Abstract
Background
Complement activation plays a role in pathogenesis of the Antiphospholipid Syndrome (APS), but the involvement of the C5b-9 membrane attack complex (MAC) is unknown. Here we studied the effects of human polyclonal antiphospholipid (aPL) antibodies on thrombosis and tissue factor (TF) up-regulation in C6 deficient (C6-/-) mice.
Methods
C6-/- or the wild-type (C3H/HeJ) C6+/+ mice were injected twice with IgG-APS (n=2) or IgM-APS (n=1) isolated from APS patients or with the corresponding control Igs (IgG-NHS or IgM-NHS). Then, the size of induced thrombi in the femoral vein were determined 72 hours after the first injection. Tissue factor was determined in homogenates of carotid arteries and in peritoneal macrophages.
Results
Thrombus sizes were significantly larger in C6+/+ treated with IgG-APS1 or with IgG-APS2 or with IgM-APS when compared with C6+/+ mice treated with IgG-NHS or with IgM-NHS, respectively. The sizes of thrombi were significantly smaller in the C6-/- mice injected with IgG-APS1, IgG-APS2 or IgM-APS (p<0.001), compared to their C6+/+ counterparts showing an important abrogation of thrombus formation in mice lacking C6. The TF expression and activity in the C6-/- mice treated with IgG-APS were diminished when compared to C6+/+ treated with the same immunoglobulins. All mice injected with IgG-APS and IgM-APS had medium-high titers of aCL and aβ2GPI antibodies.
Conclusions
These data indicate that the C6 component of the complement system mediates aPL-thrombogenic effects, underscoring an important pathogenic mechanism and indicating the possibility of inhibiting complement to ameliorate APS-related manifestations.
Introduction
The Antiphospholipid syndrome (APS) is a systemic autoimmune and inflammatory disease characterized by hypercoagulability, venous and/or arterial thromboses, pregnancy morbidity, in association with antiphospholipid antibodies (aPL), namely anticardiolipin antibodies (aCL) and/or anti-β2glycoprotein I (aβ2GPI) antibodies and/or a positive lupus anticoagulant (LA) test (1,2).
The pathogenic mechanisms of aPL-induced thrombosis are incompletely understood. APL are a heterogeneous group of antibodies that have been shown to be pathogenic in vitro and in vivo (3). Passive transfer of IgG from aPL-positive sera (IgG-APS) has been found to induce fetal loss, thrombosis and EC activation in mice, suggesting a direct pathogenic role (3-5). The data strongly suggest that in vitro aPL induce a pro-inflammatory and pro-coagulant effect on ECs and monocytes, as measured by expression of tissue factor (TF) and adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1), intercellular cell adhesion molecule-1 (ICAM-1) and E-selectin (E-sel) and platelets (enhanced activation and aggregation, thromboxane production, etc) and that these effects are mediated by p38 mitogen activated protein kinase (p38MAPK) in ECs, in monocytes and in platelets (3,6-15). These effects also require activation of nuclear factor-κ B (NF-κB) in ECs and monocytes and involve the direct interaction of β2GPI /aPL complexes with membrane receptors (i.e. TLR-4, annexin A2, Apolipoprotein E Receptor 2 (APOER2), etc). (16-21).
Two complement effector pathways are initiated by cleavage of C5: C5a and C5b, which leads to formation of the C5b-9 MAC. It is well established that activated complement fragments themselves have the capacity to bind and activate ECs, as well as to induce a prothrombotic phenotype either directly through C5b-9 MAC or through C5a receptor (C5aR)-mediated effects (22,23). Furthermore C5a and the C5b-9 MAC complex have been shown to bind to ECs and to induce TF expression and exert procoagulant effects (24,25). In addition, both complement products have been shown to activate NF-κB and p38 MAPK in various cell types (26,27). Studies performed in rats have shown that CD59, an inhibitor of C5b-9 assembly and insertion, serves a protective role in a rat model of thrombotic microangiopathy, demonstrating that C5b-9 plays a critical role in the pathogenesis of thrombosis (28).
Complement activation – involving specifically C3 and C5 – has been shown to contribute to aPL-mediated thrombosis and pregnancy loss in mice (29-34). In previous studies, our group showed that C5 activation is required for aPL-mediated thrombogenic and pro-inflammatory effects in vivo, utilizing C3 and C5 and C5a receptor deficient mice and an anti-C5 monoclonal antibody (anti-C5MoAb) (30,32). These effects were seen only when injecting in the mice complement-fixing aPL antibodies (32). However, whether C5b-9 MAC mediates aPL-prothrombotic effects in vivo is not known. Hence, here we addressed that question by examining whether thrombus formation and TF upregulation induced by aPL antibodies are affected in C6 deficient -/- mice treated with human polyclonal IgG or IgM aPL antibodies isolated from APS patients.
Material and Methods
Purification and Characterization of Immunoglobulins with APL Activity and Controls
Sera from three individuals with primary APS who fulfilled the Sapporo revised criteria (2) were used to isolate IgG and IgM with aCL and aβ2GPI activity (IgG-APS and IgM-APS, respectively). Clinical and laboratory characteristics of the APS patients are shown in Table 1. Pooled sera from ten (n=10) healthy donors [Normal Human Serum, (NHS)] was used as source of control IgG and IgM (IgG-NHS and IgM-NHS, respectively). All research subjects who donated serum signed an informed consent that was approved by the Institutional Review Board of the University of Texas Medical Branch.
Table 1.
Clinical and Laboratory Characteristics of the Patients Used as Source of immunoglobulins for the Study.
| Patient | Sex/age | Thrombosis Site (s) | Pregnancy losses | aCL IgG/IgM (GPL/MPL) | aβ2GPI IgG/IgM (SGU/SGM) | LA |
|---|---|---|---|---|---|---|
| IgG-APS1 | M/58 | 3DVT, CAPS | N/A | 134 | 135 | (+) |
| IgG-APS2 | F/34 | CAPS | No | 125 | 203 | (+) |
| IgM-APS | F/33 | N/A | Yes | 189 | 203 | (+) |
IgG-APS= Immunoglobulins obtained from patient with antiphospholipid antibodies IgG; aCL= anticardiolipin antibodies; aβ2GP I = anti-β2 Glycoprotein I antibodies; LA= lupus anticoagulant activity; GPL= standard G units for aCL; MPL= standard M units for aCL; SGU=standard G units aβ2GPI; SMU= standard units M for aβ2GPI; M=male; F=female; DVT=deep vein thrombosis; CAPS: catastrophic antiphospholipid syndrome.
IgG-APS IgG-NHS, IgM-APS and IgM-NHS were isolated from the serum of patients and controls by ion exchange chromatography using a DEAE-Sepharose CL-6B column (GE Healthcare Bio-Sciences Corp, Piscataway, New Jersey, USA), as described elsewhere (10).
The isolated IgG and IgM fractions were determined to be free of endotoxin contamination by the limulus amebocyte lysate assay (E-toxate; Sigma-Aldrich Co, St. Louis, MO). Protein concentrations were determined by the Bradford method (35).
Finally, the aCL and aβ2GPI activities in human sera, in the isolated human Igs and in the sera of the mice after treatment, were determined using an in house method for the aCL (the result were expressed in standard GPL and MPL units) (36) and by using a commercial kit for the detection of aβ2GPI antibodies (INOVA Diagnostics, Inc. San Diego, CA) (the results were expressed in SGU and SMU units).
The complement fixing activities of the IgG-APS, IgM-APS or IgG-NHS, IgM-NHS were determined by ELISA as described (37). Briefly, the Ig fractions were incubated onto a plate coated with β2GPI followed by addition of purified C1q (Quidel, San Diego, CA) at the concentration of 20 μg/ml for 60 minutes at 37°C. Then, the bound C1q was detected by its reaction with goat anti-C1q IgG labeled with biotin followed by alkaline phosphatase- streptavidin labeled rabbit anti-goat IgG (Sigma-Aldrich). The OD values of the control group did not exceed 0.3.
In vivo Experiments: Analysis of Thrombus Dynamics
C6-/- mice were kindly provided by Drs. Salmon and Girardi (Cornell University, New York, NY). C6+/+ (C3H/HeJ) mice were purchased from Jackson Laboratories (Bar Harbor, MA). All the animals were handled by trained personnel according the Institutional Animal Care and Use Committee guidelines and housed in the animal care facilities of the University of Texas Medical Branch. Mice weight was approximately 20g.
Five animals per group were injected intraperitoneally (i.p.) twice (at time 0 and 48 hours later) with 500 μg of IgG-APS or IgM-APS, or with the corresponding controls (IgG-NHS or IgM-NHS). Surgical procedures were performed to study thrombus dynamics 72 hours after the first injection.
Analysis of thrombus dynamics in the mice was assessed as described elsewhere after treatment (3,6,10,16,19-21,30,32,38). Briefly, mice were anesthetized and the right femoral vein was exposed and pinched using a standard pressure to introduce injury. Clot formation and dissolution in the trans-illuminated vein were visualized and recorded with a microscope attached to a close-circuit video system. Thrombus size (expressed in μm2) was measured three times and the mean thrombus area was computed for each group of animals. At the end of the surgical procedures a sample of blood was obtained from the mice to determine the aCL and aβ2GPI titers, as described in previous section.
Tissue Factor Activity in Carotid Homogenates and Peritoneal Macrophages
At the end of the surgical procedures, uninjured carotids were dissected and collected from each animal and placed into Tris-buffered saline-0.01% Triton X-100 buffer containing heparin (39). Peritoneal macrophages were collected by flushing the peritoneal cavity with phosphate buffered saline solution (PBS) for 5 minutes.. Then, carotids and macrophages were homogenized by sonication and the TF activity was determined using a commercial chromogenic assay (Antichrome TF; American Diagnostica, Stamford, CT)) and the results were normalized using the protein concentration as reference and expressed in pM/mg/ml.
Tissue Factor Expression by ex vivo Quantum dot Nanocrystals and Dual Photon Excitation Laser Scanning Microscopy in Peritoneal Macrophages
C6-/- mice or their corresponding wild-type (C3H/Hej) in groups of two were injected, with IgG-APS or with IgM-APS or with the corresponding controls IgG-NHS or IgM-NHS. In addition, C6-/- and C3H/HeJ mice were injected with 100μg lipopolysaccharides (LPS; Sc-3535 Santa Cruz Biotechnology) as a positive control or with sterile PBS (GIBCO, Invitrogen™) as a negative control, four hours before euthanasia.
Peritoneal macrophages were obtained after euthanasia by washing the peritoneal cavity with 5 ml of Dulbecco's Modified Eagle Medium (DMEM, GIBCO, Grand Island, NY) supplemented with polymyxin B (final concentration 20μg/ ml). The liquid obtained from the peritoneal cavity was centrifuged at 500g for 10 min at 4 °C. The pellet obtained was suspended in 50 μL of ice-cold buffer and then the cell suspension was placed on a sterile slide and into a sterile Petri plate, subsequently covered and incubated at 37°C at 5% CO2 for 60 minutes to allow the adherence of macrophages on surface. Non-adherent cells and medium were removed and washed twice with 1 ml of PBS, then cells were fixed with Buffered formaldehyde-Fresh 4% (Fischer Scientific) for 10 minutes at room temperature. The preparations were finally washed twice with 1ml of PBS and they were blocked with PBS-2% bovine serum albumin (BSA, SIGMA) free proteases for 30 minutes at room temperature to avoid non-specific binding (19).
The quantum dot immunolabeling of the TF was carried out by treatment of the preparations first with a primary antibody [rabbit anti-mouse tissue factor IgG (American Diagnostica)] diluted in PBS-2% BSA (:100 dilution) for 1.5 hours and subsequently incubated with a goat antirabbit IgG conjugated with Qdot 655 (Molecular Probes, Eugene, OR). Staining of the nuclei was done by applying a Hoechst stain (2:1000 in PBS, Molecular Probes) (40,41).
Images of Qdot fluorescence were obtained using a custom built 2-photon microscope consisting of a Zeiss 410 LSM 2- photon excitation laser scanning microscope, equipped with a near- infrared titanium-sapphire femto second laser (Spectra Physics) that was tuned and mode-locked at 750 nm. An image analysis program Metamorph software (Molecular Devices) was used to measure the average fluorescence intensity in several cells.
Statistical analyses
All statistical analyses were done used SIGMA STAT version 3.5 software (Systat Software, Inc.) All data are represented as means ± standard deviations. Comparisons between the study groups were performed with One Way ANOVA, followed by a Tukey's test in TF activity in carotid artery homogenates, TF expression in murine peritoneal macrophages and thrombus size. P values of less than 0.05 were considered statistically significant.
Results
Characterization of the Immunoglobulins Used to Inject the Mice
First, we determined the titers of aCL and aβ2GPI in the Ig preparations. All Igs isolated from APS sera were medium to high positive for aCL and aβ2GPI antibodies. The controls (IgG-NHS and IgM-NHS) were negative for aCL and an aβ2GPI antibodies (Table 2). Then, we examined the ability of the Ig preparations to fix complement after binding to β2GPI-coated plates and as described in the Methods section. While IgG-APS (1 and 2) and IgM-APS plates fixed C1q after binding to β2GPI, IgG-NHS and IgM-NHS did not. (Table 2).
Table 2.
Characterization of the Immunoglobulins Injected into the Mice.
| Immunoglobulin | aCL units | aβ2GPI | *C1q Complement-fixing activity (O.D. units at 405) |
|---|---|---|---|
| IgG-APS1 | 123 GPL | 100 SGU | 0.857 |
| IgG-APS2 | 123 GPL | 120 SGU | 0.948 |
| IgM-APS | 159 MPL | 100 SMU | 0.745 |
| IgG-NHS | < 10 | < 20 | 0.153 |
| IgM-NHS | <10 | <20 | 0.268 |
IgG-APS= IgG obtained from patient with APS
IgM-APS= IgM obtained from patient with APS
aCL= anticardiolipin antibodies
aβ2GP I = anti-β2 Glycoprotein I antibodies
LA= lupus anticoagulant activity
GPL= G phospholipid units
SGU=standard G units
MPL= M phospholipid units
SMU=standard M units
considered positive when >0.3 OD units
C6-/- Mice Are Protected from the Thrombogenic Effects of aPL Antibodies
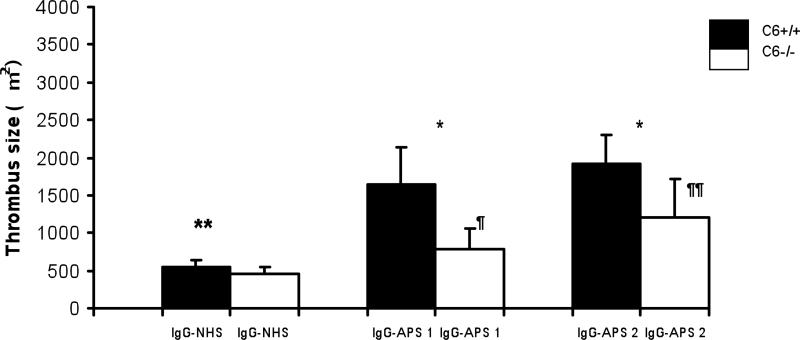
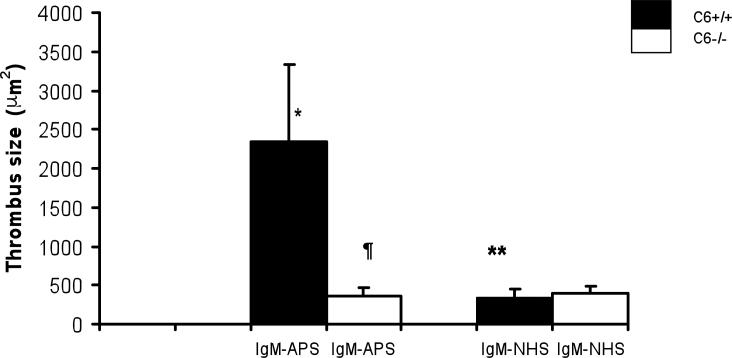
At the time of the surgical procedures the mice injected with IgG-APS or IgM-APS - whether C6 deficient or not - had medium to high titers of aCL and aβ2GPI antibodies,- (>40 SGU/SMU and >40GPL/MPL, respectively) (Tables 3 and 4). Then, we looked at the thrombogenic effects of IgG isolated from APS patients in mice lacking C6. Thrombus sizes were significantly larger in C6+/+ treated with IgG-APS1 or IgG-APS2 when compared with C6+/+ mice treated with IgG-NHS (p <0.001, and p=0.001, respectively). Importantly, the thrombus size was partially but significantly smaller in C6-/- mice injected with IgG-APS1, IgG-APS2 compared to their C6+/+ counterparts (p<0.001, 0.009) (Figure 1). Mice lacking C6 and injected with IgG-APS1 or IgG-APS2 has larger thrombi compared to the same mice (C6-/-) injected with IgG-NHS, (p=0.005 and p=0.002, respectively). Indicating the lack of C6 protected the mice partially from IgG-APS-induced thrombophilia IgM antibodies are known to fix complement with high avidity. Hence, we examined the thrombogenic effects of an IgM isolated from a patient with confirmed APS (IgM-APS), that was only positive for IgM aCL and aβ2GPI antibodies. Similarly to what was observed with IgG-APS, C6 +/+ mice injected with IgM-APS had larger thrombi when compared to the same type of mice injected with IgM-NHS (p<0.001). Importantly, the thrombus sizes in mice lacking C6 treated with IgM-APS were significantly smaller compared to the ones seen in the C6+/+ strain (p<0.001).
Table 3.
aCL and aβ2GPI Activities in Mice treated with IgG-APS and IgG-NHS.
| Strain/Treatment | aβ2GPI (SGU units) mean (± SD) | aCL (GPL units) mean (± SD) |
|---|---|---|
| C6+/+/IgG-APS 1 | 40.59 (± 9.73) | 93.86 (± 14.42) |
| C6+/+/IgG-APS 2 | 105.4 (± 26.6) | 93 (± 23.92) |
| C6-/-/IgG-APS1 | 41.04± 9.45) | 86.35± (21.11) |
| C6-/-/IgG-APS2 | 103.29 (± 4.4) | 97.2 (± 13.9) |
| C6 -/-/IgG-NHS | 3.75 (± 3.36) | 0.191 (± 0.1) |
| C6+/+/IgG-NHS | 2.6 (± 0.45) | 0.10 (± 0.1) |
aCL= anticardiolipin antibodies
aβ2GP I = anti-β2 Glycoprotein I antibodies
GPL: G phospholipid units
SGU=standard G units
Table 4.
aCL and aβ2GPI Activities in Mice treated with IgM-APS and IgM-NHS.
| aCL (MPL Units) mean (± SD) | aβ2GPI SMU mean (± SD) | Strain/Treatment |
|---|---|---|
| 45 (± 9.5) | 24 (± 5) | C6 +/+ /IgM-APS |
| 0.14 (± 0.19) | 0.8 (± 0.2) | C6 +/+ /IgM-NHS |
| 68 (± 25.3) | 62.7 (± 24) | C6 -/- / IgM-APS |
| 0.8 (± 0.85) | 2.4 (± 1.9) | C6-/- /IgM-NHS |
aCL= anticardiolipin antibodies
aβ2GP I = anti-β2 Glycoprotein I antibodies
MPL: M phospholipid units
SMU=standard M units
Figure 1. C6-/- Mice Are Protected from the Thrombogenic Effects of IgG-APS.
Five animals per group were injected i.p. with 500 μg of IgG-APS1 or with IgG-APS2 or with IgG-NHS twice, as described in the Methods section. Thrombus formation was studied after 72 hours of the first injection.
* Statistically significant different between C6-/- treated with IgG-APS1, IgG-APS2 vs. mice C6+/+ treated with IgG-APS1, IgG-APS2 respectively (p = <0.001), (p = 0.009).
** Statistically significant between C6+/+ mice treated with IgG-APS1, IgG-APS2 vs. mice C6+/+ treated with IgG-NHS respectively (p = <0.001 and p = 0.001, respectively).
¶ Statistically significant between C6-/- mice treated with IgG-APS1 vs mice C6-/- treated with IgG-NHS p=(0.005)
¶¶ Statistically significant between C6-/- mice treated with IgG-APS2 vs mice C6-/- treated with IgG-NHS (p=0.002),
Tissue Factor Activity Induced by IgG-APS is Diminished in C6-/- mice
We measured the TF activity on the monocytes and carotids harvested from the experimental animals injected with IgG-APS1 or IgG-NHS. The TF activities in carotid artery homogenates and in peritoneal macrophages in C6+/+ mice after the treatment with IgG-APS were significantly higher compared to the activities seen in the same type of mice treated with IgG-NHS (p=0.013 and p=0.010, respectively) (Table 5). In contrast, C6-/- mice injected with IgG-APS1 showed significantly lower TF activities in carotid artery and peritoneal macrophage preparations (p=0.036 and p=0.013), when, compared to TF activity in the C6+/+ mice under the same conditions (Table 5). However that effect was partial since TF activities in carotid artery homogenates and in the peritoneal macrophages of C6-/- mice injected with IgG-NHS had significantly lower TF activities in carotid and macrophage preparations when compared to mice lacking C6 injected with IgG-APS1 (p=0.01 and p=0.01, respectively)
Table 5.
TF activity in C6-/- mice is abrogated after the treatment with IgG-APS1.
| Mice/Treatment | TF carotids homogenates pM/mg protein (Mean ± SD) | TF macrophages homogenates pM/mg protein (Mean ± SD) |
|---|---|---|
| C6 +/+ /IgG-APS1 | * 51.55 ±5.66 | *28.51 ±13.37 |
| C6 +/+ /IgG-NHS | 17.3 ±0.14 | 12.64 ±4.37 |
| C6 -/- / IgG-APS1 | ¶ 29.95 ±1.8 | ¶ 14.83 ±9.17 |
| C6-/- /IgG-NHS | ¶¶19.25 N/A | ¶¶4.6575 ±1.22 |
Peritoneal macrophages and uninjured carotids were harvested from the experimental animals (five per group) and the TF activity was measured using a commercial kit as described in the methods section.
statistically significant from C6+/+ mice treated with IgG-APS1 vs C6+/+ treated with IgG-NHS (p=0.013 for TF in carotid artery homogenates and p=0.010 for TF in peritoneal macrophages, respectively).
statistically significant different from C6-/- mice treated with IgG-APS1 vs C6+/+ mice treated with IgG-APS1 (p=0.036 for TF in carotid artery homogenates and p=0.013 for peritoneal macrophages, respectively)
statistically significant different from C6-/- mice treated with IgG-APS1 vs C6+/+ mice treated with IgG-NHS (p=0.01 for TF in carotid artery homogenates and p=0.01 for TF in peritoneal macrophages, respectively)
Tissue Factor Expression in Peritoneal Macrophages is Reduced in C6-/- Mice after Treatment with IgG-APS
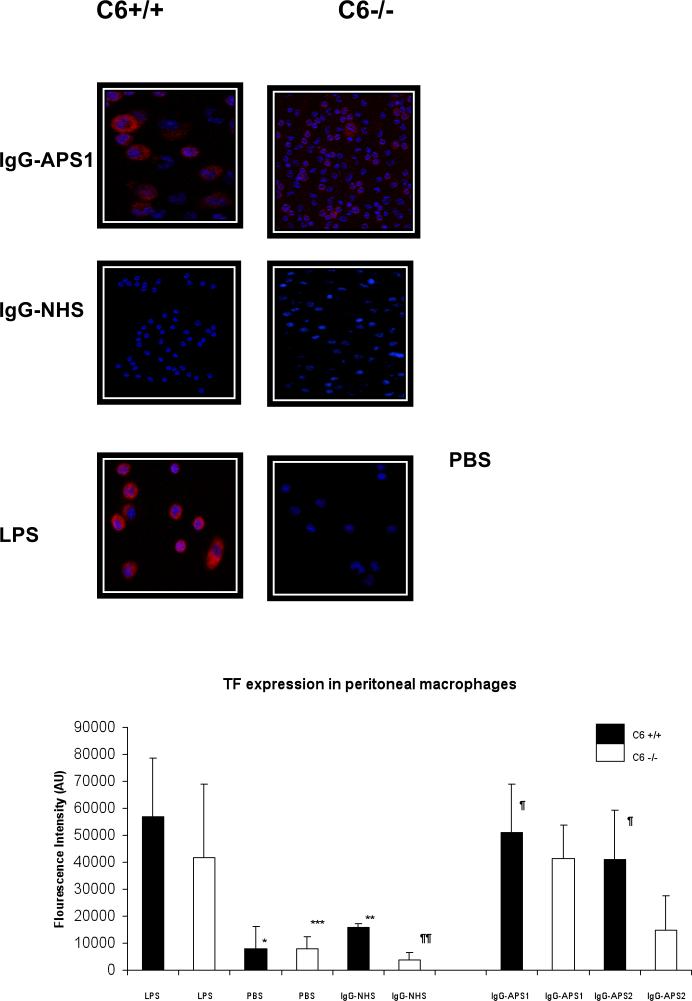
Macrophages were isolated from the peritoneal cavities of the experimental animals after treatment with IgG-APS1, IgG-APS2 or with IgG-NHS or with LPS or with PBS. TF expression in the macrophages was examined ex vivo as described in the Methods section using qdot nanocrystals and dual photon excitation scanning microscopy. Wild-type mice treated with LPS or with IgG-APS1, IgG-APS2 expressed significantly larger amounts of TF on the surface of the macrophages when compared with C6 +/+ mice injected with PBS (p<0.001). In addition, C6+/+ mice had significantly larger amounts of TF when compared to same type of mice injected with control IgG-NHS (p<0.001). C6-/- mice treated with LPS expressed significantly larger amounts of TF on the surface of the macrophages when compared with C6 -/- mice injected with PBS (p<0.001). Importantly, TF expression induced by IgG-APS1 were significantly diminished in C6-/- mice compared between C6+/+ mice treated with IgG-APS1 (p<0.001). However this effect was only partial, since the TF expression in C6-/- mice treated with IgG-NHS was significantly lower when compared to C6-/- mice treated with IgG-APS 1 or with IgG-APS2 (p<0.001) (Figure 3).
Figure 3. Tissue Factor Expression in Murine Peritoneal Macrophages Induced by IgG-APS Is Diminished in Mice Lacking C6.
Macrophages from the peritoneal cavity of the experimental animals were harvested and stained for TF using a specific Qdot bioconjugates and quantified with a dual-photon laser confocal microscope (Methods section). The upper panel shows representative images of TF expression (in red), cell nucleus (blue). Two mice were treated with LPS or PBS used as a controls of the stain.
The lower panel represents the quantitative analysis of the mentioned expression. The TF expression is shown as fluorescence intensity In AU (mean ±SD).
*Statistically significant different from mice injected with LPS vs mice injected with PBS (p<0.001)
¶Statistically significant different from C6+/+ mice injected with IgG-APS1, IgG-APS2 vs C6-/- mice injected with IgG-APS1, IgG-APS2(p<0.001)
¶¶ Statistically significant different between C6-/-mice injected with IgG-APS and C6-/- injected with IgG-NHS (p<0.001)
Discussion
In these studies we demonstrate for the first time that thrombogenic effects of human IgG and IgM from APS patients were significantly diminished in mice that lack C6 and that are subsequently not able to assemble the membrane attack complex, indicating that C5b-9 MAC is involved in aPL-mediated pathogenic effects in vivo. Several studies have shown upregulation of TF in monocytes and EC in vitro and in vivo by aPL antibodies. It is now accepted that TF is a prothrombotic biomarker in APS (7,11,12-15). Importantly, in our studies the decrease in the thrombogenic effect produced by aPL antibodies in C6 deficient mice, was associated with a significant lowering of TF activity in carotid arteries and in macrophages. In addition, the effects of aPL antibodies and controls in the various strains of mice were also studied ex vivo in experiments where TF expression was measured using quantum dot nanocrystal and dual photon excitation laser scanning microscopy, an innovative and state-of-the art technique that enables to determine not only the distribution but also the quantitation of the TF expression in the surface of the cells obtained from the mice (40-43).
The hypothesis that complement activation plays a role in the pathogenesis of aPL antibodies is based on aPL-complement fixing antibodies. Human IgMs are known to fix complement efficiently. However, human IgG subclasses differ in their capacity to fix and activate complement through the classical pathway, IgG4 being non-fixing complement antibodies. Studies have shown that approximately 70-80% of aCL antibodies in APS patient sera are able to fix complement (44). Hence it is possible that not all IgG-APS will fix complement. In a previous study, we showed that C5aR deficient mice were protected from thrombophilia and EC activation in vivo, only when mice were injected with IgM-APS but not when a non-complement fixing IgG-APS was used (32). In agreement with that study, here we showed that the abrogation of pathogenic effects (thrombosis and TF upregulation) was seen in mice lacking C6 that were injected with either IgM-APS or with any of the two IgG-APS - both of which showed C1q-binding activity to β2GPI-coated plates.
The animal model of induced thrombosis utilized in these studies has proven over the years to be effective in testing pathogenic effects of human and mouse monoclonal and polyclonal aPL antibodies in different strains of mice, including several types of knock-out animals (3,6,10,16,19,20,21,30,32,38). In addition, human aPL antibodies can be used in mice since the aminoacid sequence and structure of human and mice β2GPI - the major antigenic target of aPL antibodies - are highly homologous (45).
Interestingly, hypocomplementemia has been found in a significant proportion of patients with primary APS and was associated with thrombosis in one study and with livedo reticularis and thrombocytopenia in another publication (46-48). Recent studies have suggested that activation of the complement cascade is necessary for aPL-mediated thrombophilia and fetal loss (29-34). First, in a study by our group, we found that inhibition of the complement cascade in vivo, using the C3 convertase inhibitor complement receptor 1-related gene protein y (Crry)-Ig, blocks aPL-induced fetal loss and growth retardation and inhibit aPL-mediated thrombosis (31). Furthermore, mice deficient in complement C3 and C5 (C3-/- and C5-/-, respectively) were resistant to thrombosis, EC activation and fetal loss induced by aPL Abs (30). Furthermore, an anti-C5 MoAb reversed thrombogenic properties of aPL Abs in vivo, then confirming that involvement of C5 complement activation in aPL-induced thrombosis (30). It has also been shown that the interaction of complement component 5a (C5a) with its receptor (C5aR) is necessary for thrombosis of placental vasculature (33). Then, it was concluded that complement activation is a necessary intermediary event in the pathogenesis of thrombosis and fetal loss associated with aPL Abs. These findings were confirmed in rats by Fischetti et al, who showed that thrombus formation induced by rat antibodies to β2GPI require a priming factor – such as bacterial lipopolysaccharide (LPS)- and is complement dependent (37).
We propose the following mechanism to explain the pathogenic effects of aPL antibodies on thrombosis. First, aPL Abs bind directly to EC and other target cells such as monocytes and platelets and induce their activation and a procoagulant state, as demonstrated in vivo and in vitro studies. These include upregulation of adhesion molecules and TF expression (6-15). APL antibodies also induce platelet activation and interact with elements of the coagulation cascade (12). Interestingly and in agreement with that proposal, in these studies the abrogation of the thrombogenic effects and of the TF upregulation by the two IgG-APS in C6 deficient mice, though statistically significant - was incomplete, indicating that other pathogenic mechanisms are involved, most likely due to the direct effects of aPL antibodies through receptor(s) in target cells, as described (6-15). Then, activation of the complement cascade by aPL antibodies may amplify these effects by stimulation of the generation of potent mediators of platelet and EC activation, including C5b-9 MAC. Proinflammatory and prothrombotic effects of C5b-9 MAC have shown in numerous studies. Sublytic concentrations of the C5b-9 MAC induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through NF-κB activation (27,49). In addition, sublethal amounts of C5b-9 MAC can activate neutrophils and endothelium by upregulating adhesion molecules a nd promoting the release of cell stimulants such as hydrolytic enzymes, reactive oxygen species, arachidonic acid metabolites and cytokines (23,49). In addition, C5b-9 MAC can enhance procoagulant properties of the endothelium. In fact, C5b-9 MAC is now widely recognized as a potent promoter of coagulation. For example, C5b-9 MAC added to EC in sublytic concentration s induces release of von Willebrand factor, which in turn favors platelet adherence to the vessel wall and promotes the assembly of the prothrombinase by the exposure of phospholipids (38,39). In addition, sublytic C5b-9 MAC has been shown to stimulate the expres sion of TF on EC, which triggers a prothrombotic state through a factor VII-dependent pathway(23,49,50). Membrane deposition of C5b-9 MAC c omplexes has been shown to stimulate production of reactive oxygen metabolite s and leukotriene B4 in polymorphonuclear leukocytes and prostaglandin E2 and thromboxane in macrophages (23). Deposition of C5b-9 MAC and other comple ment proteins onto the vessel wall has been demonstrated in diseases associated with immune vasculitis and in vascular beds on infarcted myocardium and surrounding areas (51). Furthermore, disorders involving intravascular complement activation frequently exhibit thrombotic diatheses, suggesting a linkage between intravascular complement and perturbation of the thrombo-regulatory properties of the endothelium. Hence, binding of complement activation products to EC produces several biological effects enabling these cells to participate in inflammatory processes and to promote coagulation and regulate vascular tone. Promotion of coagulation is another effect exerted by the complement system on the endothelium and altogether these studies underscore the link between complement activation and thrombophilia in inflammatory diseases. Hence, It can be hypothesized that all those proinflammatory and prothrombotic effects of C5b-9 MAC may enhance the direct effects of aPL antibodies on target cells such as EC, monocytes and platelets and contribute to the thrombotic diathesis observed in APS.
In summary, the findings from this study underscore for the first time a novel pathogenic mechanism mediated by aPL antibodies involving the C5b-9 MAC and opens the possibility of utilizing complement system inhibitors for the treatment of clinical manifestation of APS, pending the successful completion of clinical studies.
Figure 2. C6-/- Mice Are Protected from the Thrombogenic Effects of IgM-APS.
Five animals per group were injected i.p. twice with 500μg of IgM-APS or with IgM-NHS . The thrombus dynamics was assessed 72 hours after the first injection, as described in the Methods section.
**Statistically significant difference between mice C6+/+ treated with IgM-APS vs. mice C6+/+ treated with IgM-NHS (p = <0.001),
*Statistically significant difference between mice C6+/+ treated with IgM-APS and C6-/- mice with IgM-APS (p <0.001).
Acknowledgements
This work was supported by an Arthritis Foundation grant, Texas Chapter. AL C-M Salary was paid with a scholarship from Consejo Nacional de Ciencia y Tecnología (CONACyT) from México.
References
- 1.Harris EN. Syndrome of the black swan. Br J Rheumatol. 1987;26:324–326. doi: 10.1093/rheumatology/26.5.324. [DOI] [PubMed] [Google Scholar]
- 2.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 3.Pierangeli SS, Colden-Stanfield M, Liu X, et al. Antiphospholipid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo. Circulation. 1999;99(15):1997–2002. doi: 10.1161/01.cir.99.15.1997. [DOI] [PubMed] [Google Scholar]
- 4.Blank M, Cohen J, Toder V, Shoenfeld Y. Induction of anti-phospholipid syndrome in naive mice with mouse lupus monoclonal and human polyclonal anti-cardiolipin antibodies. Proc Natl Acad Sci USA. 1991;88:3069–3073. doi: 10.1073/pnas.88.8.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branch DW, Dudley DJ, Mitchell MD, et al. Immunoglobulin G fractions from patients with antiphospholipid antibodies cause fetal death in BALB/c mice: a model for autoimmune fetal loss. Am J Obstet. Gynecol. 1990;163:210–216. doi: 10.1016/s0002-9378(11)90700-5. [DOI] [PubMed] [Google Scholar]
- 6.Espinola RG, Liu X, Colden-Stanfield M, Hall J, et al. E-selectin mediates pathogenic effects of antiphospholipid antibodies. J Thromb Haemost. 2002;1:843–848. doi: 10.1046/j.1538-7836.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 7.Vega-Ostertag ME, Casper K, Swerlick R, et al. Involvement of p38 MAPK in the up-regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis RHeum. 2005;52:1545–1554. doi: 10.1002/art.21009. [DOI] [PubMed] [Google Scholar]
- 8.Del Papa N, Guidali L, Sala A, et al. Endothelial cells as target for antiphospholipid antibodies - Human polyclonal and monoclonal anti-β2 glycoprotein I antibodies react in vitro with endothelial cells through adherent β2 glycoprotein I and induce endothelial activation. Arthritis Rheum. 1997;40:551–561. doi: 10.1002/art.1780400322. [DOI] [PubMed] [Google Scholar]
- 9.Simantov R, LaSala JM, Lo SK, et al. Activation of cultured vascular endothelial cells by antiphospholipid antibodies. J Clin Invest. 1995;96:2211–2219. doi: 10.1172/JCI118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierangeli SS, Espinola RG, Liu X, Harris EN. Thrombogenic effects of antiphospholipid antibodies are mediated by intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and P-selectin. Circ Res. 2001;88:245–250. doi: 10.1161/01.res.88.2.245. [DOI] [PubMed] [Google Scholar]
- 11.Cuadrado MJ, López-Pedrera C, Khamashta MA, et al. Thrombosis in primary antiphospholipid syndrome - A pivotal role for monocyte tissue factor expression. Arthritis Rheum. 1997;40:834–841. doi: 10.1002/art.1780400509. [DOI] [PubMed] [Google Scholar]
- 12.Vega-Ostertag M, Harris EN, Pierangeli SS. Intracellular events in antiphospholipid-mediated platelet activation. Arthritis Rheum. 2004;50:2911–2919. doi: 10.1002/art.20434. [DOI] [PubMed] [Google Scholar]
- 13.Forastiero RR, Martinuzzo ME, De Larranaga GF. Circulating levels of tissue factor and proinflammatory cytokines in patients with primary antiphospholipid syndrome or leprous related antiphospholipid antibodies. Lupus. 2005;14:129–136. doi: 10.1191/0961203305lu2048oa. [DOI] [PubMed] [Google Scholar]
- 14.Williams FM, Parmar K, Hughes GR, Hunt BJ. Systemic endothelial cell markers in antiphospholipid syndrome. Thromb Haemost. 2000;84:742–746. [PubMed] [Google Scholar]
- 15.Ferrara DE, Swerlick R, Casper K, et al. Fluvastatin inhibits upregulation of tissue factor expression by antiphospholipid antibodies on endothelial cells. J Thromb Haemost. 2004;2:1558–1563. doi: 10.1111/j.1538-7836.2004.00896.x. [DOI] [PubMed] [Google Scholar]
- 16.Montiel-Manzano G, Romay-Penabad Z, Papalardo de Martinez E, et al. In vivo effects of an inhibitor of nuclear factor-kappa B on thrombogenic properties of antiphospholipid antibodies. Ann N Y Acad Sci. 2007;1108:540–553. doi: 10.1196/annals.1422.057. [DOI] [PubMed] [Google Scholar]
- 17.Dunoyer-Geindre S, de Moerloose P, Galve-de Rochemonteiz B, et al. NFκB is an essential intermediate in the activation of endothelial cells by anti-β2glycoprotein I antibodies. Thromb Haemost. 2002;88:851–857. [PubMed] [Google Scholar]
- 18.Bohgaki M, Atsumi T, Yamashita Y, et al. The p38 mitogen-activated protein kinase (MAPK) pathway mediates induction of the tissue factor gene in monocytes stimulated with human monoclonal anti-β2glycoprotein I antibodies. Int Immunol. 2004;16:1632–1641. doi: 10.1093/intimm/dxh166. [DOI] [PubMed] [Google Scholar]
- 19.Romay-Penabad Z, Montiel-Manzano MG, Shilagard T, et al. Annexin A2 is involved in antiphospholipid antibody-mediated pathogenic effects in vitro and in vivo. Blood. 2009;114:3074–83. doi: 10.1182/blood-2008-11-188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierangeli SS, Vega-Ostertag ME, Raschi E, et al. Toll-like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis. 2007;66(10):1327–33. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romay-Penabad Z, Aguilar-Valenzuela R, Urbanus RT, et al. Apolipoprotein E receptor 2 is involved in the thrombotic complications in a murine model of the antiphospholipid syndrome. Blood. 2011;117:1408–14. doi: 10.1182/blood-2010-07-299099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin ML, Ros HG, Nicolescu FI, et al. Membrane attack by complement assembly and biology of terminal complement complexes. Biomembranes. 1996;4:123–129. [Google Scholar]
- 23.Hansch GM, Seitz M, Betz M. Effect of late complement components C5b-9 on human monocytes: release of prostanoids, oxygen radicals and of a factor inducing cell proliferation. Int Arch Allergy Immujnol. 1987;82:317–320. doi: 10.1159/000234216. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda K, Nagasawa K, Horiuchi T, et al. C5a induces tissue factor activity on endothelial cells. Thromb Haemost. 1997;77:394–398. [PubMed] [Google Scholar]
- 25.Saadi S, Holznecht RA, Patte CP, et al. Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med. 1995;182:1807–1814. doi: 10.1084/jem.182.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan ZK. Anaphylotoxins C5a and C3a induce nuclear factor kappa B activation in human peripheral blood monocytes. Biochim Biophys Acta. 1998;1443:90–98. doi: 10.1016/s0167-4781(98)00198-5. [DOI] [PubMed] [Google Scholar]
- 27.Chiou W, Tsai H, Yang L, Tsai W. C5a differentially stimulates the ERK1/2 and p38 MAPK phosphorylation through independent signaling pathways to induced chemotactic migration in RAW264.7 macrophages. Int Immunopharmacol. 2004;4:1329–1341. doi: 10.1016/j.intimp.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 28. CD59.
- 29.Salmon JE, Girardi G, Holers VM. Complement activation as a mediator of antiphospholipid antibody induced pregnancy loss and thrombosis. Ann Rheum Dis. 2002;61(Suppl 2):ii46–50. doi: 10.1136/ard.61.suppl_2.ii46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierangeli SS, Girardi G, Vega-Ostertag M, et al. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis and Rheumatism. 2005;52(7):2120–4. doi: 10.1002/art.21157. [DOI] [PubMed] [Google Scholar]
- 31.Holers VM, Girardi G, Mo L, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195:211–20. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romay-Penabad Z, Liu XX, Montiel-Manzano G, et al. C5a receptor-deficient mice are protected from thrombophilia and endothelial cell activation induced by some antiphospholipid antibodies. Ann N Y Acad Sci. 2007;1108:554–66. doi: 10.1196/annals.1422.058. [DOI] [PubMed] [Google Scholar]
- 33.Redecha P, Tilley R, Tencati M, et al. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110:2423–31. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10:1222–6. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- 35.Bradford MM. A refined and sensitive method for the Quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 36.Pierangeli SS, Harris EN. A protocol for determination of anticardiolipin antibodies by ELISA. Nat Protoc. 2008;3(5):840–8. doi: 10.1038/nprot.2008.48. [DOI] [PubMed] [Google Scholar]
- 37.Fischetti F, Durigutto P, Pellis V, et al. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005;106:2340–6. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 38.Pierangeli SS, Liu XW, Barker JH, et al. Induction of thrombosis in a mouse model by IgG, IgM and IgA immunoglobulins from patients with the antiphospholipid syndrome. Thromb Haemost. 1995;74:1361–7. [PubMed] [Google Scholar]
- 39.Day SM, Reeve JL, Pedersen B, et al. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105:192–198. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 40.Ferrara DE, Weiss D, Carnell PH, et al. Quantitative 3D fluorescence technique for the analysis of en face preparations of arterial walls using quantum dot nanocrystals and two-photon excitation laser scanning microscopy. Am J Physiol Regulatory Intergrative Comp Physionl. 2006;290:114–123. doi: 10.1152/ajpregu.00449.2005. [DOI] [PubMed] [Google Scholar]
- 41.Chan WC, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 42.Gao X, Nie S. Molecular profiling of single cells and tissue specimens with quantum dots. Trends in Biotech. 2003;21:371–373. doi: 10.1016/S0167-7799(03)00209-9. [DOI] [PubMed] [Google Scholar]
- 43.Chan WC, Maxwell DJ, Gao X, et al. Luminescent quantum dots for multiplexed biological detection and imaging. Curr Op Biotech. 2002;13:40–46. doi: 10.1016/s0958-1669(02)00282-3. [DOI] [PubMed] [Google Scholar]
- 44.Carbone J, Oerra M, Rodriguez-Mahou M, et al. Immunologicalabnormalities in primary APS evolving into SLE: 6 years follow-up in women with repeated pregnancy loss. Lupus. 1999;8:274–278. doi: 10.1191/096120399678847777. [DOI] [PubMed] [Google Scholar]
- 45.Tincani A, Gilburd B, Abu-Shakra M, et al. Immunization of naïve Balb/c mice with human b2glycoprotein I breaks tolerance to the murine molecule. Arthritis Rheum. 2002;46:1399–1404. doi: 10.1002/art.10304. [DOI] [PubMed] [Google Scholar]
- 46.Oku K, Atsumi T, Bohgaki M, et al. Complement activation in patients with primary antiphospholipid syndrome. Ann RHeum Dis. 2009;68:1030–5. doi: 10.1136/ard.2008.090670. [DOI] [PubMed] [Google Scholar]
- 47.Munakata Y, Saito T, Matsuda K, et al. Detection of complement-fixing antiphospholipid antibodies in association with thrombosis. Thromb Haemost. 2000;83:728–731. [PubMed] [Google Scholar]
- 48.Davis WD, Brey RL. Antiphospholipid antibodies and complement activation in patients with cerebral ischemia. Clin Exp Immunol. 1992;10:455–460. [PubMed] [Google Scholar]
- 49.Kilgore KS, Schmid E, Shanley TP, et al. Sublytic concentrations of the membrane attack complex of complement induce interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-kappa B activation. Am J Pathol. 1997;150:2019–2031. [PMC free article] [PubMed] [Google Scholar]
- 50.Wiedmer T, Esmon CT, Sims PJ. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood. 1986;68:875–880. [PubMed] [Google Scholar]
- 51.Schafer H, Mathey D, Hugo F, Bhakdi S. Deposition of the terminal C5b-9 complement complex in infracted areas of human myocardium. J Immunol. 1986;137:1945–1949. [PubMed] [Google Scholar]