co-evolutionary pattern of transglutaminase and its substrates in the seminal fluid indicate that crosslinking mouse mating plug proteins may be a response to the action of female proteases (Tseng et al. 2012).
In the mouse, unchecked proteolysis of the mating plug, due to the deletion of the seminal vesicle-expressed serine protease inhibitor, SERPINE2 (also known as protease nexin-1) in male mice led to malformed mating plugs and decreased fertility (Murer et al. 2001). Plugs formed after mating with SERPINE2 mutant males were abnormally small, soft, and fibrous compared to plugs formed by wildtype males, apparently due to proteolysis of important structural proteins of the mating plug. These defects resulted in loss of sperm from the female reproductive tract after mating. Even when sperm are able to remain in the female reproductive tract, the function of SERPINE2 as a decapacitation factor (Lu et al. 2011; Murer et al. 2001) likely contributes to the infertility seen in these mutant mice (Murer et al. 2001).
Mating plugs in Anopheles gambiae promote sperm storage. Analysis of the Anopheles mating plug revealed the presence of 5 male-derived proteases and many female-derived proteases (Rogers et al. 2009). A male accessory gland-specific transglutaminase is also required in Anopheles for semen coagulation. Crosslinking of the semen coagulum may prevent proteolysis by female proteases in mosquitoes, as in the mammalian examples above. Even though their mating systems are diverse, mating plugs in mammals and insects may play similar roles in male-female interactions.
Roles for Sfp proteolysis and inhibitors in sperm storage and sperm competition
Sperm storage is a universal phenomenon in animals with internal fertilization. The storage of sperm in specialized organs or areas within the female reproductive tract has three stages: entry of sperm into storage, maintenance of sperm within storage (for time periods between hours and decades, depending on the organism), and appropriate release of sperm for fertilization (Holt 2011).
The mating plug may act to prevent insemination by subsequent males. In Drosophila, genetic deletion of the major mating plug protein, PEBII resulted in an increase in remating by females very soon after mating, relative to females mated first to normal males (Bretman et al. 2010), though the mechanism for this function of PEBII is not known. Beyond the mating plug, Sfps play an important role in all three stages of sperm storage (Avila et al. 2011; Hung and Suarez 2010; Suarez 2008).
In Drosophila, a few Sfps have been implicated in sperm storage (Bloch Qazi and Wolfner 2003; Neubaum and Wolfner 1999; Wong et al. 2008a). These include the glycoprotein Acp36DE, an inferred proteolytic substrate (along with ovulin) of the seminal metalloprotease CG11864. Acp36DE is cleaved within the female reproductive tract after mating, though the fates of individual cleavage products are not known, nor is the function of its cleavage. Acp36DE is required for efficient storage of sperm (Bloch Qazi and Wolfner 2003; Neubaum and Wolfner 1999), possibly through its role in effecting post-mating conformational changes of the uterus (Avila and Wolfner 2009). Because females mated to males lacking Acp36DE store fewer sperm than mates of normal males, sperm from Acp36DE null males are less able to defend against displacement by sperm from a second male (Chapman et al. 2000). Acp36DE is also a component of the mating plug (Lung and Wolfner 2001). Whether any of these functions of Acp36DE are related to its processing by CG11864 has yet to be determined.
Several alleles of the D. melanogaster trypsin protease inhibitor Acp62F, another Sfp, are associated with variation in sperm competition success (Fiumera et al. 2007). Sperm competition refers to the ability of a male's sperm to resist displacement by a second male's ejaculate (defense) or to induce displacement of a previous male's ejaculate (offense). Complete deletion of the Acp62F gene results in males with improved defensive sperm competitive ability (Mueller et al. 2008). Unlike with Acp36DE, this does not appear to be related to the number of sperm stored. Instead, the improvement in “defense” may be a side effect of Acp62F's true, unknown function. For example, Acp62F may be involved in efficient release of sperm from storage; loss of this protein may make sperm harder to displace by rival sperm, but also less efficient at fertilizing eggs. While no defects in progeny-production numbers have been observed in laboratory assays of males lacking Acp62F (Mueller et al. 2008), this does not rule out a defect that results in less efficient fertilization that would not necessarily be detected by the assays used in the study. Identifying the protease(s) inhibited by Acp62F may help shed light on its role in sperm use.
Though sperm storage and sperm competition are also important in mammals, these phenomena are much more difficult to study in mammalian systems than in insects. As a first step toward identifying Sfps with roles in these two processes, Claydon et al. (Claydon et al. 2012) studied the protein turnover rate of Sfps in the mouse (Mus musculus domesticus). A relatively high protein turnover rate is a prerequisite for a male's ability to control the abundance of specific Sfps in the ejaculate in response to perceived sperm competition (Parker and Pizzari 2010). A high turnover rate would allow for a protein's abundance to be adapted on a short timescale. Six abundant seminal vesicle proteins were measured for incorporation of a dietary heavy isotope label over time, including SPIKL (a Kazal-type serine protease inhibitor), and all had higher than expected turnover rates of about 10% per day (Claydon et al. 2012). These data suggest that male mice may have the ability to change their ejaculate composition in response to social cues. A related phenomenon has been reported in flies, as male Drosophila melanogaster are able to strategically allocate specific Sfps to females based on perceived levels of sperm competition (Sirot et al. 2011b). The exact role of individual proteins, including protease inhibitors, in the context of sperm competition will require further study.
Proteolysis regulators in sperm maturation and activation
Many of the proteins important for sperm maturation and sperm activation (or capacitation) in mammals are not part of the seminal fluid per se, but rather are components of the sperm plasma membrane (Dacheux et al. 2003). On the other hand, there are protease inhibitors in the seminal fluid that are important for mammalian sperm function (Guyonnet et al. 2011). Several recent reports support a role of seminal fluid protease inhibitors in protection of the sperm from proteolysis.
A seminal vesicle-secreted protease inhibitor, Spink3 (for `serine protease inhibitor Kazal-type 3'), binds to the plasma membrane of the apical hook of mouse sperm (Ou et al. 2012). During transit through the female reproductive tract, Spink3 appears to have a protective protease inhibitor function against female-expressed trypsin (Ou et al. 2012). Spink3 may also have a function unrelated to its ability to inhibit trypsin proteases. Incubation of GST-Spink3, which was unable to inhibit trypsin activity due to the presence of GST, prevented the acrosome reaction in capacitated sperm in vitro by reducing the levels of sperm nitric oxide (NO) (Zalazar et al. 2012). Very high levels of NO inhibit sperm function, though a moderate increase in NO production is required for full sperm maturation following capacitation (de Lamirande and Lamothe 2009; Roessner et al. 2010). This non-inhibitory function of Spink3 may be required to keep sperm from completing the acrosome reaction until it reaches the ovum (Zalazar et al. 2012).
A related protease inhibitor, Spink2, is expressed in the male germ cells of mouse (Lee et al. 2011). Spink2 is required to protect the developing sperm against protease activity during spermatogenesis and is also expressed in the mouse epididymis (Lee et al. 2011). Thus, Spink2 may also have a role in protecting against proteolysis of Sfps. Another protease inhibitor, SPINKL (Spink-like), is secreted by the mouse seminal vesicle where it protects against premature capacitation (Lin et al. 2008). These examples (Spink2, Spink3, and SPINKL) demonstrate that protease inhibitors are important for regulating sperm capacitation, the acrosome reaction, and proteolysis by the female in mammals.
Proteolysis regulators are required for sperm function in other organisms as well. A serine protease and two serine protease inhibitors are important for sperm activation in nematodes (Smith and Stanfield 2011; Stanfield and Villeneuve 2006; Zhao et al. 2012). In the androdioecious species Caenorhabditis elegans, activation of male sperm occurs during insemination and involves a cellular rearrangement of the spermatid that confers motility on the mature spermatozoon(Ward and Carrel 1979). Though males are not required for reproduction in C. elegans, since hermaphrodites can self-fertilize, males' sperm are preferentially used when mating has occurred (Ward and Carrel 1979). A predicted serine protease inhibitor, SWM-1, is required to prevent premature sperm activation in males (Stanfield and Villeneuve 2006). In the absence of SWM-1, sperm become activated in the male tract and cause infertility by preventing the transfer of sperm to hermaphrodites. The importance of a serine protease inhibitor suggests a role for a serine protease(s) in C. elegans sperm activation. This was recently confirmed; a trypsin-like serine protease, TRY-5, is present in the ejaculate of C. elegans males and is required for sperm activation in the absence of hermaphrodite-specific activation factors (Smith and Stanfield 2011). Work in a related nematode species, Ascaris suum, recently identified an additional serine protease inhibitor, As_SRP-1, that is expressed in the spermatid itself and is required for the cytoskeletal changes at activation that lead to sperm motility (Zhao et al. 2012). Following sperm activation, As_SRP-1 is secreted into the seminal fluid where it prevents activation of any sperm not already activated, presumably by blocking protease activity in the seminal fluid (Zhao et al. 2012). Taken together, these studies demonstrate a crucial role of proteolysis in nematode sperm activation, and that this activity must be highly regulated both in time and space.
Trypsin-like serine proteases are also implicated in sperm activation in insects. In Manduca sexta, sperm acquire motility in the spermatophore following ejaculation, in a process dependent on proteases secreted in the male reproductive tract (Friedländer et al. 2001). In vitro experiments indicate that a trypsin protease(s) is involved in the activation of sperm motility. Similarly, a serine protease called initiatorin is required for sperm activation in another lepidopteran, the silkmoth Bombyx mori (Nagaoka et al. 2012). Initiatorin may function by activating a protein-arginine degradation cascade in the seminal fluid (Aigaki et al. 1994). As evidence of this, purified recombinant initiatorin is proteolytically active and can activate sperm in vitro (Nagaoka et al. 2012). Serine proteases are also likely to function in sperm activation in the true bugs Aquarius remigis (water striders). Miyata, et al. (Miyata et al. 2012) found a crucial role for trypsin activity in initiating conserved signaling pathways for sperm motility in this species, though it is unclear whether the required trypsin protease(s) are present in the seminal fluid.
Links between Sfp proteolysis regulators and infertility
A study of 473 Han-Chinese men found significant correlations between alleles of the epididymal serine protease inhibitor Eppin and some semen parameters, including sperm number, curvilinear velocity, and straight line velocity (Ding et al. 2010). These parameters are used to assess the quality of semen in men seeking treatment for infertility. Eppin is part of a protein complex on sperm that includes lactotransferrin, clusterin, and SgI (reviewed in O'Rand et al. 2011). Binding of Eppin to SgI is critical to the role of SgI in inhibiting sperm motility prior to semen liquefaction (Mitra et al. 2010). In support of a role for Eppin in human fertility, treatment of male macaques with anti-Eppin antibodies resulted in complete, and reversible, loss of their sperm's motility (O'Rand et al. 2004). Anti-Eppin antibodies from infertile monkeys also inhibit human sperm motility in vitro (O'Rand et al. 2009).
Studies of protein abundance in human semen have also uncovered correlations between metalloprotease expression and semen parameters. Levels of the latent form of the matrix metalloprotease MMP-9 are elevated in semen samples with low sperm concentration (Tentes et al. 2007). MMP-9 and MMP-2 are also present in canine seminal fluid (Saengsoi et al. 2011). In dogs, ejaculate volume was negatively correlated with levels of latent MMP-2 and latent MMP-9. Both the latent and activated forms of dog MMP-9 were negatively correlated with sperm concentrations, similar to the findings in humans (Tentes et al. 2007). The cause and effect relationship between these parameters remains to be determined.
Functional studies in model organisms will be crucial for determining how these proteins may affect fertility in humans. For example, the same study linking SERPINE2 in mice to mating plug malformation and infertility also compared the levels of the human homolog of SERPINE2 in semen samples between fertile and infertile men (Murer et al. 2001). In contrast to the situation in mice, infertility in men with low semen fructose levels was correlated with higher than normal levels of SERPINE2. Though these results are in opposition to the role of this protein in mice, they nevertheless still indicate an important role of SERPINE2 in fertility, and its molecular functions may be similar in both species.
Protective effects of Sfp proteolysis regulators
In mammals, Sfps are important in protecting sperm from attack by the female immune system and in promoting immune tolerance that is essential for pregnancy establishment (Moldenhauer et al. 2009; Robertson 2005). Recent work has found direct links between seminal plasma and modulation of female immune function in mice (Guerin et al. 2011). Seminal fluid serine proteases, specifically KLKs, have been implicated in activation of latent TGF-beta in the semen (Emami and Diamandis 2010). TGF-beta is secreted primarily from the prostate and additionally by other male accessory glands. It is thought to be the major contributor of seminal fluid immunosuppression, accounting for approximately half of the immune tolerance of the semen (Nocera and Chu 1993; Nocera and Chu 1995; Robertson et al. 2002). KLK14 can activate latent TGF-beta in vitro at physiologically relevant levels through cleavage of the N-terminal latency-associated pro-peptide (LAP) (Emami and Diamandis 2010). KLK14 may also cleave latent TGF-beta1-binding protein, contributing to TGF-beta activation. Emami and colleagues (Emami and Diamandis 2010) demonstrated that KLKs 1, 2, and 5 may also be involved in TGF-beta activation by nicking the LAP to more easily allow for further activation by KLK14.
Proteolysis of the seminal clot has recently been identified as a major contributor to the antibacterial activity of seminal plasma in humans (Edström et al. 2008). The presence of antibacterial molecules in the seminal fluid of mammals (Mårdh and Colleen 1975) and insects (Lung et al. 2001; Marchini et al. 1997) argues that one function of Sfps is to protect against bacterial infection of the reproductive tract. This is important because sperm may come into contact with bacteria in the vagina introduced during copulation, which may cause harm within the uterus or fallopian tubes. In one study, partially processed Semenogelin proteins had high levels of antibacterial activity, which was lost as the proteins became fully processed (Edström et al. 2008). The timescale over which this happens mirrors that of the loss of antibacterial activity in whole semen (Edström et al. 2008).
In Drosophila melanogaster, ectopic expression of one metalloprotease Sfp (CG6168) or either of two serine protease inhibitor Sfps (Spn3 and CG42537—previously annotated as CG10284) can protect females from systemic bacterial infection (Mueller et al. 2007). At least one of the protease inhibitors, Spn3, enters the female circulatory system after normal transfer from the male at mating (Ravi Ram et al. 2005). This may be important in allowing the female to better fight off infections that might otherwise divert resources from egg production or decrease the female's lifespan. The added boost from Sfps may be especially beneficial, given that female production of antimicrobial peptides in response to infection is lowered after receipt of seminal fluid (Short et al. 2012).
Toxicity of proteolysis regulators in seminal fluid
Identifying Sfps with toxic effects, as exist in some species, may help to get to the cause of certain pathologies in the male reproductive tract. Misregulation of proteolysis pathways in particular is likely to lead to damage of the male tract. Therefore, studying the regulation of Sfp proteolysis pathways and the consequences to their misregulation is a promising approach for further understanding of these pathologies.
Toxic or deleterious effects of Sfps may be due to unwanted side-effects of the main functions of those proteins, as otherwise they would be expected to be lost due to natural selection. In contrast to the protective effect of seminal liquefaction on bacterial infection, recent work has shown that Sg degradation by PSA during liquefaction may form amyloid fibers that increase the probability of HIV infection (Roan et al. 2011). PSA also has a more direct deleterious effect—it is an allergen in women with seminal plasma hypersensitivity (Weidinger et al. 2006) and potentially also in men with post-orgasmic illness syndrome (Waldinger et al. 2011). Sensitivity to PSA is likely a side-effect of other allergies, particularly allergy to dog dander. In one study, approximately 24% of women with dog epithelium allergies also had a positive skin-prick result with PSA, suggesting that at least some portion of dog allergies may be caused by a reaction to a PSA-like protein (Basagaña et al. 2008). Recently, the protein responsible for this allergic response was identified as Can f 5—a kallikrein protease also found in dog semen and urine (Basagaña et al. 2012). In one case study, a woman with seminal plasma hypersensitivity and known allergy to male, but not female, dogs, was gradually desensitized to seminal plasma by taking HI blocker medication prior to unprotected sex (Kofler et al. 2012). The existence of a highly homologous protein in dog dander suggests that Can f 5 could be used for desensitization protocols in place of human seminal plasma.
The toxic effects of some Sfps are well established in Drosophila (Chapman et al. 1995; Lung et al. 2002; Mueller et al. 2007). Ectopic expression of several Sfps, including two protease inhibitors (Acp62F and Spn2), can cause lethality when expressed in preadult flies (Mueller et al. 2007). One of these Sfps, the trypsin protease inhibitor Acp62F, localizes to the uterus and sperm storage organs, while approximately 10% of this protein enters the female's circulation (Lung et al. 2002). Though ectopic expression of Acp62 in adult virgin females did shorten their lifespan (Lung et al. 2002), females mated to males lacking this Sfp do not experience any change in lifespan (Mueller et al. 2008). This is likely because other factors (e.g., sex peptide (Wigby and Chapman 2005)) overwhelmed the effect of Acp62F in the experiment. Identifying the targets of Acp62F will likely reveal why overexpression of this protease inhibitor causes toxicity in females.
FUTURE DIRECTIONS FOR STUDIES OF SEMINAL FLUID PROTEOLYSIS REGULATORS
The proteomic studies outlined in the first section of this review (Findlay et al. 2009; Findlay et al. 2008; Fung et al. 2004; Kelleher et al. 2009; Pilch and Mann 2006; Utleg et al. 2003) have led to the identification of a large number of proteolysis regulators and their homologs in seminal fluid, but functions for the majority of those proteins are not yet known. Here, I attempt to outline some broad approaches that may lead to a better general understanding of the roles of proteolysis regulators in semen.
Substrate profiling
A first step to understanding what proteases are doing in the seminal fluid is to find what substrates they act upon. Identification of a protease as belonging to a specific class does not in itself suggest possible biological functions for that protease. Though broad substrate specificities are known for some protease families, these still need to be determined empirically for individual proteases. Several powerful methods, collectively called “degradomics” (Agard and Wells 2009), now exist to profile substrate specificities (i.e., identify particular residues cleaved by the protease) and identify native protein substrates for individual proteases.
Unbiased methods such as 1D and 2D gel-based approaches can identify large numbers of protease substrates, though not their exact cleavage sites. One such method is PROTOMAP (Dix et al. 2008). PROTOMAP combines 1D PAGE with LC-MS/MS to identify protein size changes (indicative of proteolysis) across a wide dynamic range of potential substrate sizes. PROTOMAP is especially useful when large numbers of samples have to be compared, for example, when re-constructing a timecourse of proteolytic events.
Other methods directly screen for proteolytic events. N-terminal peptide identification methods (reviewed in Agard and Wells 2009) allow for simultaneous identification of protease substrates and cleavage sites by taking advantage of the unique N-termini produced by proteolysis (Becker-Pauly et al. 2011; Prudova et al. 2010; Schilling and Overall 2008).
Recent studies using state-of-the-art mass spectrometry methods have been applied to blood plasma to identify peptides produced by proteolysis in vivo (Shen et al. 2010a; Shen et al. 2010b). These methods allow for de novo sequencing of peptides and thus identification of peptides with unexpected modifications (such as proteolysis by a non-standard enzyme). This yields high-quality peptides with low false-discovery rates (Shen et al. 2008). Similar methods should be directly applicable to degradomics in seminal plasma.
Using a combination of the above degradomic methods, investigators in a number of systems could identify substrate specificities and substrate identities for proteases in the seminal fluid. They could also be used to identify proteolytic events immediately following ejaculation or over a longer timecourse in whole seminal plasma in order to identify new members of post-mating proteolytic pathways. Degradomics could also lead to the identification of biomarkers for prostate cancer by shifting the focus from expression levels of proteins to enzyme activity levels. The currently used screening test for prostate cancer measures levels of the serine protease PSA in the blood. However, due to the high frequency of false positives from this test, there is question as to its utility as a diagnostic tool (Basch et al. 2012). Instead, focusing on the activity of proteases, such as PSA, by tracking their substrates, may yield more useful biomarkers.
Model organism studies
Questions regarding Sfp functions are not easily addressed using human samples, and this is no less true for proteases and protease inhibitors. Model organisms, such as the mouse and Drosophila melanogaster, can be used to address the roles of proteolysis regulators in the ejaculate. Single protein knockout studies are feasible in both organisms; these are most informative when the protein in question has a large fertility effect (such as the mouse SERPINE2, described earlier). However, many proteolysis regulators do not appear to have such large effects (e.g., Drosophila proteins Acp62F (Mueller et al. 2008) and CG11864 (Ravi Ram et al. 2006)), and there may be functional redundancy among proteases or inhibitors. Thus, multiple genes may need to be knocked out to study the function of a group of proteins, a task that is much more practical in Drosophila than in the mouse. In addition, mating context—such as female genotype or mating status, temperature, nutritional status, or possibly bacterial infection of either mating partner (Short et al. 2012)—may be important in the function of a particular Sfp. These questions require repeated, statistically powerful experiments that are difficult to perform using mammalian models (e.g., mouse or human) but are routine in Drosophila.
Additionally, structural analyses of specific proteases or inhibitors could also benefit from a model organism approach. Identifying crucial residues for function—including exosites (important regulatory sites outside of the catalytic site)—is easily carried out in model organisms such as Drosophila and C. elegans, while follow-up studies using mouse models and in vitro studies with human recombinant proteins will be important in establishing relevance in humans.
Unexplored territory: Inactive protease homologs and non-inhibitory serpins
An fascinating result from Drosophila is the number of inactive homologs of serine proteases and non-inhibitory serpins that are present in the seminal fluid (Table 3). The majority of the predicted Sfp serine proteases have mutations in essential active site residues. In addition, five out of the nine predicted serpins appear to be non-inhibitory based on the absence of a conserved inhibitory loop found in serpins (Garrett et al. 2009). Many non-catalytic protease homologs in the seminal fluid of Drosophila show signatures of positive selection (Wong et al. 2008b), possibly reflecting newly acquired adaptive roles in fertility.
Inactive protease homologs may nevertheless play a role in proteolysis pathways. They may bind the substrates of other proteases without cleaving them, acting as competitive inhibitors. Inactive protease homologs may also have regulatory roles. For example, the non-catalytic rhomboid protease homologs (iRhoms) are transmembrane proteins required for shuttling EGFR ligands to the ER-associated degradation pathway in Drosophila (Zettl et al. 2011) and for shuttling the metalloprotease TACE out of the ER to the cell surface in mice (Adrain et al. 2012). Similar regulatory roles (such chaperones or transport proteins) may be important in the seminal fluid for Sfp function.
In Drosophila, at least one inactive serine protease homolog is part of a post-mating reproductive pathway, though the molecular mechanism of this protein is still unknown. The inactive serine protease homolog CG9997, an Sfp, is part of the pathway that binds sex peptide to sperm and allows for the long-term maintenance of sex peptide's functions in the female (Ravi Ram and Wolfner 2007; Ravi Ram and Wolfner 2009) (Figure 1).
Noninhibitory serpins have many roles in mammals outside of the seminal fluid (Whisstock et al. 2010). Noninhibitory serpins act as molecular chaperones, hormone transporters, and tumor suppressors (Whisstock et al. 2010). Whether any Sfp noninhibitory serpins, in Drosophila (see Table 3) or other organisms, share any of these functions should be investigated.
Missing pieces of the puzzle: Female substrates/regulators/interactors of male proteases
Finally, it will be important to identify female protein interactors, substrates, and regulators of seminal fluid-mediated proteolysis. In Drosophila, many genes and proteins are differentially regulated in the female in response to mating (Lawniczak and Begun 2007; Mack et al. 2006; McGraw et al. 2004). At least 5 genes are specifically upregulated in the absence of the seminal fluid protease inhibitor Acp62F (McGraw et al. 2008). Thus, Acp62F may have an important function in pathways leading to regulation of those genes.
Female-expressed proteolysis regulators are good candidates for interactors of male-derived proteases and protease inhibitors, as they may act together in post-mating proteolysis networks. Proteases in particular are an enriched class of genes that are upregulated in Drosophila females in response to mating (Lawniczak and Begun 2007; Mack et al. 2006; McGraw et al. 2008; McGraw et al. 2004). Also in Drosophila melanogaster, secreted proteins in the spermathecae (one of the two types of sperm storage organs) are significantly enriched for serine proteases (Prokupek et al. 2009). At least one of these proteases, Send2, is expressed in response to mating (Schnakenberg et al. 2011) and may function in sperm storage or regulation of proteolytic pathways. A mating-responsive female protease has also been identified in the mouse (Daimon and Wada 2005). The metalloprotease MMP-9 (which is also a component of seminal fluid) is expressed at higher levels in the uteruses of mated females compared to virgins; removal of male seminal vesicles prior to mating abolishes this increase (Daimon and Wada 2005). As this increase in MMP-9 expression is coincident with neutrophil infiltration to the uterus, increased MMP-9 activity may be required for implantation success (Daimon and Wada 2005).
In the mosquito Anopheles gambiae, a genomic cluster of serine protease genes was recently reported as expressed in the female reproductive tract (Mancini et al. 2011). Evolutionary analysis of these genes revealed signatures of directional selection at specific codons. Rapid evolution of lineage-specific female reproductive-expressed proteases has also been observed in Drosophila mojavensis and its sister species, D. arizonae (Kelleher and Pennington 2009; Kelleher et al. 2007). Together, these results suggest that female protease genes could be evolving in response to interactions with male proteins. They may function to counter-act the effects of Sfps, as a result of sexual conflict (see discussions in Kelleher et al. 2007; Lawniczak and Begun 2007). They may also mediate sperm-egg fusion through breakdown of plasma membrane components, protect sperm from proteolysis, regulate male-derived proteases, or activate protease cascades important in regulation of post-mating processes.
As mentioned earlier in this review, at least two protease pathways are known that begin in the male and are completed in the female: the seminase/CG11864 pathway in Drosophila (LaFlamme et al. 2012; Ravi Ram et al. 2006) and the seminal clot liquefaction pathway in humans (Pampalakis and Sotiropoulou 2007). These pathways, and others not yet identified, may require the action of female-derived proteases, co-factors for male-derived proteolysis pathway components, or specific environmental conditions of the female reproductive tract (e.g.: pH or ion concentrations) for their regulation.
CONCLUSIONS
The recent advances in proteomics of the seminal fluid have led to exciting discoveries that will drive a more detailed understanding of the roles of Sfps. However, identifying functions for each Sfp is a challenge: many may have redundant functions, or their effects may only be visible in certain contexts. By taking a pathway-centered approach, we may better be able to define the broad roles of Sfps and identify patterns that span taxa, as well as species-specific functions. As the studies outlined here have shown, a focus on proteolysis regulators is a good first step to identifying Sfp-mediated pathways and their functions in fertility.
Acknowledgements
We thank A. Clark, D. Barbash and J. Mezey for comments on this manuscript, members of the Wolfner lab for helpful discussions and anonymous reviewers for helpful suggestions. We thank NIH grant R01-HD038921 (to MFW) and NSF predoctoral fellowship to BAL for support.
Grant-sponsorship: We thank NIH grant R01-HD038921 (to MFW) and an NSF predoctoral fellowship (to BAL) for support.
Abbreviations
- KLK
kallikrein-like serine protease
- LAP
latency-associated pro-peptide
- MMP
matrix metalloprotease
- NO
nitric oxide
- PAGE
poly-acrylamide gel electrophoresis
- PCI
protein-C inhibitor
- PSA
prostate-specific antigen
- SFP
seminal fluid protein
- Sg
semenogelin
- Spink
serine protease-inhibitor, Kazal-type
- TF
thromboplastin
- TGM4
transglutaminase 4
REFERENCES
- Adrain C, Zettl M, Christova Y, Taylor N, Freeman M. Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science. 2012;335(6065):225–228. doi: 10.1126/science.1214400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agard NJ, Wells JA. Methods for the proteomic identification of protease substrates. Curr Opin Chem Biol. 2009;13(5–6):503–509. doi: 10.1016/j.cbpa.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigaki T, Kasuga H, Nagaoka S, Osanai M. Purification and partial amino acid sequence of initiatorin, a prostatic endopeptidase of the silkworm, Bombyx mori. Insect Biochem Mol Biol. 1994;24(10):969–975. doi: 10.1016/0965-1748(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Andrés JA, Maroja LS, Bogdanowicz SM, Swanson WJ, Harrison RG. Molecular evolution of seminal proteins in field crickets. Mol Biol Evol. 2006;23(8):1574–1584. doi: 10.1093/molbev/msl020. [DOI] [PubMed] [Google Scholar]
- Avila FW, Ravi Ram K, Bloch Qazi MC, Wolfner MF. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics. 2010;186(2):595–600. doi: 10.1534/genetics.110.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annu Rev Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Wolfner MF. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc Natl Acad Sci USA. 2009;106(37):15796–15800. doi: 10.1073/pnas.0904029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroles JF, Carbone MA, Stone EA, Jordan KW, Lyman RF, Magwire MM, Rollmann SM, Duncan LH, Lawrence F, Anholt RRH, Mackay TFC. Systems genetics of complex traits in Drosophila melanogaster. Nat Genet. 2009;41(3):299–307. doi: 10.1038/ng.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroles JF, LaFlamme BA, Stone EA, Wolfner MF, Mackay TFC. Functional genome annotation of Drosophila seminal fluid proteins using transcriptional genetic networks. Genet Res (Camb. 2011;93(6):387–395. doi: 10.1017/S0016672311000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo RVDM, Dias DBS, Bretãs JAC, Mazzoni CJ, Souza NA, Albano RM, Wagner G, Davila AMR, Peixoto AA. The Transcriptome of Lutzomyia longipalpis (Diptera: Psychodidae) Male Reproductive Organs. PLoS ONE. 2012;7(3):e34495. doi: 10.1371/journal.pone.0034495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer B, Heazlewood JL, Taylor NL, Eubel H, Millar AH. The seminal fluid proteome of the honeybee Apis mellifera. Proteomics. 2009;9(8):2085–2097. doi: 10.1002/pmic.200800708. [DOI] [PubMed] [Google Scholar]
- Basagaña M, Bartolomé B, Pastor C, Torres F, Alonso R, Vivanco F, Cisteró-Bahíma A. Allergy to human seminal fluid: cross-reactivity with dog dander. J Allergy Clin Immunol. 2008;121(1):233–239. doi: 10.1016/j.jaci.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Basagaña M, Bartolome B, Pastor-Vargas C, Mattsson L, Lidholm J, Labrador-Horrillo M. Involvement of Can f 5 in a Case of Human Seminal Plasma Allergy. International archives of allergy and immunology. 2012;159(2):143–146. doi: 10.1159/000336388. [DOI] [PubMed] [Google Scholar]
- Basch E, Oliver TK, Vickers A, Thompson I, Kantoff P, Parnes H, Loblaw DA, Roth B, Williams J, Nam RK. Screening for prostate cancer with prostate-specific antigen testing: american society of clinical oncology provisional clinical opinion. J Clin Oncol. 2012;30(24):3020–3025. doi: 10.1200/JCO.2012.43.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-Pauly C, Barré O, Schilling O, Auf dem Keller U, Ohler A, Broder C, Schütte A, Kappelhoff R, Stöcker W, Overall CM. Proteomic analyses reveal an acidic prime side specificity for the astacin metalloprotease family reflected by physiological substrates. Mol Cell Proteomics. 2011;10(9):M111.009233. doi: 10.1074/mcp.M111.009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begun DJ, Lindfors HA. Rapid evolution of genomic Acp complement in the melanogaster subgroup of Drosophila. Mol Biol Evol. 2005;22(10):2010–2021. doi: 10.1093/molbev/msi201. [DOI] [PubMed] [Google Scholar]
- Behrendt N, editor. Matrix Proteases in Health and Disease. Wiley-VCH; Weinheim, Germany: 2012. p. 416. [Google Scholar]
- Bloch Qazi MC, Wolfner MF. An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. J Exp Biol. 2003;206(Pt 19):3521–3528. doi: 10.1242/jeb.00585. [DOI] [PubMed] [Google Scholar]
- Bond JS, Beynon RJ. The astacin family of metalloendopeptidases. Protein Sci. 1995;4(7):1247–1261. doi: 10.1002/pro.5560040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgoño CA, Diamandis EP. The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer. 2004;4(11):876–890. doi: 10.1038/nrc1474. [DOI] [PubMed] [Google Scholar]
- Bretman A, Lawniczak MKN, Boone J, Chapman T. A mating plug protein reduces early female remating in Drosophila melanogaster. J Insect Physiol. 2010;56(1):107–113. doi: 10.1016/j.jinsphys.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Carnahan SJ, Jensen-Seaman MI. Hominoid seminal protein evolution and ancestral mating behavior. Am J Primatol. 2008;70(10):939–948. doi: 10.1002/ajp.20585. [DOI] [PubMed] [Google Scholar]
- Chapman HA, Riese RJ, Shi GP. Emerging roles for cysteine proteases in human biology. Annu Rev Physiol. 1997;59:63–88. doi: 10.1146/annurev.physiol.59.1.63. [DOI] [PubMed] [Google Scholar]
- Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci USA. 2003;100(17):9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373(6511):241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- Chapman T, Neubaum DM, Wolfner MF, Partridge L. The role of male accessory gland protein Acp36DE in sperm competition in Drosophila melanogaster. Proc Biol Sci. 2000;267(1448):1097–1105. doi: 10.1098/rspb.2000.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NL, Aagaard JE, Swanson WJ. Evolution of reproductive proteins from animals and plants. Reproduction. 2006;131(1):11–22. doi: 10.1530/rep.1.00357. [DOI] [PubMed] [Google Scholar]
- Clark NL, Swanson WJ. Pervasive adaptive evolution in primate seminal proteins. PLoS Genet. 2005;1(3):e35. doi: 10.1371/journal.pgen.0010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss A, Persson M, Lilja H, Lundwall Å . Three genes expressing Kunitz domains in the epididymis are related to genes of WFDC-type protease inhibitors and semen coagulum proteins in spite of lacking similarity between their protein products. BMC Biochem. 2011;12:55. doi: 10.1186/1471-2091-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claydon AJ, Ramm SA, Pennington A, Hurst JL, Stockley P, Beynon RJ. Heterogenous turnover of sperm and seminal vesicle proteins in the mouse revealed by dynamic metabolic labelling. Mol Cell Proteomics. 2012;11(6):M111.014933. doi: 10.1074/mcp.M111.014993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux J-L, Gatti JL, Dacheux F. Contribution of epididymal secretory proteins for spermatozoa maturation. Microsc Res Tech. 2003;61(1):7–17. doi: 10.1002/jemt.10312. [DOI] [PubMed] [Google Scholar]
- Daimon E, Wada Y. Role of neutrophils in matrix metalloproteinase activity in the preimplantation mouse uterus. Biol Reprod. 2005;73(1):163–171. doi: 10.1095/biolreprod.104.038539. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Chapman T. Identification of genes expressed in the accessory glands of male Mediterranean Fruit Flies (Ceratitis capitata) Insect Biochem Mol Biol. 2006;36(11):846–856. doi: 10.1016/j.ibmb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Lamothe G. Reactive oxygen-induced reactive oxygen formation during human sperm capacitation. Free Radic Biol Med. 2009;46(4):502–510. doi: 10.1016/j.freeradbiomed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Dean MD, Findlay GD, Hoopmann MR, Wu CC, Maccoss MJ, Swanson WJ, Nachman MW. Identification of ejaculated proteins in the house mouse (Mus domesticus) via isotopic labeling. BMC Genomics. 2011;12:306. doi: 10.1186/1471-2164-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cera E, editor. Proteases in Health and Disease. 1 ed. Elsevier, Inc; San Diego, CA: 2011. p. 328. [Google Scholar]
- Ding X, Zhang J, Bian Z, Xia Y, Lu C, Gu A, Li Y, Song L, Wang S, Wang X. Variants in the Eppin gene show association with semen quality in Han-Chinese population. Reprod Biomed Online. 2010;20(1):125–131. doi: 10.1016/j.rbmo.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Dix MM, Simon GM, Cravatt BF. Global mapping of the topography and magnitude of proteolytic events in apoptosis. Cell. 2008;134(4):679–691. doi: 10.1016/j.cell.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanitskaya EV, Liu H, Chen S, Kubli E. The hydroxyproline motif of male sex peptide elicits the innate immune response in Drosophila females. FEBS J. 2007;274(21):5659–5668. doi: 10.1111/j.1742-4658.2007.06088.x. [DOI] [PubMed] [Google Scholar]
- Domsalla A, Melzig MF. Occurrence and properties of proteases in plant latices. Planta Med. 2008;74(7):699–711. doi: 10.1055/s-2008-1074530. [DOI] [PubMed] [Google Scholar]
- Dorus S, Evans PD, Wyckoff GJ, Choi SS, Lahn BT. Rate of molecular evolution of the seminal protein gene SEMG2 correlates with levels of female promiscuity. Nat Genet. 2004;36(12):1326–1329. doi: 10.1038/ng1471. [DOI] [PubMed] [Google Scholar]
- Dottorini T, Nicolaides L, Ranson H, Rogers DW, Crisanti A, Catteruccia F. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proc Natl Acad Sci USA. 2007;104(41):16215–16220. doi: 10.1073/pnas.0703904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle U, Sampson N, Zenzmaier C, Schwärzler P, Berger P. Seminal plasma enhances and accelerates progesterone-induced decidualisation of human endometrial stromal cells. Reprod Fertil Dev. 2012;24(3):517–522. doi: 10.1071/RD10296. [DOI] [PubMed] [Google Scholar]
- Duncan MW, Thompson HS. Proteomics of semen and its constituents. Proteomics Clin Appl. 2007;1(8):861–875. doi: 10.1002/prca.200700228. [DOI] [PubMed] [Google Scholar]
- Edström AML, Malm J, Frohm B, Martellini JA, Giwercman A, Mörgelin M, Cole AM, Sørensen OE. The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins. J Immunol. 2008;181(5):3413–3421. doi: 10.4049/jimmunol.181.5.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LL, Carney GE. Mating alters gene expression patterns in Drosophila melanogaster male heads. BMC Genomics. 2010;11:558. doi: 10.1186/1471-2164-11-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N, Diamandis EP. Human kallikrein-related peptidase 14 (KLK14) is a new activator component of the KLK proteolytic cascade. Possible function in seminal plasma and skin. J Biol Chem. 2008;283(6):3031–3041. doi: 10.1074/jbc.M707253200. [DOI] [PubMed] [Google Scholar]
- Emami N, Diamandis EP. Potential role of multiple members of the kallikrein-related peptidase family of serine proteases in activating latent TGF beta 1 in semen. Biol Chem. 2010;391(1):85–95. doi: 10.1515/BC.2010.007. [DOI] [PubMed] [Google Scholar]
- Farady CJ, Craik CS. Mechanisms of macromolecular protease inhibitors. Chembiochem. 2010;11(17):2341–2346. doi: 10.1002/cbic.201000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández JA, Heeb MJ. Role of protein C inhibitor and tissue factor in fertilization. Semin Thromb Hemost. 2007;33(1):13–20. doi: 10.1055/s-2006-958457. [DOI] [PubMed] [Google Scholar]
- Fernández JA, Heeb MJ, Radtke KP, Griffin JH. Potent blood coagulant activity of human semen due to prostasome-bound tissue factor. Biol Reprod. 1997;56(3):757–763. doi: 10.1095/biolreprod56.3.757. [DOI] [PubMed] [Google Scholar]
- Findlay GD, Maccoss MJ, Swanson WJ. Proteomic discovery of previously unannotated, rapidly evolving seminal fluid genes in Drosophila. Genome Res. 2009;19(5):886–896. doi: 10.1101/gr.089391.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GD, Yi X, Maccoss MJ, Swanson WJ. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol. 2008;6(7):e178. doi: 10.1371/journal.pbio.0060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumera AC, Dumont BL, Clark AG. Associations between sperm competition and natural variation in male reproductive genes on the third chromosome of Drosophila melanogaster. Genetics. 2007;176(2):1245–1260. doi: 10.1534/genetics.106.064915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedländer M, Jeshtadi A, Reynolds S. The structural mechanism of trypsin-induced intrinsic motility in Manduca sexta spermatozoa in vitro. J Insect Physiol. 2001;47(3):245–255. doi: 10.1016/s0022-1910(00)00109-8. [DOI] [PubMed] [Google Scholar]
- Fung KYC, Glode LM, Green S, Duncan MW. A comprehensive characterization of the peptide and protein constituents of human seminal fluid. Prostate. 2004;61(2):171–181. doi: 10.1002/pros.20089. [DOI] [PubMed] [Google Scholar]
- Garrett M, Fullaondo A, Troxler L, Micklem G, Gubb D. Identification and analysis of serpin-family genes by homology and synteny across the 12 sequenced Drosophilid genomes. BMC Genomics. 2009;10:489. doi: 10.1186/1471-2164-10-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu Rev Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- Greenspan L, Clark AG. Associations between Variation in X Chromosome Male Reproductive Genes and Sperm Competitive Ability in Drosophila melanogaster. Int J Evol Biol. 2011;2011:214280. doi: 10.4061/2011/214280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb D, Sanz-Parra A, Barcena L, Troxler L, Fullaondo A. Protease inhibitors and proteolytic signalling cascades in insects. Biochimie. 2010;92(12):1749–1759. doi: 10.1016/j.biochi.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA. Seminal Fluid Regulates Accumulation of FOXP3+ Regulatory T Cells in the Preimplantation Mouse Uterus Through Expanding the FOXP3+ Cell Pool and CCL19-Mediated Recruitment. Biol Reprod. 2011;85(2):397–408. doi: 10.1095/biolreprod.110.088591. [DOI] [PubMed] [Google Scholar]
- Guyonnet B, Dacheux F, Dacheux J-L, Gatti J-L. The epididymal transcriptome and proteome provide some insights into new epididymal regulations. J Androl. 2011;32(6):651–664. doi: 10.2164/jandrol.111.013086. [DOI] [PubMed] [Google Scholar]
- Haerty W, Jagadeeshan S, Kulathinal RJ, Wong A, Kristipati RR, Sirot LK, Levesque L, Artieri CG, Wolfner MF, Civetta A, Singh RS. Evolution in the fast lane: rapidly evolving sex-related genes in Drosophila. Genetics. 2007;177(3):1321–1335. doi: 10.1534/genetics.107.078865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz Y, Lung O, Frongillo EA, Wolfner MF. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr Biol. 2000;10(2):99–102. doi: 10.1016/s0960-9822(00)00288-8. [DOI] [PubMed] [Google Scholar]
- Heifetz Y, Vandenberg LN, Cohn HI, Wolfner MF. Two cleavage products of the Drosophila accessory gland protein ovulin can independently induce ovulation. Proc Natl Acad Sci USA. 2005;102(3):743–748. doi: 10.1073/pnas.0407692102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heutinck KM, ten Berge IJM, Hack CE, Hamann J, Rowshani AT. Serine proteases of the human immune system in health and disease. Mol Immunol. 2010;47(11–12):1943–1955. doi: 10.1016/j.molimm.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Holt WV. Mechanisms of sperm storage in the female reproductive tract: an interspecies comparison. Reprod Domest Anim. 2011;46(Suppl 2):68–74. doi: 10.1111/j.1439-0531.2011.01862.x. [DOI] [PubMed] [Google Scholar]
- Hung PH, Suarez SS. Regulation of sperm storage and movement in the ruminant oviduct. Society of Reproduction and Fertility supplement. 2010;67:257–266. doi: 10.7313/upo9781907284991.022. [DOI] [PubMed] [Google Scholar]
- Jensen-Seaman MI, Li W-H. Evolution of the hominoid semenogelin genes, the major proteins of ejaculated semen. J Mol Evol. 2003;57(3):261–270. doi: 10.1007/s00239-003-2474-x. [DOI] [PubMed] [Google Scholar]
- Kanwar KC, Yanagimachi R, Lopata A. Effects of human seminal plasma on fertilizing capacity of human spermatozoa. Fertil Steril. 1979;31(3):321–327. [PubMed] [Google Scholar]
- Kelleher E, Pennington J. Protease Gene Duplication and Proteolytic Activity in Drosophila Female Reproductive Tracts. Mol Biol Evol. 2009;26(9):2125–2134. doi: 10.1093/molbev/msp121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher E, Swanson WJ, Markow TA. Gene duplication and adaptive evolution of digestive proteases in Drosophila arizonae female reproductive tracts. PLoS Genet. 2007;3(8):e148. doi: 10.1371/journal.pgen.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher ES, Watts TD, LaFlamme BA, Haynes PA, Markow TA. Proteomic analysis of Drosophila mojavensis male accessory glands suggests novel classes of seminal fluid proteins. Insect Biochem Mol Biol. 2009;39(5–6):366–371. doi: 10.1016/j.ibmb.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Kelly VC, Kuy S, Palmer DJ, Xu Z, Davis SR, Cooper GJ. Characterization of bovine seminal plasma by proteomics. Proteomics. 2006;6(21):5826–5833. doi: 10.1002/pmic.200500830. [DOI] [PubMed] [Google Scholar]
- King M, Eubel H, Millar AH, Baer B. Proteins within the seminal fluid are crucial to keep sperm viable in the honeybee Apis mellifera. J Insect Physiol. 2011;57(3):409–414. doi: 10.1016/j.jinsphys.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Kingan SB, Tatar M, Rand DM. Reduced polymorphism in the chimpanzee semen coagulating protein, semenogelin I. J Mol Evol. 2003;57(2):159–169. doi: 10.1007/s00239-002-2463-0. [DOI] [PubMed] [Google Scholar]
- Kofler L, Kofler H, Mattsson L, Lidholm J. A case of dog-related human seminal plasma allergy. Eur Ann Allergy Clin Immunol. 2012;44(2):89–92. [PubMed] [Google Scholar]
- Kotłowska M, Kowalski R, Glogowski J, Jankowski J, Ciereszko A. Gelatinases and serine proteinase inhibitors of seminal plasma and the reproductive tract of turkey (Meleagris gallopavo) Theriogenology. 2005;63(6):1667–1681. doi: 10.1016/j.theriogenology.2004.07.020. [DOI] [PubMed] [Google Scholar]
- LaFlamme BA, Ravi Ram K, Wolfner MF. The Drosophila melanogaster Seminal Fluid Protease “Seminase” Regulates Proteolytic and Post-Mating Reproductive Processes. PLoS Genet. 2012;8(1):e1002435. doi: 10.1371/journal.pgen.1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawniczak MKN, Begun DJ. Molecular population genetics of female-expressed mating-induced serine proteases in Drosophila melanogaster. Mol Biol Evol. 2007;24(9):1944–1951. doi: 10.1093/molbev/msm122. [DOI] [PubMed] [Google Scholar]
- Lee B, Park I, Jin S, Choi H, Kwon JT, Kim J, Jeong J, Cho B-N, Eddy EM, Cho C. Impaired spermatogenesis and fertility in mice carrying a mutation in the Spink2 gene expressed predominantly in testes. J Biol Chem. 2011;286(33):29108–29117. doi: 10.1074/jbc.M111.244905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja H, Abrahamsson PA, Lundwall A. Semenogelin, the predominant protein in human semen. Primary structure and identification of closely related proteins in the male accessory sex glands and on the spermatozoa. J Biol Chem. 1989;264(3):1894–1900. [PubMed] [Google Scholar]
- Lilja H, Oldbring J, Rannevik G, Laurell CB. Seminal vesicle-secreted proteins and their reactions during gelation and liquefaction of human semen. J Clin Invest. 1987;80(2):281–285. doi: 10.1172/JCI113070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Lee R, Hwu Y, Lu C, Chu S, Chen Y, Chang W, Li S. SPINKL, a Kazal-type serine protease inhibitor-like protein purified from mouse seminal vesicle fluid, is able to inhibit sperm capacitation. Reproduction. 2008 doi: 10.1530/REP-07-0375. [DOI] [PubMed] [Google Scholar]
- Lu C-H, Lee RK-K, Hwu Y-M, Chu S-L, Chen Y-J, Chang W-C, Lin S-P, Li S-H. SERPINE2, a serine protease inhibitor extensively expressed in adult male mouse reproductive tissues, may serve as a murine sperm decapacitation factor. Biol Reprod. 2011;84(3):514–525. doi: 10.1095/biolreprod.110.085100. [DOI] [PubMed] [Google Scholar]
- Lundwall A, Brattsand M. Kallikrein-related peptidases. Cell Mol Life Sci. 2008;65(13):2019–2038. doi: 10.1007/s00018-008-8024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung O, Kuo L, Wolfner MF. Drosophila males transfer antibacterial proteins from their accessory gland and ejaculatory duct to their mates. J Insect Physiol. 2001;47(6):617–622. doi: 10.1016/s0022-1910(00)00151-7. [DOI] [PubMed] [Google Scholar]
- Lung O, Tram U, Finnerty CM, Eipper-Mains MA, Kalb JM, Wolfner MF. The Drosophila melanogaster seminal fluid protein Acp62F is a protease inhibitor that is toxic upon ectopic expression. Genetics. 2002;160(1):211–224. doi: 10.1093/genetics/160.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung O, Wolfner MF. Drosophila seminal fluid proteins enter the circulatory system of the mated female fly by crossing the posterior vaginal wall. Insect Biochem Mol Biol. 1999;29(12):1043–1052. doi: 10.1016/s0965-1748(99)00078-8. [DOI] [PubMed] [Google Scholar]
- Lung O, Wolfner MF. Identification and characterization of the major Drosophila melanogaster mating plug protein. Insect Biochem Mol Biol. 2001;31(6–7):543–551. doi: 10.1016/s0965-1748(00)00154-5. [DOI] [PubMed] [Google Scholar]
- Luo L-Y, Shan SJC, Elliott MB, Soosaipillai A, Diamandis EP. Purification and characterization of human kallikrein 11, a candidate prostate and ovarian cancer biomarker, from seminal plasma. Clin Cancer Res. 2006;12(3 Pt 1):742–750. doi: 10.1158/1078-0432.CCR-05-1696. [DOI] [PubMed] [Google Scholar]
- Lwaleed B, Jackson C, Greenfield R, Stewart A, Delves G, Birch B, Cooper A. Seminal tissue factor revisited. Int J Androl. 2006;29(2):360–367. doi: 10.1111/j.1365-2605.2005.00608.x. [DOI] [PubMed] [Google Scholar]
- Lwaleed BA, Goyal A, Greenfield RS, Cooper AJ. Seminal thrombin-activatable fibrinolysis inhibitor: a regulator of liquefaction. Blood Coagul Fibrinolysis. 2007;18(5):449–454. doi: 10.1097/MBC.0b013e328136c18a. [DOI] [PubMed] [Google Scholar]
- Lwaleed BA, Greenfield R, Stewart A, Birch B, Cooper AJ. Seminal clotting and fibrinolytic balance: a possible physiological role in the male reproductive system. Thromb Haemost. 2004;92(4):752–766. doi: 10.1160/TH04-03-0142. [DOI] [PubMed] [Google Scholar]
- Lwaleed BA, Greenfield RS, Birch BR, Cooper AJ. Does human semen contain a functional haemostatic system? A possible role for Tissue Factor Pathway Inhibitor in fertility through semen liquefaction. Thromb Haemost. 2005;93(5):847–852. doi: 10.1160/TH04-09-0600. [DOI] [PubMed] [Google Scholar]
- Mack PD, Kapelnikov A, Heifetz Y, Bender M. Mating-responsive genes in reproductive tissues of female Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103(27):10358–10363. doi: 10.1073/pnas.0604046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini E, Tammaro F, Baldini F, Via A, Raimondo D, George P, Audisio P, Sharakhov IV, Tramontano A, Catteruccia F, Della Torre A. Molecular evolution of a gene cluster of serine proteases expressed in the Anopheles gambiae female reproductive tract. BMC Evol Biol. 2011;11:72. doi: 10.1186/1471-2148-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini D, Marri L, Rosetto M, Manetti AG, Dallai R. Presence of antibacterial peptides on the laid egg chorion of the medfly Ceratitis capitata. Biochem Biophys Res Commun. 1997;240(3):657–663. doi: 10.1006/bbrc.1997.7694. [DOI] [PubMed] [Google Scholar]
- Mårdh PA, Colleen S. Antimicrobial activity of human seminal fluid. Scand J Urol Nephrol. 1975;9(1):17–23. doi: 10.3109/00365597509139907. [DOI] [PubMed] [Google Scholar]
- Marshall JL, Huestis DL, Garcia C, Hiromasa Y, Wheeler S, Noh S, Tomich JM, Howard DJ. Comparative proteomics uncovers the signature of natural selection acting on the ejaculate proteomes of two cricket species isolated by postmating, prezygotic phenotypes. Mol Biol Evol. 2011;28(1):423–435. doi: 10.1093/molbev/msq230. [DOI] [PubMed] [Google Scholar]
- Marshall JL, Huestis DL, Hiromasa Y, Wheeler S, Oppert C, Marshall SA, Tomich JM, Oppert B. Identification, RNAi knockdown, and functional analysis of an ejaculate protein that mediates a postmating, prezygotic phenotype in a cricket. PLoS ONE. 2009;4(10):e7537. doi: 10.1371/journal.pone.0007537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, Clark AG, Wolfner MF. Post-mating gene expression profiles of female Drosophila melanogaster in response to time and to four male accessory gland proteins. Genetics. 2008;179(3):1395–1408. doi: 10.1534/genetics.108.086934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, Gibson G, Clark AG, Wolfner MF. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr Biol. 2004;14(16):1509–1514. doi: 10.1016/j.cub.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Mitra A, Richardson RT, O'Rand MG. Analysis of recombinant human semenogelin as an inhibitor of human sperm motility. Biol Reprod. 2010;82(3):489–496. doi: 10.1095/biolreprod.109.081331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H, Thaler CD, Haimo LT, Cardullo RA. Protease activation and the signal transduction pathway regulating motility in sperm from the water strider Aquarius remigis. Cytoskeleton (Hoboken, NJ) 2012;69(4):207–220. doi: 10.1002/cm.21012. [DOI] [PubMed] [Google Scholar]
- Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. 2009;182(12):8080–8093. doi: 10.4049/jimmunol.0804018. [DOI] [PubMed] [Google Scholar]
- Mommens M, Wojtczak M, Ciereszko A, Babiak I. Seminal plasma proteins of Atlantic halibut (Hippoglossus hippoglossus L.) Fish Physiol Biochem. 2008;34(4):349–355. doi: 10.1007/s10695-007-9194-x. [DOI] [PubMed] [Google Scholar]
- Moura AA, Souza CE, Stanley BA, Chapman DA, Killian GJ. Proteomics of cauda epididymal fluid from mature Holstein bulls. J Proteomics. 2010;73(10):2006–2020. doi: 10.1016/j.jprot.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Mueller JL, Kristipati RR, McGraw LA, Bloch Qazi MC, Siggia ED, Clark AG, Aquadro C, Wolfner MF. Cross-species comparison of Drosophila male accessory gland protein genes. Genetics. 2005;171(1):131–143. doi: 10.1534/genetics.105.043844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JL, Linklater JR, Kristipati RR, Chapman T, Wolfner MF. Targeted gene deletion and phenotypic analysis of the Drosophila melanogaster seminal fluid protease inhibitor Acp62F. Genetics. 2008;178(3):1605–1614. doi: 10.1534/genetics.107.083766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JL, Page JL, Wolfner MF. An ectopic expression screen reveals the protective and toxic effects of Drosophila seminal fluid proteins. Genetics. 2007;175(2):777–783. doi: 10.1534/genetics.106.065318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JL, Ripoll DR, Aquadro C, Wolfner MF. Comparative structural modeling and inference of conserved protein classes in Drosophila seminal fluid. Proc Natl Acad Sci USA. 2004;101(37):13542–13547. doi: 10.1073/pnas.0405579101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer V, Spetz JF, Hengst U, Altrogge LM, de Agostini A, Monard D. Male fertility defects in mice lacking the serine protease inhibitor protease nexin-1. Proc Natl Acad Sci USA. 2001;98(6):3029–3033. doi: 10.1073/pnas.051630698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka S, Kato K, Takata Y, Kamei K. Identification of the sperm-activating factor initiatorin, a prostatic endopeptidase of the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2012;42(8):571–582. doi: 10.1016/j.ibmb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Neubaum DM, Wolfner MF. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics. 1999;153(2):845–857. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocera M, Chu TM. Transforming growth factor beta as an immunosuppressive protein in human seminal plasma. Am J Reprod Immunol. 1993;30(1):1–8. doi: 10.1111/j.1600-0897.1993.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Nocera M, Chu TM. Characterization of latent transforming growth factor-beta from human seminal plasma. Am J Reprod Immunol. 1995;33(4):282–291. doi: 10.1111/j.1600-0897.1995.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Novak S, Smith TA, Paradis F, Burwash L, Dyck MK, Foxcroft GR, Dixon WT. Biomarkers of in vivo fertility in sperm and seminal plasma of fertile stallions. Theriogenology. 2010;74(6):956–967. doi: 10.1016/j.theriogenology.2010.04.025. [DOI] [PubMed] [Google Scholar]
- O'Rand MG, Widgren EE, Beyler S, Richardson RT. Inhibition of human sperm motility by contraceptive anti-eppin antibodies from infertile male monkeys: effect on cyclic adenosine monophosphate. Biol Reprod. 2009;80(2):279–285. doi: 10.1095/biolreprod.108.072942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rand MG, Widgren EE, Hamil KG, Silva EJ, Richardson RT. Epididymal protein targets: a brief history of the development of epididymal protease inhibitor as a contraceptive. J Androl. 2011;32(6):698–704. doi: 10.2164/jandrol.110.012781. [DOI] [PubMed] [Google Scholar]
- O'Rand MG, Widgren EE, Sivashanmugam P, Richardson RT, Hall SH, French FS, VandeVoort CA, Ramachandra SG, Ramesh V, Jagannadha Rao A. Reversible immunocontraception in male monkeys immunized with eppin. Science. 2004;306(5699):1189–1190. doi: 10.1126/science.1099743. [DOI] [PubMed] [Google Scholar]
- Osanai M, Chen P. A comparative study on the arginine degradation cascade for sperm maturation of Bombyx mori and Drosophila melanogaster. Amino Acids. 1993;5(3):341–350. doi: 10.1007/BF00806952. [DOI] [PubMed] [Google Scholar]
- Ou C-M, Tang J-B, Huang M-S, Sudhakar Gandhi PS, Geetha S, Li S-H, Chen Y-H. The mode of reproductive-derived Spink (serine protease inhibitor Kazal-type) action in the modulation of mammalian sperm activity. Int J Androl. 2012;35(1):52–62. doi: 10.1111/j.1365-2605.2011.01159.x. [DOI] [PubMed] [Google Scholar]
- Page MJ, Di Cera E. Serine peptidases: classification, structure and function. Cell Mol Life Sci. 2008;65(7–8):1220–1236. doi: 10.1007/s00018-008-7565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MJ, Di Cera E, Begley TP. Wiley Encyclopedia of Chemical Biology. 2007. Serine Proteases and Serine Protease Inhibitors; pp. 1–29. [Google Scholar]
- Pampalakis G, Sotiropoulou G. Tissue kallikrein proteolytic cascade pathways in normal physiology and cancer. Biochim Biophys Acta. 2007;1776(1):22–31. doi: 10.1016/j.bbcan.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Park M, Wolfner MF. Male and female cooperate in the prohormone-like processing of a Drosophila melanogaster seminal fluid protein. Dev Biol. 1995;171(2):694–702. doi: 10.1006/dbio.1995.1315. [DOI] [PubMed] [Google Scholar]
- Parker GA, Pizzari T. Sperm competition and ejaculate economics. Biological reviews of the Cambridge Philosophical Society. 2010;85(4):897–934. doi: 10.1111/j.1469-185X.2010.00140.x. [DOI] [PubMed] [Google Scholar]
- Peng J, Chen S, Büsser S, Liu H, Honegger T, Kubli E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol. 2005;15(3):207–213. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7(5):R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiani A. Complexity of seminal fluid: a review. Behavioral Ecology and Sociobiology. 2006;60:289–310. [Google Scholar]
- Polgár L. Mechanisms of Protease Action. Taylor & Francis, Inc; 1989. p. 223. [Google Scholar]
- Prokupek AM, Kachman SD, Ladunga I, Harshman LG. Transcriptional profiling of the sperm storage organs of Drosophila melanogaster. Insect Mol Biol. 2009;18(4):465–475. doi: 10.1111/j.1365-2583.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- Prudova A, Auf dem Keller U, Butler GS, Overall CM. Multiplex N-terminome analysis of MMP-2 and MMP-9 substrate degradomes by iTRAQ-TAILS quantitative proteomics. Mol Cell Proteomics. 2010;9(5):894–911. doi: 10.1074/mcp.M000050-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto MH, Huanca W, Adams GP. Ovulation-inducing factor: a protein component of llama seminal plasma. Reprod Biol Endocrinol. 2010;8(44) doi: 10.1186/1477-7827-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram K, Ji S, Wolfner MF. Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochem Mol Biol. 2005;35(9):1059–1071. doi: 10.1016/j.ibmb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Ravi Ram K, Sirot LK, Wolfner MF. Predicted seminal astacin-like protease is required for processing of reproductive proteins in Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103(49):18674–18679. doi: 10.1073/pnas.0606228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram K, Wolfner MF. Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genet. 2007;3(12):e238. doi: 10.1371/journal.pgen.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram K, Wolfner MF. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc Natl Acad Sci USA. 2009;106(36):15384–15389. doi: 10.1073/pnas.0902923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ, Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Research. 2012;40(Database issue):D343–350. doi: 10.1093/nar/gkr987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichhart JM, Gubb D, Leclerc V. The Drosophila serpins: multiple functions in immunity and morphogenesis. Meth Enzymol. 2011;499:205–225. doi: 10.1016/B978-0-12-386471-0.00011-0. [DOI] [PubMed] [Google Scholar]
- Richer MJ, Keays CA, Waterhouse J, Minhas J, Hashimoto C, Jean F. The Spn4 gene of Drosophila encodes a potent furin-directed secretory pathway serpin. Proc Natl Acad Sci USA. 2004;101(29):10560–10565. doi: 10.1073/pnas.0401406101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roan NR, Müller JA, Liu H, Chu S, Arnold F, Stürzel CM, Walther P, Dong M, Witkowska HE, Kirchhoff F, Münch J, Greene WC. Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection. Cell Host Microbe. 2011;10(6):541–550. doi: 10.1016/j.chom.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322(1):43–52. doi: 10.1007/s00441-005-1127-3. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Ingman WV, O'Leary S, Sharkey DJ, Tremellen KP. Transforming growth factor beta--a mediator of immune deviation in seminal plasma. J Reprod Immunol. 2002;57(1–2):109–128. doi: 10.1016/s0165-0378(02)00015-3. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Martínez H, Kvist U, Ernerudh J, Sanz L, Calvete JJ. Seminal plasma proteins: what role do they play? Am J Reprod Immunol. 2011;66(Suppl 1):11–22. doi: 10.1111/j.1600-0897.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- Roessner C, Paasch U, Glander H-J, Grunewald S. Activity of nitric oxide synthase in mature and immature human spermatozoa. Andrologia. 2010;42(2):132–137. doi: 10.1111/j.1439-0272.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- Rogers DW, Baldini F, Battaglia F, Panico M, Dell A, Morris HR, Catteruccia F. Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol. 2009;7(12):e1000272. doi: 10.1371/journal.pbio.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Jiang H, Kanost MR, Wang Y. Serine proteases and their homologs in the Drosophila melanogaster genome: an initial analysis of sequence conservation and phylogenetic relationships. Gene. 2003;304:117–131. doi: 10.1016/s0378-1119(02)01187-3. [DOI] [PubMed] [Google Scholar]
- Saengsoi W, Shia W-Y, Shyu C-L, Wu J-T, Warinrak C, Lee W-M, Cheng F-P. Detection of matrix metalloproteinase (MMP)-2 and MMP-9 in canine seminal plasma. Anim Reprod Sci. 2011;127(1–2):114–119. doi: 10.1016/j.anireprosci.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Schilling O, Overall CM. Proteome-derived, database-searchable peptide libraries for identifying protease cleavage sites. Nat Biotechnol. 2008;26(6):685–694. doi: 10.1038/nbt1408. [DOI] [PubMed] [Google Scholar]
- Schnakenberg SL, Matias WR, Siegal ML. Sperm-storage defects and live birth in Drosophila females lacking spermathecal secretory cells. PLoS Biol. 2011;9(11):e1001192. doi: 10.1371/journal.pbio.1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PK, Tripathi LP, Jensen LJ, Gahnim M, Mason C, Furlong EE, Rodrigues V, White KP, Bork P, Sowdhamini R. Enhanced function annotations for Drosophila serine proteases: a case study for systematic annotation of multi-member gene families. Gene. 2008;407(1–2):199–215. doi: 10.1016/j.gene.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Shen Y, Liu T, Tolić N, Petritis BO, Zhao R, Moore RJ, Purvine SO, Camp DG, Smith RD. Strategy for degradomic-peptidomic analysis of human blood plasma. J Proteome Res. 2010a;9(5):2339–2346. doi: 10.1021/pr901083m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Tolić N, Hixson KK, Purvine SO, Anderson GA, Smith RD. De novo sequencing of unique sequence tags for discovery of post-translational modifications of proteins. Anal Chem. 2008;80(20):7742–7754. doi: 10.1021/ac801123p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Tolić N, Liu T, Zhao R, Petritis BO, Gritsenko MA, Camp DG, Moore RJ, Purvine SO, Esteva FJ, Smith RD. Blood peptidome-degradome profile of breast cancer. PLoS ONE. 2010b;5(10):e13133. doi: 10.1371/journal.pone.0013133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SM, Wolfner MF, Lazzaro BP. Female Drosophila melanogaster suffer reduced defense against infection due to seminal fluid components. J Insect Physiol. 2012;58(9):1192–1201. doi: 10.1016/j.jinsphys.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman GA, Whisstock JC, Bottomley SP, Huntington JA, Kaiserman D, Luke CJ, Pak SC, Reichhart J-M, Bird PI. Serpins flex their muscle: I. Putting the clamps on proteolysis in diverse biological systems. J Biol Chem. 2010;285(32):24299–24305. doi: 10.1074/jbc.R110.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, Hardstone MC, Helinski MEH, Ribeiro JMC, Kimura M, Deewatthanawong P, Wolfner MF, Harrington LC. Towards a semen proteome of the dengue vector mosquito: protein identification and potential functions. PLoS Negl Trop Dis. 2011a;5(3):e989. doi: 10.1371/journal.pntd.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, LaFlamme BA, Sitnik JL, Rubinstein CD, Avila FW, Chow CY, Wolfner MF. Molecular social interactions: Drosophila melanogaster seminal fluid proteins as a case study. Adv Genet. 2009;68:23–56. doi: 10.1016/S0065-2660(09)68002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, Wolfner MF, Wigby S. Protein-specific manipulation of ejaculate composition in response to female mating status in Drosophila melanogaster. Proc Natl Acad Sci USA. 2011b;108(24):9922–9926. doi: 10.1073/pnas.1100905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Stanfield GM. TRY-5 is a sperm-activating protease in Caenorhabditis elegans seminal fluid. PLoS Genet. 2011;7(11):e1002375. doi: 10.1371/journal.pgen.1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South A, Sirot LK, Lewis SM. Identification of predicted seminal fluid proteins in Tribolium castaneum. Insect Mol Biol. 2011;20(4):447–456. doi: 10.1111/j.1365-2583.2011.01083.x. [DOI] [PubMed] [Google Scholar]
- Stanfield GM, Villeneuve AM. Regulation of sperm activation by SWM-1 is required for reproductive success of C. elegans males. Curr Biol. 2006;16(3):252–263. doi: 10.1016/j.cub.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Suarez SS. Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol. 2008;52(5–6):455–462. doi: 10.1387/ijdb.072527ss. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kise H, Nishioka J, Hayashi T. The interaction among protein C inhibitor, prostate-specific antigen, and the semenogelin system. Semin Thromb Hemost. 2007;33(1):46–52. doi: 10.1055/s-2006-958461. [DOI] [PubMed] [Google Scholar]
- Swanson WJ, Clark AG, Waldrip-Dail HM, Wolfner MF, Aquadro C. Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proc Natl Acad Sci USA. 2001;98(13):7375–7379. doi: 10.1073/pnas.131568198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nat Rev Genet. 2002;3(2):137–144. doi: 10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- Szecsi PB. The aspartic proteases. Scand J Clin Lab Invest Suppl. 1992;210:5–22. [PubMed] [Google Scholar]
- Takemori N, Yamamoto M-T. Proteome mapping of the Drosophila melanogaster male reproductive system. Proteomics. 2009;9(9):2484–2493. doi: 10.1002/pmic.200800795. [DOI] [PubMed] [Google Scholar]
- Tentes I, Asimakopoulos B, Mourvati E, Diedrich K, Al-Hasani S, Nikolettos N. Matrix metalloproteinase (MMP)-2 and MMP-9 in seminal plasma. J Assist Reprod Genet. 2007;24(7):278–281. doi: 10.1007/s10815-007-9129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston RJ, Korn N, Froman DP, Bodine AB. Proteolytic enzymes in seminal plasma of domestic turkey (Meleagris gallopavo) Biol Reprod. 1993;48(2):393–402. doi: 10.1095/biolreprod48.2.393. [DOI] [PubMed] [Google Scholar]
- Tseng H-C, Tang J-B, Gandhi PSS, Luo C-W, Ou C-M, Tseng C-J, Lin H-J, Chen Y-H. Mutual adaptation between mouse transglutaminase 4 and its native substrates in the formation of copulatory plug. Amino Acids. 2012;42(2–3):951–960. doi: 10.1007/s00726-011-1009-9. [DOI] [PubMed] [Google Scholar]
- Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, Turk D. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012;1824(1):68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardy A. The role of controlled proteolysis in cell-cycle regulation. Eur J Biochem. 1996;240(2):307–313. doi: 10.1111/j.1432-1033.1996.0307h.x. [DOI] [PubMed] [Google Scholar]
- Utleg AG, Yi EC, Xie T, Shannon P, White JT, Goodlett DR, Hood L, Lin B. Proteomic analysis of human prostasomes. Prostate. 2003;56(2):150–161. doi: 10.1002/pros.10255. [DOI] [PubMed] [Google Scholar]
- Wagstaff BJ, Begun DJ. Molecular population genetics of accessory gland protein genes and testis-expressed genes in Drosophila mojavensis and D. arizonae. Genetics. 2005;171(3):1083–1101. doi: 10.1534/genetics.105.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldinger MD, Meinardi MMHM, Zwinderman AH, Schweitzer DH. Postorgasmic Illness Syndrome (POIS) in 45 Dutch Caucasian Males: Clinical Characteristics and Evidence for an Immunogenic Pathogenesis (Part 1) The journal of sexual medicine. 2011;8(4):1164–1170. doi: 10.1111/j.1743-6109.2010.02166.x. [DOI] [PubMed] [Google Scholar]
- Walker MJ, Rylett CM, Keen JN, Audsley N, Sajid M, Shirras AD, Isaac RE. Proteomic identification of Drosophila melanogaster male accessory gland proteins, including a pro-cathepsin and a soluble gamma-glutamyl transpeptidase. Proteome science. 2006;4:9. doi: 10.1186/1477-5956-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JR, Harrison RG. EST analysis of male accessory glands from Heliconius butterflies with divergent mating systems. BMC Genomics. 2008;9:592. doi: 10.1186/1471-2164-9-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JR, Harrison RG. Combined EST and proteomic analysis identifies rapidly evolving seminal fluid proteins in Heliconius butterflies. Mol Biol Evol. 2010;27(9):2000–2013. doi: 10.1093/molbev/msq092. [DOI] [PubMed] [Google Scholar]
- Ward S, Carrel JS. Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev Biol. 1979;73(2):304–321. doi: 10.1016/0012-1606(79)90069-1. [DOI] [PubMed] [Google Scholar]
- Weidinger S, Mayerhofer A, Raemsch R, Ring J, Köhn F-M. Prostate-specific antigen as allergen in human seminal plasma allergy. J Allergy Clin Immunol. 2006;117(1):213–215. doi: 10.1016/j.jaci.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Weiss BL, Stepczynski JM, Wong P, Kaufman WR. Identification and characterization of genes differentially expressed in the testis/vas deferens of the fed male tick, Amblyomma hebraeum. Insect Biochem Mol Biol. 2002;32(7):785–793. doi: 10.1016/s0965-1748(01)00161-8. [DOI] [PubMed] [Google Scholar]
- Whisstock JC, Silverman GA, Bird PI, Bottomley SP, Kaiserman D, Luke CJ, Pak SC, Reichhart J-M, Huntington JA. Serpins flex their muscle: II. Structural insights into target peptidase recognition, polymerization, and transport functions. J Biol Chem. 2010;285(32):24307–24312. doi: 10.1074/jbc.R110.141408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr Biol. 2005;15(4):316–321. doi: 10.1016/j.cub.2005.01.051. [DOI] [PubMed] [Google Scholar]
- Williams SA, Xu Y, De Marzo AM, Isaacs JT, Denmeade SR. Prostate-specific antigen (PSA) is activated by KLK2 in prostate cancer ex vivo models and in prostate-targeted PSA/KLK2 double transgenic mice. Prostate. 2010;70(7):788–796. doi: 10.1002/pros.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak M, Dietrich GJ, Ciereszko A. Transferrin and antiproteases are major proteins of common carp seminal plasma. Fish Shellfish Immunol. 2005;19(4):387–391. doi: 10.1016/j.fsi.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Wong A, Albright S, Giebel J, Ravi Ram K, Ji S, Fiumera A, Wolfner M. A role for Acp29AB, a predicted seminal fluid lectin, in female sperm storage in Drosophila melanogaster. Genetics. 2008a;180(2):921–931. doi: 10.1534/genetics.108.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A, Turchin MC, Wolfner MF, Aquadro C. Evidence for positive selection on Drosophila melanogaster seminal fluid protease homologs. Mol Biol Evol. 2008b;25(3):497–506. doi: 10.1093/molbev/msm270. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang Q. Seminal fluid reduces female longevity and stimulates egg production and sperm trigger oviposition in a moth. J Insect Physiol. 2011;57(3):385–390. doi: 10.1016/j.jinsphys.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Yamamoto M-T, Takemori N. Proteome profiling reveals tissue-specific protein expression in the male reproductive system of Drosophila melanogaster. Fly. 2010;4(1):36–39. doi: 10.4161/fly.4.1.10838. [DOI] [PubMed] [Google Scholar]
- Yapici N, Kim Y-J, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451(7174):33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev. 2001;22(2):184–204. doi: 10.1210/edrv.22.2.0424. [DOI] [PubMed] [Google Scholar]
- Zalazar L, Saez Lancellotti TE, Clementi M, Lombardo C, Lamattina L, De Castro R, Fornés MW, Cesari A. SPINK3 modulates mouse sperm physiology through the reduction of nitric oxide level independently of its trypsin inhibitory activity. Reproduction. 2012;143(3):281–295. doi: 10.1530/REP-11-0107. [DOI] [PubMed] [Google Scholar]
- Zettl M, Adrain C, Strisovsky K, Lastun V, Freeman M. Rhomboid family pseudoproteases use the ER quality control machinery to regulate intercellular signaling. Cell. 2011;145(1):79–91. doi: 10.1016/j.cell.2011.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sun W, Zhang P, Chi H, Zhang M-J, Song C-Q, Ma X, Shang Y, Wang B, Hu Y, Hao Z, Hühmer AF, Meng F, L'hernault SW, He S-M, Dong M-Q, Miao L. Nematode sperm maturation triggered by protease involves sperm-secreted serine protease inhibitor (Serpin) Proc Natl Acad Sci USA. 2012;109(5):1542–1547. doi: 10.1073/pnas.1109912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziecik AJ, Waclawik A, Kaczmarek MM, Blitek A, Jalali BM, Andronowska A. Mechanisms for the establishment of pregnancy in the pig. Reprod Domest Anim. 2011;46(Suppl 3):31–41. doi: 10.1111/j.1439-0531.2011.01843.x. [DOI] [PubMed] [Google Scholar]
- Zitka O, Kukacka J, Krizkova S, Huska D, Adam V, Masarik M, Prusa R, Kizek R. Matrix metalloproteinases. Curr Med Chem. 2010;17(31):3751–3768. doi: 10.2174/092986710793213724. [DOI] [PubMed] [Google Scholar]