Abstract
BACKGROUND.
Androgen modulation of angiogenesis in prostate cancer may be not directly mediated by androgen receptor (AR) as AR is not detected in the prostatic endothelial cells.
METHODS.
We examined the paracrine stimulation of cell proliferation by prostate tumor cells and its modulation by androgen and estrogens in a murine endothelial cell line (MEC) that does not express AR.
RESULTS.
Tumor cell conditioned media (TCM) collected from LAPC-4 or LNCaP prostatic tumor cells produced a time- and concentration-dependent induction of cell growth in MECs, which was parallel to the VEGF concentration in the TCM. This TCM-induced cell growth in MECs was enhanced by the treatment of prostatic tumor cells with dihydrotestosterone (DHT). Both the TCM-stimulation and DHT-enhancement effects in MECs were completely blocked by SU5416, a specific VEGF receptor antagonist. Co-administration of 17α-estradiol or 17β-estradiol with DHT in prostatic tumor cells completely inhibited the DHT-enhancement effect while treatment with DHT, 17α-estradiol or 17β-estradiol did not produce any significant direct effect in MECs. Moreover, administration of 17α-estradiol or 17β-estradiol in xenograft animals with LAPC-4 or LNCaP prostate tumor significantly decreased the microvessel number in the tumor tissues.
CONCLUSIONS.
Our study indicated that prostate tumor cells regulate endothelial cell growth through a paracrine mechanism, which is mainly mediated by VEGF; and DHT is able to modulate endothelial cell growth via tumor cells, which is inhibited by 17α-estradiol and 17β-estradiol. Thus, both17α-estradiol and 17β-estradiol are potential agents for anti-angiogenesis therapy in androgen-responsive prostate cancer.
Keywords: dihydrotestosterone, estrogen, prostate cancer cells, endothelial cells, VEGF
INTRODUCTION
Angiogenesis plays a crucial role in the survival, proliferation, and metastatic potential of prostate cancer through providing nutrients and oxygen [1]. Previous studies have demonstrated a link between androgen action and angiogenesis in the human prostate cancer, and androgen-responsive xenograft animal models [2-6]. Angiogenesis in the prostate seems even particularly sensitive to androgens [7-9]. It is well documented that the majority of androgen actions is mediated by activation of androgen receptor (AR) [10]. However, the prostate vascular endothelium may not be the direct target as AR expression has not been detected in the endothelial cells from normal prostate tissues or prostate carcinomas [11-13]. It appears that this androgenic effect on angiogenesis may be mediated through an indirect action via the surrounding epithelial cells and/or stromal cells.
The mechanisms of androgen actions on epithelial–endothelial crosstalk in the normal and malignant prostatic microenvironment are not fully understood. Growing evidence suggests that paracrine signals may be important mediators [14]. Prostate tumor cells and stromal cells can release a variety of soluble angiogenic factors, such as vascular endothelial growth factor (VEGF-A), angiopoietins, transforming growth factors (TGF), platelet-derived growth factor, tumor necrosis factor-α, and fibroblast growth factor (FGF) [15,16], which generate a supporting microenvironment for angiogenesis [17]. VEGF-A is the key pro-angiogenic factor secreted by prostate epithelial cells and tumor cells, which was found to be accompanied by androgen-induced vascular changes [3-5,18]. Thus, VEGF-A may be a particularly attractive candidate as the paracrine mediator of androgen action on prostatic angiogenesis.
The possible pro-angiogenic androgen action on endothelial cells has major implications for androgen blockade therapy of prostate cancer. Beyond conventional approaches, such as androgen deprivation therapy (ADT) via surgical or medical castration [19-21], current efforts are being made to not only inhibit the direct androgen action on tumor cells but also block intratumoral paracrine signaling [17]. Estrogens have been used for the treatment of advanced prostate cancer presumably via inhibition of testosterone production by a negative feedback of the hypothalamic–pituitary-gonadal axis [22,23]. Recently, we have demonstrated that estrogens via estrogen receptors (ERs) can directly inhibit AR transactivation in the prostate cancer cells, resulting in an inhibition of androgen-induced gene expression, cell proliferation and tumor growth [24,25]. Moreover, 17α-estradiol (αE2), a stereoisomer of 17β-estradiol (βE2) with less classic estrogenic activity, has been shown to inhibit androgen-induced gene expression, cell proliferation and tumor growth in xenograft mice with prostate cancer [24-26]. Based on these data, we have hypothesized that androgens such as DHT stimulate angiogenesis via a paracrine mechanism by directly acting on prostate tumor cells, which is inhibited by αE2 and βE2.
In the present study, we have tested this hypothesis by using in vitro cell cultures of MECs, LAPC-4 and LNCaP prostate cells, and in vivo xenograft animal models with subcutaneous prostate cancer. We demonstrated that similar to prostate endothelium, MECs did not express AR, and DHT failed to stimulate MEC cell growth directly. However, DHT stimulated MEC cell proliferation by regulating the production of paracrine factors in prostate tumor cells, which was inhibited by both αE2 and βE2. Furthermore, both αE2 and βE2 significantly inhibited angiogenesis in xenograft prostate tumors. These results suggest that blockade of AR transactivation in prostate tumor cells not only directly inhibits tumor cell growth but also decreases tumor angiogenesis via a paracrine mechanism.
MATERIALS AND METHODS
Chemicals, Antibodies, and Reagents
DHT, αE2, βE2, and SU-5416 were purchased from Sigma Co. (St. Louis, MO), and dissolved in ethanol at a stock concentration of 10−2 M, R1881 (methytriennolone) was obtained from Dupont NEN Life Science Products (Boston, MA). The SuperSript III Platinum® Two-Step qRT-PCR Kit was obtained from Invitrogen (Carlsbad, CA). CD31 antibody (PECAM-1, sc-1506) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Culture
LNCaP prostate tumor cells (ATCC, Rockville, MD) and LAPC-4 prostate tumor cells (a gift from Dr. C. Sawyer) were cultured in RPMI-1640 and Isove’s modified Eagle’s media (IMEM, Sigma Co.), respectively, with supplements as previously described [24-26]. MECs (sEnd.1, obtained from Dr. Steven Gross of Weill Cornell Medical College) were cultured in low glucose Dulbecco’s modified Eagle’s media (DMEM, Sigma) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 50 units/ml of penicillin, and 50 μg/ml of streptomycin. This cell line was originally established from a mouse skin capillary endothelioma induced by infection with a retrovirus harboring an insert that encodes polyoma middle T antigen [27,28].
Preparation of Tumor Cell Conditioned Media (TCM)
LAPC-4 (8.0 × 106 cells/flask) and LNCaP prostate tumor cells (3.8 × 106 cells/flask) were cultured in growth media in T-25 flasks as described [24,26]. When cells reached nearly 100% confluence of monolayer, the growth media were removed, washed three times with IMEM or RPMI-1640 conditioned media (CM) which was supplemented with 2 mM L-glutamine, 50 units/ml of penicillin, and 50 μg/ml of streptomycin. The cells were then incubated with 3 ml CM for 24, 48, or 72 hr. TCM was then collected and centrifuged at 3,000 rpm for 15 min at 4°C. The supernatants were filtered through a 0.2 μm filter, aliquoted and stored at −80°C until being used. All TCMs were normalized with cell number by the addition of CM.
For collection of hormone-treated TCM, when LAPC-4 and LNCaP cells grew to nearly 100% confluence, cells were washed three times with serum-free CM and treated with 3 ml CM plus vehicle control, DHT, αE2, or βE2 alone or in combination at doses indicated for each experiment for 48 hr. TCMs were then collected, processed, and normalized as described above.
Cell Proliferation Assay
For the determination of viable cells, MECs were plated in 96-well plates at a density of about 2,000–2,500 cells/well in phenol-red free DMEM supplemented with 5% stripped fetal bovine serum (Gemini Bio-Products, Calabasas, CA), 2 mM L-glutamine, 50 units/ml of penicillin, and 50 μg/ml of streptomycin. Fourteen hours after passage, cells were treated with TCM as indicated in each experiment for 48 hr. The number of viable cells was determined by using the Cell Titer One Solution Cell Proliferation Assay kit (Promega, Madison, WI) according to the manufacturer’s instruction.
Immunohistochemical Analysis of CD31 in Xenograft Tumor Tissues
Xenograft animal models with LAPC-4 or LNCaP prostatic tumor were prepared, monitored and treated with a placebo (20 mg cholesterol), αE2 (2 mg αE2 plus 18 mg cholesterol) or βE2 (2 mg βE2 plus 18 mg cholesterol) pellet for 4 weeks as previously described [24,25]. At the end of the 4 weeks, animals were euthanized by CO2 inhalation. Tumors were dissected and a portion of tumor tissue was fixed in 10% buffered formalin for 24 hr and then transferred to 70% ethanol before embedding. The tissues were embedded in paraffin and cut to 4-μm sections with a microtome. The animal study was approved by the institutional animal care and use committee, and done in accordance with accepted standards of humane animal care.
For CD31 immunohistochemistry, sections were deparaffinized in Histo-Clear (National Diagnostic, Atlanta, GA) and rehydrated in a graded series of ethanol concentrations. Endogenous peroxidase was blocked by 0.03% hydrogen peroxide in Phosphate-Buffered Saline (PBS) for 15 min. After being extensively washed in tap water, antigen retrieval was performed using 10 mM sodium citrate buffer, pH 6.0. After being rinsed in PBS, the sections were incubated with anti-CD31 antibody (sc-1506) diluted 1:200 in antibody diluent (Dako, Carpinteria, CA) at room temperature for 60 min, followed by sequential incubation with biotinylated secondary anti-goat immunoglobulin diluted 1:200 in 0.5% rabbit serum (VECTOR Laboratories, Burlingame, CA) at room temperature for 30 min. The Ag-Ab reaction was visualized with the Vectastain avidin-biotin complex (ABC) kit (Vector Laboratories) using 3,3′-diaminobenzidine tetrahydrochloride as a substrate. The sections were counterstained with hematoxylin and examined under a light microscope. CD31+ vessels with lumens were counted in five randomly chosen fields at 200× magnification. To define a blood vessel, a lumen was not necessary. Areas containing the densest neovascularization were identified and microvessels from five fields per section were counted simultaneously by two independent observers, of whom one was blinded to treatment conditions. Two sections of each individual tumor were counted and the number of microvessels was an average of the 10 fields from these two sections.
Determination of VEGF Concentration in TCM
The VEGF levels in TCM were determined by electrochemiluminescence detection using a MSD 96-Well Multi-Array Human VEGF Tissue Culture Assay kit from the Meso Scale Discovery (Gaithersburg, MD) according to the manufacturer’s instruction as described [29].
RT-PCR and Real-Time PCR
Total cellular RNA was isolated from harvested cells using TriPure Isolation Reagent (Roche Diagnostics, Indianapolis, IN), and the concentrations of RNA were determined by ultraviolet absorbance at 260 nm. RNA samples were subjected to a reverse transcription using the SuperSript™ III Platinum® Two-Step qRT-PCR Kit (Invitrogen) following the manufacturer’s instruction. PCR amplification was carried out according to the protocol from Promega in a solution containing 1.5 mM MgCl2, 0.5 μM of each primer, 200 μM dNTPs, 2.5 units of GoTaq® Flexi DNA Polymerase (Promega), and 2.5 μl of template from the first step RT reaction. PCR primers used are listed in Table I. The PCR conditions were 94°C for 2 min, and then 35 cycles of 94°C for 30 sec, 63°C for 30 sec for mouse ERα, or 60°C for 30 sec for mouse ERβ, mouse VEGFR1 and VEGFR2, and 72°C for 30 sec, following a final cycle of 72°C for 5 min. The PCR products were fractionated in a 2% agarose gel and visualized by ethidium bromide staining. Mouse testis or mouse ovary was used as positive controls, and yeast tRNA was used as a negative control of RT-PCR.
TABLE I.
Primers Used For RT-PCR and Real-Time PCR
| Gene | Primers |
|---|---|
| mAR | FP:
5′-AGGAACTCGATCGCATCATT-3′ RP: 5′-TGGAAATAGATGGGCTTGAC-3′ |
| mERα | FP:
5′-TACGAAGTGGGCATGATGAA-3′ RP: 5′-AAGGACAAGGCAGGGCTATT-3′ |
| mERβ | FP:
5′-CAAGAGAACCAGTGGGCACAC-3′ RP: 5′-CAGCCAATCATGTGCACCAG-3′ |
| mVEGFR1 | FP:
5′-CCCAGCAACTTAGGAAACA-3′ RP: 5′-CACCACCAATGTGCTAACCG-3′ |
| mVEGFR2 | FP:
5′-GCCTACAAGTGCTCGTACCG-3′ RP: 5′-CAGAGGCGATGAATGGTGATCT-3′ |
| hVEGF | FP:
5′-CGAAGTGGTGAAGTTCATGGATG-3′ RP: 5′-TTCTGTATCAGTCTTTCCTGGTGAG-3′ |
| hGAPDH | FP:
5′-GAAGGTGAAGGTCGGAGTC-3′ RP: 5′-GAAGATGGTGATGGGATTTC-3′ |
Real-time PCR was performed using the comparative CT method according to the instructions of the manufacturer on the ABI Prism 7900 Sequence Detection System (PE Applied Biosystems, Foster City, CA) in the institutional core facility as described [29]. Real-time PCR conditions were set according to the protocol from Invitrogen. PCR primers used are listed in Table I. Glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The difference between each sample was calculated by following the instructions of the manufacturer (PE Applied Biosystems), and the data are expressed as fold of the corresponding control group.
Statistical Analysis
The data are presented as means ± SEM. One-way analysis of variance (ANOVA) following post hoc Student–Newman–Keuls test was used to determine the difference among multiple groups. A P-value less than 0.05 was considered as statistically significant.
RESULTS
TCM From Prostate Cancer Cells Produced a Dose- and Time-Dependent Induction of Cell Proliferation in MECs
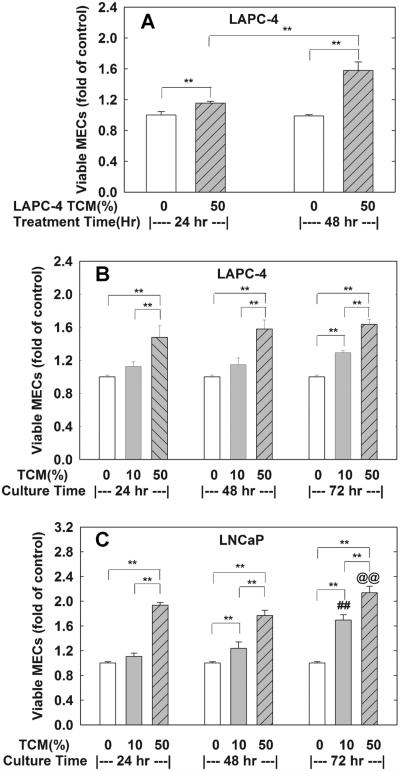
To determine whether TCM from LAPC-4 and LNCaP prostate cancer cells induce cell proliferation in MECs, TCMs collected from LAPC-4 or LNCaP at 24, 48, and 72 hr of incubation were administrated to MECs at various concentrations and the number of viable MECs was determined at 24 or 48 hr of treatment. Treatment of MECs with 50% TCM collected from 48-hr LAPC-4 cell cultures produced a time-dependent induction of cell proliferation as evidenced by the increase in viable cell number as shown in Figure 1A. The increase in viable cell number in MECs treated with 50% TCM for 48 hr is significant higher than those treated for 24 hr (58% vs. 15%). Based on these results, TCM treatment of MECs for 48 hr was selected for all subsequent experiments. In Figure 1B,C, treatment with various concentrations of TCMs collected at different time points of prostate tumor cell cultures produced a concentration-dependent induction of cell proliferation. Treatment with 50% TCM from LAPC-4 (Fig. 1B and Table II) resulted in a significant increase in viable cell number by 47%, 58%, and 63% for TCM collected at 24, 48, and 72 hr of cell cultures, respectively. However, only the TCM collected at 72 hr significantly induced cell proliferation when treated with a 10% dose in MECs (Fig. 1B). Similarly, treatment with both of 10% and 50% TCMs from 48- and 72-hr LNCaP cell culture significantly induced MECs cell proliferation (Fig. 1C). The number of viable cells was increased by 94%, 86%, and 115% in MECs treated with a 50% TCM concentration collected from 24, 48, and 72-hr LNCaP cell cultures, respectively.
Fig. 1.
MECs cell proliferation is induced by prostate tumor conditioned media (TCM). TCM were collected from LAPC-4 and LNCaP prostate tumor cell cultures after incubating for 24, 48, and 72 hr, respectively, as described in the Materials and Methods Section. In (A), MECs plated in 96-well plates were treated with control media (0), or 50% TCM collected from 48-hr LAPC-4 cell cultures for 24 or 28 hr. In (B,C), MECs plated in 96-well plates were treated for 48 hr with control media (0), or 10%, or 50% of TCM collected from 24, 48, and 72-hr LAPC-4 (B) or LNCaP (C) cell cultures. The viable cells number was determined at the end of experiments, and the data are expressed as fold of corresponding media control of each experiment. Results are means ± SEM of three independent triplicate experiments. **P < 0.01 between the indicated groups; ##P < 0.01 compared to corresponding 10% TCM-24 hr and 10% TCM-48 hr group; and @@P < 0.01 compared to corresponding 50% TCM-24 hr and 50% TCM-48 hrgroup.
TABLE II.
TCM Stimulation of MEC Cell Growth and VEGF Concentration in TCM Collected From LAPC-4 Cells
| Times of TCM collection
(hr) |
MECs viable cell number (fold
of control) |
VEGF concentration
(ng/ml) |
||
|---|---|---|---|---|
| 10% TCM | 25% TCM | 50% TCM | ||
| 0 | 1.00 ± 0.02 | 1.00 ± 0.02 | 1.00 ± 0.02 | 0 |
| 24 | 1.12 ± 0.06 | 1.07 ± 0.00 | 1.48 ± 0.14**,## | 12.43 |
| 48 | 1.15 ± 0.08 | 1.19 ± 0.02* | 1.58 ± 0.11**,## | 28.13 |
The data are the mean ± SEM from three independent experiments except VEGF concentrations in TCM, which is a duplicate assay of a single sample.
P < 0.05 compared to 0 hr control in the same TCM concentration group.
P < 0.01 compared to 0 hr control in the same TCM concentration group.
P < 0.01 compared to 10% and 25% TCM concentration collected at the same time.
Treatment With DHT in LAPC-4 and LNCaP Cells Further Enhanced TCM Induction of MEC Cell Proliferation
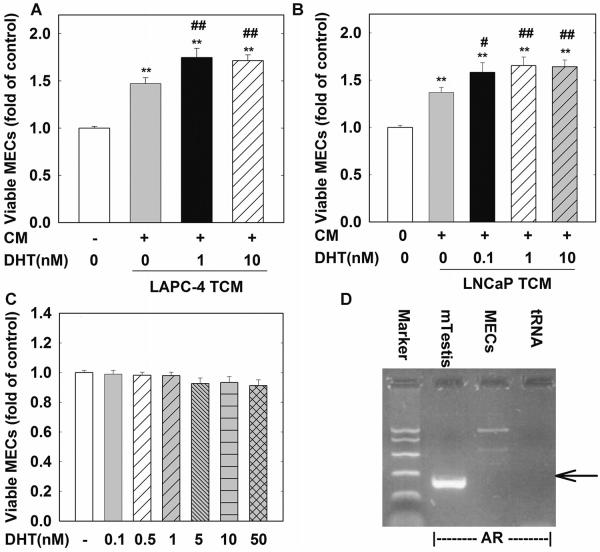
To assess the effect of DHT in MECs, MECs were treated with various doses of DHT for various times. As shown in Figure 2C, treatment with DHT at doses ranging from 0.1 to 50 nM for 48 hr failed to stimulate MEC cell proliferation, presumably due to the lack of AR expression in these cells (Fig. 2D).
Fig. 2.
DHT acting on prostate tumor cells further enhances TCM-induced cell proliferation in MECs. In (A,B), MECs were seeded in 96-well plate and treated with TCMs collected from LAPC-4 and LNCaP cells treated with vehicle control (0), or DHT as indicated for 48 hr. In (C) MECs were treated with DHT at doses ranging from 0.1 to 50 nM for 48 hr. The data are the means ± SEM of four independent triplicate experiments. (D) is a representative RT-PCR analysis of mouse AR gene expression in MECs. Mouse testis (mTestis) and tRNA were used as positive and negative control, respectively. **P < 0.01 and *P < 0.05 compared to untreated control; ##P < 0.01 and #P < 0.05 compared to TCM-vehicle control.
To determine whether DHT affects MEC cell proliferation via a paracrine mechanism through the modulation of prostate tumor cells, LAPC-4 or LNCaP cells were treated with vehicle control or various doses of DHT for 48 hr and TCMs were collected and processed as described in the Materials and Methods Section. As shown in Figure 2A, TCMs collected from LAPC-4 cells treated with 1 or 10 nM DHT produced a further 27% (P < 0.01) and 24% (P < 0.01) increase in MEC viable cell number compared to vehicle-treated LAPC-4 TCM, respectively. Similar effects were observed for TCMs collected from LNCaP cells treated with 0.1, 1, or 10 nM DHT (Fig. 2B).
Concomitant Administration of αE2 and βE2 With DHT in LAPC-4 Cells Inhibitedthe Paracrine Effectof DHT on Stimulation of MEC Cell Proliferation
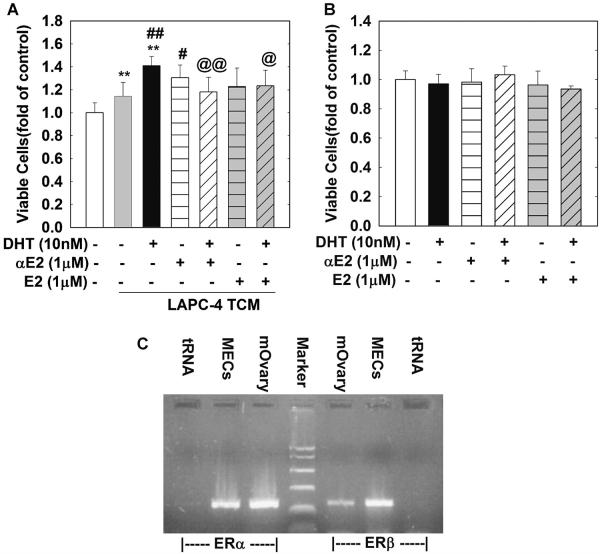
Our previous studies clearly demonstrated that both αE2 and βE2 inhibit DHT-induced gene expression and cell growth in LAPC-4 and LNCaP cells [24,25]. To assess whether αE2 and βE2 can attenuate DHT-enhanced MEC cell growth through a paracrine mechanism by acting on LAPC-4 and LNCaP cells, MECs cells were treated with TCMs from LAPC-4 cells treated with DHT (10 nM), αE2 (1 μM) or βE2 (1 μM) alone or in combination for 48 hr. As shown in Figure 3A, TCM from LAPC-4 cells treated with DHT (10 nM) produced an approximately 41% increase in viable cell number compared to control CM (P < 0.01), and a 27% increase compared to TCM from LAPC-4 cells treated with vehicle control (P < 0.01). This DHT effect was significantly inhibited by the concomitant treatment of LAPC-4 cells with either αE2 (1 μM, P < 0.01) or βE2 (1 μM, P < 0.01). Moreover, both βE2 (1 μM) and αE2 (1 μM) failed to directly alter cell proliferation in MECs (Fig. 2B) even though estrogen receptor a and b were expressed in MECs (Fig. 3C).
Fig. 3.
Co-administration of αE2 or βE2 with DHT in LAPC-4 cells inhibits DHT enhancement of TCM-induced MEC cell proliferation. In (A) MECs were plated in 96-well plates and treated for 48 hr with TCMs collected from LAPC-4 cells treated with a vehicle control, or αE2 (1 μM), or βE2 (1 μM) in the presence or absence of DHT as indicated. In (B) MECs were plated in 96-well plates and treated with vehicle control, DHT (10 nM), αE2 (1 μM), or βE2 (1 μM) alone or in combination for 48 hr. (C) is a representative RT-PCR analysis of mouse ERα and ERβ expression in MECs. Mouse ovary (mOvary) and tRNA were used as positive and negative control, respectively. The data are the means ± SEM SEM of three independent triplicate experiments. **P < 0.01 compared to vehicle control; ##P < 0.01 and #P < 0.05 compared to TCM-vehiclegroup; and @@P < 0.01 and @P < 0.05 compared to TCM-DHT10 nM group.
VEGF in TCM Was a Major Factor Responsible for the Paracrine Stimulation of MEC Cell Proliferation by TCM
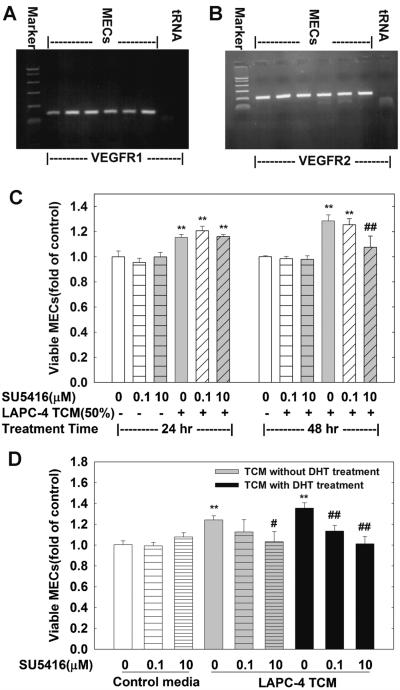
To elucidate what factor(s) in TCM might stimulate MEC cell proliferation, the levels of VEGF in TCM were determined since VEGF is a major growth factor of endothelial cells. The VEGF concentrations in TCM were increased with an increase in cell culture duration of LAPC-4 cells (Table II). The levels of VEGF are 12.43 and 28.13 ng/ml in TCM collected from 24 and 48-hr LAPC-4 cell cultures. Moreover, both VEGF receptor 1 (VEGFR1) and 2 (VEGFR2) were expressed in MECs (Fig. 4A,B), suggesting that VEGF may induce MECs cell proliferation through acting on VEGFR1 and 2.
Fig. 4.
SU5416 blocks TCM-induced MEC cell growth. (A,B) are representative RT-PCR analysis of mouse VEGFR1 and VEGFR2 gene expressionin MECs, and tRNA was used as a negative control. In (C) MECs were plated in 96-well plates and treated for 24 or 48 hr with or without various doses of SU5416 and 50% TCM collected from LAPC-4 cells. In (D) MECs were plated in 96-well plates and treated for 48 hr with or without various doses of SU5416 and 50% TCM collected from LAPC-4 cells treated with or without DHT for 48 hr as indicated. The data are the means ± SEM of three independent experiments. P < 0.01 compared to the corresponding vehicle control; and ##P < 0.01, #P < 0.05 compared to the corresponding TCM treatment.
To address the functional importance of VEGF in TCM-induced MECs cell growth, SU5416 (semaxanib), a selective synthetic inhibitor of the VEGFRs, was co-administrated with 50% TCM collected from 48-hr LAPC-4 cell cultures. At doses ranging from 0.1 to 10 μmol/L, SU5146 produced a time- and dose-dependent inhibition of TCM-induced increases in MEC viable cell number (Fig. 4C,D). SU5416 at 10 μmol/L completely inhibited TCM-induced MEC growth at 48 hr, indicating that the TCM-induced cell growth is mediated, at least in part, through the VEGF-VEGFRs pathway in the system.
To determine whether VEGF is also responsible for the DHT paracrine enhancement of MEC cell proliferation, SU5416 was co-administrated with 50% TCM from LAPC-4 cells treated with 10 nM DHT (DHT-TCM) for 48 hr. Similar to the observation with TCM, SU5416 completely blocked DHT-TCM stimulation of MEC cells in a dose-dependent manner as shown in Figure 4D.
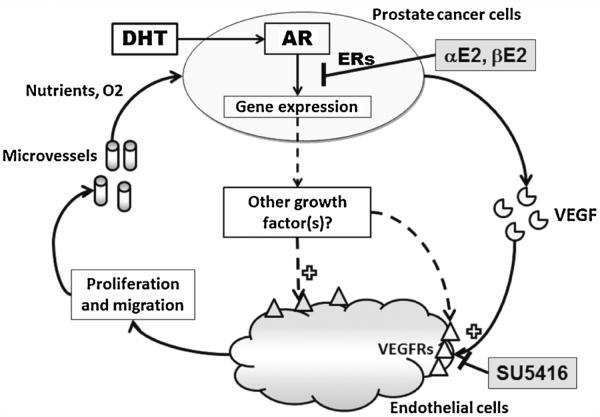
Although VEGF is a major factor responsible for the induction of both TCM and DHT-TCM in MEC growth, the enhancement of MEC growth by DHT-TCM was not associated with an increase in VEGF concentration in DHT-TCM (Tables III and IV). DHT also failed to alter VEGF mRNA levels in both LAPC-4 and LNCaP cells (Table IV). Moreover, treatment with αE2, or βE2 in LAPC-4 and LNCaP cells neither altered the cellular VEGF mRNA levels nor the VEGF concentrations in TCM (Table IV). These data collectively suggest that other factor(s) in addition to VEGF was involved in the paracrine enhancement of MEC cell growth by DHT-TCM, which might be modulated by αE2 and βE2 via inhibition of AR transactivation activity in prostate cancer cells (Fig. 6).
TABLE III.
VEGF Concentrations in TCM Collected From LAPC-4 and LNCaP Cells Treated With or Without DHT
| Treatment | TCM from LAPC-4, VEGF concentration (ng/ml) |
TCM from LNCaP, VEGF concentration (ng/ml) |
|---|---|---|
| Control | 28.10 ± 2.93 | 46.69 ± 5.51 |
| DHT 0.1 nM | N/A | 51.18 ± 8.86 |
| DHT 1 nM | 28.44 ± 2.88 | 53.61 ± 5.85 |
| DHT 10 nM | 31.01 ± 3.39 | 54.01 ± 6.23 |
The data are the mean ± SEM from three independent experiments.
TABLE IV.
The Effects of DHT, αE2, and βE2 on Cellular VEGF mRNA and TCM VEGF Concentrations in LAPC-4 and LNCaP Cells
| LAPC-4 |
LNCaP |
|||
|---|---|---|---|---|
| Treatment | VEGF mRNA level (fold of control) |
VEGF in TCM (ng/ml) |
VEGF mRNA level (fold of control) |
VEGF in TCM (ng/ml) |
| Control | 1.00 ± 0.01 | 21.56 ± 1.38 | 1.00 ± 0.03 | 46.51 ± 6.27 |
| DHT 10 nM | 1.10 ± 0.08 | 22.34 ± 1.09 | 1.00 ± 0.18 | 53.78 ± 6.80 |
| αE2 1 μM | 1.18 ± 0.08 | 21.27 ± 1.96 | 0.90 ± 0.05 | 39.58 ± 2.36 |
| βE2 1 μM | 1.08 ± 0.06 | 22.19 ± 1.46 | 0.90 ± 0.09 | 53.53 ± 6.82 |
| αE2 + DHT | 1.11 ± 0.10 | 21.48 ± 0.62 | 1.12 ± 0.19 | 45.13 ± 5.83 |
| βE2 + DHT | 1.08 ± 0.08 | 24.34 ± 2.05 | 1.03 ± 0.18 | 55.89 ± 6.26 |
The data are the mean ± SEM from three to four independent experiments.
Fig. 6.
An illustration of the paracrine stimulation of endothelial cell proliferation and angiognenisis by prostate cancer cells and its modulation by hormones.
αE2 and βE2 Decreased Microvessel Numbers in Xenograft LAPC-4 or LNCaP Prostate Tumor Tissues
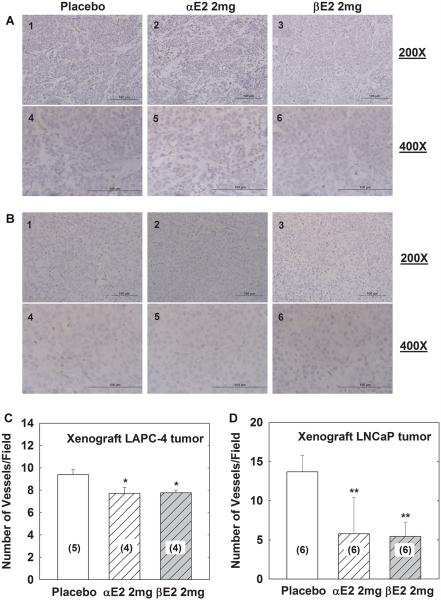
To evaluate the in vivo effectiveness of αE2 and βE2 in modulating tumor angiogenesis, xenograft animals with LAPC-4 or LNCaP prostate tumor were treated with placebo, αE2, or βE2 for 4 weeks as previously described [24,25]. After 4-week treatment, xenograft tumors were dissected, fixed, embedded, sectioned and immunoblotted with anti-CD31 antibody to evaluate angiogenesis in tumor tissues. As shown in Figure 5, treatment with either αE2 or βE2 significantly decreased the microvessel numbers by approximately 16% and 17% compared to placebo in xenograft LAPC-4 tumor tissues(Fig. 5A,C, P < 0.05), and by approximately 58% and 60% compared to placebo in xenograft LNCaP tumor tissues, respectively (Fig. 5B,D, P < 0.01).
Fig. 5.
αE2 and βE2 decrease microvessel number in tumor tissues from xenograft animals with LAPC-4 or LNCaP prostate tumor. (A,B) show representative images in 200× and 400× magnification of CD31 immunohistochemistry of tumor tissues from xenograft animals with LAPC-4 or LNCaP prostatic tumor, respectively. The scale bar on the bottom of each image is 100 μm. The number of microvessels (MV) was quantitated as indicated in the Materials and Methods Section, and the data are presented as mean ± SEM in (C) and (D) for LAPC-4 and LNCaP xenograft tumor tissues, respectively. **P < 0.01 and *P < 0.05 compared to the corresponding placebo group. The number in the parenthesis is the sample size.
Consistent with in vitro cell culture studies, neither αE2 nor βE2 changed VEGF mRNA levels in xenograft LAPC-4 and LNCaP tumor tissues as shown in Table V.
TABLE V.
Effect of αE2 and βE2 on VEGF Gene Expression in Xenograft Tumor Tissues of LAPC-4 or LNCaP Prostate Cancer
| Treatment | Xenograft LAPC-4 tumor tissue, VEGF mRNA level (fold of control) |
Xenograft LNCaP tumor tissue, VEGF mRNA level (fold of control) |
|---|---|---|
| Placebo | 1.00 ± 0.12 (5) | 1.00 ± 0.17 (6) |
| αE2 2 mg | 1.13 ± 0.20 (4) | 1.34 ± 0.27 (6) |
| βE2 2 mg | 1.18 ± 0.09 (4) | 1.07 ± 0.23 (6) |
The data are the mean ± SEM and the number in parenthesis indicates the sample size.
DISCUSSION
Prostate cancer is no longer viewed as a disease of abnormally proliferating epithelial cells alone but rather as multicellular “organ” that consists of a variety of cells as well as a scaffold of noncellular matrix [17]. Different cell crosstalk in the tumor plays a significant role in prostate cancer growth, progression, and metastasis [17]. Angiogenesis or new vessel formation, involving in endothelial cell proliferation and migration, can be activated by the tumor cells via secretory paracrine factors [30], or direct cell transformation [31], which is essential for tumor cell survival, growth and metastasis through providing nutrients and oxygen (Fig. 6) [1]. In the present study, we have demonstrated that prostate tumor cells are able to stimulate endothelial cell proliferation through a paracrine mechanism (Fig. 1). Moreover, androgens and estrogens can indirectly regulate endothelial cell growth via interacting in prostate tumor cells (Figs. 2 and 3).
It is well documented that androgens action via AR plays critical roles in prostate development, growth and pathogenesis of benign prostate hyperplasia and prostate cancer [32]. Studies have also demonstrated a link between androgen action and angiogenesis in androgen-responsive human prostate cancer xenografts [2,6]. The associated collapse of tumor angiogenesis and reduction of endothelial cells were found after castration, which were normalized by androgen replacement [7-9,33]. Androgen actions on endothelial cell growth/angiogenesis are not only directly mediated by AR, but also by a variety of paracrine factors secreted by surrounding cancer cells and/or stromal cells if the endothelial cells are AR-negative [34]. In our study, we have observed that DHT produced a further enhancement of MEC cell proliferation through prostate tumor cells (Fig. 2). These data support that DHT is able to stimulate AR-negative endothelial cell proliferation through a paracrine mechanism acting on prostate tumor cells.
Targeted therapy to AR may block the androgen action in prostatic microenvironment, which is expected to be clinically promising. Estrogens have been used as an androgen ablation therapy of prostate cancer for more than a half of century, presumably by inhibiting testosterone biosynthesis via a negative feedback of the hypothalamus-pituitary-gonadal axis [22,23]. In addition, our team and others have demonstrated that ER ligands, such as αE2 and βE2, may directly modulate androgen actions in the prostate cell in a receptor-isoform, ligand, and co-regulator specific manner [10,23-25]. In the present study, we have further shown that αE2 and βE2 were able to inhibit DHT enhancement of MEC cell proliferation indirectly through the modulation of DHT actions in prostate cancer cells (Fig. 3A). This inhibitory effect of αE2 and βE2 was further evaluated using xenograft animal models with LAPC-4 and LNCaP prostatic tumors. Treatment with an effective dose of αE2 or βE2 for 4 weeks in mice with either xenograft LAPC-4 or LNCaP tumor produced a significant decrease in the microvessels number in tumor tissues (Fig. 5), as well as in tumor growth and/or circulating PSA concentration and tumor cellular PSA mRNA levels as previously demonstrated [24,25]. Taken together, our previous studies [24,25] and current demonstrations suggest that androgens and estrogens may interact together to directly regulate gene expression and cell proliferation in prostate tumor cells, which further affects endothelial cell growth/angiogenesis in prostate tumors via a paracrine mechanism.
It has been documented that the modulation of DHT actions by αE2 or βE2 in prostate tumor cells is mediated through interacting with ERs [24-26,35]. As ERβ is the predominant ER isoform expressed in LAPC-4 and LNCaP cells while ERα is undetectable [24,25], the effects of αE2 and βE2 could be mainly mediated through ERβ. This concept is supported by findings that prostate epithelial cells are proliferated more rapidly in ERβ knockout mice and in ERβ siRNA transfected LAPC-4 cells [36,37]. Moreover, Qiao et al. have reported that estrogen could directly modulate androgen-induced gene expression via ERβ in cell transfection analysis [24-26].
It is worthy to note that αE2 or βE2 alone, or in combination with DHT did not directly produce any effect on MECs cell growth (Fig. 3A), even though MECs are expressing ERα and ERβ (Fig. 3B). Previous studies have shown that estrogen produced viable effect on endothelial cells [8,26,38,39], a major factor is the tissue-specific expression of estrogen receptor modulators [40,41]. As the profile of estrogen receptor modulators in the MECs is not analyzed, the reason for this discrepancy remains to be elucidated.
How tumor cells affect endothelial cells has been actively investigated. Interactions between epithelial cells and endothelial cells in the normal and malignant prostatic microenvironment involve a number of soluble factors and their receptors, such as VEGF, FGF, and members of the platelet-derived growth factor or TGF-β families [15-17]. Understanding what secretory factor(s) responsible for the paracrine stimulation of endothelial cell proliferation by prostate tumor cells is imperative in the development of strategies and agents for prostate cancer therapy. VEGF, with more than seven family members described to date, is the essential growth factor proven to be specific for both normal and tumor-associated angiogenesis via interacting with a set of cellular membrane tyrosine-kinase receptors, including VEGFR1, VEGFR2, and VEGFR3 [42]. Anti-VEGF therapy using numerous approaches ranging from antibodies that bind and block VEGF or VEGFR2, to small molecules that block VEGF2 kinase activity, and to genetic ablation of VEGF in tumor cells has been shown to ultimately blunt tumor growth [43]. VEGFA, the primary member of VEGF family, can induce endothelial cell proliferation, migration, sprouting and tubule formation through acting on VEGFR1 and VEGFR2 [42,44,45]. In the present study, we demonstrated that both LAPC-4 and LNCaP cells expressed VEGF-A mRNA (Table IV) and a large quantity of VEGF was secreted in the cell culture media (Tables II-IV). Furthermore, co-administration of SU5416, a specific VEGFR inhibitor with a high affinity to VEGFR2 (IC50 of VEGFR1 around 0.2 mol/L and IC50 of VEGFR2 about 0.08 mol/L) [46], resulted in a dose- and time-dependent inhibition of TCM-induced cell proliferation in MECs (Fig. 2C). These data collectively suggest that VEGF secreted from prostate cancer cells stimulates MEC cell proliferation via interacting with VEGFRs, mainly VEGFR2.
VEGF also appears to play a significant role in androgen regulation of vascular growth in the prostate as a reduction of VEGF expression and an induction of endothelial cell apoptosis were observed in prostate tumor tissues following castration [3], and androgen-induced prostate neo-vascularization and prostate regeneration in castrated mice were inhibited by blocking VEGF signals with soluble VEGF receptors [47,48]. Consistent with these reports, we also observed in the present study that blocking VEGF signaling by SU5416 completely inhibited the induction of MEC cell proliferation by DHT-treated TCM (Fig. 4D). However, neither VEGF concentrations in TCM nor mRNA levels in prostate tumor cells were significantly altered by the treatment of DHT, αE2, or βE2 alone or in combination in the present experiment (Tables III and IV) although DHT treatment further enhanced the paracrine action of prostate tumor cells on the induction of endothelial cell proliferation. Moreover, unlike SU5614 that completely blocked both TCM and DHT-TCM induced cell proliferation in MECs (Fig. 4C,D), αE2 and βE2 only blocked the DHT enhancement of TCM-induced endothelial cell proliferation without inhibiting TCM action (Fig. 3A). These data suggest that DHT-TCM induced endothelial cell proliferation involves other paracrine factor(s) in addition to VEGF signaling pathway, and alternatively, DHT-induced paracrine factor(s) also acts through VEGFR2 as illustrated in Figure 6. The identification of this DHT-induced paracrine factor(s) in the prostate tumor cells would further facilitate our understanding of the interaction between tumor cells and endothelial cells, and the functional significance of androgen actions in prostate cancer growth and progress, which is ongoing in our laboratory.
CONCLUSIONS
Our present study has demonstrated that prostate cancer cells can stimulate endothelial cell proliferation/microvessel formation through a paracrine mechanism. Most interestingly, androgens and estrogens via interacting on prostate cancer cells, are able to affect endothelial cell functions even though they lack direct actions. Although VEGF-VEGFR signal pathway is the major or permissive factor in mediating the paracrine action of prostate tumor cells on endothelial cell proliferation, other factor(s) that remains to be identified is involved in the androgen–estrogen regulation of tumor cell and endothelial cell interaction. Since αE2 and βE2 produce anti-angiogenic action in both in vitro and in vivo system, they are warranted further preclinical and clinical investigations as anti-prostate cancer agents.
ACKNOWLEDGMENTS
NIH UL1 RR024996, National Natural Science Foundation of China, 30873126. We are very grateful to Dr. Liu for his technical supervision in immunocytochemistry; to Dr. Charles Sawyer in Memorial Sloan-Kettering Cancer Center for providing LAPC-4 cell line, and to Dr. Steve Gross in Weill Cornell Medical College for providing MECs cell line.
Footnotes
Yuan Zhao present address is A visiting fellow from the Department of Cardiovascular Surgery of the Second Xiangya Hospital, Central South University, Changsha, China.
REFERENCES
- 1.van Moorselaar RJ, Voest EE. Angiogenesis in prostate cancer: Its role in disease progression and possible therapeutic approaches. Mol Cell Endocrinol. 2002;197:239–250. doi: 10.1016/s0303-7207(02)00262-9. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK, Safabakhsh N, Sckell A, Chen Y, Jiang P, Benjamin L, Yuan F, Keshet E. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: Role of vascular endothelial growth factor. Proc Natl Acad Sci USA. 1998;95:10820–10825. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart RJ, Panigrahy D, Flynn E, Folkman J. Vascular endothelial growth factor expression and tumor angiogenesis are regulated by androgens in hormone responsive human prostate carcinoma: Evidence for androgen dependent destabilization of vascular endothelial growth factor transcripts. J Urol. 2001;165:688–693. doi: 10.1097/00005392-200102000-00095. [DOI] [PubMed] [Google Scholar]
- 4.Joseph I, Nelson J, Denmeade S, Isaacs J. Androgens regulate vascular endothelial growth factor content in normal and malignant prostatic tissue. Clin Cancer Res. 1997;3:2507–2511. [PubMed] [Google Scholar]
- 5.Okihara K, Watanabe H, Kojima M. Kinetic study of tumor blood flow in prostatic cancer using power Doppler imaging. Ultrasound Med Biol. 1999;25:89–94. doi: 10.1016/s0301-5629(98)00140-9. [DOI] [PubMed] [Google Scholar]
- 6.Joseph IB, Isaacs JT. Potentiation of the antiangiogenic ability of linomide by androgen ablation involves down-regulation of vascular endothelial growth factor in human androgen-responsive prostatic cancers. Cancer Res. 1997;57:1054–1057. [PubMed] [Google Scholar]
- 7.Franck-Lissbrant I, Haggstrom S, Damber JE, Bergh A. Testosterone stimulates angiogenesis and vascular regrowth in the ventral prostate in castrated adult rats. Endocrinology. 1998;139:451–456. doi: 10.1210/endo.139.2.5683. [DOI] [PubMed] [Google Scholar]
- 8.Shabsigh A, Chang DT, Heitjan DF, Kiss A, Olsson CA, Puchner PJ, Buttyan R. Rapid reduction in blood flow to the rat ventral prostate gland after castration: Preliminary evidence that androgens influence prostate size by regulating blood flow to the prostate gland and prostatic endothelial cell survival. Prostate. 1998;36:201–206. doi: 10.1002/(sici)1097-0045(19980801)36:3<201::aid-pros9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Shabisgh A, Tanji N, D’Agati V, Burchardt M, Rubin M, Goluboff ET, Heitjan D, Kiss A, Buttyan R. Early effects of castration on the vascular system of the rat ventral prostate gland. Endocrinology. 1999;140:1920–1926. doi: 10.1210/endo.140.4.6644. [DOI] [PubMed] [Google Scholar]
- 10.Zhu YS, Imperato-McGinley JL. 5alpha-reductase isozymes and androgen actions in the prostate. Ann N Y Acad Sci. 2009;1155:43–56. doi: 10.1111/j.1749-6632.2009.04115.x. [DOI] [PubMed] [Google Scholar]
- 11.Lissbrant IF, Lissbrant E, Damber JE, Bergh A. Blood vessels are regulators of growth, diagnostic markers and therapeutic targets in prostate cancer. Scand J Urol Nephrol. 2001;35:437–452. doi: 10.1080/003655901753367532. [DOI] [PubMed] [Google Scholar]
- 12.Prins GS, Birch L, Greene GL. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991;129:3187–3199. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- 13.Gao J, Arnold JT, Isaacs JT. Conversion from a paracrine to an autocrine mechanism of androgen-stimulated growth during malignant transformation of prostatic epithelial cells. Cancer Res. 2001;61:5038–5044. [PubMed] [Google Scholar]
- 14.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 15.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–C970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 16.Schmid MC, Varner JA. Myeloid cell trafficking and tumor angiogenesis. Cancer Lett. 2007;250:1–8. doi: 10.1016/j.canlet.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlou M, Tzelepi V, Efstathiou E. Therapeutic targeting of the prostate cancer microenvironment. Nat Rev Urol. 2010;7:494–509. doi: 10.1038/nrurol.2010.134. [DOI] [PubMed] [Google Scholar]
- 18.Richard C, Kim G, Koikawa Y, Salm SN, Tsujimura A, Wilson EL, Moscatelli D. Androgens modulate the balance between VEGF and angiopoietin expression in prostate epithelial and smooth muscle cells. Prostate. 2002;50:83–91. doi: 10.1002/pros.10035. [DOI] [PubMed] [Google Scholar]
- 19.Ko YJ, Balk SP. Targeting steroid hormone receptor pathways in the treatment of hormone dependent cancers. Curr Pharm Biotechnol. 2004;5:459–470. doi: 10.2174/1389201043376616. [DOI] [PubMed] [Google Scholar]
- 20.Carroll PR, Kantoff PW, Balk SP, Brown MA, D’Amico AV, George DJ, Grossfeld GD, Johnson CS, Kelly WK, Klotz L, Lee WR, Lubeck DP, Mcleod DG, Oh WK, Pollack A, Sartor O, Smith MR, Hart C. Overview consensus statement. Newer approaches to androgen deprivation therapy in prostate cancer. Urology. 2002;60:1–6. doi: 10.1016/s0090-4295(02)01559-5. [DOI] [PubMed] [Google Scholar]
- 21.Knudsen KE, Scher HI. Starving the addiction: New opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15:4792–4798. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bush TL, Barrett-Connor E. Noncontraceptive estrogen use and cardiovascular disease. Epidemiol Rev. 1985;7:89–104. [PubMed] [Google Scholar]
- 23.Oh WK. The evolving role of estrogen therapy in prostate cancer. Clin Prostate Cancer. 2002;1:81–89. doi: 10.3816/cgc.2002.n.009. [DOI] [PubMed] [Google Scholar]
- 24.Qiao Y, Zhang ZK, Cai LQ, Tan C, Imperato-McGinley JL, Zhu YS. 17alpha-estradiol inhibits LAPC-4 prostatic tumor cell proliferation in cell cultures and tumor growth in xenograft animals. Prostate. 2007;67:1719–1728. doi: 10.1002/pros.20656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao Y, Wang L, Cai LQ, Tan C, Imperato-McGinley J, Zhu YS. Inhibition of aberrant androgen receptor induction of prostate specific antigen gene expression, cell proliferation and tumor growth by 17alpha-estradiol in prostate cancer. J Urol. 2011;185:305–314. doi: 10.1016/j.juro.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu YS, Cai LQ, You X, Cordero JJ, Huang Y, Imperato-McGinley J. Androgen-induced prostate-specific antigen gene expression is mediated via dihydrotestosterone in LNCaP cells. J Androl. 2003;24:681–687. doi: 10.1002/j.1939-4640.2003.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 27.Williams RL, Courtneidge SA, Wagner EF. Embryonic lethalities and endothelial tumors in chimeric mice expressing polyoma virus middle T oncogene. Cell. 1988;52:121–131. doi: 10.1016/0092-8674(88)90536-3. [DOI] [PubMed] [Google Scholar]
- 28.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol. 2008;294:H1530–H1540. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai J, Hong Y, Weng C, Tan C, Imperato-McGinley J, Zhu YS. Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. Am J Physiol Heart Circ Physiol. 2011;300:H1210–H1221. doi: 10.1152/ajpheart.01210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Cozzi PJ. Angiogenesis as a strategic target for prostate cancer therapy. Med Res Rev. 2010;30:23–66. doi: 10.1002/med.20161. [DOI] [PubMed] [Google Scholar]
- 31.Niu YN, Xia SJ. Stroma-epithelium crosstalk in prostate cancer. Asian J Androl. 2009;11:28–35. doi: 10.1038/aja.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu YS. Molecular basis of steroid action in the prostate. Cellsci Rev. 2005;1:27–55. doi: 10.1901/jaba.2005.1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.English HF, Drago JR, Santen RJ. Cellular response to androgen depletion and repletion in the rat ventral prostate: Autoradiography and morphometric analysis. Prostate. 1985;7:41–51. doi: 10.1002/pros.2990070106. [DOI] [PubMed] [Google Scholar]
- 34.Wang GM, Kovalenko B, Huang Y, Moscatelli D. Vascular endothelial growth factor and angiopoietin are required for prostate regeneration. Prostate. 2007;67:485–499. doi: 10.1002/pros.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu YS, Cai LQ, Huang Y, Fish J, Wang L, Zhang ZK, Imperato-McGinley JL. Receptor isoform and ligand-specific modulation of dihydrotestosterone-induced prostate specific antigen gene expression and prostate tumor cell growth by estrogens. J Androl. 2005;26:500–508. doi: 10.2164/jandrol.05002. 509–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson JA. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci USA. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling S, Komesaroff P, Sudhir K. Cellular mechanisms underlying the cardiovascular actions of oestrogens. Clin Sci (Lond) 2006;111:107–118. doi: 10.1042/CS20050084. [DOI] [PubMed] [Google Scholar]
- 39.Morales DE, McGowan KA, Grant DS, Maheshwari S, Bhartiya D, Cid MC, Kleinman HK, Schnaper HW. Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation. 1995;91:755–763. doi: 10.1161/01.cir.91.3.755. [DOI] [PubMed] [Google Scholar]
- 40.Kawagoe J, Ohmichi M, Tsutsumi S, Ohta T, Takahashi K, Kurachi H. Mechanism of the divergent effects of estrogen on the cell proliferation of human umbilical endothelial versus aortic smooth muscle cells. Endocrinology. 2007;148:6092–6099. doi: 10.1210/en.2007-0188. [DOI] [PubMed] [Google Scholar]
- 41.Zaitseva M, Yue DS, Katzenellenbogen JA, Rogers PA, Gargett CE. Estrogen receptor-alpha agonists promote angiogenesis in human myometrial microvascular endothelial cells. J Soc Gynecol Investig. 2004;11:529–535. doi: 10.1016/j.jsgi.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 43.Aragon-Ching JB, Dahut WL. VEGF inhibitors and prostate cancer therapy. Curr Mol Pharmacol. 2009;2:161–168. doi: 10.2174/1874467210902020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gale NW, Thurston G, Davis S, Wiegand SJ, Holash J, Rudge JS, Yancopoulos GD. Complementary and coordinated roles of the VEGFs and angiopoietins during normal and pathologic vascular formation. Cold Spring Harb Symp Quant Biol. 2002;67:267–273. doi: 10.1101/sqb.2002.67.267. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson U, Alitalo K. Structure, expression and receptor-binding properties of novel vascular endothelial growth factors. Curr Top Microbiol Immunol. 1999;237:41–57. doi: 10.1007/978-3-642-59953-8_3. [DOI] [PubMed] [Google Scholar]
- 46.Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O’Reilly T, Persohn E, Rosel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch KH, Schneider MR, Drevs J, Martiny-Baron G, Totzke F. PTK787/ ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178–2189. [PubMed] [Google Scholar]
- 47.Wang GM, Kovalenko B, Wilson EL, Moscatelli D. Vascular density is highest in the proximal region of the mouse prostate. Prostate. 2007;67:968–975. doi: 10.1002/pros.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lissbrant IF, Hammarsten P, Lissbrant E, Ferrara N, Rudolfsson SH, Bergh A. Neutralizing VEGF bioactivity with a soluble chimeric VEGF-receptor protein flt(1-3)IgG inhibits testosterone-stimulated prostate growth in castrated mice. Prostate. 2004;58:57–65. doi: 10.1002/pros.10312. [DOI] [PubMed] [Google Scholar]