Abstract
Glutamate, the principal excitatory neurotransmitter of the brain, participates in a multitude of physiologic and pathologic processes, including learning and memory. Glutathione, a tripeptide composed of the amino acids glutamate, cysteine, and glycine, serves important cofactor roles in antioxidant defense and drug detoxification, but glutathione deficits occur in multiple neuropsychiatric disorders. Glutathione synthesis and metabolism are governed by a cycle of enzymes, the γ-glutamyl cycle, which can achieve intracellular glutathione concentrations of 1-10 millimolar. Because of the considerable quantity of brain glutathione and its rapid turnover, we hypothesized that glutathione may serve as a reservoir of neural glutamate. We quantified glutamate in HT22 hippocampal neurons, PC12 cells and primary cortical neurons after treatment with molecular inhibitors targeting three different enzymes of the glutathione metabolic cycle. Inhibiting 5-oxoprolinase and γ-glutamyl transferase, enzymes that liberate glutamate from glutathione, leads to decreases in glutamate. In contrast, inhibition of γ-glutamyl cysteine ligase, which uses glutamate to synthesize glutathione, results in substantial glutamate accumulation. Increased glutamate levels following inhibition of glutathione synthesis temporally precede later effects upon oxidative stress.
Keywords: Glutathione, Glutamate, Neurons, Antioxidants, Glutamyl cycle, Neurotransmitter
1. Introduction
Glutamate plays essential roles in many physiological functions and is the major excitatory transmitter in the central nervous system [1,2]. Glutamate participates in many important physiological processes, such as developmental plasticity and long-term potentiation, as well as in pathological conditions such as epilepsy, cerebral ischemia, amyotrophic lateral sclerosis, Alzheimer's disease, Parkinson's disease and schizophrenia [3].
Glutathione, a tripeptide of glutamate, cysteine and glycine, displays high intracellular concentrations, 1-10 mM, making it the most abundant low molecular weight thiol of bacteria, plant and animal cells [4-7]. As such, it participates in a number of critical cellular processes, including the metabolism of endogenous compounds such as estrogens, prostaglandins, leukotrienes, and xenobiotic drugs. Glutathione is a well known antioxidant agent, protecting cells from oxidative or other forms of stress, largely as a cofactor for the glutathione peroxidase and S-tranferase enzyme families [8-10].
Glutathione synthesis is governed by the enzymes of the γ-glutamyl cycle (Supplemental Fig. 1), for which glutamate is specifically added and liberated at discrete steps [5,11]. Deficiencies in enzymes of the cycle—5-oxoprolinase (OPLAH), γ-glutamyl cysteine ligase (GCL), glutathione synthetase, and γ-glutamyl transferase (GGT)—have been associated with neurologic, psychiatric and cognitive symptomatology [12,13]. Multiple investigators have also identified decreases in glutathione in neuropsychiatric disorders such as Parkinson's and Alzheimer's disease, and schizophrenia [14,15]. Substantial proportions of glutamate neurotransmitter pools (50-60%) have long thought to be derived from the glutamine-glutamate shuttle [16,17], with much smaller amounts arising from glycolysis [18-20]. Given the import of glutamate in normal brain physiology and pathologic states, it would be advantageous for neuronal populations to have a glutamate buffering reservoir. Because glutathione exists at high levels in brain, is one third glutamate, and has a short half-life, we hypothesized that the γ-glutamyl cycle serves as a reservoir of neuronal glutamate. Using selective inhibitors of different steps of the cycle, we now show that glutathione serves as a source for a major portion of glutamate in HT22 hippocampal neurons, PC12 cells and primary cortical neurons.
2. Materials and methods
2.1. Cell culture and reagents
PC12 were maintained in Dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 5% horse serum, 50 U/mL penicillin and streptomycin, and 200 mM L-glutamine (Invitrogen). HT22 were maintained in DMEM supplemented with 10% FBS, 50 U/mL penicillin and streptomycin and 200mM L-glutamine. Cells were maintained at 37 °C in 5% CO2/95% atmosphere and medium replaced every 3 days. For glutathione and glutamate quantification, cell viability analysis and oxidative stress assay, cells were sub-cultured in 6-well plates (5 × 105 cells/well for PC12 and 2 × 105 cells/well for HT22). For longer exposure experiments (greater than 24 hours), cells were seeded in 24-well plates (5 × 104 cells per well for PC12 and 5 × 103 cells per well for HT22) and grown for 48 hours before experiments. Acivicin was obtained from BIOMOL. 2-imidazolidone-4-carboxylate (2I4C) and buthionine sulfoximine (BSO) were obtained from Sigma. All animal procedures related to were approved by the Johns Hopkins University Animal Care and Use Committee. Dissociated cortical neuron cultures from Sprague-Dawley rats were prepared as described previously [21].
2.2. Cell viability
3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT) was used to evaluate cell viability. Cells in grown in 24 well plates were treated 2 hours with MTT (final concentration 50 ug/mL), and media was replaced with 200 uL of dimethylsulfoxide to solubilize formazan. Sample absorbance at 570 nm was determined by microplate spectrophotometer and cell viability was expressed as percentage relative to controls.
2.3. Measurement of glutathione
Total and oxidized glutathione was determined by the Tieze method with minor modifications [22]. Cells were washed in PBS, scraped and suspended in phosphate buffer then sonicated. A portion of lysate was suspended in 0.1% N-lauroylsarcosine and used for analysis of protein content by Bradford assay [23]. The remaining solution was centrifuged 15 minutes at 20,000g and the soluble fraction was used for detection of glutathione content. Proteins in the remaining fraction were precipitated with 50 mg/mL metaphosphoric acid, removed by centrifugation, and supernatants neutralized with 200 mM triethanolamine. For oxidized glutathione measurement, an aliquot of the supernatant was incubated 60 minutes at room temperature with 10 mM 2-vinylpyridine (2-VP) to scavenge reduced glutathione. The rate of increase in absorbance at 415 nm, which measures the reduction of 5-5′-dithiobis(2-nitrobenzoic acid) by glutathione, reflects the total glutathione content (or oxidized glutathione when 2-VP is added). The concentration of total and oxidized glutathione content in cells was calculated by a calibration curve with standards. Glutathione content was normalized to total cellular protein per assay and expressed as percentage relative to control, generally 97% reduced and 3% oxidized glutathione.
2.4. Measurement of glutamate
Cells were collected in phosphate buffer (100 mM sodium phosphate, 1 mM ethylenediaminetetraacetate, pH 7.5) and sonicated and lysates centrifuged to remove insoluble debris. Determination of glutamate was performed by Kusakabe's method [24]. 2-10 nmol of L-glutamate standards and the cell lysate were mixed with reaction buffer (final concentrations of 36.8 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (pH 7.1), 2.19×10−5 U L-glutamate oxidase, 1 U peroxidase, 0.8 mM 3.5-dimethoxy-N-ethyl-N-(2-hydroxy-3-sulphopropyl) aniline (sodium salt) (DAOS), 0.8 mM aminoantipyrine). After incubating at 37 °C for 30 minutes, the mixture was measured at a wavelength of 570 nm with a microplate spectrophotometer. The concentration of L-glutamate was then calculated using a standard calibration curve, normalized to protein sample concentrations, and levels expressed as percentage relative to controls.
2.5. Measurement of Reactive Oxygen Species (ROS)
2-[6-(4′hydroxy)phenoxy-3H-xanthen-3-on-9-yl] benzoic acid (HPF) [25] was obtained from Cayman and prepared fresh as a 10 mM stock solution in ethanol. HT22 and PC12 cells were seeded onto 12-well plastic dish at a density 8 × 104 cells and 2 × 105 in 1 mL medium. After maintenance in culture media for the aforementioned treatment periods, the cells were loaded with 5 uM HPF in media and incubated for 30 minutes at 37 °C in the dark. As a positive control, cells were treated with 500 uM hydrogen peroxide and 100 uM ferrous sulfate for 1 hour. Cells were washed and resuspended in HEPES based buffer. HPF fluorescence was quantified with a Perkin Elmer LS55 fluorescent spectrophotometer using excitation/emission wavelengths of 488 nm and 515 nm, respectively.
3. Results
3.1. Inhibiting the liberation of glutamate from glutathione leads to decreases in neuronal glutamate
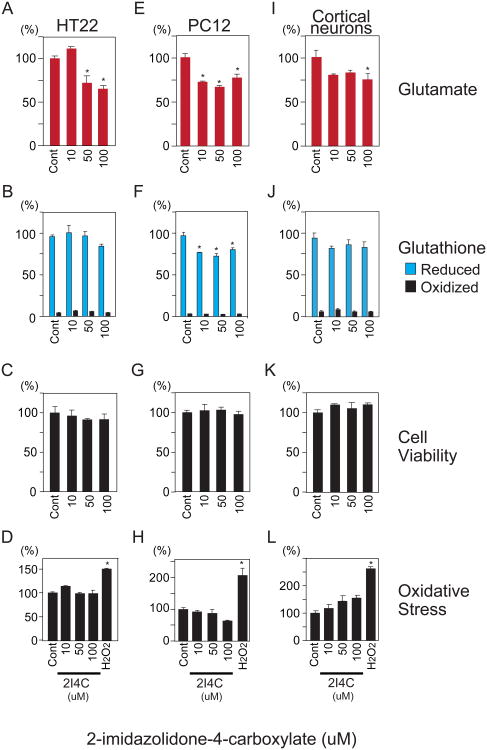
To test the hypothesis that glutathione may be a significant reservoir for glutamate, HT22 hippocampal neurons, PC12 cells and primary cortical neurons were treated with three molecular inhibitors targeting three distinct enzymes of the glutathione metabolic cycle: 2I4C, acivicin and BSO. Enzyme expression was confirmed in the target cell types via immunoblotting (Supplementary Fig. 2). Glutathione and glutamate were quantified by Ellman's procedure and glutamate oxidase methods, respectively [26,27]. 2I4C blocks OPLAH, the enzyme that liberates glutamate from 5-oxoproline (Supplementary Fig. 1) [28]. If glutathione were to serve as a substantial glutamate reservoir, then 2I4C treatment would be expected to produce significant decreases in total neuronal glutamate. Treatment of HT22 hippocampal neurons, PC12 and primary cortical neurons with low to mid-micromolar concentrations of 2I4C resulted in dose dependent 25-30% decreases in glutamate (Fig. 1A,E,I). Reduced glutathione levels were no significantly altered in HT22 and cortical neurons (Fig. 1B,J) but decreased 25% in PC12 cells (Fig. 1F). This is consistent with a shorter half life of glutathione in PC12 cells, as noted with BSO treatment (Fig. 3F). Under these circumstances, glutathione would continue to be consumed by the families of glutathione peroxidases and S-transferases that utilize it as a substrate.
Fig. 1.
Inhibition of 5-oxoprolinase by 2I4C decreases neuronal glutamate levels. HT22, PC12 and cortical neurons were treated with L-2-imidazolidone-4-carboxylate (2I4C), a specific inhibitor of 5-oxoprolinase (OPLAH), for 24 hours. (A,E,I) Total neuronal glutamate was decreased 25-30% by 2I4C relative to control treatment. (B,F,J) Effect of 2I4C on reduced and oxidized glutathione. (C,G,K) Decreased neuronal glutamate by 2I4C was not associated with altered cell viability as determined by MTT assay. (D,H,L) Effect of 2I4C on oxidative stress. Reactive oxygen species were determined by HPF fluorescent probe after 24 hour treatment of HT22, PC12 and cortical neurons with 2I4C. One hour treatment with 500 uM hydrogen peroxide (H2O2) was used as a control. Data represent mean ± standard error of triplicates, representative of 3 independent experiments. *, p<0.05 vs. control by 1-way ANOVA with Tukey-Kramer post hoc test.
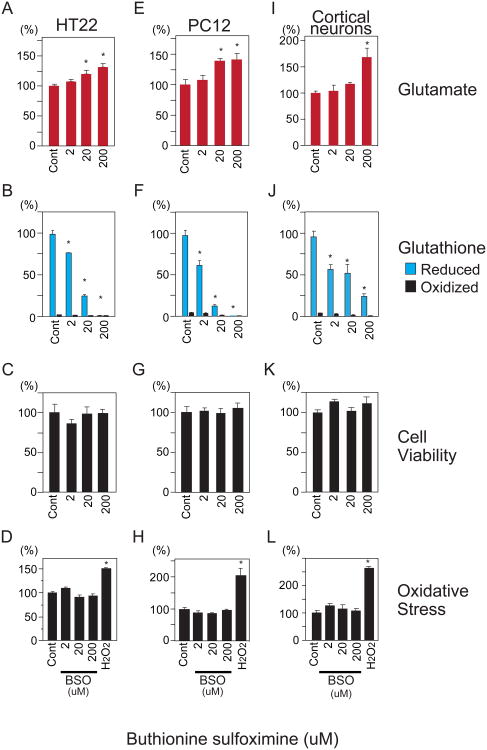
Fig. 3.
Buthionine sulfoximine (BSO) inhibition of γ-glutamyl cysteine ligase (GCL) augments glutamate levels. HT22, PC12 and primary cortical neurons were treated with BSO for 24 hours after which glutamate, glutathione, cell viability and oxidative stress levels were determined. (A,E,I) BSO increases glutamate in a dose-dependent manner in HT22, PC12 and cortical neurons. (B,F,J) Decrease in glutathione following GCL inhibition by BSO. (C,G) Effect of BSO treatment (24 hours) upon cell viability of HT22, PC12 and cortical neurons. (D,H,L) Effect of BSO on oxidative stress at 24 hours. Following 24 hour treatment with BSO, oxidative stress was assessed by HPF fluorescence. One hour exposure to 500 uM hydrogen peroxide (H2O2) was used as a control. Data represent means ± standard error of triplicate determinations. *, p<0.05 vs. control by 1-way ANOVA with Tukey-Kramer post hoc test.
The 2I4C-elicited decline in glutamate levels was not attributable to cell death, as cell viability measured by the MTT assay (Fig. 1C,G,K) was unaffected. We also assessed whether decreases in neuronal glutamate produced by 2I4C were accompanied by oxidative stress. Reactive oxygen species, including Fenton reaction derived hydroxyl radicals, were measured by HPF. This molecule becomes fluorescence following oxidation, and offers greater sensitivity than earlier generation probes whose intrinsic fluorescence is decreased by oxidation. Treatment with 2I4C for 24 hours did not augment oxidative stress, whereas a 1 hour exposure to 500 uM hydrogen peroxide robustly augmented HPF fluorescence (Fig. 1D,H,L). Oxidized glutathione, which serves as a marker of pharmacological and oxidative stress, but was also unaffected by 2I4C treatment (Fig. 1B,F,J).
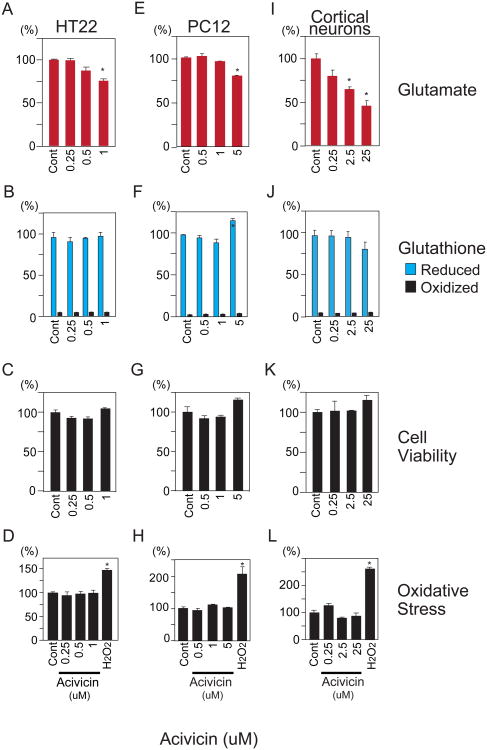
To further explore a role for the γ-glutamyl cycle as a glutamate reservoir, we sought to inhibit the activity of GGT by acivicin, an enzymatic step upstream of the liberation of glutamate from the cycle (Supplementary Fig. 1). GGT can generate a γ-glutamyl amino acid or glutamate, depending upon whether an amino acid or water is used as an acceptor [29]. Accordingly, we examined whether inhibition of GGT by acivicin influences cellular glutamate. HT22, PC12 and cortical neurons treated with low micromolar acivicin concentrations exhibited 25-50% reductions in total glutamate, with effects most pronounced in primary cortical neurons (Fig. 2A,E,I). Glutathione levels increased in PC12 cells at the 5 uM dose (Fig. 2F). This may be explained by induction of GCLC, the rate-limiting step for glutathione synthesis, by acivicin in PC12, but not HT22 neurons (Supplementary Fig. 3). This induction of GCLC specifically occurred at the 5 uM dose associated with increased Glutathione levels in PC12 cells. Cell viability, reactive oxygen species and oxidized glutathione were unaffected by acivicin treatment (Fig. 2B-D,F-H,J-L).
Fig. 2.
Acivicin, an inhibitor of γ-glutamyl transferase, decreases glutamate in HT22, PC12 and cortical neurons. (A,E,I) 24 hour treatment with acivicin results in diminished glutamate levels. (B,F,J) Effect of acivicin upon reduced and oxidized glutathione in HT22 and PC12 and cortical neurons. (C,G,K) Decreased neuronal glutamate by 2I4C was not associated with altered cell viability as determined by MTT assay. (D,H,L) Effect of acivicin on oxidative stress. HT22, PC12 and cortical neurons were treated 24 hours with 2I4C and oxidative stress was assessed by HPF fluorescence. One hour exposure to 500 uM hydrogen peroxide (H2O2) was used as a control. Data represent mean ± standard error of triplicates, representative of 3 independent experiments. *, p<0.05 by 1-way ANOVA with Tukey-Kramer post hoc test.
3.2. Blocking conversion of glutamate to glutathione leads to glutamate accumulation
We targeted the rate-limiting enzyme of the γ-glutamyl cycle, GCL, with a highly specific inhibitor, BSO. Inhibition of GCL leads to glutathione depletion owing to its short half life (0.5-4 hours), including its utilization as a cofactor for over 20 enzymes [5,11]. If the γ-glutamyl cycle were a significant glutamate reservoir, then BSO should promote accumulation of reservoir glutamate (Supplemental Fig. 1). BSO treatment of HT22, PC12 and primary cortical neurons resulted in concentration-dependent glutamate increases of 25-50% (Fig. 3A,E,I), similar to the glutamate decrease elicited by 2I4C and acivicin. As expected, BSO led to decreases in total glutathione in HT22, PC12 and primary cortical neurons (Fig. 3B,F,J).
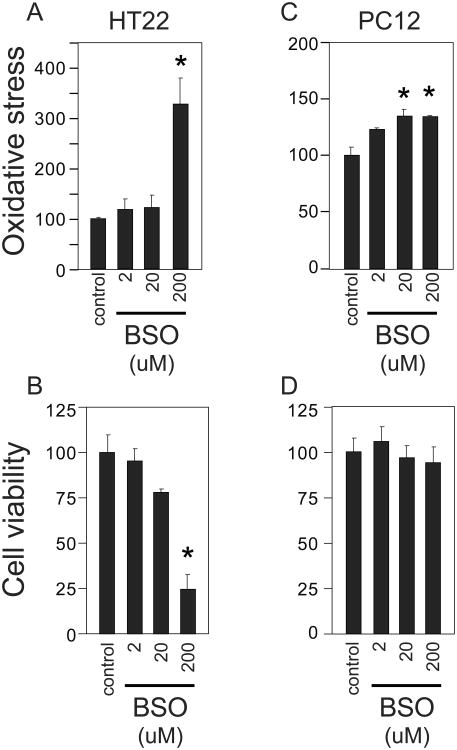
Because BSO depletes glutathione, we examined whether the observed increases in glutamate were associated with oxidative stress or cytotoxicity. The 24 hour BSO treatment that increases glutamate did not significantly alter cell viability (Fig. 3C,G,K) or oxidative stress (Fig. 3D,H,L). BSO treatment ultimately increases oxidative stress as would be expected following glutathione depletion. BSO treatment of HT22, PC12 cells for 60 hours results in an increase in reactive oxygen species (Fig. 4A,C). HT22, but not PC12 cells, experienced a significant reduction in cell viability following the 60 hour BSO treatment (Fig. 4B,D). Thus BSO inhibition of GCL is associated with an increase in glutamate that is temporally independent of later oxidative stress associated with glutathione depletion.
Fig. 4.
Extended treatment of PC12 and HT22 cells with BSO increases oxidative stress. (A,B) Cells were treated with BSO for 60 hours and relative oxidative stress was quantified by HPF fluorescence. (C,D) Effect of 60 hour BSO treatment upon cell viability in HT22 and PC12. Data represent means ± standard error of triplicate determinations. *, p<0.05 vs. control by 1-way ANOVA with Tukey-Kramer post hoc test.
4. Discussion
In the present study we provide evidence that glutathione may serve as a physiologic reservoir of glutamate in neuronal cell types. We targeted three distinct enzymatic steps of the γ-glutamyl cycle in HT22, PC12 and primary cortical neurons. 2I4C and acivicin, acting at steps upstream of glutamate liberation from glutathione, led to 25-50% decreases in glutamate. BSO, which blocks utilization of glutamate to synthesize glutathione, leads to 25-50% increases in total glutamate. While acivicin can also target glutaminase and transglutaminase 2 [30,31], our findings are unlikely to be explained solely such by off-target effects, as a reservoir function for glutathione is supported by three inhibitors acting at three distinct enzymatic steps in three different cell types. Higher doses of acivicin did lead to increases in glutathione in PC12 cells, but not HT22 or primary cortical neurons (Fig. 2f). This may be explained by differential effects upon GCL, the rate-limiting step for glutathione synthesis; acivicin increases the catalytic subunit of GCL in PC12 cells, but not HT22 neurons (Supplementary Fig. 3).
The antioxidant and cytoprotective functions of glutathione are well established. However the three cell types we studied tolerated perturbations of the glutathione cycle leading to 25-50% changes in glutamate over 24 hours, without accentuating oxidative stress or cytotoxicity. Depletion of glutathione for 60 hours eventually promoted oxidative stress (Fig. 4). The large concentrations of intracellular glutathione may facilitate buffering or stabilization of neuronal glutamate levels without impacting upon its cytoprotective functions.
A reservoir function of glutathione to modulate glutamate levels implies a tight relation between glutathione and glutamate. However, acivicin and 2I4C, which block liberation of glutamate from glutathione, would not necessarily lead to increases of glutathione. Although we did not address this specifically in our experiments, any glutathione increases induced by enzymatic inhibition may be offset by its continued consumption by over 20 enzymes using it as a substrate (Fig. 1F). In addition, we did not characterize the levels of additional intermediates of the glutathione cycle, such as 5-oxoproline or gamma-glutamyl amino acids, whose levels may be affected to a greater degree than glutathione. Flux of glutamate out of the glutathione cycle may also lead to compensatory changes in activity or protein expression of glutathione cycle enzymes or transmembrane transporters that supply substrates to the pathway.
A glutamate reservoir role for glutathione has particular relevance to neurobiology. The γ-glutamyl cycle was elucidated over a decade prior to the general acceptance of glutamate as a neurotransmitter [32]. Cytosolic glutamate levels in neurons are typically reported as 1-10 mM, which overlap neuronal glutathione concentrations ranging from 0.2 to1 mM, though some have suggested concentrations as high as 10 mM [7,33-37]. We are presently exploring roles for the glutathione cycle regulating glutamate neurotransmission. Alterations in neuronal glutamate levels, perhaps by fluxes to and from a glutathione reservoir, might impact the filling of glutamate vesicles. Indeed, the vesicular glutamate transporters have a much lower affinity for glutamate, ∼1 mM, than plasma membrane transporters such as GLT1/EAAT2 whose Km is 4-40 uM [2,38]. Thus, fluxes of cytosolic glutamate to and from the γ-glutamyl cycle may influence glutamate neurotransmission. Localized glutathione synthesis would be expected to have an even more pronounced effect, and it has been suggested that non-cell body areas contain more glutathione [39]. Furthermore, as glutamate neurotransmitter typically does not saturate postsynaptic receptors, modest impacts upon release may influence synaptic strength [33,37,40,41]. Thus, increasing intracellular glutamate concentration in presynaptic terminals leads to greater excitatory post synaptic currents (EPSC) amplitudes [33,37].
Major amounts of glutamate neurotransmitter (50-60%) have long thought to be derived from the action of the glutamine-glutamate shuttle [16,17], with substantially smaller amounts of glutamate transmitter derived from glycolysis [18-20]. While the glutamine-glutamate shuttle contributes in a major way to GABA neurotransmitter (glutamate is a GABA precursor), other sources may be more relevant for glutamate [42]. “Phasic” axons that fatigue in release of glutamate neurotransmitter have lower glutamate levels than “tonic” glutamate axons with greater glutamate levels [35]. Glutamine levels are similar in both, suggesting that substantial reservoirs of glutamate exist in neurons independent of glutamine. Nicoll and colleagues [43] demonstrated that the shuttle is fairly slow to replenish neuronal glutamate. After inhibition of glutamine recycling, there was an abrupt decrease, but quick recovery of excitatory neurotransmission, independent of the shuttle. The authors speculated that neurons might be utilizing an ill-defined endogenous source of glutamate [43]. The conventional glutathione cycle may not provide de novo glutamate, as this both enters and exits the cycle. However the large quantities of intracellular glutathione, up to 20 mM, are also consistent with a reservoir function of glutamate rather than a de novo biosynthetic source provided by glycolysis or glutamine precursor. Future efforts will characterize the contribution of these pathways to filling the glutathione pool.
Supplementary Material
Highlights.
Glutathione is a reservoir of significant amount of neuronal glutamate.
Acivicin and 2I4C treatments decrease neuronal glutamate.
Buthionine sulfoximine increases neuronal glutamate.
Glutathione reservoir function is independent of antioxidant function.
Acknowledgments
Support by NIH NINDS 1K08NS057824 (TWS), NIH MH 092443 (AS), NIH MH 18501 (SHS), NIH MH 084018 (AS) and NARSAD Young Investigator Award (TWS). We thank Yukiko Lema for guidance in manuscript preparation.
Footnotes
Conflict of interest: The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reis HJ, Guatimosim C, Paquet M, et al. Neuro-transmitters in the central nervous system & their implication in learning and memory processes. Curr Med Chem. 2009;16:796–840. doi: 10.2174/092986709787549271. [DOI] [PubMed] [Google Scholar]
- 2.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoyama K, Watabe M, Nakaki T. Regulation of Neuronal Glutathione Synthesis. J Pharmacol Sci. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- 5.Forman HJ, Zhang H, Rinna A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janáky R, Cruz-Aguado R, Oja SS, et al. Glutathione in the Nervous System: Roles in Neural Function and Health and Implications for Neurological Disease. In: Lajtha A, Oja SS, Schousboe A, et al., editors. Handbook of Neurochemistry and Molecular Neurobiology. Springer; US: 2007. pp. 347–399. [Google Scholar]
- 7.Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 8.Chinta SJ, Kumar MJ, Hsu M, et al. Inducible Alterations of Glutathione Levels in Adult Dopaminergic Midbrain Neurons Result in Nigrostriatal Degeneration. J Neurosci. 2007;27:13997–14006. doi: 10.1523/JNEUROSCI.3885-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 10.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 11.Meister A. Selective modification of glutathione metabolism. Science. 1983;220:472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- 12.Njalsson R, Ristoff E, Carlsson K, et al. Genotype, enzyme activity, glutathione level, and clinical phenotype in patients with glutathione synthetase deficiency. Hum Genet. 2005;116:384–389. doi: 10.1007/s00439-005-1255-6. 389. [DOI] [PubMed] [Google Scholar]
- 13.Ristoff E, Larsson A. Patients with genetic defects in the gamma-glutamyl cycle. Chem Biol Interact. 1998;111-112:113–121. doi: 10.1016/s0009-2797(97)00155-5. [DOI] [PubMed] [Google Scholar]
- 14.Ballatori N, Krance SM, Notenboom S, et al. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390:191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Do KQ, Cabungcal JH, Frank A, et al. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Hertz L. Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Prog Neurobiol. 1979;13:277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- 17.Sanacora G, Zarate CA, Krystal JH, et al. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamberger AC, Chiang GH, Nylén ES, et al. Glutamate as a CNS transmitter. I. Evaluation of glucose and glutamine as precursors for the synthesis of preferentially released glutamate. Brain Res. 1979;168:513–530. doi: 10.1016/0006-8993(79)90306-8. [DOI] [PubMed] [Google Scholar]
- 19.Kanai Y, Hediger M. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch. 2004;447:469–479. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- 20.Thanki CM, Sugden D, Thomas AJ, et al. In Vivo Release from Cerebral Cortex of [14C]Glutamate Synthesized from [U-14C]Glutamine. J Neurochem. 1983;41:611–617. doi: 10.1111/j.1471-4159.1983.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi-Takagi A, Takaki M, Graziane N, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 23.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Kusakabe H, Midorikawa Y, Fujishima T, et al. Purification and properties of a new enzyme, L-glutamate oxidase, from Streptomyces sp X-119-6 grown on wheat bran. Agric Biol Chem. 1983;47:1323–1328. [Google Scholar]
- 25.Setsukinai K, Urano Y, Kakinuma K, et al. Development of Novel Fluorescence Probes That Can Reliably Detect Reactive Oxygen Species and Distinguish Specific Species. J Biol Chem. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- 26.Chapman J, Zhou M. Microplate-based fluorometric methods for the enzymatic determination of -glutamate: application in measuring -glutamate in food samples. Anal Chim Acta. 1999;402:47–52. [Google Scholar]
- 27.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protocols. 2007;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 28.Van Der Werf P, Stephani RA, Meister A. Accumulation of 5-oxoproline in mouse tissues after inhibition of 5-oxoprolinase and administration of amino acids: evidence for function of the gamma-glutamyl cycle. Proc Natl Acad Sci U S A. 1974;71:1026–1029. doi: 10.1073/pnas.71.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keillor JW, Castonguay R, Lherbet C, et al. Gamma Glutamyl Transpeptidase Substrate Specificity and Catalytic Mechanism. Methods Enzymol. 2005;401:449–467. doi: 10.1016/S0076-6879(05)01027-X. [DOI] [PubMed] [Google Scholar]
- 30.Hausch F, Halttunen T, Maki M, et al. Design, synthesis, and evaluation of gluten peptide analogs as selective inhibitors of human tissue transglutaminase. Chem Biol. 2003;10:225–231. doi: 10.1016/s1074-5521(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 31.Steckel J, Roberts J, Philips FS, et al. Kinetic properties and inhibition of Acinetobacter glutaminase-asparaginase. Biochem Pharmacol. 1983;32:971–977. doi: 10.1016/0006-2952(83)90613-5. [DOI] [PubMed] [Google Scholar]
- 32.Orrego F, Villanueva S. The chemical nature of the main central excitatory transmitter: A critical appraisal based upon release studies and synaptic vesicle localization. Neuroscience. 1993;56:539–555. doi: 10.1016/0306-4522(93)90355-j. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa T, Sahara Y, Takahashi T. A Single Packet of Transmitter Does Not Saturate Postsynaptic Glutamate Receptors. Neuron. 2002;34:613–621. doi: 10.1016/s0896-6273(02)00692-x. [DOI] [PubMed] [Google Scholar]
- 34.Pileblad E, Eriksson PS, Hansson E. The presence of glutathione in primary neuronal and astroglial cultures from rat cerebral cortex and brain stem. J Neural Transm Gen Sect. 1991;86:43–49. doi: 10.1007/BF01250374. [DOI] [PubMed] [Google Scholar]
- 35.Shupliakov O, Atwood HL, Ottersen OP, et al. Presynaptic glutamate levels in tonic and phasic motor axons correlate with properties of synaptic release. J Neurosci. 1995;15:7168–7180. doi: 10.1523/JNEUROSCI.15-11-07168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun X, Shih AY, Johannssen HC, et al. Two-photon Imaging of Glutathione Levels in Intact Brain Indicates Enhanced Redox Buffering in Developing Neurons and Cells at the Cerebrospinal Fluid and Blood-Brain Interface. J Biol Chem. 2006;281:17420–17431. doi: 10.1074/jbc.M601567200. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita T, Ishikawa T, Takahashi T. Developmental Increase in Vesicular Glutamate Content Does Not Cause Saturation of AMPA Receptors at the Calyx of Held Synapse. J Neurosci. 2003;23:3633–3638. doi: 10.1523/JNEUROSCI.23-09-03633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liguz-Lecznar M, Skangiel-Kramska J. Vesicular glutamate transporters (VGLUTs): the three musketeers of glutamatergic system. Acta Neurobiol Exp Wars. 2007;67:207–218. doi: 10.55782/ane-2007-1649. [DOI] [PubMed] [Google Scholar]
- 39.Slivka A, Mytilineou C, Cohen G. Histochemical evaluation of glutathione in brain. Brain Res. 1987;409:275–284. doi: 10.1016/0006-8993(87)90712-8. [DOI] [PubMed] [Google Scholar]
- 40.Otis TS. Vesicular Glutamate Transporters In Cognito. Neuron. 2001;29:11–14. doi: 10.1016/s0896-6273(01)00176-3. [DOI] [PubMed] [Google Scholar]
- 41.Pozo K, Goda Y. Unraveling Mechanisms of Homeostatic Synaptic Plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang SL, Carlson GC, Coulter DA. Dynamic Regulation of Synaptic GABA Release by the Glutamate-Glutamine Cycle in Hippocampal Area CA1. J Neurosci. 2006;26:8537–8548. doi: 10.1523/JNEUROSCI.0329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kam K, Nicoll R. Excitatory Synaptic Transmission Persists Independently of the Glutamate Glutamine Cycle. J Neurosci. 2007;27:9192–9200. doi: 10.1523/JNEUROSCI.1198-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.