Abstract
Optic neuritis is an inflammatory demyelination of optic nerve often occurring in multiple sclerosis (MS) patients. Mice with experimental autoimmune encephalomyelitis (EAE), an MS model, develop optic neuritis, but it is detected histologically after sacrifice, limiting the ability to monitor progression or treatment in vivo. We examined whether pupillary light responses measured by pupillometry can identify eyes with optic neuritis in EAE mice. C57BL/6 mice were exposed to unilateral light flashes of increasing intensity at 10 second intervals (4.7, 37, and 300 μW/cm2). Pupillary responses were recorded with a commercially available pupillometer. EAE was then induced by immunization with myelin oligodendrocyte glycoprotein. Pupillometry was repeated up to 17 days post-immunization, and responses were correlated with optic nerve inflammation. By day 17 post-immunization, 90% of EAE eyes had optic nerve inflammation. EAE eyes had significantly reduced pupillary constriction compared to control eyes. Mice exhibited more than a 25% decrease in pupillary constriction in at least one eye by days 13-15 post-immunization. In some eyes, pupil responses decreased prior to onset of detectable inflammation. Results show that pupillometry detects decreased optic nerve function in experimental optic neuritis, even in the absence of histological detection. Measuring pupillary constriction allows in vivo identification and functional assessment of eyes with optic neuritis that will be useful in evaluating potential therapies over time. Furthermore, results demonstrate that decreased visual function occurs early in optic neuritis, before optic nerve inflammation reaches its peak level.
Keywords: Optic neuropathy, pupillometry, multiple sclerosis, EAE
1. Introduction
Optic neuritis, an inflammatory demyelinating disease of the optic nerve, often occurs in patients with the central nervous system demyelinating disease MS (Noseworthy et al., 2000; Arnold, 2005; Optic Neuritis Study Group, 2008). Experimental optic neuritis occurs in the MS model EAE, induced by immunization of rodents with myelin antigens that result in monophasic, relapsing, or chronic optic nerve inflammation (Potter and Bigazzi, 1992; Meyer et al., 2001; Shao et al., 2004; Shindler et al., 2006, 2008), with distinct disease courses depending on the specific antigen and type of strain used.
EAE optic neuritis models have provided important insight into mechanisms of retinal ganglion cell (RGC) loss induced by inflammation, and have provided a means of examining potential therapies designed to preserve RGCs damaged during optic neuritis (Guy et al., 1998; Hobom et al., 2004; Shindler et al., 2007, 2008; Dutt et al., 2010). However, the development of optic neuritis is variable, with bilateral optic neuritis occurring in some animals, and unilateral or no optic neuritis in others (Shindler et al., 2006, 2008). Because detection of optic neuritis depends on histological evaluation of optic nerves, analysis of effects of optic neuritis are complicated by the possibility that inflammation may resolve by the time tissues are harvested, or inflammation may occur in focal lesions along the optic nerve that may be missed when tissues are sectioned. Efficient, reliable methods of detecting which eyes in EAE mice have signs of optic neuritis in vivo will reduce the chances of missing the optic nerve inflammation, and can facilitate longitudinal studies of the effects of experimental optic neuritis on visual function.
Pupillometry measures dynamic changes in pupillary diameter in response to light stimuli, providing a functional assessment of RGC axonal pathways. Pupillary responses have been used as important measures of optic nerve function in optic neuritis and MS patients (Ellis, 1979; Beck et al., 1992; Surakka et al., 2008). Pupillometry has been used recently to characterize normal murine pupillary light responses and the role of melanopsin-containing RGCs (Zhu et al., 2007; Hussain et al., 2009). A potential role for pupillometry in monitoring optic nerve function in EAE mice was suggested (Hussain et al., 2009), but has not been reported.
In the current study, pupillary light responses were recorded in mice with chronic EAE. The degree of pupil constriction in each eye in response to three different light intensities was measured and compared to responses in control mice. The degree of inflammation observed in histological sections of optic nerves was evaluated to determine whether inflammation correlated with reduced pupillary responses. Results suggest that pupillometry can be used as a sensitive, non-invasive method of detecting eyes with experimental optic neuritis, and suggest that even mild or focal inflammation below the level of detection by gross histology can result in decreased optic nerve function in this model.
2. Materials and methods
2.1 Mice
Seven week old female C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Animals were housed at animal facilities at the University of Pennsylvania. All treatments conformed to Institutional Animal Care and Use Committee guidelines and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2 RGC labeling
RGCs were labeled using previously published methods (Shindler et al., 2006, 2008). Briefly, mice were anesthetized intraperitoneally with 2 mg ketamine (Sigma, St. Louis, MO) and 1 mg xylazine (Sigma). Heads were shaved, a mediosagittal incision was made in the scalp, and holes were drilled through the skull. 2.5 μl of 1.25% hydroxystilbamidine (Fluorogold; Molecular Probes, Eugene, OR) in sterile phosphate buffered saline (PBS) was injected stereotactically into each superior colliculus.
2.3 Pupillometry
Pupil responses were elicited and recorded using a Neuroptics Pupillometer (San Clemente, CA, USA) for rodents (Hussain et al., 2009). This device uses a dual-camera system to emit infrared wavelengths and allow for pupil fixation in the pigmented mouse. Cameras are positioned in front of the right and left eye of non-anesthetized mice, and single light flashes with varying intensities are generated in one eye. The light stimulus is presented to the eye being tested by a circumferential configuration of eight 5 mm white light emitting diodes, within an enclosed cone, whose opening is directed to the eye. This provides equal illumination to all segments of the iris. Changes in pupillary diameter of both eyes are tracked and recorded through threshold detection. The Neuroptics software samples at 15 Hz frequency, and the pulse duration of light stimuli is 100ms with a relaxation time of 9,900ms. Three light intensities, low, medium and high (4.7, 37, and 300 μW/cm2, respectively) were presented at varying time points in three separate experimental paradigms. A series of five flashes of light were used to stimulate each eye, and the percent constriction of each pupil was determined by averaging the change in pupil diameter following each flash of light. Mice were dark-adapted overnight prior to pupillometry, and pupil constriction was measured following each light flash as the maximal percent decrease in pupillary size from the dark-adapted pupil diameter. Resting (dark-adapted) pupil diameter did not vary significantly between baseline (prior to disease induction) and subsequent time points along the EAE disease course.
2.4 Induction and monitoring of EAE
EAE was induced one week after RGC labeling. Mice (n = 5/experiment) were anesthetized and injected subcutaneously at two sites on the back with a total of 200 μg of myelin oligodendrocyte glycoprotein peptide (MOG; Genscript, Piscataway, NJ, USA) emulsified in Complete Freund's Adjuvant (CFA; Difco, Detroit, MI, USA) containing 2.5 mg/ml mycobacterium tuberculosis (Difco). Control mice (n = 5/experiment) were injected with an equal volume of PBS and CFA. 200 ng pertussis toxin (List Biological, Campbell, CA, USA) in 0.2 ml PBS was injected intraperitoneally on days 0 (day of immunization) and 2 in all mice. Severity of EAE was scored using a previously published 5-point scale (Shindler et al., 2006): no disease = 0; partial tail paralysis = 0.5; tail paralysis or waddling gait = 1.0; partial tail paralysis and waddling gait = 1.5; tail paralysis and waddling gait = 2.0; partial limb paralysis = 2.5; paralysis of one limb = 3.0; paralysis of one limb and partial paralysis of another = 3.5; paralysis of two limbs = 4.0; moribund state = 4.5; death = 5.0.
2.5 Histopathologic evaluation of optic nerves
Isolated optic nerves were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 5μm longitudinal sections. Following hematoxylin and eosin (H&E) staining, a blinded investigator examined each section for the presence of inflammatory cells. The degree of inflammation observed was scored using a previously described 4 point scale (Shindler et al., 2006, 2008): No infiltrating inflammatory cells = 0, mild cellular infiltration = 1, moderate infiltration = 2, severe infiltration = 3, and massive infiltration = 4. Each eye was analyzed individually as optic neuritis occurs as an independent event in EAE (Shindler et al., 2006).
2.6 Statistics
The average pupillometry reading in each eye under each light intensity stimulus was calculated for the statistical comparison. The pupillary responses to light intensity stimulus were compared between EAE mice and control mice by using generalized linear models, with inter-eye correlation between paired eyes of the same mouse adjusted for by using generalized estimating equations (Liang and Zeger, 1986). All data analysis was performed using SAS V9.2 (SAS Inc, Cary, NC), and two-sided p-value <0.05 was considered statistically significant.
3. Results
3.1 Incidence of optic neuritis in chronic EAE
In a prior study, most optic nerves in mice with chronic EAE developed histological evidence of inflammation by day 17 post-immunization (Shao et al., 2004). We found similar results, and graded the degree of optic nerve inflammation on a relative scale. 7-8 week old female C57BL/6 mice were immunized with MOG on day 0 and observed daily for the development of EAE. EAE symptoms of tail and hind limb paralysis began by day 12-13 post-immunization, peaking and leveling off by day 15 (Fig. 1A). On day 17, mice were euthanized and optic nerves were isolated (n = 10/group). H & E staining of longitudinal optic nerve sections demonstrated increased cellularity consistent with inflammatory cell infiltration in EAE optic nerves as compared to controls (Fig. 1B-D). 90% of optic nerves from EAE mice developed histological evidence of optic neuritis, with a variable degree of inflammation as scored by a blinded investigator – 40% mild, 30% moderate, 20% severe (Fig. 1E).
Fig. 1.
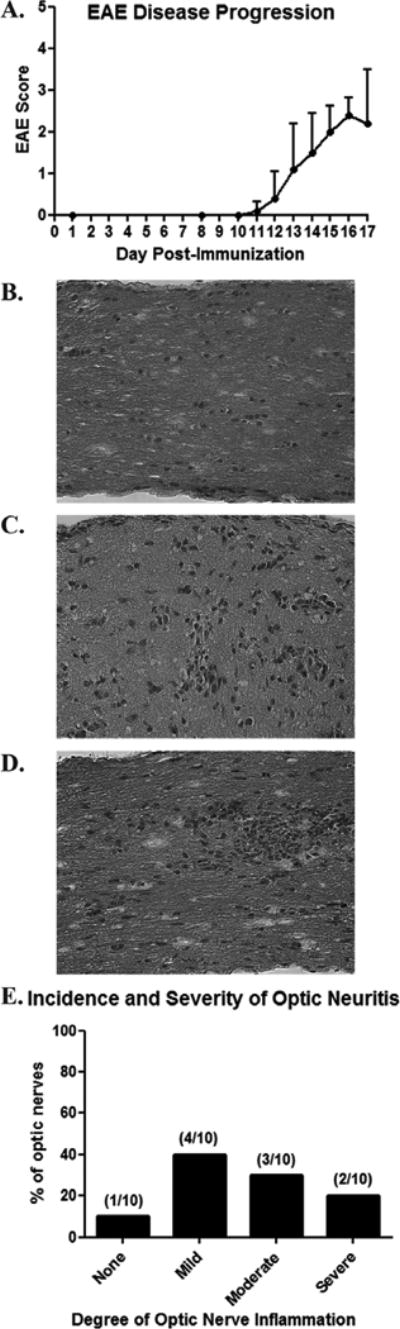
High incidence of optic neuritis occurs in chronic murine EAE. C57BL/6 mice were immunized on day 0 with MOG to induce EAE. (A) Clinical tail and limb paralysis, hallmarks of EAE inflammation in spinal cord, began by days 12-13 post-immunization in all mice (n = 5), with severity increasing through at least day 15. (B) Longitudinal section of an optic nerve from a control, non-EAE mouse stained by H&E shows the normal cellularity. (C) An optic nerve from a day 17 post-immunization EAE mouse demonstrates mild inflammation, with small scattered foci of increased cellularity. (D) Moderate inflammation, with a focal area containing marked cellular infiltrate, is shown. (E) Histological examination of 10 EAE optic nerves 17 days post-inflammation demonstrates 90% of nerves develop optic neuritis, with varying degrees of inflammation. Original magnification X40 (B-D).
3.2 Decreased pupillary light responses in EAE mice with optic nerve inflammation
Baseline pupillometry measurements were taken in EAE and control mice prior to immunization. Repeat pupillometry was performed prior to sacrifice on day 17. There was no significant difference in the degree of pupillary constriction in response to the high intensity light stimulus between EAE and control mice (Fig. 2A). However, in response to both medium (Fig. 2B) and low (Fig. 2C) intensity light stimuli, pupillary constriction was significantly decreased in EAE mouse eyes measured at day 17 post-immunization, as compared to control eyes. .
Fig. 2.
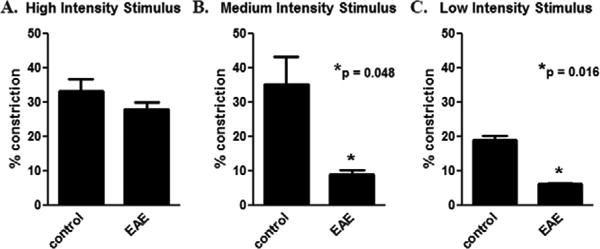
Pupillary light responses are reduced in EAE eyes. Pupillometry was performed in C57BL/6 mice before immunization and again 17 days later, after onset of EAE and optic neuritis. Pupil responses are recorded as the average percentage area constriction (pupil area prior to light stimulus – small pupil size post-stimulus/pupil area prior to light stimulus) in response to a single light flash in each eye (n = 10). (A) A 300 μW/cm2 light stimulus induced a 33.2 ± 3.5% constriction of the pupils in control mice, with no significant difference in EAE mice. (B) A 37 μW/cm2 light stimulus induced a 35.3 ± 8.1% constriction of the pupils in control mice, and a significantly smaller 9.1 ± 1.2% constriction in EAE mice. (C) A 4.7 μW/cm2 light stimulus induced an 18.9 ± 1.5% constriction of the pupils in control mice, and a significantly smaller 6.2 ± 0.4% constriction in EAE mice.
3.3 Time course of pupillary responses in experimental optic neuritis
Above results show that pupillary responses are decreased by the time overt inflammation is manifest in most optic nerves. We next examined the time course of pupil responses following induction of EAE to determine whether pupillometry can be used to detect the early onset of optic neuritis. Baseline pupillometry was performed in EAE and control mice prior to immunization, and was repeated at several time points through day 17. The high intensity light stimulus failed to produce significant differences in pupil response between EAE and control eyes (Fig. 3A) at any time point. For the medium (Fig. 3B) and low (Fig. 3C) light intensity stimuli, significant decreases in the percentage area of pupillary constriction was observed in EAE mouse eyes as compared to controls prior to day 17, as early as day 15 post-immunization.
Fig. 3.
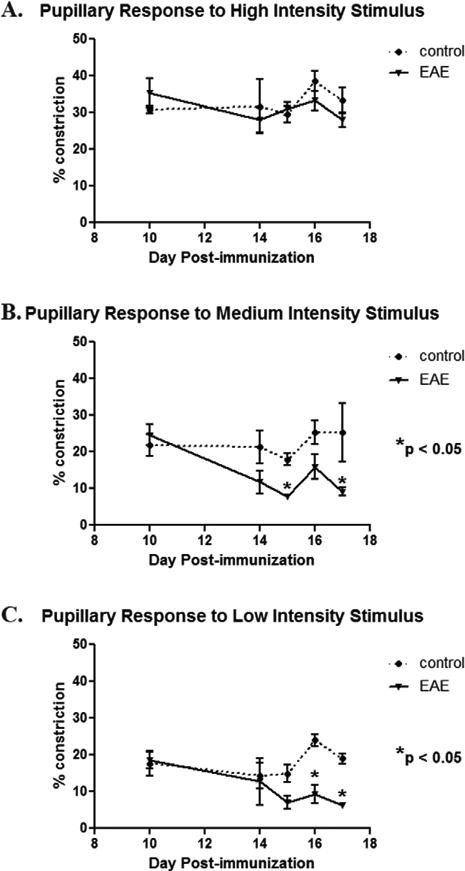
Pupillary light responses decrease by day 15 post-immunization in EAE mice. Pupillometry was repeated on multiple days after immunization with MOG to induce EAE in C57BL/6 mice. (A) Similar to findings at day 17, no difference in the degree of pupillary constriction induced by a 300 μW/cm2 light stimulus was found at any time point between control and EAE mouse eyes (n = 10). (B) Pupil constriction in response to a 37 μW/cm2 stimulus was no different between control and EAE eyes at early time points, with decreasing pupillary responses developing gradually in EAE mice and reaching statistical significance at day 15 post-immunization. (C) Similar to the medium intensity stimulus, pupil constriction in response to a 4.7 μW/cm2 stimulus was significantly decreased by day 16 post-immunization in EAE eyes as compared to control eyes.
In order to examine the relation between initial decreases in pupillary light response and histological evidence of optic nerve inflammation, a separate group of mice were immunized to induce EAE. Pupillometry was performed prior to immunization, and repeated daily beginning 10 days after immunization. Mice were sacrificed on the first day that a 25% or greater decrease in pupillary constriction from baseline was detected in at least one eye in response to the low light intensity stimulus. Amongst five mice, two developed decreased pupillary constriction on day 13, two on day 14, and one on day 15 post-immunization. After sacrifice, optic nerve sections showed varying levels of inflammation (Fig. 4), with no inflammation observed in 60% of optic nerves, including nerves from eyes that had decreased pupillary response (Fig. 4A,B). In 5 mice, histologic evidence of optic nerve inflammation was detected bilaterally in 1 mouse, unilaterally in 2 mice, and undetectable bilaterally in 2 mice.
Fig. 4.
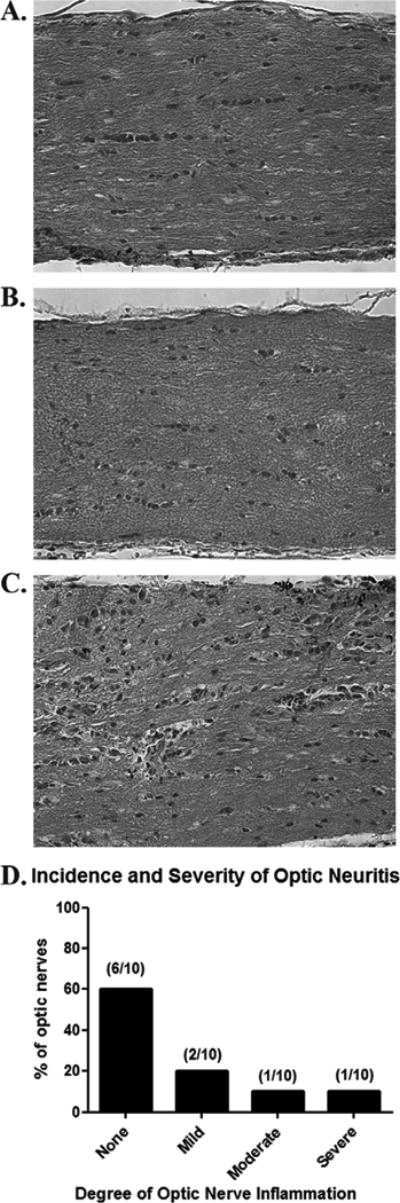
Decreased pupillary light responses precede histological evidence of optic neuritis. Baseline pupillometry was performed using a 4.7 μW/cm2 stimulus prior to induction of EAE, and was repeated daily beginning 10 days post-immunization. Mice (n = 5) were sacrificed on the first day that one or both eyes demonstrated a 25% or greater decrease in pupillary constriction compared to baseline. (A,B) Normal optic nerve histology, without evidence of inflammation, is shown from two representative EAE mouse eyes that had marked (greater than 25%) decreases in pupillary light response. (C) Mild inflammation observed in another EAE eye that had decreased pupillary constriction. (D) While 8 of 10 eyes from EAE mice had 25% or greater decrease in pupillary constriction at the time of sacrifice, only 4 eyes demonstrated varying levels of inflammation on histological evaluation, with 60% of eyes exhibiting no detectable inflammation. Original magnification X40 (A-C).
4. Discussion
Results demonstrate that almost all eyes develop optic neuritis in the C57BL/6 MOG-induced chronic EAE model, similar to previous studies using histological parameters to detect optic nerve inflammation (Shao et al., 2004; Quinn et al, 2011). By the time that detectable inflammation is present, 17 days after EAE induction, there is a significant decrease in the pupillary light reflex reflecting decreased optic nerve function. These findings suggest that this non-invasive method can be used as a surrogate measure of the presence of optic nerve inflammation in experimental optic neuritis.
While the lowest and middle level light intensity stimuli used in the current studies led to reduced pupillary responses in EAE mice, pupil responses to a high intensity stimulus were not reduced in EAE mice as compared to controls. It is possible that at higher light intensities there is sufficient signal generated to saturate the pupillary light reflex and drive maximal constriction even in nerves that are partially dysfunctional due to active inflammation. Alternatively, preserved pupil responses in EAE mice may be due to high intensity stimuli triggering the recently described intrinsic iris photoresponse (Xue et al, 2011). Results suggest that using lower light intensities, such as the 4.7 μW/cm2 stimulus used here, is likely the best method for evaluating pupillary responses in murine models of optic neuropathy. The specific degree of pupillary constriction will need to be assessed in different EAE optic neuritis models induced in other genetic backgrounds, as pupillary responses have been shown to vary depending on mouse strain and gender (Manno, 2009).
The fact that responses to lower light intensity stimuli correlated best with optic nerve inflammation is encouraging for potential use of this method in longitudinal studies. While we did not observe any significant decrease in pupillary response over time in control mice in these short-term studies, light-induced toxicity to photoreceptors can occur at high levels of light exposure (Wenzel et al., 2005; Santos et al., 2010). It is possible that light-induced damage may accumulate over time with repeated measures, and needs to be examined further, but it is less likely to occur using lower light intensity stimuli.
Interestingly, when mice were sacrificed at earlier time points, at the first onset of measureable decline in pupillary light responses, inflammation was detected in histological sections of optic nerve from only a subset of eyes. In fact, some eyes with more than a 25% decrease in pupillary constriction had no inflammation detected in the optic nerve. This suggests that pupillometry may be able to detect eyes early during the initial onset of optic neuritis, with the amount of inflammatory cells infiltrating the nerve either being too few to detect an increase in cellularity in histological sections, or alternatively there may only be small focal areas of inflammation that might be skipped during the process of sectioning the nerves. While the data at these early time points alone might raise the possibility that changes measured on pupillometry may be non-specific, the significant decrease in pupil constriction measured just a few days later (by day 17) in eyes with optic nerve inflammation suggests that this method is indeed identifying those eyes that are developing optic neuritis. This suggests that optic neuritis can induce significant visual impairment even with very mild levels of inflammation, and if patients present early after onset of symptoms, there may be an opportunity to intervene to limit the ultimate amount of inflammation that will develop. Pupillary responses have been used to assess optic nerve function in optic neuritis and MS patients (Ellis, 1979; Beck et al., 1992; Surakka et al., 2008), and recent studies demonstrated that decreased pupillary responses correlate with structural changes in the retina, specifically thinning of the retinal nerve fiber layer which contains RGC axons (Salter et al., 2009). We previously demonstrated significant RGC loss occurs in EAE eyes with optic neuritis (Shindler et al, 2008; Quinn et al, 2011). Together, results suggest pupillometry may be useful not only for detecting eyes with optic neuritis, but also for predicting RGC loss.
Pupillometry provides a non-invasive, quantifiable measure of optic nerve function that facilitates identification of eyes with optic neuritis in the EAE model of MS. Results suggest that low light intensity stimuli can be used to detect optic neuritis eyes and monitor optic nerve function over time. In addition to detecting disease onset, pupillometry provides a promising method for evaluating changes in optic nerve function that can be used to evaluate new treatments in experimental optic neuritis, and likely will be useful in models of other optic nerve disorders as well.
High incidence of optic neuritis occurs in EAE model of multiple sclerosis
Common detection by histology has limited reliability/can only be done post-sacrifice
Reduced pupillary responses detected by pupillometry correlate with optic neuritis
Pupillometry detects optic neuritis early, even without histological signs of inflammation
Pupillometry allows simple, reliable in vivo detection of experimental optic neuritis
Acknowledgments
This work was supported by Grants EY015098 (KS), EY019014 (KS), P30 Core Grant for Vision Research EY01583 (GSY), and EY017024 (DC) from the National Institutes of Health, Research to Prevent Blindness (KS), National Multiple Sclerosis Society Grant RG 4214-A-1 (KS), Hope for Vision Foundation (DC), Howard Hughes Medical Institute (KR), Fidelity Charitable Trust (DC), and the F. M. Kirby Foundation (KS and DC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold AC. Evolving management of optic neuritis and multiple sclerosis. Am J Ophthalmol. 2005;139:1101–1108. doi: 10.1016/j.ajo.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Beck RW, Cleary PA, Anderson MM, Jr, Keltner JL, Shults WT, Kaufman DI, Buckley EG, Corbett JJ, Kupersmith MJ, Miller NR, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. New Eng J Med. 1992;326:581–588. doi: 10.1056/NEJM199202273260901. [DOI] [PubMed] [Google Scholar]
- Dutt M, Tabuena P, Ventura E, Rostami AM, Shindler KS. Timing of corticosteroid therapy is critical to prevent retinal ganglion cell loss in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2010;51:1439–1445. doi: 10.1167/iovs.09-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis CJ. The afferent pupillary defect in acute optic neuritis. J Neurol Neurosurg Psychiatry. 1979;42:1008–1017. doi: 10.1136/jnnp.42.11.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Qi X, Hauswirth WW. Adeno-associated viral-mediated catalase expression suppresses optic neuritis in experimental allergic encephalomyelitis. Proc Natl Acad Sci. 1998;95:13847–13852. doi: 10.1073/pnas.95.23.13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobom M, Storch MK, Weissert R, Maier K, Radhakrishnan A, Kramer B, Bähr M, Diem R. Mechanisms and time course of neuronal degeneration in experimental autoimmune encephalomyelitis. Brain Pathol. 2004;14:148–157. doi: 10.1111/j.1750-3639.2004.tb00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain RZ, Hopkins SC, Frohman EM, Eagar TN, Cravens PC, Greenberg BM, Vernino S, Stüve O. Direct and consensual murine pupillary reflex metrics: Establishing normative values. Auton Neurosci. 2009;151:164–167. doi: 10.1016/j.autneu.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Manno FAM., 3rd Pupillometry in mice: sex and strain-dependent phenotypes of pupillary functioning. Optom Vis Sci. 2009;86:895–899. doi: 10.1097/OPX.0b013e3181adfde9. [DOI] [PubMed] [Google Scholar]
- Meyer R, Weissert R, Diem R, Storch MK, de Graaf KL, Kramer B, Bahr M. Acute neuronal apoptosis in a rat model of multiple sclerosis. J Neurosci. 2001;21:6214–6220. doi: 10.1523/JNEUROSCI.21-16-06214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. New Eng J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65:727–732. doi: 10.1001/archneur.65.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter NT, Bigazzi PE. Acute optic neuritis associated with immunization with the CNS myelin proteolipid protein. Invest Ophthalmol Vis Sci. 1992;33:1717–1722. [PubMed] [Google Scholar]
- Quinn T, Dutt M, Shindler KS. Optic neuritis and retinal ganglion cell loss in a chronic murine model of multiple sclerosis. Front Neurol. 2011;2:50. doi: 10.3389/fneur.2011.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter AR, Conger A, Frohman TC, Zivadinov R, Eggenberger E, Calabresi P, Cutter G, Balcer L, Frohman EM. Retinal architecture predicts pupillary reflex metrics in MS. Mult Scler. 2009;15:479–486. doi: 10.1177/1352458508100503. [DOI] [PubMed] [Google Scholar]
- Santos AM, Martin-Oliva D, Ferrer-Martin RM, Tassi M, Calvente R, Sierra A, Carrasco MC, Marín-Teva JL, Navascués J, Cuadros MA. Microglial response to light-induced photoreceptor degeneration in the mouse retina. J Comp Neurol. 2010;518:477–492. doi: 10.1002/cne.22227. [DOI] [PubMed] [Google Scholar]
- Shao H, Huang Z, Sun SL, Kaplan HJ, Sun D. Myelin/oligodendrocyte glycoprotein-specific T-cells induce severe optic neuritis in the C57BL/6 mouse. Invest Ophthalmol Vis Sci. 2004;45:4060–4065. doi: 10.1167/iovs.04-0554. [DOI] [PubMed] [Google Scholar]
- Shindler KS, Guan Y, Ventura E, Bennett J, Rostami A. Retinal ganglion cell loss induced by acute optic neuritis in a relapsing model of multiple sclerosis. Mult Scler. 2006;12:526–532. doi: 10.1177/1352458506070629. [DOI] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Dutt M, Rostami AM. Inflammatory demyelination induces axonal injury and retinal ganglion cell apoptosis in experimental optic neuritis. Exp Eye Res. 2008;87:208–213. doi: 10.1016/j.exer.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Rex TS, Elliott P, Rostami A. SIRT1 activation confers neuroprotection in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2007;48:3602–3609. doi: 10.1167/iovs.07-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surakka J, Ruutiainen J, Romberg A, Puukka P, Kronholm E, Karanko H. Pupillary function in early multiple sclerosis. Clin Auton Res. 2008;18:150–154. doi: 10.1007/s10286-008-0471-2. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC, Merbs SL, Welsbie DS, Yoshioka T, Weissgerber P, Stolz S, Flockerzi V, Freichel M, Simon MI, Clapham DE, Yau KW. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479:67–73. doi: 10.1038/nature10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tu DC, Denner D, Shane T, Fitzgerald CM, Van Gelder RN. Melanopsin-dependent persistence and photopotentiation of murine pupillary light responses. Invest Ophthalmol Vis Sci. 2007;48:1268–1275. doi: 10.1167/iovs.06-0925. [DOI] [PubMed] [Google Scholar]


