Abstract
Multicellular aggregates of cells, termed spheroids, are of interest for studying tumor behavior and for evaluating the response of pharmacologically active agents. Spheroids more faithfully reproduce the tumor macrostructure found in vivo compared to classical 2D monolayers. We present a method for embedding spheroids within collagen gels followed by quantitative and qualitative whole spheroid and single cell analyses enabling characterization over the length scales from molecular to macroscopic. Spheroid producing and embedding capabilities are demonstrated for U2OS and MDAMB 231 cell lines, of osteosarcoma and breast adenocarncinoma origin, respectively. Finally, using the MDA-MB-231 tumor model, the chemotherapeutic response between paclitaxel delivery as a bolus dose, as practiced in the clinic, is compared to delivery within an expansile nanoparticle. The expansile nanoparticle delivery route provides a superior outcome and the results mirror those observed in a murine xenograft model. These findings highlight the synergistic beneficial results that may arise from the use of a drug delivery system, and the need to evaluate both drug candidates and delivery systems in the research and pre-clinical screening phases of a new cancer therapy development program.
Keywords: spheroid, drug delivery, tumor mimic, cancer model, collagen, 3D cell culture, cell migration
INTRODUCTION
Three dimensional (3D) in vitro cell culture models are being adopted as preclinical tools for studying tumor behavior and drug response [1]. This paradigm shift is in response to a growing body of evidence that 3D systems promote greater in vivo-like cell behavior than their two-dimensional (2D) counterparts due to recreating more of the characteristic traits of the native tumor environment [2, 3]. As such, these models are proving more predictive than monolayer based systems. The majority of these 3D tumor cell models are prepared by either: a) growing cells on non-adherent surfaces or in suspension to induce cell clustering; b) seeding cells within a preformed polymer scaffold [4-7]; or c) embedding cells within a hydrogel to promote cell-cluster formation along with cell-matrix attachments [8, 9].
With regards to the latter technique, several polymer compositions including Matrigel™ [10, 11], collagen [12], and hyaluronic acid [13] are being used to create 3D scaffolds in an effort to recreate the native extracellular matrix (ECM)-like environments in vitro [14-17]. These systems promote differential cell behavior when compared to 2D systems, but fail to reproduce the tumor macrostructure found in vivo [3, 18]. Clinical tumors usually consist of a singular structure with metabolically active cells at the surface and a necrotic core, while cell clusters in the 3D matrices are substantially smaller and numerous. Solid tumors also possess mass transport limitations stemming from decreased surface area-to-volume ratios and longer diffusion lengths, which are not present in single cells or small cell clusters [18, 19].
To address these challenges, several methods of creating large cell clusters (>350 μm) are reported in the literature [20-22]. These techniques eliminate or minimize the surface attachment sites for cells, forcing them to interact principally with each other, and include spinning flasks, hanging drops, and agarose coated plates. The resulting clusters, or spheroids, are of a similar size to small tumors. Unlike clinical tumors, they exist in an attachment-free microenvironment with very different mechanical and biochemical properties than the native ECM [23]. This is an important caveat to their use, as matrix attachments via integrins and substrate mechanics play crucial roles in cell differentiation and survival [24]. The interplay between the ECM and the tumor drastically affects drug response, epigenetic state, and metastasis in cancer [2, 18]. Therefore, there is a need for additional methods to prepare stable and reproducible models which mimic the native tumor environment while being large enough for comparison to patient tumors.
In order to simultaneously study and model key cellular parameters that regulate form and function including cell adhesion, cell-ECM interaction, biochemical state, mechanical properties, and tumor macrostructure, we present a scalable and reproducible method for embedding and manipulating cancer cell spheroids inside a 3D collagen gel. It builds upon previous spheroid and spheroid-collagen models,[25-30] and enables individual spheroid manipulation along with quantitative and qualitative whole spheroid and single cell analyses. Specifically, we describe the formation of human osteosarcoma and breast adenocarcinoma multicellular spheroids and subsequent embeddingwithin a collagen matrix (Figure 1). We hypothesize that a multicellular spheroid contained in an ECM derived matrix will respond differently to the first-line chemotherapeutic agent paclitaxel based on its delivery route in contrast to that observed in a 2D monolayer system. Herein, we report the effects of matrix stiffness, cell seeding number, cell type, and chemotherapeutic treatment on a collagen embedded spheroid.
Figure 1.
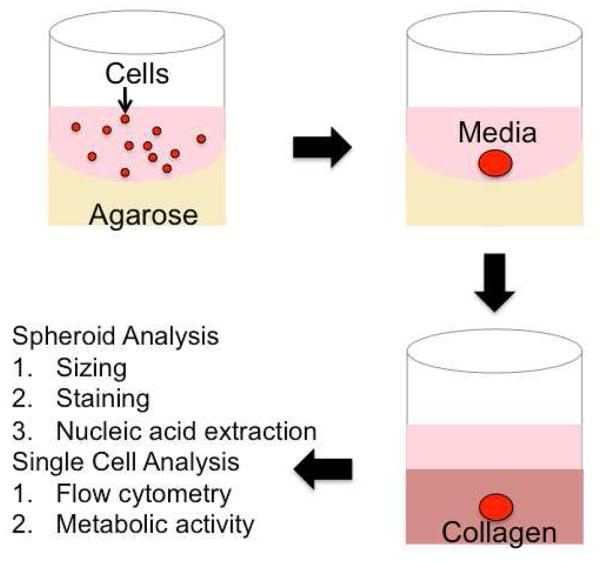
Creation of Embedded Spheroids: Spheroid formation is encouraged by placing a suspension of cells (red) in media (pink) on agarose (yellow) coated wells. After 72 hours, a spheroid is formed, and then transferred into a collagen gel.
MATERIALS AND METHODS
CELL CULTURE
Experiments were performed on the pediatric osteosarcoma cell line U2OS and/or breast adenocarcinoma cell line MDA-MB-231 (ATCC, Manassas, VA). Both cell lines express high levels of E-Cadherin, readily form spheroids, and are well characterized, including their protein expression and secretion profiles as well as have been extensively studied in cancer research applications [12, 31, 32]. Cells were cultured in complete RPMI (U2OS) or DMEM (MDA-MB-231) media supplemented with 10% fetal calf serum and 1% penicillin-streptomycin solution (10,000 IU/mL penicillin; 10,000 μg/mL streptomycin). Cell cultures were maintained in 2D monolayers in a humidified incubator at 37°C, 5% C02.
SPHEROID FORMATION
Cell aggregation was induced by growing cell suspensions in agarose-coated 96 well plates. Briefly, 1.5% (wt/vol) agarose solution was made by combining 0.15 g agarose (Sigma-Aldrich, St. Louis, MO) with 10 mL PBS. This solution was microwaved until agarose dissolution (~ 30s) and kept on a hot plate during the well coating process, to prevent premature gelation. To prepare the 96-well plate, 70 μL of the hot agarose solution was pipetted into each well and allowed to cool for 20 minutes. Separately, cell monolayers were detached from their culture flask via a standard typsinization protocol. Cells were counted and re-suspended in media to the desired concentration. Next, 100 μL of the cell suspension was added to each agarose-coated well. After 72 hours, the resulting cell aggregates were lifted via gentle pipetting and were immediately seeded into collagen gels as described below.
COLLAGEN EMBEDDING
Spheroids were transferred into an un-polymerized collagen gel following a published procedure [12]. High Density Rat Tail Type I collagen (BD Biosciences) was diluted to 10 mg/mL in 0.02 N acetic acid and combined 1:1 with a buffer solution (100 mM HEPES in 2x PBS, pH 7.3). This mixture was further diluted with PBS to the experimental collagen concentration (2-5 mg/mL). This solution self-polymerizes into a gel after 1 hour at 37 °C. Tumor cell spheroids wer e added to 100uL of unpolymerized collagen solution in each well of a 96 well plate. After 1 hour, 100 uL of media or media with drug was added on top of the gel. This media was removed and replaced every 24 hours.
TRACKING SPHEROID GROWTH
After collagen embedding, spheroids were imaged every 24 hours for the duration of the experiment. Images were acquired on a DMI600B microscope (Leica, Solms, Germany) with an ImagEM EM-CCD Camera (Hamamatsu Photonics, Hamamatsu, Japan) in a spinning disc confocal setup (Yokogawa, Tokyo, Japan). Imaging was done using Micro-Manager 1.4 Software (http://www.micro-manager.org). Resulting images were analyzed with ImageJ software to measure spheroid diameter.
DISAGGREGATION OF SPHEROIDS FOR ASSESSMENT OF METABOLIC ACTIVITY
MDA-MB-231 spheroids were transferred into collagen (4 mg/mL) for 24 hours prior to disaggregation by collagenase I treatment (2.38 mg/mL) (Life Technologies, Carlsbad, CA) in Hank’s Buffered Salt Solution at 37°C for one hour. Disaggregated cells from both spheroids and monolayer culture were seeded for 12 hours in 96 well plates. Cell viability was assessed via the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2- (4-sulfophenyl)-2H-tetrazolium) cell proliferation assay (Sigma, St. Louis, MO). Metabolic activity was calculated as the percentage of the positive control absorbance at 490 nm.
LIVE/DEAD STAINING
MDA-MB-231 spheroids were stained with Calcein AM and Eithidium homodimer-1 (Molecular Probes, Eugene, OR), for live and dead cells, respectively 72 hours after transferring to collagen (4 mg/mL). Spheroids were either imaged as previously described with the addition of laser excitation of the sample at 488 and 561 nm or disaggregated for fluorescent activated cell sorting (FACS). In addition to spheroids, MDA-MB 231 monolayers were stained for use as live and dead (after treatment with 70% methanol) controls. Cells were fixed with 4% formaldehyde prior to FACS with appropriate filters. Data was analyzed with FlowJo (Tree Star, Ashland, OR). Control samples were gated for live and dead populations, and then applied to the spheroid sample to calculate a percent alive and dead.
NUCLEIC ACID EXTRACTION
Nucleic acid was extracted from fully embedded spheroid samples using the Trizol Reagent without additional purification or disaggregation (Life Technologies, Carlsbad, CA). Subsequent RNA concentration was then measured with a NanoDrop Lite (Thermo Scientific, Wilmington, DE). Ten MDA-MB 231 spheroids (20,000 cells each) yielded 4.735 μg RNA.
NANOPARTICLE PREPARATION
Paclitaxel-loaded expansile nanoparticles (Pax-eNP) were prepared as previously described [33]. Briefly an oil-in-water mini-emulsion technique was used to polymerize monomer (5-methyl-2-(2,4,6-trimethoxyphenyl)-1,3-dioxan-5-yl)methyl methacrylate with crosslinker , 1,4-phenylene bis(2-methylacrylate) and the addition of (5% wt/wt) paclitaxel (MP Biomedicals, Solon OH). A non-loaded fluorescently labeled eNP was made in a similar manner using a covalently incorporated rhodamine co-monomer. Paclitaxel was selected for study because of its clinical use in the treatment of metastatic breast cancer and other solid tumor malignancies [34]. The mechanism of action is through binding and subsequent stabilization of microtubules which prevents dynamic reorganization [35]. Due to insolubility the compound is clinically dissolved in Cremophor El (1:1 solution of ethanol and polyoxyethylated castor oil) for in vivo administration [36].
SPHEROID TREATMENT WITH DIFFERENT DRUG DELIVERY METHODS
MDA-MB-231 spheroids were made with 20,000 cells and subsequently embedded in collagen gels (4 mg/mL) for 24 hours. Spheroids were exposed to either 100 ng/mL or 1000 ng/mL paclitaxel delivered via bolus dose (paclitaxel cremophor/ethanol) in the media or via Pax-eNP. In both cases, cells were incubated with the drug treatment for 24 hours with monitoring of spheroid size over the following week. An analogous experiment was performed with fluorescently labeled eNPs, and the spheroid was imaged after treatment for 24 hours upon excitation at 561 nm.
RESULTS
SPHEROID SIZE IS LINEARLY DEPENDENT ON CELL SEEDING NUMBER
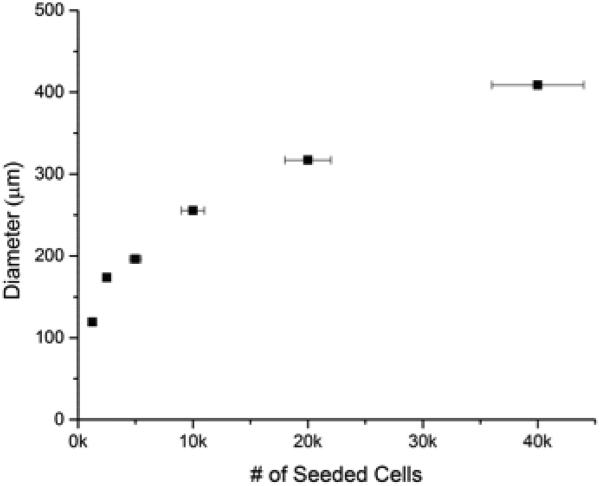
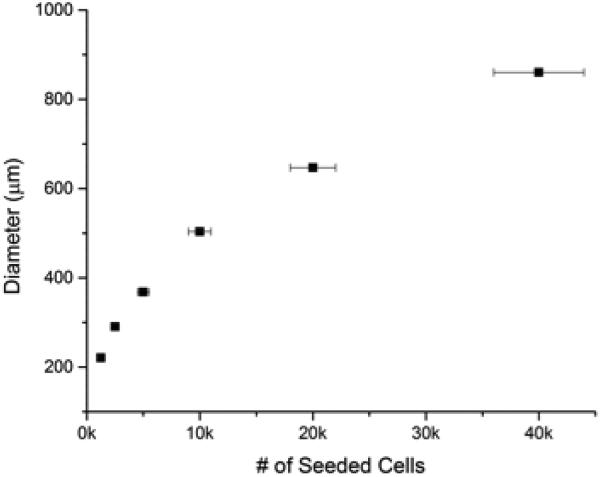
To demonstrate the scalability and reproducibility of spheroid formation, we investigated the dependency of cell seeding number on subsequent spheroid diameter. Figure 2 shows the resulting trend-line for both the U2OS and MDA-MB-231 cell lines. The results demonstrate the fidelity and reproducibility of our method. In both cases, the data was fit to a power-law (R2 >0.98) with very small sample-to-sample error (<3% in U2OS cells and <5% in MDA-MB-231 cells). The horizontal error bars represent the cell counting errors arising from using a standard hemocytometer, which is estimated at less than 10%. The results indicate that homogenous samples are reproducibly prepared and that spheroid size can be precisely controlled.
Figure 2.
Controllable Spheroid Size: Spheroid size can be controlled by varying cell seeding number. Sizing based on cell seeding number was demonstrated with both bone (top) and breast (bottom) cancer cells.
COLLAGEN GEL PROPERTIES AFFECT SPHEROID GROWTH
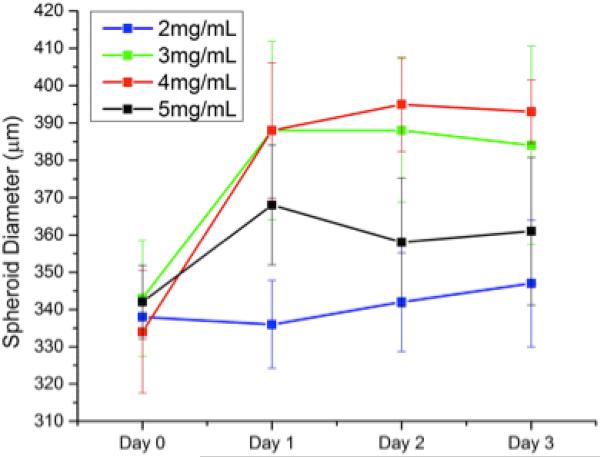
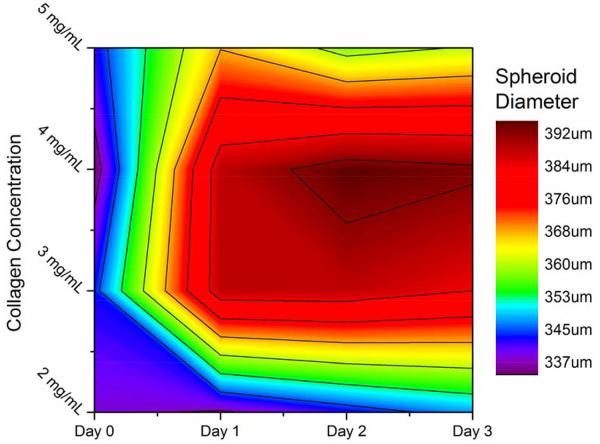
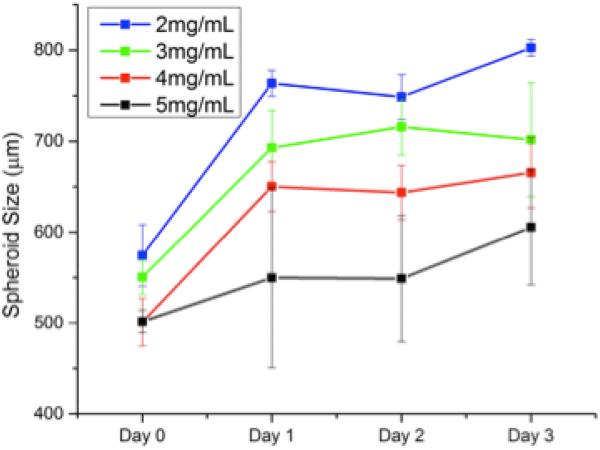
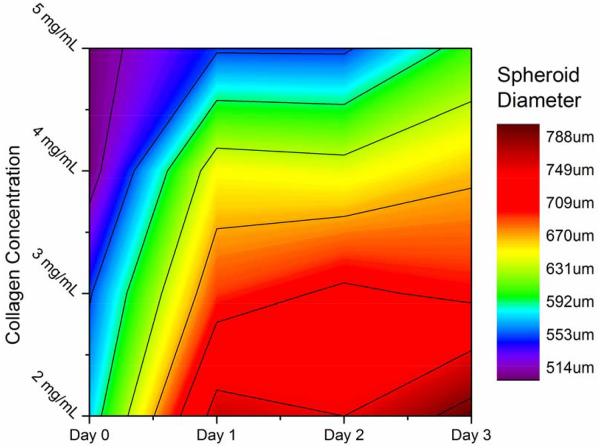
Embedding spheroids inside a matrix represents an important step forward in in vitro cancer research; however the matrix must be tunable to reflect the highly variable properties of the in vivo ECMs. Our chosen scaffold, type 1 collagen gels, can be prepared with different collagen content to directly control the pore size, attachment ligand density, and mechanical properties of the gels [37]. We therefore investigated the effect of changing the collagen gel content surrounding the embedded spheroids by seeding spheroids in collagen gels with collagen content ranging from 2-5 mg/mL. These gels possess linear elastic moduli that vary over an order of magnitude, (~10-200 kPa) enabling us to expose the spheroids to a wide range of mechanical environments [38, 39]. Figures 3 and 4 show the line and contour plot of both U2OS and MDA-MB-231 spheroid growth, respectively, over 72 hours in different collagen gels. In figures 3 and 4, the X-axis in the top figure represents time and the legend entries represent different gel collagen densities. In figures 3b and 4b, the colored XY-plane represents the spheroid size under those conditions. The spectrum, from red to purple indicates increasing spheroid diameter. In general, U2OS spheroids showed a standard deviation in diameter of 3-5% while the MDA-MB-231 spheroids showed slightly higher error of approximately 6-8%. By three days, there was a significant difference in the size of spheroids within the different collagen gels. In U2OS cells, spheroids grow the largest in 4 mg/mL collagen gels while MDA-MB-231 cells exhibit optimal growth in 2mg/mL gels.
Figure 3.
U2OS Spheroid Growth in Collagen: Bone cancer spheroids grown in collagen gels with varying mechanical properties demonstrated the most growth in 3-4 mg/mL, growing less in both stiffer and weaker gels.
Figure 4.
MDA-MB 231 Spheroid Growth in Collagen: Breast cancer spheroids grown in collagen gels with varying mechanical properties demonstrated the most growth in the weakest collagen gels.
TUMOR LIKE CHARACTERISTICS
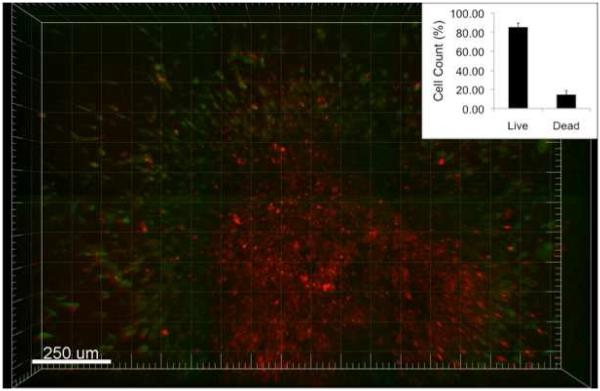
For live/dead staining, the Ethidium homodimer-1 fluorescence was used to indicate a compromised cell membrane with subsequent binding to nucleic acids and Calcein AM staining demonstrated metabolically active cells. After 72 hours of growth within collagen, this live/dead staining revealed a pattern of metabolic activity cells surrounding a core of dead cells. Figure 5 demonstrates a spheroid with a metabolically active ring of cells on the periphery combined with non-viable cell within the core. To quantitatively look at cell death, the same dyes were utilized via FACs. The population of disaggregated spheroids had 85.50±4.23% live cells and 14.50 ±4.23% dead cells.
Figure 5.
Pattern of Metabolic Activity: A spheroid in collagen was stained with a calcein based stain (green) to show metabolically active cells and an ethidium based stain (red) to demonstrate cells with a compromised membrane. The red staining on the interior indicates a mostly dead core, whereas the green cells shows a metabolically active outer ring of cells. The inset quantitatively demonstrates that 85.5% of the spheroid is alive after 3 days via FACS.
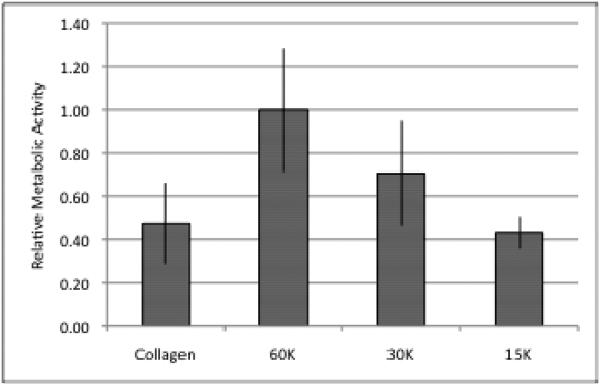
Next, the metabolic activity of spheroids embedded within collagen for 24 hours was assessed after being disaggregated and seeded in a monolayer. To account for cell growth during spheroid formation, cells were counted after disaggregation. After 96 hours, spheroids seeded with 20,000 cells had grown to 60,125 ± 8,819 cells (n=4). Figure 6 shows a comparison of MTS metabolic activity of cells grown in a spheroid compared to cells grown solely in a monolayer. The cells from the spheroid exhibited a significantly lower MTS activity value (p<<0.05) being comparable to only 30,000 cells grown in a monolayer.
Figure 6.
Metabolic Activity of Spheroids: Disaggregated spheroids were plated in a monolayer and the metabolic activity was compared to cells grown only in a monolayer. Although there were approximately 60,000 cells, the overall metabolic level is comparable to 30,000 cells.
SPHEROID RESPONSE TO DIFFERENT DRUG DELIVERY METHODS
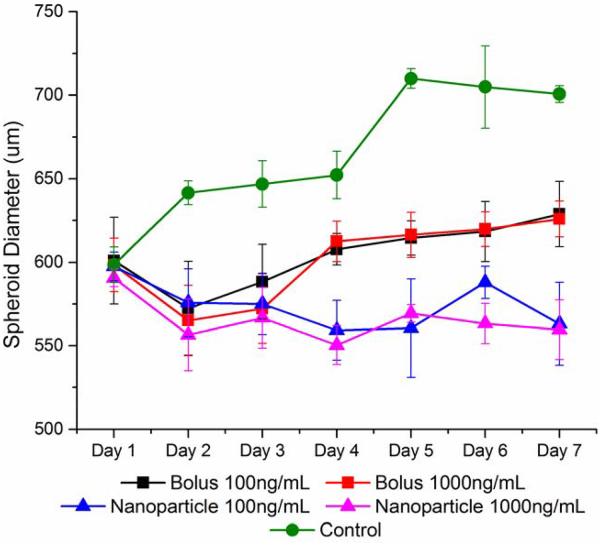
We treated MDA-MB-231 spheroids with paclitaxel delivered either as a bolus or within expansile nanoparticles (eNPs) [33, 40]. Expansile nanoparticles are designed to directly transport the encapsulated drug into the cell using macropinocytosis, where pH-mediated drug release occurs in the endosome [41]. Figure 7 shows that eNPs have fully penetrated the spheroid within 24 hours (eNPs are labeled red). As shown in Figure 8, both treatments showed a statistically significant (p < 0.05) spheroid size reduction when compared to control samples. Interestingly, the decrease in spheroid size in response to paclitaxel treatment was similar for both the low and high dose (100ng/mL versus 1000ng/mL) for a given delivery method, however, Pax-eNP resulted in significantly greater size reduction in spheroid diameter as compared to treatment with bolus paclitaxel.
Figure 7.
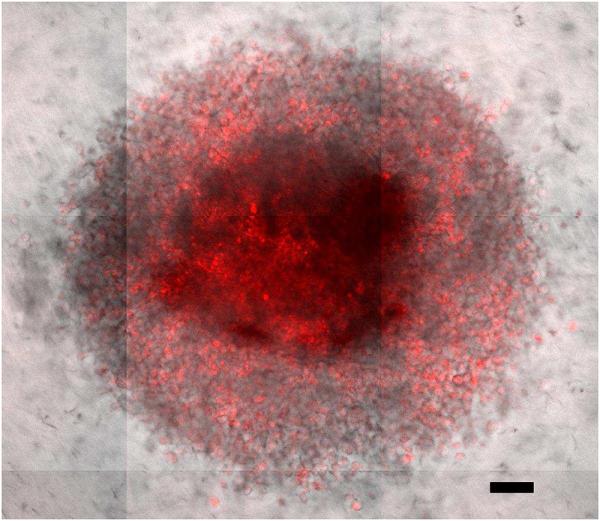
Nanoparticle Penetration: Within 24 hours fluorescently labeled nanoparticles were able to fully penetrate a 20,000 cell MDA-MB 231 spheroid. Scale bar is 100 μm.
Figure 8.
Spheroid Treatment with Nanoparticles: Paclitaxel, a chemotherapeutic drug was delivered via bolus dose or nanoparticles for 24 hours before the treatment was removed. The nanoparticle delivery method was the most effective at reducing spheroid size.
DISCUSSION
Over the last two decades, the identification of new anticancer agents for clinical evaluation has relied on the successful completion of in vitro cell toxicity assays, tumor bearing small animal models, and large animal biodistribution/toxicity studies. Animals do provide a physiological model for evaluating drug efficacy and toxicity, but are expensive, labor intensive, and low throughput. In contrast, the rapid and high throughput in vitro models used to analyze cellular and multicellular responses are based on 2D monolayers, and possess a number of limitations including artificial and unrealistic mechanical environments with a lack of ECM, tumor macrostructure, and diffusion gradients [18]. The results obtained with these 2D monolayer models, therefore, do not always reflect or predict in vivo performance. Thus, 3D cell spheroid models are being used to more accurately represent the native tumor environment (and mirror the findings observed in preclinical tumor bearing animals or patients). Building on the successes of previous 3D spheroids, [25-30] we extend this methodology and report a scalable method for preparation of single spheroids of controllable size incorporated into collagen gels as a tumor model.
Using cell lines derived from two different human carcinomas (osteosarcoma, U2OS, and adenocarcinoma, MDA-MB-231, cell lines), we demonstrate the ability to accurately control the size of multicellular spheroids based on cell seeding. Osteosarcoma and adenocarcinoma spheroids are prepared ranging from ≈ 115 to 395 and 200 to 875 μm in diameter, respectively, based on initial cell seeding number (Figure 2). As the spheroid is embedded in collagen, a suitable matrix is provided for cell ingrowth and interaction with cells, as observed in vivo. The collagen gel also provides a means to further control tumor-matrix interactions through alteration of the mechanical properties of the collagen gel. Osteosarcoma and adenocarcinoma spheroids perform strikingly differently in collagen gels varying in modulus from ~10 to 200 kPa. As shown in Figures 3 and 4, U2OS and MDA-MB-231 spheroids with diameters ranging from ≈ 335 to 395 and 550 to 800 μm, respectively are observed based on the mechanical properties of the collagen gels. The largest osteosarcoma spheroids are present in the stiffest gel while the largest adenocarcinoma spheroids are found in the softer gel. The preference of bone and breast cancer cells for stiffer and softer gels, respectively, indicates an optimal mechanical environment for these cells, and is consistent with other reports [42-44].
Optical imaging of the single intact spheroid enables evaluation of spheroid shape, morphology, and surface characteristics as well as analysis after histological or classical optical live/dead stains. Figure 5 shows an optical image of a MDA-MB-231 spheroid after Calcein AM/Ethidium homodimer-1 staining, highlighting the metabolically active ring of cells on the periphery along with the non-viable cells located within the core. Observation of these tumor hallmarks reflects the metabolic and proliferative gradients present in this model [3, 11]. The combination of decreased metabolic gradients and growth reduces overall metabolic activity in 3D-culture compared to its 2D-monolayer counterpart [3, 11]. To further assess this effect in our model, collagen embedded MDA-MB-231 spheroids were disaggregated, seeded in a monolayer, and analyzed for metabolic activity. As shown in Figure 6, cells from the spheroid display a relatively lower MTS activity value compared to those grown in 2D. In fact, 60,000 spheroid-grown cells exhibit the activity of approximately 30,000 cells grown in a monolayer. However, an MTS focuses solely on metabolic level of live cells. To ascertain the population of dead cells, FACs was used, confirming that after 3 days in collagen a portion (14.50 ±4.23%) of the spheroid is dead. This latter result documents that our spheroid model is amenable to both single cell analyses (e.g., cell uptake, metabolic activity, DNA studies), and whole spheroid experiments. Furthermore, nucleic acid extraction allows insights into global changes in genetic expression.
A key feature of this system is the ability to investigate the complexity of cancer treatment by providing a model solid tumor as opposed to conducting studies on a monolayer. One potential application of this model is as a tool for evaluating drug delivery devices and quantifying their impact on tumor size. Penetration through the collagen and the spheroid as well as cellular uptake must be carefully considered when treating large cell masses, but these delivery challenges do not arise in monolayer cell cultures. We therefore hypothesized that similar to tumors in vivo, where pharmacological kinetics play an important role in drug efficacy,[45] collagen embedded spheroids will show a differential drug response when the delivery method is varied. For these studies, we compared paclitaxel delivery as a bolus dose, as is practiced in the clinic, versus loaded within an expansile nanoparticle [33]. The expansile nanoparticle is designed to localize to the tumor and once inside the tumor cell or ECM where the pH is mildly acidic (pH = 5.5-6.5), the particle will expand or swell to release the paclitaxel resulting in focused localized tumor delivery.
A striking difference in efficacy, as measured by spheroid growth inhibition, is observed between these two paclitaxel delivery methods (Figure 8). Although bolus delivery does slow growth, the effect is significantly more pronounced with delivery via expansile nanoparticles. A second set of experiments examines the uptake of rhodamine labeled eNPs in the spheroid using confocal microscopy and shows the eNPs dispersed throughout the spheroid after diffusing through the collagen and into the spheroid (Figure 7). This result is similar to eNPs in vivo, where eNPs are present within the tumor and release drug locally over time as opposed to the bolus delivery of paclitaxel where the tumor is only transiently exposed to drug. Interestingly, when these two paclitaxel delivery modes are tested in the same cell line in a 2D monolayer there is no difference in response between bolus and eNP delivered paclitaxel [40]. In contrast, our findings using the 3D spheroids mirror the results obtained using an established human triple negative breast cancer fat-pad tumor mouse model [40]. A bolus dose of paclitaxel results in a small reduction in tumor size, while the Pax-eNP treatment significantly reduces tumor growth after 6 weeks (p<0.05). The spheroid model better replicates the observed in vivo response of the two different paclitaxel delivery methods. These results demonstrate the importance of evaluating drug candidates along with the method of delivery as synergistic beneficial results may arise from the combination.
Several studies report that cells grown in monolayer are more sensitive to radiation exposure when compared to in vivo tumors [46-48]. Equal doses of radiation to a malignant cell line had a greater effect when the tumor cells were grown as a monolayer rather than in a murine tumor model [46]. This discrepancy is attributed to increased cellular robustness due to cell-cell proximity, paracrine signaling, and a hypoxic core [49, 50]. Spheroids with 3D cell contacts and hypoxic regions, which reduce the presence of reactive oxygen species, are more predictive of in vivo radiation response than are cell monolayer models [49-52]. When a human melanoma line was treated with radiation both as in vitro spheroids and in vivo murine tumors, delay in cell growth within spheroids correlated with survival with in vivo tumors [51]. In addition to radiation, spheroids are being used as a testing model for multiple experimental treatments such as photodynamic therapy [53], gene therapy [54], and radioimmunotherapy [55]. These examples highlight the need for and relevance of using 3D spheroids for assessing existing treatment methods and exploring novels treatments. Although a step forward, these spheroid-based experiments disregard contributions from the extracellular matrix. The collagen embedded spheroid model would further increase the relevance of the spheroid system as a tool for therapy assessment and discovery.
CONCLUSION
While no tumor model fully recreates the in vivo system, the presented model combines the favorable attributes of a large spheroid structure with an extracellular matrix. It builds on the successes of previous 3D spheroid models and extends the methodology for preparation of single spheroids of controllable size incorporated into a collagen gel as a tumor model. The key features of this tumor model are: 1) a multicellular spheroid of controllable size; 2) a collagen based ECM structure surrounding the spheroid; and 3) a spheroid with a metabolically active outer layer and necrotic core. Our technique remains easily scalable for high throughput applications and the combination of whole spheroid techniques with single cell analysis allows in-depth investigations into basic cancer cell behavior from the macroscopic to single molecule scales. Using this tumor model, we observed performance differences in terms of chemotherapeutic response based on the mode of paclitaxel delivery, which unlike the monolayer experiments mirrored the results obtained in an in vivo animal study. In summary, the 3D biomimetic culture platform described here will facilitate the study of basic cancer cell behavior, the evaluation of new anticancer agents, the role of drug delivery devices in optimizing the anti-tumoral response, and the development of simulation software for data modeling and predictions.
Acknowledgements
This work was supported in part by BU, BWH, National Science Foundation (DMR-1006601 to MWG and DMR-1206635 to MHZ), the Boston University’s Nanomedicine Program and Cross-Disciplinary Training in Nanotechnology for Cancer (NIH R25 CA153955), and the Boston University T32 Grant entitled Translational Research in Biomaterials (NIH T32EB006359).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hutmacher DW. Biomaterials offer cancer research the third dimension. Nature Mater. 2010;9:90–3. doi: 10.1038/nmat2619. [DOI] [PubMed] [Google Scholar]
- 2.Fallica B, Makin G, Zaman MH. Bioengineering approaches to study multidrug resistance in tumor cells. Integr Biol. 2011;3(5):529–39. doi: 10.1039/c0ib00142b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro - a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;15(5):405–12. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Dhiman HK, Ray AR, Panda AK. Characterization and evaluation of chitosan matrix for in vitro growth of MCF-7 breast cancer cell lines. Biomaterials. 2004;25(21):5147–54. doi: 10.1016/j.biomaterials.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 5.Dhiman HK, Ray AR, Panda AK. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials. 2005;26(9):979–86. doi: 10.1016/j.biomaterials.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Sun L, Maffini MV, Soto A, Sonnenschein C, Kaplan DL. A complex 3D human tissue culture system based on mammary stromal cells and silk scaffolds for modeling breast morphogenesis and function. Biomaterials. 2010;31(14):3920–9. doi: 10.1016/j.biomaterials.2010.01.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, et al. Engineering tumors with 3D scaffolds. Nature Methods. 2007;4(10):855–60. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 8.Pampaloni F, Reynaud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nature Rev Mol Cell Biol. 2007;8:839–45. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 9.Martin KJ, Patrick DR, Bissell MJ, Fournier MV. Prognostic breast cancer signature identified from 3D culture model accurately predicts clinical outcome across independent datasets. PLoS ONE. 2008;3(8):e2994. doi: 10.1371/journal.pone.0002994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver VM, Petersen OW, Wang F, Larabell C, Briand P, Damsky C, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137(1):231–45. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JB, Stein R, O’Hare MJ. Three-dimensional in vitro tissue culture models of breast cancer - a review. Breast Cancer Res Treat. 2004;85(3):281–91. doi: 10.1023/B:BREA.0000025418.88785.2b. [DOI] [PubMed] [Google Scholar]
- 12.Fallica B, Maffei JS, Villa S, Makin G, Zaman M. Alteration of cellular behavior and response to PI3K pathway inhibition by culture in 3D collagen gels. PLoS ONE. 2012;7(10):e48024. doi: 10.1371/journal.pone.0048024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurski LA, Jha AK, Zhang C, Jia X, Farach-Carson MC. Hyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cells. Biomaterials. 2009;30(30):6076–85. doi: 10.1016/j.biomaterials.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103(4):655–63. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Delivery Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 16.Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials. 2010;31(32):8494–506. doi: 10.1016/j.biomaterials.2010.07.064. [DOI] [PubMed] [Google Scholar]
- 17.Carletti E, Motta A, Migliaresi C. Scaffolds for tissue engineering and 3D cell culture. In: Haycock JW, editor. 3D Cell Culture. Springer; 2011. pp. 17–39. [DOI] [PubMed] [Google Scholar]
- 18.Ghajar CM, Bissell MJ. Tumor engineering: the other face of tissue engineering. Tissue Eng Part A. 2010;16(7):2153–6. doi: 10.1089/ten.tea.2010.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedrich J, Ebner R, Kunz-Schughart LA. Experimental anti-tumor therapy in 3-D: spheroids-old hat or new challenge? Int J Radiat Biol. 2007;83(11-12):849–71. doi: 10.1080/09553000701727531. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland RM, McCredie JA, Inch WR. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J Natl Cancer Inst. 1971;46(1):113–20. [PubMed] [Google Scholar]
- 21.Timmins N, Dietmair S, Nielsen L. Hanging-drop multicellular spheroids as a model of tumour angiogenesis. Angiogenesis. 2004;7:97–103. doi: 10.1007/s10456-004-8911-7. [DOI] [PubMed] [Google Scholar]
- 22.Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng. 2003;83:173–80. doi: 10.1002/bit.10655. [DOI] [PubMed] [Google Scholar]
- 23.Santini MT, Rainaldi G, Indovina PL. Apoptosis, cell adhesion and the extracellular matrix in the three-dimensional growth of multicellular tumor spheroids. Crit Rev Oncol Hematol. 2000;36:75–87. doi: 10.1016/s1040-8428(00)00078-0. [DOI] [PubMed] [Google Scholar]
- 24.St. Croix B. Cell adhesion and drug resistance in cancer : current opinion in oncology. Curr Opin Oncol. 1997;9:549–56. doi: 10.1097/00001622-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Stein AM, Demuth T, Mobley D, Berens M, Sander LM. A mathematical model of glioblastoma tumor spheroid invasion in a three-dimensional in vitro experiment. Biophys J. 2007;92(1):356–65. doi: 10.1529/biophysj.106.093468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell HS, Wharton SB, Leaver HA, Whittle IR. Effects of N-6 essential fatty acids on glioma invasion and growth: experimental studies with glioma spheroids in collagen gels. J Neurosurg. 1999;91(6):989–96. doi: 10.3171/jns.1999.91.6.0989. [DOI] [PubMed] [Google Scholar]
- 27.Yip D, Cho CH. A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem Biophys Res Commun. 2013;433:327–32. doi: 10.1016/j.bbrc.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Wiercinska E, Naber HP, Pardali E, van der Pluijm G, van Dam H, ten Dijke P. The TGF-β/Smad pathway induces breast cancer cell invasion through the up-regulation of matrix metalloproteinase 2 and 9 in a spheroid invasion model system. Breast Cancer Res Treat. 2011;128(3):657–66. doi: 10.1007/s10549-010-1147-x. [DOI] [PubMed] [Google Scholar]
- 29.Tamaki M, McDonald W, Amberger VR, Moore E, Del Maestro RF. Implantation of C6 astrocytoma spheroid into collagen type I gels: invasive, proliferative, and enzymatic characterizations. J Neurosurg. 1997;87(4):602–9. doi: 10.3171/jns.1997.87.4.0602. [DOI] [PubMed] [Google Scholar]
- 30.Vamvakidou AP, Mondrinos MJ, Petushi SP, Garcia FU, Lelkes PI, Tozeren A. Heterogeneous breast tumoroids: An in vitro assay for investigating cellular heterogeneity and drug delivery. J Biomol Screen. 2007;12(1):13–20. doi: 10.1177/1087057106296482. [DOI] [PubMed] [Google Scholar]
- 31.Xue B, Wen C, Shi Y, Zhao D, Li C. Human NRAGE disrupts E-cadherin/β-catenin regulated homotypic cell–cell adhesion. Biochem Biophys Res Commun. 2005;336:247–51. doi: 10.1016/j.bbrc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 32.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–85. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colby A, Colson Y, Grinstaff MW. Microscopy and tunable resistive pulse sensing characterization of the swelling of pH-responsive, polymeric expansile nanoparticles. Nanoscale. 2013;5:3496–504. doi: 10.1039/c3nr00114h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst. 1990;82(15):1247–59. doi: 10.1093/jnci/82.15.1247. [DOI] [PubMed] [Google Scholar]
- 35.Foa R, Norton L, Seidman AD. Taxol (paclitaxel): a novel anti-microtubule agent with remarkable anti-neoplastic activity. Int J Clin Lab Res. 1994;24(1):6–14. doi: 10.1007/BF02592403. [DOI] [PubMed] [Google Scholar]
- 36.Lorenz W, Reimann H-J, Schmal A, Dormann P, Schwarz B, Neugebauer E, et al. Histamine release in dogs by Cremophor El® and its derivatives: oxethylated oleic acid is the most effective constituent. Agents Actions. 1977;7(1):63–7. doi: 10.1007/BF01964882. [DOI] [PubMed] [Google Scholar]
- 37.Zaman MH, Trapani LM, Sieminski AL, MacKellar D, Gong H, Kamm RD, et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. PNAS. 2006;103(29):10889–94. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osborne CS, Barbenel JC, Smith D, Savakis M, Grant DMH. Investigation into the tensile properties of collagen/chondroitin-6-sulphate gels: the effect of crosslinking agents and diamines. Med Biol Eng Comput. 1998;36:129–34. doi: 10.1007/BF02522870. [DOI] [PubMed] [Google Scholar]
- 39.Sheu M-T, Huang J-C, Yeh G-C, Ho H-O. Characterization of collagen gel solutions and collagen matrices for cell culture. Biomaterials. 2001;22:1713–9. doi: 10.1016/s0142-9612(00)00315-x. [DOI] [PubMed] [Google Scholar]
- 40.Liu R, Gilmore DM, Zubris KAV, Xu X, Catalano PJ, Padera RF, et al. Biomaterials. 2012. Prevention of nodal metastases in breast cancer following the lymphatic migration of paclitaxel-loaded expansile nanoparticles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zubris KAV, Liu R, Colby A, Schultz MD, Colson YL, Grinstaff MW. In vitro activity of paclitaxel-loaded polymeric expansile nanoparticles in breast cancer cells. Biomacromolecules. 2013;14(6):2074–82. doi: 10.1021/bm400434h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mylona E, Dailiana Z, Trepat X, Lagoudakis M, editors. European Symposium on Biomedical Engineering. 2008. Substrate rigidity dictates phenotype, survival, and mechanics of primary human osteosarcoma cells. [Google Scholar]
- 43.PaszeK MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Yoshikawa HY, Cui J, Kratz K, Matsuzaki T, Nakabayashi S, Marx A, et al. Quantitative evaluation of adhesion of osteosarcoma cells to hydrophobic polymer substrate with tunable elasticity. J Phys Chem B. 2012;116(28):8024–30. doi: 10.1021/jp212385p. [DOI] [PubMed] [Google Scholar]
- 45.Bachur NR. Oral Complications of Cancer Chemotherapy. Springer; 1983. Pharmacology of chemotherapeutic agents; pp. 13–22. [Google Scholar]
- 46.Bristow RG, Hill RP. Comparison between in vitro radiosensitivity and in vivo radioresponse in murine tumor cell lines II: in vivo radioresponse following fractionated treatment and in vitro/in vivo correlations. Int J Radiat Oncol Biol Phys. 1990;18:331–45. doi: 10.1016/0360-3016(90)90098-5. [DOI] [PubMed] [Google Scholar]
- 47.Malaise EP, Fertil B, Chavaudra N, Guichard M. Distribution of radiation sensitivities for human tumor cells of specific histological types: Comparison of in vitro to in vivo data. Int J Radiat Oncol Biol Phys. 1986;12:617–24. doi: 10.1016/0360-3016(86)90071-4. [DOI] [PubMed] [Google Scholar]
- 48.Brzozowska K, Pinkawa M, Eble MJ, Müller W-U, Wojcik A, Kriehuber R, et al. In vivo versus in vitro individual radiosensitivity analysed in healthy donors and in prostate cancer patients with and without severe side effects after radiotherapy. Int J Radiat Biol. 2012;88:405–13. doi: 10.3109/09553002.2012.666002. [DOI] [PubMed] [Google Scholar]
- 49.T. Santini GR. Multicellular tumour spheroids in radiation biology. Int J Radiat Biol. 1999;75:787–99. doi: 10.1080/095530099139845. [DOI] [PubMed] [Google Scholar]
- 50.Kunz-Schughart LA. Multicellular tumor spheroids: intermediates between monolayer culture and in vivo tumor. Cell Biol Int. 1999;23:157–61. doi: 10.1006/cbir.1999.0384. [DOI] [PubMed] [Google Scholar]
- 51.Rofstad EK, Wahl A, Brustad T. Radiation response of multicellular spheroids initiated from five human melanoma xenograft lines. Relationship to the radioresponsiveness in vivo. Br J Radiol. 1986;59:1023–9. doi: 10.1259/0007-1285-59-706-1023. [DOI] [PubMed] [Google Scholar]
- 52.Olive PL, Durand RE. Drug and radiation resistance in spheroids: cell contact and kinetics. Cancer Metastasis Rev. 1994;13:121–38. doi: 10.1007/BF00689632. [DOI] [PubMed] [Google Scholar]
- 53.Hirschberg H, Sun C-H, Tromberg BJ, Yeh AT, Madsen SJ. Enhanced cytotoxic effects of 5-aminolevulinic acid-mediated photodynamic therapy by concurrent hyperthermia in glioma spheroids. J Neurooncol. 2004;70(3):289–99. doi: 10.1007/s11060-004-9161-7. [DOI] [PubMed] [Google Scholar]
- 54.Boyd M, Mairs S, Stevenson K, Livingstone A, Clark A, Ross S, et al. Transfectant mosaic spheroids: a new model for evaluation of tumour cell killing in targeted radiotherapy and experimental gene therapy. J Gene Med. 2002;4(5):567–76. doi: 10.1002/jgm.293. [DOI] [PubMed] [Google Scholar]
- 55.Ballangrud ÖM, Yang W-H, Palm S, Enmon R, Borchardt PE, Pellegrini VA, et al. Alpha-particle emitting atomic generator (actinium-225)-labeled trastuzumab (Herceptin) targeting of breast cancer spheroids efficacy versus HER2/neu Expression. Clin Cancer Res. 2004;10(13):4489–97. doi: 10.1158/1078-0432.CCR-03-0800. [DOI] [PubMed] [Google Scholar]