Abstract
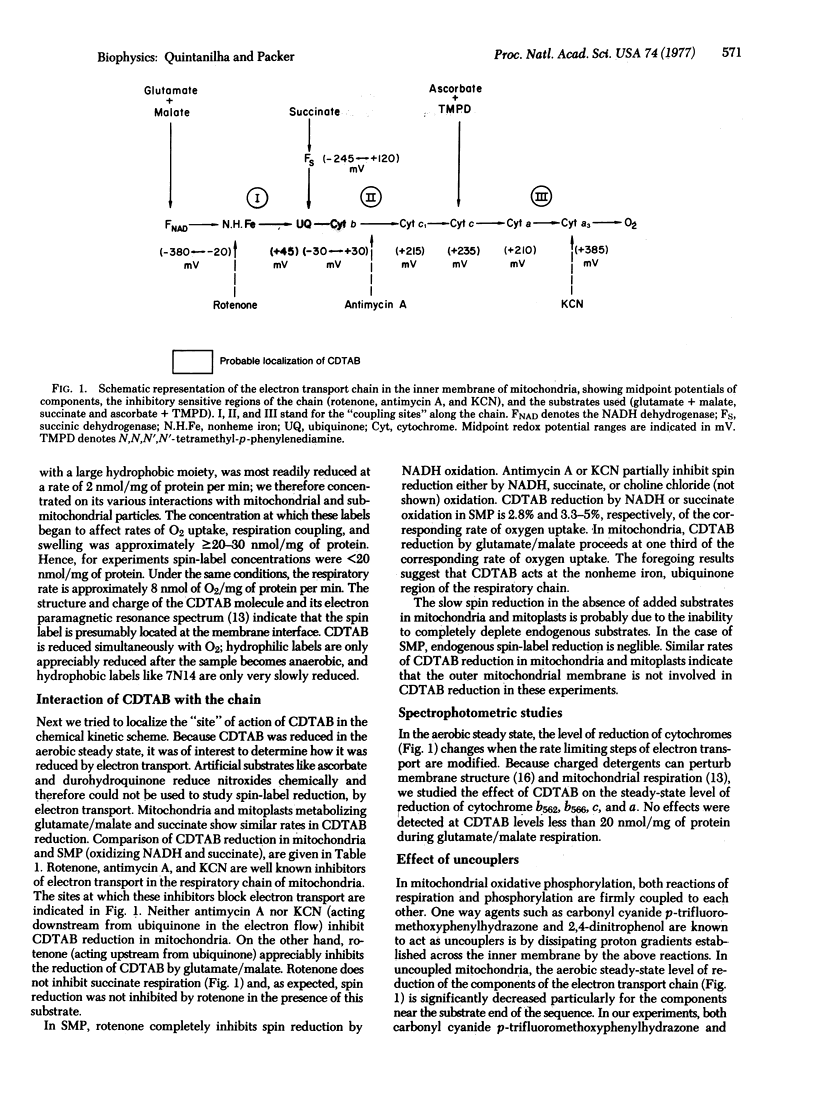
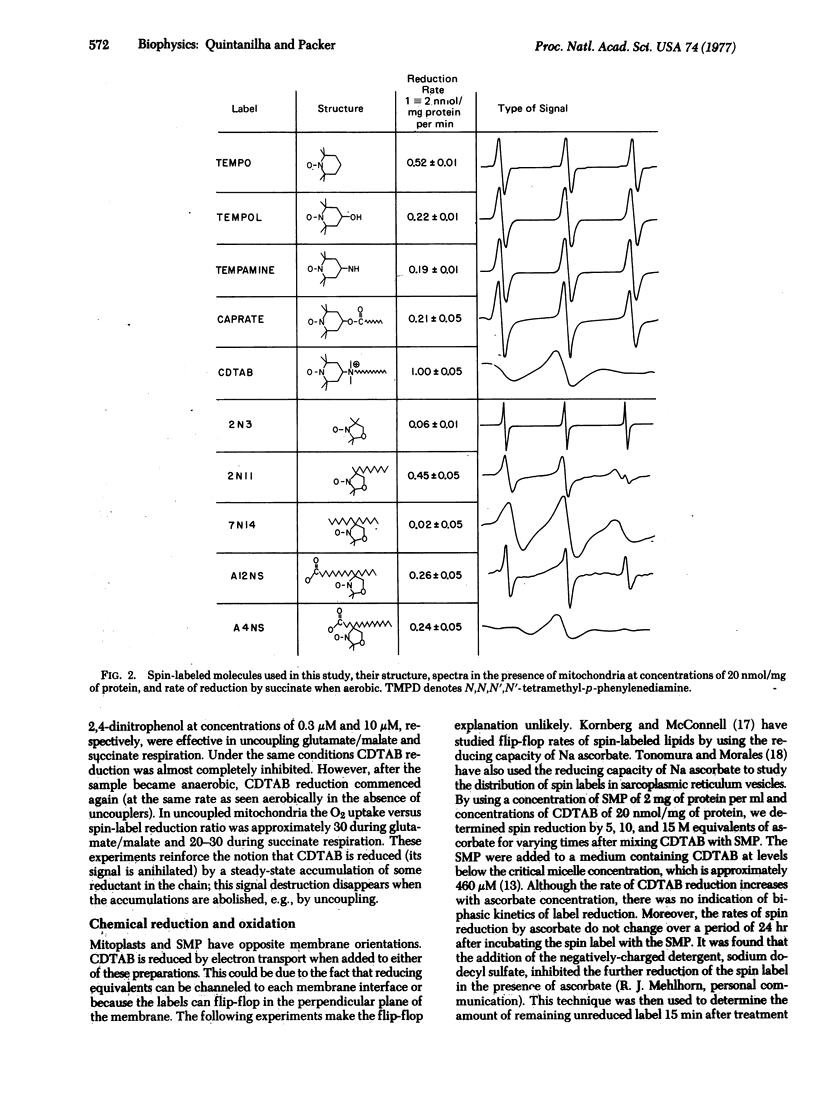
The relative rates of reduction of several spin-labeled molecules that partition differently across the hy-drophobic-interface of inner membranes from rat liver mitochondria were investigated. Spin labels localized either deep in the hydrophobic region or in the aqueous phase are only slowly reduced; however a spin-labeled analogue of the cationic detergent cetyltrimethylammonium bromide that partitions at the interface is rapidly reduced by coupled electron transport. Chemical studies on the reduction and oxidation of the spin label show that loss of signal is due to reduction and not destruction of the label. No evidence was found for flip-flop of the label in submitochondrial preparations. Spin reduction of respiring mitochondria, mitoplasts, or inverted submitochondrial preparations is inhibited by rotenone but is relatively insensitive to antimycin A and KCN. Because the midpoint potentials of the spin labels were found to be similar to that of ubiquinone, it is concluded that reducing equivalents of mitochondrial electron transport from this region of the chain are channeled to either membrane interface.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Bragadin M. A., Tamburro A. M., Santato M. Site-directed spin labeling of the mitochondrial membrane. Synthesis and utilization of the adenosine triphosphatase inhibitor (N-(2, 2, 6, 6-tetramethyl-piperidyl-1-oxyl)-N'-(cyclohexyl)-carbodiimide). J Biol Chem. 1973 Aug 10;248(15):5520–5526. [PubMed] [Google Scholar]
- Baldassare J. J., Robertson D. E., McAfee A. G., Ho C. A spin-label study of energy-coupled active transport in Escherichia coli membrane vesicles. Biochemistry. 1974 Dec 3;13(25):5210–5214. doi: 10.1021/bi00722a025. [DOI] [PubMed] [Google Scholar]
- FLEISCHER S., BRIERLEY G., KLOUWEN H., SLAUTTERBACK D. B. Studies of the electron transfer system. 47. The role of phospholipids in electron transfer. J Biol Chem. 1962 Oct;237:3264–3272. [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Ohnishi T. Properties of the S-3 iron-sulphur centre of succinate dehydrogenase in the intact respiratory chain of beef heart mitochondria. FEBS Lett. 1975 Jun 15;54(2):167–171. doi: 10.1016/0014-5793(75)80067-6. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., McConnell H. M. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 1971 Mar 30;10(7):1111–1120. doi: 10.1021/bi00783a003. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mehlhorn R. J., Packer L. Inactivation and reactivation of mitochondrial respiration by charged detergents. Biochim Biophys Acta. 1976 Mar 12;423(3):382–397. doi: 10.1016/0005-2728(76)90195-x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 1975 Aug 1;56(1):1–6. doi: 10.1016/0014-5793(75)80098-6. [DOI] [PubMed] [Google Scholar]
- Palmer J. M., Hall D. O. The mitochondrial membrane system. Prog Biophys Mol Biol. 1972;24:125–176. doi: 10.1016/0079-6107(72)90006-5. [DOI] [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H., Schepler K. L., Dunham W. R., Sands R. H. Interaction of ubisemiquinone with a paramagnetic component in heart tissue. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2886–2890. doi: 10.1073/pnas.72.8.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancliff R. C., Williams M. A., Utsumi K., Packer L. Essential fatty acid deficiency and mitochondrial function. Arch Biochem Biophys. 1969 May;131(2):629–642. doi: 10.1016/0003-9861(69)90438-x. [DOI] [PubMed] [Google Scholar]
- Tinberg H. M., Melnick R. L., Maguire J., Packer L. Studies on mitochondrial proteins. II. Localization of components in the inner membrane: labeling with diazobenzenesulfonate, a non-penetrating probe. Biochim Biophys Acta. 1974 Apr 12;345(1):118–128. doi: 10.1016/0005-2736(74)90251-x. [DOI] [PubMed] [Google Scholar]
- Tinberg H. M., Packer L., Keith A. D. Role of lipids in mitochondrial energy coupling: evidence from spin labeling and freeze-fracture electron microscopy. Biochim Biophys Acta. 1972 Nov 17;283(2):193–205. doi: 10.1016/0005-2728(72)90235-6. [DOI] [PubMed] [Google Scholar]
- Tonomura Y., Morales M. F. Change in state of spin labels bound to sarcoplasmic reticulum with change in enzymic state, as deduced from ascorbate-quenching studies. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3687–3691. doi: 10.1073/pnas.71.9.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Pereira J., Mehlhorn R., Keith A. D., Packer L. Changes in membrane lipid structure of illuminated chloroplasts: studies with spin-labeled and freeze-fractured membranes. Arch Biochem Biophys. 1974 Jan;160(1):90–99. doi: 10.1016/s0003-9861(74)80012-3. [DOI] [PubMed] [Google Scholar]


