Abstract
Chronic cannabis use is associated with residual negative effects on measures of executive functioning. However, little previous work has focused specifically on executive processes involved in performance monitoring in frequent cannabis users. The present study investigated event-related potential (ERP) correlates of performance monitoring in chronic cannabis users. The error-related negativity (ERN) and error positivity (Pe), ERPs sensitive to performance monitoring, were recorded from 30 frequent cannabis users (mean usage=5.52 days/week) and 32 cannabis-naïve control participants during a speeded stimulus discrimination task. The “oddball” P3 ERP was recorded as well. Users and controls did not differ on the amplitude or latency of the ERN; however, Pe amplitude was larger among users. Users also showed increased amplitude and reduced latency of the P3 in response to infrequent stimuli presented during the task. Among users, urinary cannabinoid metabolite levels at testing were unrelated to ERP outcomes. However, total years of cannabis use correlated negatively with P3 latency and positively with P3 amplitude, and age of first cannabis use correlated negatively with P3 amplitude. The results of this study suggest that chronic cannabis use is associated with alterations in neural activity related to the processing of motivationally-relevant stimuli (P3) and errors (Pe).
Keywords: Cannabis, event-related potentials, error-related negativity, error positivity, P3
Introduction
Cannabis is the most commonly used illicit substance in the United States, and approximately 6.9 million Americans use cannabis on 20 or more days per month (Substance Abuse and Mental Health Services Administration, 2011). Repeated exposure to delta-9-tetrahydrocannabinol (THC), the primary psychoactive phytocannabinoid in cannabis, is associated with downregulation of the endogenous cannabinoid type 1 (CB1) receptors in the human brain (Villares, 2007). CB1 receptors are found in high concentrations throughout the frontal cortex (Herkenham et al., 1990; Pattij et al., 2008), a brain region critical for executive cognitive functioning. Previous research has found that chronic cannabis use is associated with residual (i.e. non-acute) negative effects on tests of executive functioning, including attention, concentration, response inhibition, cognitive flexibility, verbal fluency, and decision making (for a review, see Crean et al., 2011). To date, however, relatively little work has focused specifically on executive processes involved in performance monitoring in the context of chronic cannabis use. This represents a significant gap in the literature, as deficits in behavioral monitoring or control have been identified previously as contributors to continued substance use despite adverse consequences (Garavan and Hester, 2007; Garavan and Stout, 2005). The goal of the present study was to address this gap in the literature. To this end, we used event-related potentials (ERPs) to examine the neural correlates of error monitoring in chronic cannabis users and cannabis-naïve controls.
Error monitoring and processing are critical components of behavioral regulation. Such processes may signal the need for additional cognitive control processes, such as increased attention to task-relevant stimuli, thereby facilitating behavioral adaptation and control (Botvinick et al., 2001). The ability to monitor behavior effectively has important implications for substance abuse. For instance, a drug user who is impaired in terms of the ability to self-monitor his or her behavior might fail to establish connections between actions (e.g. drug use) and negative outcomes (e.g. loss of a job, relationships, or freedom), which could contribute to increased use and a transition from casual use to abuse or dependence (Garavan and Hester, 2007). Impaired behavioral monitoring also could compromise the ability to recognize internal cues (i.e. stressors) which lead to substance use, thereby contributing to greater relapse risk.
Event related potentials associated with performance monitoring
The error-related negativity (ERN) and error positivity (Pe) are two ERPs which are sensitive to neural processes involved in error detection and processing. To date, no studies have examined the associations between the ERN/Pe and chronic cannabis use, although previous research has shown that the ERN is disrupted in regular users of other drugs of abuse, such as cocaine (Franken et al., 2007; Sokhadze et al., 2008) and alcohol (Padilla et al., 2011; Schellekens et al., 2010). The ERN is a negative-voltage, response-locked ERP which occurs approximately 50–100 ms after an incorrect response and is maximally negative over fronto-central scalp electrode sites (Gehring et al., 1993). The ERN likely reflects the activity of a system involved in monitoring conflict between simultaneously active, but competing, response options (Botvinick et al., 2001; Botvinick et al., 2004; Carter and Van Veen, 2007; Van Veen and Carter, 2002; Yeung et al., 2004). The neural generator of the ERN is believed to be the anterior cingulate cortex (ACC; Dehaene et al., 1994; Van Veen and Carter, 2002). The ACC has been implicated in multiple processes related to behavioral monitoring and control, including response selection, conflict monitoring, and error detection (Bush et al., 2000). Functional magnetic resonance imaging (fMRI) studies of chronic cannabis users have found evidence of ACC hypoactivity during cognitive control tasks, such as a Stroop task (Eldreth et al., 2004; Gruber and Yurgelun-Todd, 2005) and a go/no-go task (Hester et al., 2009). While there have been no previous studies of the residual effects of chronic cannabis use on ERN amplitude or latency, acute cannabis administration is associated with reduced ERN amplitude to errors relative to placebo (Spronk et al., 2011). This finding is in line with reports of decreased behavioral regulation, a potential consequence of impaired performance monitoring, following acute cannabis administration (McDonald et al., 2003; Ramaekers et al., 2006).
The Pe (Falkenstein et al., 1991; Falkenstein et al., 2000) is a positive-voltage ERP which follows the ERN and peaks at centroparietal sites approximately 200–400 ms after the commission of an incorrect response (Falkenstein et al., 2000). The Pe has been less well-studied than the ERN, and its functional significance is less clear (Overbeek et al., 2005). Research by Falkenstein and colleagues (2000) provided partial support for the notion that the Pe represents further post-error processing independent of the ERN. In addition, recent research has shown that the Pe, unlike the ERN, is sensitive to participants’ awareness that they have committed an error (Endrass et al., 2007; Nieuwenhuis et al.,2001; Shalgi et al., 2009). The neural generator of the Pe is unclear as well, but candidate generators include the parietal cortex (Falkenstein et al., 2000) and rostral ACC (Van Veen and Carter, 2002; Herrmann et al., 2004). No previous studies have reported on acute or residual effects of cannabis use on the Pe.
The P3
Several authors have noted that the Pe is sensitive to the salience and motivational significance of the error to the individual, similar to the well-known “oddball” P3 ERP (Endrass et al., 2007; Nieuwenhuis et al., 2001; Ridderinkhof et al., 2009). The “oddball” P3, also known as the P3b, is a positive-going wave that peaks at 300 ms post-stimulus and is most commonly observed following presentation of infrequent target stimuli during stimulus detection tasks (Polich, 2004; Polich and Criado, 2006). The amplitude of this component is believed to reflect attention and memory processes involved in updating stimulus representations (Polich, 2004; Polich and Criado, 2006), whereas its latency represents stimulus evaluation speed (McCarthy and Donchin, 1981). While substance abuse generally is associated with reduced P3 amplitude (Ceballos et al., 2009; Singh and Basu, 2009), studies of this component in cannabis users have produced mixed results. Previous studies reported increased (Skosnik et al., 2008), decreased (Kempel et al., 2003), and equivalent (De Sola et al., 2008; Patrick et al., 1995; Solowij et al., 1995) P3 amplitude among chronic cannabis users relative to non-users. Similarly, P3 latency has been found to be increased (Kempel et al., 2003; Solowij et al., 1995) and decreased (De Sola et al., 2008) in cannabis users. Differences in participant characteristics (e.g. duration of cannabis use, history of other drug abuse) could contribute to discrepancies in results across studies but this possibility has not been well-studied to date. The P3 and Pe share several properties, including timing and scalp topography, which has led to speculation that these components may represent similar cognitive processes (Davies et al., 2001; Ridderinkhof et al., 2009). Hester and colleagues (2005) reported greater activation bilaterally in preferontal and parietal cortices during errors of which participants were aware versus those of which they were unaware. The authors concluded that these results support the notion that the Pe and P3 represent similar processes, as prefrontal and parietal generators are believed to underlie the P3 as well (Soltani and Knight, 2000).
Purpose of the present study
The primary purpose of the present investigation was to compare chronic cannabis users with cannabis-naive control participants on ERP correlates of performance monitoring (the ERN and Pe). If cannabis users show aberrations in those ERPs then this might suggest that neural activity related to behavioral monitoring is disrupted in those individuals, similar to previous observations in chronic users of other substances of abuse (Franken et al., 2007; Padilla et al., 2011; Schellekens et al., 2010; Sokhadze et al., 2008). This could have important implications for improving our understanding of how individual differences in these processes influence decisions to use cannabis. We also compared the groups on the stimulus-locked P3, as previous studies of this potential in chronic cannabis users have produced inconsistent results. A previous study of chronic users found that heavier cannabis use is associated with greater decreases in neuropsychological performance across multiple domains, including executive function (Bolla et al., 2002). Therefore, we also examined intercorrelations among cannabis use variables (age of first use, total years of use, quantity, and frequency), urinary levels of THC and THC’s inactive and active metabolites (THC-COOH and OH-THC respectively), and ERP and behavioral outcomes for the cannabis-using group to explore whether individual differences in the duration or intensity of cannabis use might be associated with similar changes on ERP or behavioral outcomes related to executive functioning. Based upon previous reports (Eldreth et al., 2004; Gruber and Yurgelun-Todd, 2005; Hester et al., 2009) we hypothesized that, relative to controls, cannabis users would demonstrate impaired performance monitoring as evidenced by reduced ERN and Pe amplitudes in response to errors on a continuous performance task. We also expected that users would exhibit reduced P3 amplitude as well in light of previous reports which have suggested that the Pe and P3 share similar properties (Endrass et al., 2007; Nieuwenhuis et al., 2001; Ridderinkhof et al., 2009). However, we considered this hypothesis tentative because studies of the P3 in chronic cannabis users and non-using controls have produced conflicting results (De Sola et al., 2008; Kempel et al., 2003; Patrick et al., 1995; Skosnik et al., 2008; Solowij et al., 1995).
Methods and materials
Participants
Participants were recruited from the local university and surrounding community and completed the present study as part of a larger study of cannabis use, executive functioning, and neural processing. Thirty-three cannabis-naive controls (13 female) and 33 cannabis users (6 female) completed the study. Data from one control were excluded due to equipment failure. Data were excluded from three cannabis users who reported using cannabis less than 24 h prior to participation to minimize the potential influence of recent cannabis use on study outcomes. Thus, the final sample consisted of 32 controls and 30 cannabis users (see Table 1). All participants provided informed consent and were paid $10 per hour for their participation. The study was approved by the local institutional review board.
Table 1.
Demographics and substance use data for the control and cannabis-using groups. Values are provided as means (standard deviation (SD)) unless otherwise noted.
| Outcome | Controls (n=32) | Cannabis users (n=30) |
p |
|---|---|---|---|
| Female (n) | 12 | 6 | 0.17a |
| Age | 20.84 (2.95) | 20.20 (2.86) | 0.39a |
| Education | 13.97 (1.49) | 13.07 (1.28) | 0.01b |
| Estimated verbal IQ | 107.14 (4.48) | 106.86 (4.41) | 0.81b |
| Current tobacco users (n) | 0 | 8 | 0.002a |
| Average drinks/week (past one month) | 0.69 (1.34) | 3.80 (4.07) | <0.001b |
| Average no. of days using alcohol/week (past one month) | 0.48 (0.88) | 1.65 (1.31) | <0.001b |
| Age of first cannabis use | – | 16.00 (1.93) | – |
| Total years of cannabis use | – | 4.20 (3.71) | – |
| Joints/week consumed | – | 17.32 (14.84) | – |
| Days/week using cannabis | – | 5.52 (1.74) | – |
| Duration of abstinence from cannabis prior to EEG (hours) | – | 45.60 (33.60) | – |
EEG: electroencephalogram; IQ: intelligence quotient.
Chi square test;
Analysis of variance (ANOVA).
Formal inclusion criteria were as follows: (a) For the cannabis group: current cannabis consumption at the rate of at least once per week during the past month, no other illicit substance use during the past three months, and no current or past diagnosis of any DSM-IV Axis I or II disorder except cannabis abuse or dependence (including no current or past abuse of or dependence upon any other illicit substance) as determined via the Structured Clinical Interview for DSM-IV Axis I and II Disorders (SCID-I and SCID-II; First et al., 1997; First et al., 2002); (b) For the control group: no history of any illicit substance use (including cannabis) and no history of psychiatric illness (Axis I or II); (c) For all participants: ages 18–35 years, completion of high school education, no history of cardiovascular disease, hearing problems, neurological disease, learning disability, or head injury resulting in loss of consciousness, and self-reported alcohol consumption less than or equal to three standard drinks per day for males and two standard drinks per day for females. Some members of the cannabis using group reported past experimental use of other illicit substances, as follows (the number in parentheses represents the number of participants in the cannabis-using group who reported any prior use of each substance): sedatives (9), ecstasy (3,4-methylenedioxymethamphetamine (MDMA)) (5), stimulants (11), opiates (7), hallucinogens (13), salvia divinorum (12), and inhalants (4). Cannabis users were asked to abstain from cannabis use for 24 hours prior to participating in the study to minimize the possible effects of acute cannabis intoxication. Cannabis and alcohol use patterns were assessed as described previously (Fridberg et al., 2011; Skosnik et al., 2007). Briefly, a locally developed substance-use questionnaire based on a time-line follow-back approach and the SCID-I module E were used to ascertain current and past patterns of drug and alcohol use. All participants were asked to report the quantity (average number of alcoholic drinks) per week over the one-month period prior to their participation and frequency (average number of drinking occasions) per week over that same period. For the cannabis-using group, measures of frequency (days/week using cannabis) and quantity of cannabis consumption (joints/week) were determined via the questionnaire for the month prior to the test session. Age of first cannabis use and total years of use were estimated to provide measures of total lifetime cannabis use. Self-reported recent drug use was corroborated using urine drug screens (Q10-1, Proxam) and quantitative urinary cannabinoid analysis. Participants included in the control group tested negative for all drugs, whereas those included in the cannabis-using group tested positive for cannabis (THC-COOH) only. All participants were administered the American National Adult Reading Test (ANART; Strauss et al., 2006) as an estimate of verbal IQ (Gladsjo et al., 1999).
XO continuous performance task (XOCPT)
The XOCPT is a simple two-choice discrimination task designed to elicit response conflict by requiring participants to suppress a well-rehearsed, prepotent motor response in favor of an alternative response during rare trials. Participants were instructed that they would complete a simple test of attention in which they would have to quickly discriminate between “X” and “O” stimuli presented rapidly on the screen. Speed and accuracy were emphasized equally in the task instructions.
Stimuli and procedure
Each trial of the XOCPT started with the presentation of a fixation point (asterisk) in the center of the computer screen for 350 ms. The fixation point was then replaced by a letter stimulus (“X” or “O”) for 80 ms. Participants were then permitted up to 800 ms to indicate whether the letter presented was an “X” or an “O”. Following a response, a blank screen was presented for 450, 500, 550, 600, or 650 ms prior to the start of the next trial. Participants indicated the identity of the target letter stimulus by pressing a key using the index (for an “X”) and middle (for an “O”) fingers of their dominant hand. One stimulus was assigned to be the rare stimulus prior to the start of the task. The identity of the rare stimulus was counterbalanced across participants. The frequent stimulus was presented on 80% of trials, whereas the rare stimulus was presented on 20% of trials. This difference in stimulus frequency was intended to produce a prepotent response bias to the frequent stimulus which had to be suppressed in favor of an alternate response during presentations of the infrequent stimulus. Participants were instructed to make their responses as quickly and accurately as possible. In order to increase task difficulty and encourage rapid responding, participants did not receive feedback after each response.
Participants completed a block of 30 practice trials, during which both stimuli were presented an equal number of times, prior to the start of the actual task to familiarize them with the input scheme and response time requirements. The XOCPT consisted of four blocks of 125 discrimination trials. The rare stimulus was presented on 25 (20%) trials per block, whereas the frequent stimulus was presented on 100 (80%) trials per block. Trials were presented randomly within each block. Rest periods were offered following blocks 1, 2, and 3. The presentation computer recorded participants’ response time and accuracy for each trial. Seven control participants completed a shorter version of the task which consisted of four blocks of 100 trials each. All other task parameters were identical, and there were no differences in performance outcomes between participants that completed the shorter and longer tasks (ps>0.40). Therefore, the data were collapsed across the shorter and longer tasks for all subsequent analyses. The task took approximately 8 min to complete.
Electroencephalogram (EEG) data collection and processing
EEG was recorded continuously (band pass 0.1–100 Hz; sampling rate 1000 Hz) from the scalp using a 37-channel Ag/AgCl electrode cap with a nose reference (Compumedics Neuroscan, USA). Scalp electrodes were placed according to the international 10–20 system. Additional electrodes were placed above and below the participant’s left eye to measure the vertical electrooculogram (VEOG), and a forehead electrode served as the ground. Electrode impedances were maintained below 10 kΩ. Participants completed the EEG recording session in a sound-attenuated room while sitting upright in a chair. Stimuli were presented on a Power Macintosh computer using SuperLab stimulus presentation software. Participants made their responses using a computer keyboard. Stimuli were presented on a 17-inch cathode ray tube monitor centered 70 cm away from the participants.
Data analysis focused on midline electrodes (FCz, Cz, CPz, Pz, Oz) since most studies of the ERN, Pe, and P3 have reported on those sites. EEG data were filtered using a 0.05–40 Hz (12 dB/octave roll-off) band pass filter. Data were segmented from 200 ms before each response to 800 ms after each response for the ERN/Pe, and from 200 ms before each stimulus to 800 ms after each stimulus for the P3. The average value from the 200 ms baseline period was subtracted from all sample points in the epoch for baseline correction. Eyeblinks were statistically removed from the data using the method of Gratton and Coles (1983) and any epoch containing voltages in excess of ±100 µV were excluded from further analysis. Epochs containing correct and incorrect responses, and those containing rare and frequent stimuli were averaged separately. The average of the incorrect response trials was used to identify the ERN and Pe, whereas the average of the rare stimuli trials was used to identify the P3. The peak latency of all ERPs was measured at the electrode site where the waveform of interest, grand-averaged across all participants, was largest. For each participant, ERPs were measured as the mean of the maximally-negative voltage and the surrounding 20 data points (i.e. the maximal value±10 data points) within a predetermined time window, relative to the incorrect response (ERN/Pe) or presentation of the rare stimulus (P3). ERN amplitude was measured at FCz between −50 and 150 ms relative to the response. Pe was measured at CPz between 100 ms and 450 ms post-response. P3 was measured at CPz between 280 ms and 600 ms post-stimulus. After preprocessing, the mean (standard deviation (SD)) number of epochs included in the analyses of the ERN and Pe was 31.34 (24.52) for controls and 31.23 (14.51) for cannabis users. The mean (SD) number of epochs included in the analyses of the P3 was 98.13 (2.69) for controls and 95.87 (6.89) for cannabis users.
Quantitative urinalysis
THC, THC-COOH, and OH-THC
The samples were initially analyzed for THC and THC-COOH by gas chromatography-mass spectrometry (GC-MS) using the extraction described by Foltz et al. (1983) and the GC-MS conditions described by Huang et al. (2001). The assay had an analytical range of 0.5 to 100 ng/mL with 1.0 mL aliquots. To ensure measurement of both analytes, the urine samples were analyzed for THC on a 1.0 mL aliquot and THC-COOH on a 0.1 mL aliquot. For THC, the aliquots were pretreated with β-glucuronidase for 18 h at 37°C. For THC-COOH, the samples were not pretreated, but the analysis preparation involves an extraction step that includes the addition of 0.2 N sodium hydroxide. Under these basic conditions, THC-COOH is freed from its glucuronide conjugate. Duplicate calibrators (1.0 mL with both THC-COOH and THC) were at 0.5, 1.0, 2.5, 5, 10, 25, 50 and 100 ng/mL. Duplicate 1.0 mL (with both THC-COOH and THC) quality control samples (QCs) were included at 1.5, 10 and 80 ng/mL. Triplicate 0.1 mL dilution QCs were included at 200 ng/mL.
Subsequently, the method was improved by using gas chromatography-tandem mass spectrometry (GC-MS/MS) with the addition of 11-hydroxy-THC (OH-THC) to the assay. This assay had a quantitative range of 0.1 to 100 ng/mL with a 1.0 mL aliquot. All samples were reanalyzed to determine OH-THC using the β-glucuronidase pretreatment. Besides obtaining the OH-THC concentration, some samples with THC or THC-COOH results less than the lower limit of quantitation in the initial analysis now had quantifiable results.
Creatinine
Creatinine was determined using a microplate colormetric test based on the Jaffe reaction where picric acid reacts with creatinine to form a colored product. Samples were diluted 20-fold (0.025 mL plus 0.475 mL water). Duplicate 0.5 mL calibrators were run at 2, 4, 6, 8, 10, 12 and 15 mg/dL. Due to sample dilution, the calibration range was 40–300 mg/dL. Triplicate diluted QCs were included at 25 and 100 mg/dL. Samples outside the calibration range were repeated using a smaller or larger dilution as needed. THC, THC-COOH, and OH-THC concentrations were normalized by creatinine levels to account for differing levels of urine dilution across subjects (THC/Cr, THC-COOH/Cr, and OH-THC/Cr respectively).
General procedure
The study took place over two testing sessions conducted on separate days. During the first session, participants provided informed consent and were administered the SCID-I and SCID-II psychiatric interviews, ANART, and additional pencil and paper measures of personality which were part of the larger study battery and are not presented here. Upon arrival to the laboratory for the second testing session, participants completed the drug use history questionnaire and urine screen. The urine sample was packaged and submitted for quantitative urinalysis. Next, participants completed the EEG recording procedure, followed by additional computer-based measures of cognition which were part of the larger study battery.
Data analysis
Cannabis user and control groups were compared on demographic, behavioral, and ERP outcomes via analysis of variance (ANOVA). Aside from cannabis use, users and controls differed on some demographic and substance use variables (see Table 1). Specifically, the user group was slightly less educated (13.07 years for users, 13.97 years for controls, p=0.01), contained a higher proportion of current tobacco users (eight in the user group, none in the control group, p=0.002), consumed more alcohol (mean 3.80 drinks/week for users, 0.69 drinks/week for controls, p<0.001), and used alcohol on more days per week (mean 1.65 days/week for users, 0.48 days/week for controls, p<0.001). Since the groups differed on years of education, tobacco, and alcohol use, we calculated Spearman correlation coefficients to determine whether individual differences in these variables were related to differences in ERP outcomes within each group. Finally, we used Spearman correlations to explore potential associations between quantity and frequency of cannabis use and ERP outcomes. Alpha was set to 0.05 for all analyses, and all tests were two-tailed. PASW Statistics 18.0 was used for all analyses.
Results
XOCPT task performance
Cannabis users and controls performed similarly on the XOCPT overall, as evidenced by a similar number of total errors on the task (see Table 2). There were no between-group differences in errors to frequent or infrequent stimuli. However, response times (RTs) to correct (p=0.001) and incorrect (p=0.005) trials were significantly faster among cannabis users relative to controls.
Table 2.
Behavioral performance on the XO continuous performance task (XOCPT).
| Group | |||
|---|---|---|---|
| Control | Cannabis users | pa | |
| Total errors | 28.13 (12.68) | 32.07 (15.02) | 0.27 |
| Errors to frequent stimuli | 5.91 (4.84) | 5.57 (6.03) | 0.81 |
| Errors to infrequent stimuli | 22.22 (11.06) | 26.50 (12.05) | 0.15 |
| Mean RT for correct trials | 277.73 (36.70) | 242.86 (41.22) | 0.001 |
| Mean RT for incorrect trials | 210.77 (43.88) | 178.98 (40.95) | 0.005 |
RT: response time.
One-way analysis of variance (ANOVA).
ERP results
ERN
The ERN was maximal at FCz across the entire participant sample, so group comparisons focused on latency and amplitude at that site. Response-locked ERP waveforms at FCz to incorrect trials for the user and control groups are shown in Figure 1(a). ANOVA revealed that ERN latency at FCz did not differ significantly between users and controls, F(1,60)=0.70, p=0.41. Furthermore, ERN amplitude at FCz did not differ between the groups, F(1,60)=1.11, p=0.30.
Figure 1.
Response-locked event-related potentials (ERPs) for incorrect response trials at (a) FCz and (b) CPz. The error-related negativity (ERN) and error positivity (Pe) are labeled.
Pe
The Pe was largest at CPz across all subjects. Response-locked ERPs to incorrect trials at CPz are shown in Figure 1(b). Pe latency did not differ significantly between the groups, F(1,60)=0.96, p=0.33. However, Pe amplitude at CPz was significantly larger among the cannabis using group relative to controls, F(1,60)=5.68, p=0.02 (Figure 1(b)).
P3
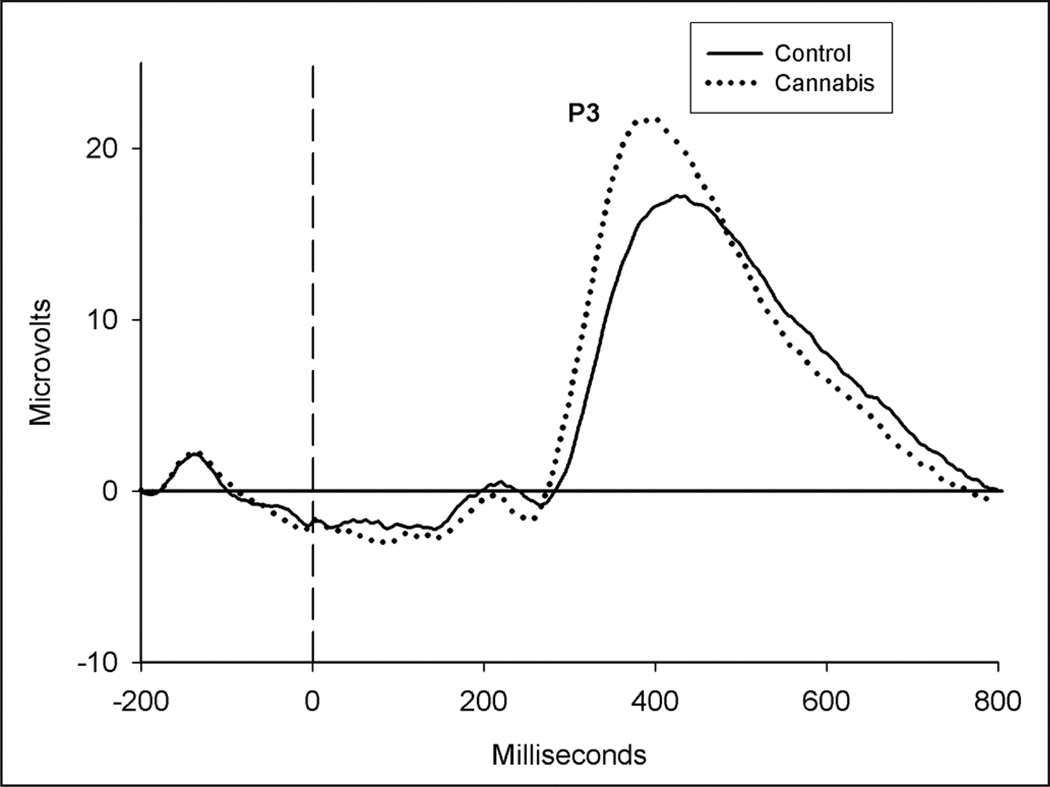
The stimulus-locked P3 was elicited by infrequent stimuli and was largest at CPz across all subjects. P3 latency to rare stimuli was significantly shorter among cannabis users relative to controls, F(1,60)=13.54, p=0.001. In addition, the effect of group was significant for P3 amplitude, such that average P3 amplitude to infrequent stimuli was larger in the cannabis using group versus to the control group, F(1,60)=6.26, p=0.015 (see Figure 2).
Figure 2.
Stimulus-locked event-related potentials (ERPs) to rare stimuli at CPz. The P3 is labeled.
Associations between sex, education, alcohol and tobacco use variables, and ERP outcomes
Men made up a larger proportion of the cannabis-using group than the control group, although this difference was not statistically significant (Table 1). Regardless, we re-analyzed our ERP outcome measures using ANOVA with the factors group and sex to explore potential sex effects in our data. Sex was not a significant contributor to any ERP outcome (all Fs<0.86, all ps>0.36). Cannabis users and controls differed significantly with regard to total years of education, tobacco use, and alcohol use (Table 1). Therefore, we used Spearman correlations to determine whether individual differences in these variables were related to differences in ERP outcomes within each group. Education and alcohol use quantity and frequency were unrelated to the amplitude or latency of the ERN, Pe, or P3 in the control (all rs<0.28, all ps>0.12) and cannabis user (all rs<0.34, all ps>0.07) groups. With regard to tobacco use, there was a trend for cannabis users who used tobacco to exhibit shorter P3 latencies than cannabis users who did not use tobacco, F(1,28)=3.83, p=0.06, but no other comparisons approached significance (all ps<0.37).
Correlations among urinary cannabinoid metabolite concentrations, cannabis use variables, and ERP and behavioral outcomes
Spearman rank-order correlations between cannabinoid metabolite levels, cannabis use variables (quantity and frequency of use, age of first use, and total years of use) and ERP outcomes for the cannabis-using group are shown in Table 3. Quantity of cannabis use (joints consumed/week) correlated positively with THC-COOH: creatinine levels (p<0.05) and OH-THC: creatinine levels (p<0.05). THC-COOH: creatinine levels and OH-THC: creatinine levels correlated positively with cannabis use frequency (days using cannabis/week) (ps<0.01) and negatively with duration of abstinence from cannabis prior to the EEG session (ps<0.001). With regard to ERP outcomes, P3 latency correlated negatively with total years of cannabis use (p<0.05). P3 amplitude correlated negatively with age of first cannabis use (p<0.05) and positively with total years of use (p<0.05). Pe latency correlated positively with P3 latency (p<0.01), and Pe amplitude correlated positively with P3 amplitude (p<0.001) (see Table 3).
Table 3.
Spearman correlations among cannabis use variables and event-related potential (ERP) outcomes for the cannabis-using group (n=30).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | THC/creatinine ratio | – | ||||||||||||
| 2. | THC-COOH/creatinine ratio | 0.61a | – | |||||||||||
| 3. | OH-THC/creatinine ratio | 0.48b | 0.84a | – | ||||||||||
| 4. | Joints/week | 0.23 | 0.39c | 0.39c | – | |||||||||
| 5. | Frequency of cannabis use (days/week) | 0.30 | 0.42c | 0.41c | 0.68a | – | ||||||||
| 6. | Age of first cannabis use | −0.24 | −0.16 | −0.14 | −0.36d | −0.34d | – | |||||||
| 7. | Total years of cannabis use | 0.33d | 0.27 | 0.25 | 0.24 | 0.46b | −0.73a | – | ||||||
| 8. | Duration of abstinence from THC (hours) | −0.26 | −0.56a | −0.64a | −0.49b | −0.32d | −0.03 | −0.03 | – | |||||
| 9. | ERN latency | 0.14 | 0.06 | 0.03 | −0.03 | −0.06 | −0.17 | 0.08 | −0.11 | – | ||||
| 10. | ERN amplitude | −0.18 | 0.07 | 0.02 | 0.01 | −0.10 | −0.13 | −0.26 | 0.11 | −0.18 | – | |||
| 11. | Pe latency | −0.24 | −0.05 | 0.09 | 0.05 | 0.09 | −0.05 | −0.02 | −0.16 | 0.13 | −0.10 | – | ||
| 12. | Pe amplitude | −0.05 | −0.11 | −0.07 | 0.07 | 0.14 | −0.04 | −0.04 | −0.06 | −0.30 | 0.05 | 0.04 | – | |
| 13. | P3 latency | −0.12 | −0.20 | −0.26 | −0.12 | −0.28 | 0.15 | −0.36c | 0.02 | 0.18 | 0.17 | 0.42 c | 0.14 | – |
| 14. | P3 amplitude | −0.05 | −0.01 | −0.07 | 0.21 | 0.27 | −0.39c | 0.40c | 0.07 | −0.20 | −0.05 | −0.11 | 0.57a | −0.23 |
ERN: error-related negativity; Pe: error positivity; THC: delta-9-tetrahydrocannabinol.
p < 0.001;
p < 0.01;
p < 0.05;
p < 0.10.
Finally, we conducted additional exploratory correlation analyses to determine whether individual differences in error rates and RTs on the XOCPT among cannabis users were related to recent cannabis use as determined via urinary metabolite concentrations. There were significant correlations between number of errors to rare stimulus trials and THC-COOH: creatinine levels (rs= −0.37, p=0.04) and OH-THC: creatinine levels (rs= −0.42, p=0.02). No other correlations were significant (all ps>0.06).
Discussion
Summary of results
The primary purpose of the present investigation was to compare chronic cannabis users with cannabis-naïve control participants on the ERN and Pe, ERP correlates of performance monitoring. We also compared users and controls on the amplitude and latency of the P3, which are believed to represent processes associated with attention/memory and stimulus evaluation, respectively. Users and controls committed a similar number of errors on a speeded discrimination task, the XOCPT, but RTs during correct and incorrect trials were significantly faster among members of the user group. Contrary to our expectations, users and controls did not differ in terms of the amplitude or latency of an ERP correlate of response-monitoring, the response-locked ERN, following incorrect trials on the XOCPT. In addition, Pe amplitude following errors was significantly larger among users relative to controls, while Pe latency did not differ between the groups. Similarly, the user group demonstrated larger stimulus-locked P3 amplitude and shorter P3 latency relative to controls to rare stimuli. Exploratory correlation analyses revealed that cannabis usage variables were unrelated to ERN and Pe amplitude or latency. Greater total years of cannabis use were associated with shorter P3 latency and greater P3 amplitude, and greater age of first cannabis use was associated with lower P3 amplitudes. Higher urinary cannabinoid metabolite levels, reflecting recent cannabis use, were related to increased self-reported cannabis use and fewer errors on the XOCPT, but were unrelated to ERP outcomes.
Significance of the ERN in cannabis users and comparison with previous studies
A limitation of the ERP technique is that the precise neuroanatomical generator of components such as the ERN cannot be determined conclusively from waveforms recorded at the scalp. However, prior studies incorporating both EEG and fMRI have identified the ACC as the putative neural generator of the ERN (for a review, see Taylor et al., 2007). Although we are unaware of any previous research comparing ERN amplitude and latency in chronic cannabis users versus non-using controls, previous fMRI research found reduced ACC activity in heavy cannabis users relative to non-users (Eldreth et al., 2004; Gruber and Yurgelun- Todd, 2005; Hester et al., 2009). There are several possible explanations for the apparent discrepancy between the results of those studies and the findings of the present investigation. For instance, previous investigations of ACC activity in cannabis users relied upon Stroop (Eldreth et al., 2004; Gruber and Yurgelun-Todd, 2005) and go/no-go (Hester et al., 2009) tasks which may have been more cognitively demanding and more sensitive to subtle deficits in ACC functioning associated with chronic cannabis use than the XOCPT. Those studies also relied upon smaller (9≤n≤16) samples of older users (mean age approximately 25 years versus 20 years in the current study) who may have used more heavily than users in the present study. Frequency (Bolla et al., 2002) and duration (Solowij et al., 2002) of cannabis use have been shown to correlate negatively with cognitive performance among chronic users. However, we did not observe significant correlations between self-reported cannabis usage variables or THC metabolites and ERN amplitude or latency. It is possible that disruptions in ACC functioning and, consequently, ERN deficits may emerge after longer periods of frequent cannabis use, and that users in the present study had not used for a duration sufficient to observe such effects. For instance, the study by Gruber and Yurgelun-Todd (2005) examined neural correlations of performance monitoring in cannabis users who were slightly older (mean age=26.8 years) and had used cannabis over a longer period of time (mean age of onset=14.1 years) than those in the present study.
Association between the Pe and P3 in cannabis users
We found that chronic cannabis use was associated with greater peak response-locked Pe amplitudes and stimulus-locked P3 amplitudes. The precise functional significance of the Pe is unclear, but the Pe is similar to the P3 in terms of scalp topography, timing, and functional properties (Overbeek et al., 2005). Indeed, Pe amplitude and latency correlated significantly with P3 amplitude and latency within participants in the present study. Pe amplitude is large following trials in which participants are aware of the fact that they committed an error (aware errors), and absent following trials of which they are unaware of an error (unaware errors) (Endrass et al., 2007; Nieuwenhuis et al., 2001; Shalgi et al., 2009). This suggests that the Pe is sensitive to the motivational significance of an error (Overbeek et al., 2005). That is, aware errors presumably would be considered more salient, and would be more likely to be associated with behavioral change (e.g. correcting one’s error or exercising caution to avoid similar errors in the future) than unaware errors. P3 amplitude is sensitive to motivational significance as well, as P3 amplitude is significantly larger following relevant target stimuli versus irrelevant non-target stimuli (Polich and Criado, 2006). Thus, both the Pe and P3 may reflect neural activity sensitive to the motivational properties of an event.
While these results are consistent with the notion that the Pe and P3 covary, they do not demonstrate conclusively that both components reflect the same underlying cognitive processes, nor do they explain why the amplitude of these components would be increased in chronic cannabis users. One possible explanation for these findings is that increased Pe/P3 amplitude among cannabis users reflects “compensatory” neural activity during trials where errors are likely, or in response to the actual commission of an error. Evidence for this position comes from previous neuroimaging studies which found more diffuse patterns of blood-oxygen-level dependent (BOLD) activity during inhibitory control tasks among users versus controls, despite similar error rates between the groups (Eldreth et al., 2004; Gruber and Yurgelun-Todd, 2005; Hester et al., 2009). A similar effect has been observed in adolescent cannabis users as well (Tapert et al., 2007). In addition, a recent study of frequent users of MDMA and cannabis found evidence of greater BOLD response in frontal and parietal regions during successful inhibitions, and in temporal, frontal, and cingulate regions following incorrect responses on a go/no-go task (Roberts and Garavan, 2010). Cannabis users may need to devote more cognitive resources to perform equivalently to controls on inhibitory control tasks, which may be reflected in between-group differences in neural activity to stimulus characteristics and response outcomes. This effect could be more pronounced in heavier users, which could explain why longer use (i.e., total years) was associated with shorter P3 latency and greater P3 amplitude, and why higher urinary metabolite levels were associated with relatively better performance (i.e., fewer errors) on the XOCPT task among cannabis users in the current study. In addition, we speculate that compensatory processing among cannabis users could explain why ERN amplitudes were similar between cannabis users and controls in the present study, whereas Pe amplitudes differed between the groups. Cannabis users and controls may not have differed in terms of their ability to detect the presence of errors, resulting in equivalent ERN amplitudes between the groups. However, users may have needed to devote extra resources to avoid committing errors on the task, relative to controls. The extra attention paid by users to their performance on the task may have resulted in increased post-error activity when those errors did occur, as users may have been more attentive to those errors than controls. Future EEG or fMRI studies could manipulate the difficulty of an inhibitory control task to determine whether increases in difficulty are associated with increases in compensatory neural activity among cannabis users.
The possibility that cannabis users show compensatory neural activity in response to demands to monitor their behavior represents a promising avenue for future research, but there are other plausible explanations for the observed increased amplitudes of the Pe and P3 among cannabis users in the present study. One possibility is that cannabis users were more anxious then controls, or experienced higher levels of arousal during the task. Increased state anxiety is associated with increased P3 amplitude (Grillon and Ameli, 1994), and persons who meet diagnostic criteria for an anxiety disorder show greater P3 amplitude relative to non-anxious individuals (Enoch et al., 2001). Similarly, Hajcak and colleagues (2003) reported that greater autonomic arousal (skin conductance response) was associated with greater Pe amplitude following errors on a Stroop task. While no participant in the present study met DSM-IV criteria for any anxiety disorder, we did not assess state anxiety or subjective arousal during the task. A future study could study psychophysiological processes related to behavioral monitoring in cannabis users under varying conditions of anxiety or arousal to determine the extent to which differences in state anxiety affect the pattern of results obtained in the present study.
P3 latency
Peak P3 latency was faster among the user group relative to the control group in the present study, and total years of cannabis was negatively correlated with P3 latency among users. P3 latency is believed to reflect neural activity related to stimulus processing speed or cognitive efficiency, independent of motor reaction time (Polich and Criado, 2006), such that faster or more efficient processing is associated with shorter peak latency (Polich, 2007). Like P3 amplitude, previous studies of P3 latency in chronic cannabis users have produced mixed results, with some studies reporting increased (Solowij et al., 1995; Kempel et al., 2003) and decreased (De Sola et al., 2008) P3 latency in those individuals. Similar to the present results, the study by de Sola and colleagues (2008) found that greater lifetime cannabis use was associated with shorter peak P3 latency; however, that study examined this association in a mixed sample which included 14 individuals who used MDMA and cannabis in addition to 13 individuals who used cannabis only. The present results suggest that reduced peak P3 latency may be observed in response to motivationally-relevant stimuli among chronic cannabis users who do not use other drugs heavily. One possible explanation for the present finding is that cannabis users were more efficient at processing infrequent (“oddball”) stimuli during the XOCPT task than were non-users. This is in line with the finding that users’ RTs were significantly faster during the task, despite equivalent levels of behavioral performance (i.e. errors). Of note, a previous study reported faster RTs among current cannabis users relative to past users and non-using controls during an attentional inhibition (negative priming) task (Skosnik et al., 2001). However, in contrast to the present study, users’ faster RTs in that study were accompanied by greater attentional disinhibition on the task.
Alternatively, cannabis users in the present study may have had a tendency to respond more impulsively than non-users on the XOCPT, which would result in faster RTs among members of the former group. Impulsivity has been identified as a potential cause and consequence of chronic drug abuse (De Wit, 2009). However, the associations between impulsivity and chronic cannabis use specifically are unclear. Some studies have found evidence of greater impulsivity and impaired response inhibition among chronic cannabis users after a period of abstinence, while others have found no such effects (for a review, see Crean et al., 2011). Most recently, a study of a relatively large sample (n=65) of young (mean age=20.3±2.0 years), non-treatment-seeking chronic cannabis users found no differences between those individuals and age-matched non-users on several measures sensitive to impulsive decision making and response inhibition (Gonzalez et al., 2012). As noted previously, users and controls did not differ significantly in terms of their error rates on the XOCPT in the present study. If greater impulsivity was the reason for faster RTs among users, then we might expect them to commit more errors on the task as well as to have greater difficulty inhibiting the prepotent motor response on rare target trials. At present the association between chronic cannabis use and impulsivity is unclear, and additional research on this topic is warranted.
Comparisons with acute administration studies
While the current study focused on the ERN, Pe, and P3 in cannabis users who were not acutely intoxicated at the time of testing, previous research has examined the effect of acute THC exposure on these components. Spronk and colleagues (2011) reported a significant reduction in ERN amplitude to errors on a speeded response task following acute THC administration relative to placebo, suggesting that behavioral monitoring as indexed by the ERN is impaired during acute cannabis intoxication. The active dose and placebo groups did not differ in terms of their performances on the task. However, the authors did not report on the effects of THC on Pe amplitude or latency in that study. Several studies have found that acute THC administration is associated with reduced P3 amplitude in response to target stimuli on working memory or choice reaction time tasks (Hart et al., 2010; Ilan et al., 2005; Ilan et al., 2004; Roser et al., 2008) as well as on traditional “oddball” target detection tasks (D’Souza et al., 2012) suggesting that acute intoxication disrupts cortical processes involved in context updating and attention. Previous studies have reported mixed results with regard to the P3 in chronic cannabis users (De Sola et al., 2008; Kempel et al., 2003; Patrick et al., 1995; Solowij et al., 1995) although at least one study has reported increased P3 in users relative to controls during a visual oddball task (Skosnik et al., 2008). The results of the present study suggest that the residual effects of chronic cannabis use on the ERN, Pe, and P3 may differ from those of acute THC administration. This is in line with previous research which has found differences between the acute and residual effects of cannabis use on neurocognitive functioning (for a recent review, see Crean et al., 2011). Future studies which compare directly the effects of acute cannabis intoxication versus those of non-acute (residual) use on the ERN, Pe, and P3 would improve our understanding of the effects of cannabis use upon of processes related to performance monitoring, attention, and memory.
Limitations
The results of the present study should be interpreted in the light of some potential limitations. We were not able to quantify the concentrations of the different cannabinoids (e.g. THC, cannabidiol) which were present in the cannabis consumed by the user group. Different preparations of cannabis (e.g. marijuana versus sinsemilla versus hashish) vary considerably in terms of potency (Mehmedic et al., 2010) and consequently may differ in terms of their effects upon neurocognition. In addition, quantity and frequency of cannabis use was based on self-report and may have been susceptible to individual differences in accuracy of reporting. However, all participants were screened for recent drug use prior to the EEG recording session, and urinary metabolite levels correlated with self-reported cannabis consumption (joints/week) and frequency of use. In addition, because cannabis users were tested after abstaining from cannabis use for 24 h, it is unclear whether the present results represent the residual effects of chronic cannabis use (Pope et al., 2002) or consequences of cannabis withdrawal (Budney et al., 2004). A future study examining ERPs related to performance monitoring in cannabis users who vary in terms of abstinence duration would provide further clarification on this issue. Another limitation of the study is that cannabis users and controls differed in terms of tobacco and alcohol use. However, there were no significant differences in ERP outcomes between cannabis users who used tobacco and those who did not use tobacco, overall alcohol consumption was low in both groups, and alcohol use quantity and frequency were unrelated to ERP outcomes. It will be important for future studies to consider further how individual difference variables (e.g. a history of polysubstance abuse, age, chronicity of use) contribute to differences in ERP outcomes across studies and within samples of heavy cannabis users; such an analysis would require a larger sample of cannabis users who varied widely on the specific characteristics of interest. In addition, our correlation analyses were exploratory and we did not correct for multiple comparisons. Future research should attempt to reproduce the pattern of associations shown in Table 3. Finally, the cross-sectional nature of the study precludes a causal interpretation of the present results. Chronic cannabis use may lead to alterations in the Pe and P3; alternatively, individual differences in the neural processes underlying those components may be predispose individuals to use cannabis heavily.
Conclusions
To our knowledge, this is the first study which has examined the non-acute effects of frequent cannabis use on the ERN and Pe. The results suggest that chronic cannabis use is associated with alterations in neural activity related to the processing of motivationally-relevant stimuli (P3) and errors (Pe). No group differences were found in terms of the amplitude or latency of the ERN, an ERP sensitive to response conflict and error monitoring. The increased Pe amplitude in response to errors among cannabis users in the present study is in line with previous neuroimaging studies which found evidence of altered neural activity during performance monitoring in heavy cannabis users relative to controls (Eldreth et al., 2004; Gruber and Yurgelun-Todd, 2005; Hester et al., 2009). Heavy cannabis users may recruit compensatory processes to effectively regulate their behavior and monitor their performance (Hester et al., 2009; Tapert et al., 2007), although additional research is needed to confirm this hypothesis and rule out alternate explanations (e.g. higher anxiety or arousal). Recent cannabis use, assessed via urinary levels of THC, THC-COOH, and OH-THC, was unrelated to ERP outcomes but associations between ERP measures and self-reported cannabis-use variables (age of first cannabis use, total years of use) were observed. Further research is needed to further clarify associations between stimulus and error salience, P3, and Pe amplitude and latency in cannabis users, as well as the relation between chronicity of cannabis use and ERP correlates of performance monitoring in chronic users.
Acknowledgements
The authors would like to thank David Moody and David Andrenyak for performing the THC, OH-THC, and THC-COOH analysis, which was supported by National Institute on Drug Abuse contract N01DA-9-7767 to PDS. They would also like to thank Margaret Brumbaugh and Jennifer Vollmer for their help collecting data for this project.
Funding
This work was supported in part by a National Institute on Alcohol Abuse and Alcoholism training grant fellowship (2 T32 AA007462-26) to DJF, as well as grants from the National Institute on Drug Abuse (1 R03 DA019630-01; 1 R21 DA023097-01A1) and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award to PDS.
Footnotes
Conflict of interest
The authors declare that there are no conflict of interest.
References
- Bolla KI, Brown K, Eldreth D, et al. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, et al. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, et al. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingular cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, Bauer LO, Houston RJ. Recent EEG and ERP findings in substance abusers. Clin EEG Neurosci. 2009;40:122–128. doi: 10.1177/155005940904000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An evidence-based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Dywan J, et al. Error-negativity and positivity as they relate to other ERP indices of attentional control and stimulus processing. Biol Psychol. 2001;56:191–206. doi: 10.1016/s0301-0511(01)00080-1. [DOI] [PubMed] [Google Scholar]
- De Sola S, Tarancón T, Peña-Casanova J, et al. Auditory event-related potentials (P3) and cognitive performance in recreational ecstasy polydrug users: Evidence from a 12-month longitudinal study. Psychopharmacology. 2008;200:425–437. doi: 10.1007/s00213-008-1217-5. [DOI] [PubMed] [Google Scholar]
- De Wit H. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychol Sci. 1994;5:303–305. [Google Scholar]
- D’Souza DC, Fridberg DJ, Skosnik PD, et al. Dose-Related Modulation of Event-Related Potentials to Novel and Target Stimuli by Intravenous Δ9-THC in Humans. Neuropsychopharmacology. 2012;37:1632–1646. doi: 10.1038/npp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, et al. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Endrass T, Reuter B, Kathmann N. ERP correlates of conscious error recognition: Aware and unaware errors in an antisaccade task. Eur J Neurosci. 2007;26:1714–1720. doi: 10.1111/j.1460-9568.2007.05785.x. [DOI] [PubMed] [Google Scholar]
- Enoch M-A, White KV, Harris CR, et al. Alcohol use disorders and anxiety disorders: Relation to the P300 event-related potential. Alcohol Clin Exp Res. 2001;25:1293–1300. [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, et al. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, et al. ERP components on reaction errors and their functional significance: A tutorial. Biol Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington, DC: American Psychiatric Press, Inc.; 1997. [Google Scholar]
- First MB, Spitzer RL, Miriam G, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Foltz RL, Mcginnis KM, Chinn DM. Quantitative measurement of Δ9-tetrahydrocannabinol and two major metabolites in physiological specimens using capillary column gas chromatography negative ion chemical ionization mass spectrometry. Biol Mass Spectrom. 1983;10:316–323. doi: 10.1002/bms.1200100503. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Van Strien JW, Franzek EJ, et al. Error-processing deficits in patients with cocaine dependence. Biol Psychol. 2007;75:45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Fridberg DJ, Vollmer JM, O’donnell BF, et al. Cannabis users differ from non-users on measures of personality and schizotypy. Psychiatry Res. 2011;186:46–52. doi: 10.1016/j.psychres.2010.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, et al. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- Gladsjo JA, Heaton RK, Palmer BW, et al. Use of oral reading to estimate premorbid intellectual and neuropsychological functioning. J Int Neuropsychol Soc. 1999;5:247–254. doi: 10.1017/s1355617799533079. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, et al. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. J Clin Exp Neuropsychol. 2012;34:962–976. doi: 10.1080/13803395.2012.703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R. P300 assessment of anxiety effects on processing novel stimuli. Int J Psychophysiol. 1994;17:205–217. doi: 10.1016/0167-8760(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: A pilot investigation. Brain Res Cogn Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Mcdonald N, Simons RF. To err is autonomic: Error-elated brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40:895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ilan AB, Gevins A, et al. Neurophysiological and cognitive effects of smoked marijuana in frequent users. Pharmacol Biochem Behav. 2010;96:333–341. doi: 10.1016/j.pbb.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Römler J, Ehlis A-C, et al. Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe) Brain Res Cogn Brain Res. 2004;20:294–299. doi: 10.1016/j.cogbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Hester R, Foxe JJ, Molholm S, et al. Neural mechanisms involved in error processing: A comparison of errors made with and without awareness. Neuroimage. 2005;27:602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Moody DE, Andrenyak DM, et al. Simultaneous determination of delta9-tetrahydrocannabinol and 11-nor-9-carboxy-delta9-tetrahydrocannabinol in human plasma by solid-phase extraction and gas chromatography-negative ion chemical ionization-mass spectrometry. J Anal Toxicol. 2001;25:531–537. doi: 10.1093/jat/25.7.531. [DOI] [PubMed] [Google Scholar]
- Ilan AB, Gevins A, Coleman M, et al. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol. 2005;16:487–496. doi: 10.1097/00008877-200509000-00023. [DOI] [PubMed] [Google Scholar]
- Ilan AB, Smith ME, Gevins A. Effects of marijuana on neuro-physiological signals of working and episodic memory. Psychopharmacology (Berl) 2004;176:214–222. doi: 10.1007/s00213-004-1868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempel P, Lampe K, Parnefjord R, et al. Auditory-evoked potentials and selective attention: Different ways of information processing in cannabis users and controls. Neuropsychobiology. 2003;48:95–101. doi: 10.1159/000072884. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: A comparison of P300 latency and reaction time. Science. 1981;211:77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, et al. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- Mehmedic Z, Chandra S, Slade D, et al. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55:1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, et al. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Overbeek TJM, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing: On the functional significance of the Pe vis-à-vis the ERN/Ne. 2005;19:319–329. [Google Scholar]
- Padilla M, Colrain I, Sullivan E, et al. Electrophysiological evidence of enhanced performance monitoring in recently abstinent alcoholic men. Psychopharmacology. 2011;213:81–91. doi: 10.1007/s00213-010-2018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick G, Straumanis JJ, Struve FA, et al. Auditory and visual P300 event related potentials are not altered in medically and psychiatrically normal chronic marihuana users. Life Sci. 1995;56:2135–2140. doi: 10.1016/0024-3205(95)00199-g. [DOI] [PubMed] [Google Scholar]
- Pattij T, Wiskerke J, Schoffelmeer ANM. Cannabinoid modulation of executive functions. Eur J Pharmacol. 2008;585:458–463. doi: 10.1016/j.ejphar.2008.02.099. [DOI] [PubMed] [Google Scholar]
- Polich J. Clinical application of the P300 event-related brain potential. Phys Med Rehabil Clin N Am. 2004;15:133–161. doi: 10.1016/s1047-9651(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, et al. Cognitive measures in long-term cannabis users. J Clin Pharmacol. 2002;42:S41–S47. doi: 10.1002/j.1552-4604.2002.tb06002.x. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Van Ruitenbeek P, et al. High-Potency Marijuana Impairs Executive Function and Inhibitory Motor Control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ramautar JR, Wijnen JG. To Pe or not to Pe: A P3-like ERP component reflecting the processing of response errors. Psychophysiology. 2009;46:531–538. doi: 10.1111/j.1469-8986.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- Roberts GMP, Garavan H. Evidence of increased activation underlying cognitive control in ecstasy and cannabis users. Neuroimage. 2010;52:429–435. doi: 10.1016/j.neuroimage.2010.04.192. [DOI] [PubMed] [Google Scholar]
- Roser P, Juckel G, Rentzsch J, et al. Effects of acute oral Δ9-tetrahydrocannabinol and standardized cannabis extract on the auditory P300 event-related potential in healthy volunteers. Eur Neuropsychopharm. 2008;18:569–577. doi: 10.1016/j.euroneuro.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Schellekens AFA, De Bruijn ERA, Van Lankveld CaA, et al. Alcohol dependence and anxiety increase error-related brain activity. Addiction. 2010;105:1928–1934. doi: 10.1111/j.1360-0443.2010.03065.x. [DOI] [PubMed] [Google Scholar]
- Shalgi S, Barkan I, Deouell LY. On the positive side of error processing: Error-awareness positivity revisited. Eur J Neurosci. 2009;29:1522–1532. doi: 10.1111/j.1460-9568.2009.06690.x. [DOI] [PubMed] [Google Scholar]
- Singh SM, Basu D. Clinical study: The P300 event-related potential and its possible role as an endophenotype for studying substance use disorders: A review. Addict Biol. 2009;14:298–309. doi: 10.1111/j.1369-1600.2008.00124.x. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Edwards CR, O’Donnell BF, et al. Cannabis use disrupts eyeblink conditioning: Evidence for cannabinoid modulation of cerebellar-dependent learning. Neuropsychopharmacology. 2007;33:1432–1440. doi: 10.1038/sj.npp.1301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skosnik PD, Park S, Dobbs L, et al. Affect processing and positive syndrome schizotypy in cannabis users. Psychiatry Res. 2008;157:279–282. doi: 10.1016/j.psychres.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Spatz-Glenn L, Park S. Cannabis use is associated with schizotypy and attentional disinhibition. Schizophr Res. 2001;48:83–92. doi: 10.1016/s0920-9964(00)00132-8. [DOI] [PubMed] [Google Scholar]
- Sokhadze E, Stewart C, Hollifield M, et al. Event-related potential study of executive dysfunctions in a speeded reaction task in cocaine addiction. J Neurother. 2008;12:185–204. doi: 10.1080/10874200802502144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Michie PT, Fox AM. Differential impairments of selective attention due to frequency and duration of cannabis use. Biol Psychiatry. 1995;37:731–739. doi: 10.1016/0006-3223(94)00178-6. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Soltani M, Knight RT. Neural Origins of the P300. Crit Rev Neurobiol. 2000;14:199–224. [PubMed] [Google Scholar]
- Spronk D, Dumont GJH, Verkes RJ, et al. Acute effects of Delta-9-Tetrahydrocannabinol on performance monitoring in healthy volunteers. Front Behav Neurosci. 2011;5:59. doi: 10.3389/fnbeh.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Shermann EMS, Spreen O. A Compendium of Neuropsychological Tests. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) US Department of Health and Human Services. Rockville, Maryland: SAMHSA; 2011. Results from the 2010 National Survey on Drug Use and Health: Summary of national findings. [Google Scholar]
- Tapert S, Schweinsburg A, Drummond S, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: Recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145:323–334. doi: 10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]