Abstract
MuSK (muscle-specific kinase) is a receptor tyrosine kinase that plays a central signaling role in formation of neuromuscular junctions (NMJs). MuSK is activated in a complex spatio-temporal manner to cluster acetylcholine receptors on the postsynaptic (muscle) side of the synapse and to induce differentiation of the nerve terminal on the presynaptic side. The ligand for MuSK is LRP4 (low-density lipoprotein receptor-related protein-4), a transmembrane protein in muscle, whose binding affinity for MuSK is potentiated by agrin, a neuronally derived heparan-sulfate proteoglycan. In addition, Dok7, a cytoplasmic adaptor protein, is also required for MuSK activation in vivo. This review focuses on the physical interplay between these proteins and MuSK for activation and downstream signaling, which culminates in NMJ formation.
Introduction
MuSK is a receptor tyrosine kinase that plays an important role in the formation and stabilization of neuromuscular junctions (NMJs). MuSK was originally identified in a PCR-based screen for tyrosine kinases in the electric organ of Torpedo californica, which is a rich source of neuromuscular synaptic molecules (Jennings et al., 1993). MuSK is an abbreviation for muscle-specific kinase. However, it is now understood that MuSK is expressed in mammalian tissues other than skeletal muscle, including excitatory neurons in the central nervous system (Garcia-Osta et al., 2006) and sperm (Kumar et al., 2006).
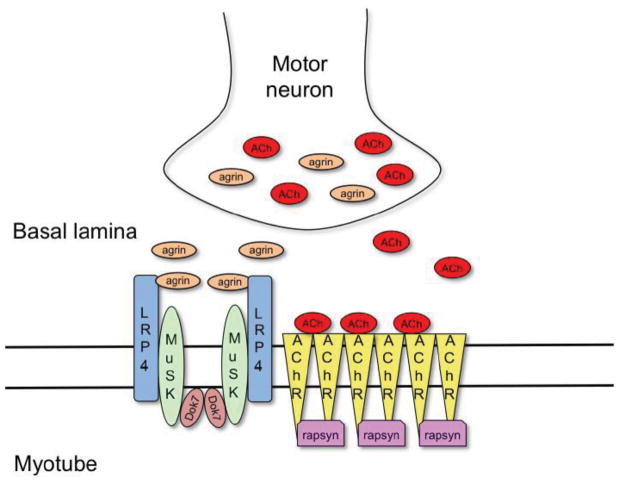
Formation of NMJs involves a complex signaling process, both spatially and temporally, between motor neurons and muscle myotubes, the end result of which is the clustering of acetylcholine receptors (AChRs) on the postsynaptic side of the junction and a differentiated nerve terminal on the presynaptic side (Burden, 2002). MuSK plays a central role in this process. Mice that lack MuSK do not form NMJs and die at birth because they fail to breathe (DeChiara et al., 1996). Other key proteins in NMJ formation (Burden, 2011) include a neuronally derived heparan-sulfate proteoglycan, agrin (Glass et al., 1996), and three muscle proteins: low-density lipoprotein receptor-related protein-4 (LRP4) (Kim et al., 2008; Zhang et al., 2008), downstream of kinase-7 (Dok7) (Okada et al., 2006) and rapsyn (Burden et al., 1983; Neubig et al., 1979) (Figure 1). In short (discussed in detail below), LRP4 serves as a cis-acting (in muscle) transmembrane ligand for MuSK, agrin acts as an allosteric regulator of LRP4’s interaction with MuSK, Dok7 functions as a cytoplasmic activator of MuSK, and rapsyn binds directly to AChRs to facilitate clustering.
Figure 1. Schematic representation of the key components involved in the formation of the neuromuscular junction.
Motor neurons secret agrin, a heparan-sulfate proteoglycan, into the synaptic basal lamina, which binds to LRP4 on the myotube surface and activates the receptor tyrosine kinase MuSK. MuSK activation is also driven by Dok7, a dimeric cytoplasmic adaptor protein. Activated MuSK phosphorylates downstream signaling components, which leads to rapsyn-dependent clustering of acetylcholine receptors (AChRs). The neurotransmitter acetylcholine, secreted by motor neurons, binds to the clustered AChRs and initiates muscle contraction.
Failure to form proper NMJs (lack of NMJs is lethal), or to maintain them, leads to neuromuscular-transmission pathologies such as myasthenia gravis and congenital myasthenic syndromes (CMS). Myasthenia gravis is an autoimmune disorder in which auto-antibodies against AChR, MuSK and LRP4 have been identified (Higuchi et al., 2011; Hoch et al., 2001; Pevzner et al., 2012; Vincent et al., 2003). The MuSK auto-antibodies belong to the IgG4 subtype, which cannot activate complement, and are thus believed to directly interfere with agrin/LRP4-dependent MuSK signaling (Klooster et al., 2012; Niks et al., 2008). CMS is a genetic disorder in which mutations are present in genes encoding agrin, MuSK, Dok7, rapsyn or AChR (or possibly other proteins) (Engel, 2012). The CMS mutations impair NMJ formation/stabilization either through a decrease in protein expression or by compromising protein function. CMS mutations identified in MuSK include a frameshift mutation (c220insC) and the missense mutations V790M, M605I, A727V and P31L (Chevessier et al., 2004; Maselli et al., 2010). In this review, we focus on the structural and mechanistic aspects of MuSK, which can help us to understand the role of MuSK in NMJ formation and how mutations in or auto-antibodies against MuSK cause disease.
MuSK Structure
MuSK is a type I, single-pass transmembrane glycoprotein of ~120 kDa (in mammals, including glycosylation). The extracellular region (ectodomain) comprises three immunoglobulin-like domains (Ig1-3) and a cysteine-rich domain (CRD) with sequence and structural similarities to the CRD of Frizzled proteins, the receptors for Wnts. In certain species (fish, amphibians and birds), the CRD is followed by a Kringle domain (three disulfide bridges). A single transmembrane helix connects the ectodomain to the cytoplasmic region, which contains the tyrosine kinase domain (Figure 2a).
Figure 2. MuSK architecture and structure.
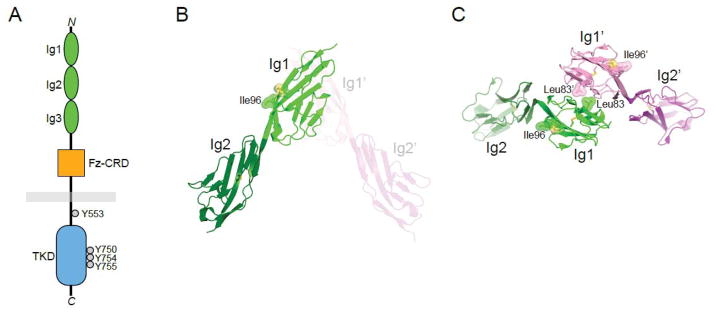
(A) Schematic diagram of MuSK. The extracellular region comprises three immunoglobulin (Ig)-like domains and a Frizzled-like cysteine-rich domain (Fz-CRD). The cytoplasmic domain contains the tyrosine kinase domain (TKD). Autophosphorylation (in trans) occurs on Tyr553 in the juxtamembrane region and Tyr750/754/755 in the activation loop of the kinase domain. (B) Crystal structure of Ig1-2 (PDB ID: 2IEP), highlighting in Ig1 a solvent-exposed disulfide bridge and adjacent Ile96. The second protomer in the crystallographic asymmetric unit is shown in light colors, and the domains are labeled with a prime (’). (C) Dimer of Ig1-2 mediated by Leu83 in Ig1. The view is approximately 90° from that in (B), from the top. Coloring and labeling as in (B).
X-ray crystallographic studies of MuSK have revealed the atomic structures of Ig1-2 (Stiegler et al., 2006), the CRD (Stiegler et al., 2009) and the tyrosine kinase domain (Till et al., 2002). The crystal structure of Ig1-2 showed that these two domains are disposed in an end-to-end linear fashion with essentially no intervening linker; the last β strand of Ig1 leads directly into the first β strand of Ig2 (Figure 2b). Sequence analysis suggests that this linear arrangement extends through Ig3.
Two features of Ig1 are noteworthy. First, a disulfide bridge is present on the surface of Ig1 (Figure 2b), which is in addition to the canonical disulfide bridge linking the two β sheets. When the two cysteine residues of the surface-exposed disulfide bridge were mutated, the receptor was not trafficked to the plasma membrane, and biochemical data indicated that Ig1 was not folded properly (Stiegler et al., 2006). Mutation of a nearby surface-exposed isoleucine residue (Ile96) abrogated the ability of MuSK to be activated by agrin. Later, it was shown that Ile96 is part of the LRP4 binding site on MuSK (Zhang et al., 2011b), which could include the disulfide bridge as well. The second feature of note in Ig1 is a hydrophobic patch on the surface opposite that of Ile96, centered at Leu83. In the crystal structure, this patch mediates an Ig1-2 dimer (Figure 2c). Whether this dimer forms in the context of the full-length receptor on the cell surface is not known, but mutation of Leu83 (or nearby Met48) led to loss of agrin-mediated activation of MuSK (Stiegler et al., 2006).
Following a linker of approximately ten residues (which may or may not be a flexible tether), the MuSK CRD adopts the fold of a Frizzled CRD (Stiegler et al., 2009), with one important exception, as highlighted in the recent crystal structure of Wnt8 bound to the Frizzled-8 CRD (Janda et al., 2012): the binding pocket for the lipid moiety in Wnts is absent in the MuSK CRD. Although there is evidence that Wnts bind directly to the MuSK CRD (Jing et al., 2009; Zhang et al., 2012), they evidently do so non-canonically.
The cytoplasmic juxtamembrane segment of MuSK (~40 residues) links the transmembrane helix to the tyrosine kinase domain. Within this segment lies Tyr553, autophosphorylation of which is crucial for MuSK activation (Herbst and Burden, 2000) (discussed below). Tyr553 resides in an NPXY (where X is any amino acid) sequence motif, which, when phosphorylated, recruits Dok7, an adaptor protein that contains pleckstrin-homology (PH) and phosphotyrosine-binding domains (Bergamin et al., 2010; Okada et al., 2006).
During receptor activation, the tyrosine kinase domain of MuSK undergoes autophosphorylation of three tyrosine residues (Tyr750, Tyr754 and Tyr755) in the activation loop in the kinase domain. A crystal structure of the basal, unphosphorylated form of the MuSK kinase domain (Till et al., 2002) revealed an autoinhibited active site very similar to that observed for the insulin receptor kinase (IRK) (Hubbard et al., 1994), which also contains three phosphorylatable tyrosine residues in the activation loop. The second tyrosine residue in the loop—Tyr754 in MuSK, Tyr1162 in IRK—is bound in its own active site, serving as a pseudosubstrate. Tyr754 cannot be phosphorylated in cis because the ATP binding site is simultaneously blocked by the beginning of the activation loop.
MuSK Activation
In comparison to most RTKs, activation of MuSK is complex, involving at least three other proteins: agrin, derived from motor neurons, and LRP4 and Dok7, expressed in the plasma membrane and cytoplasm, respectively, of muscle myotubes. Agrin was initially thought to be the ligand for MuSK, yet a direct interaction could not be observed, which suggested the existence of a so-called myotube-associated specificity component (MASC) (Glass et al., 1996). MASC was eventually shown to be the transmembrane protein LRP4 (Kim et al., 2008; Zhang et al., 2008). LRP4 consists of a large extracellular region comprising eight so-called LDLa repeats, two epidermal growth factor (EGF)-like domains and four β-propeller domains, each with an accompanying C-terminal EGF-like domain (Figure 3a). A single transmembrane helix is followed by a relatively short (~160 residues) cytoplasmic region, which was shown to be dispensable for NMJ formation (Gomez and Burden, 2011).
Figure 3. LRP4 architecture and agrin interaction.
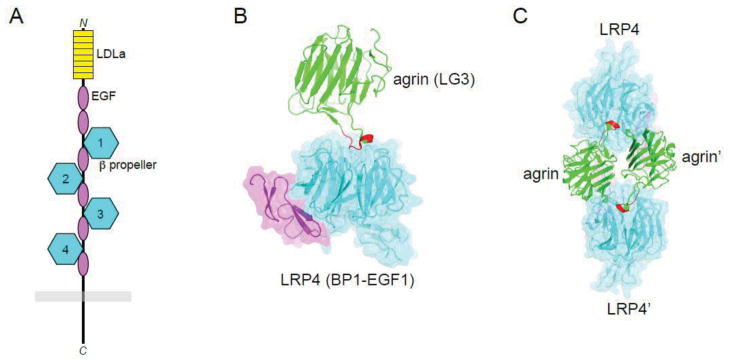
(A) Schematic diagram of LRP4. The extracellular region consists of eight LDLa repeats, two EGF-like domains, and four β propeller-EGF units. The short cytoplasmic region is devoid of folded domains. (B) Crystal structure of agrin (LG3 domain) bound to the first β propeller-EGF unit of LRP4 (PDB ID: 3V64). Coloring of LRP4 as in (A). The z8 splicing insert of agrin is colored red. (C) 2:2 complex of agrin and LRP4 observed in the crystal structure. Same coloring as in (B). The domains in the second 1:1 agrin:LRP4 complex are labeled with a prime.
LRP4 interacts with MuSK even in the absence of agrin. This basal interaction, along with the action of Dok7 (discussed below), is sufficient for partial activation of MuSK, which is important for pre-patterning of AChRs in myotubes prior to innervation (Arber et al., 2002; Kummer et al., 2006). Agrin binds to the first β-propeller domain of LRP4, which evidently induces a conformational change in LRP4 and enhances the interaction between LRP4 and MuSK (Zhang et al., 2011a; Zong et al., 2012). Hence, agrin acts as an allosteric regulator of LRP4’s interaction with MuSK.
A crystal structure of a complex between agrin (LG3 domain) and Lrp4 (first β-propeller domain) revealed that residues in the neuronal-specific z8 splicing insert of agrin mediate the interaction with LRP4 (Zong et al., 2012) (Figure 3b). The structure also suggests that a 2:2 agrin:LRP4 complex, mediated by an agrin dimer (Figure 3c), could recruit two MuSK receptors into the complex for trans-phosphorylation of MuSK. Regarding the interaction between LRP4 and MuSK, the first three β-propeller domains of LRP4 are implicated, as is Ig1 of MuSK (Zhang et al., 2011a), particularly Ile96, as described above.
The details of the constitutive interaction between LRP4 and MuSK, how that interaction is modulated upon agrin binding to LRP4 and how the interaction between LRP4 and MuSK facilitates MuSK trans-phosphorylation are all outstanding questions. In addition to the interaction of LRP4 with MuSK, LRP4 self-associates (Kim et al., 2008; Zhang et al., 2011a), which is likely to be important for MuSK trans-phosphorylation/activation.
A cis-acting transmembrane protein (LRP4) serving as a ligand distinguishes MuSK from most other RTKs, whose ligands are typically soluble (and small) protein growth factors, e.g., epidermal growth factor. In addition to this, MuSK requires a cytoplasmic protein, Dok7, for activation (Okada et al., 2006). Dok7 is an adaptor protein possessing N-terminal PH and PTB domains followed by a long unstructured region containing two sites of tyrosine phosphorylation. The PTB domain of Dok7 binds directly to phosphorylated Tyr553 (and the surrounding residues) in the juxtamembrane region of MuSK, and the PH domain facilitates this interaction by binding to phosphoinositides in the plasma membrane (Bergamin et al., 2010). Importantly, the PH domain also mediates dimerization of Dok7, which, through PTB-domain binding to the juxtamembrane region of MuSK, juxtaposes two MuSK kinase domains for trans-phosphorylation of the three tyrosine residues (Tyr750, Tyr754 and Tyr755) in the activation loop (Bergamin et al., 2010). Activation-loop phosphorylation relieves autoinhibition in the kinase domain, thereby activating MuSK. These data explain why mutation of Tyr553 alone results in loss of activation-loop phosphorylation and MuSK activation (Herbst and Burden, 2000).
This Dok7-mediated MuSK activation mechanism is premised on the assumption that Tyr553 in the MuSK juxtamembrane region is autophosphorylated (in trans, to create a docking site for Dok7) prior to activation-loop phosphorylation and full kinase activation. Evidence that a heterologous kinase (other than MuSK) is responsible for the phosphorylation of Tyr553 comes from mutation of the conserved lysine (Lys608) in the MuSK kinase domain, which abrogates all tyrosine phosphorylation on MuSK (Zhou et al., 1999). Like most tyrosine kinases, the MuSK kinase domain possesses low basal (unphosphorylated activation loop) activity, which is sufficient to allow trans-autophosphorylation of the juxtamembrane region and activation loop upon juxtaposition of two receptors via dimerization. By analogy with the insulin receptor kinase, only when the MuSK activation loop is phosphorylated on both Tyr754 and Tyr755 (second and third sites) will the kinase domain be fully activated (Hubbard, 1997). In the context of the soluble MuSK cytoplasmic domain, Tyr553 and Tyr754 are the preferred auto-phosphorylation sites, and Tyr750 and Tyr755 are autophosphorylated more slowly (Till et al., 2002). Adventitious (non-agrin-stimulated) interactions of MuSK in the muscle cell membrane (potentiated by LRP4) are hypothesized to result in a sub-stoichiometric phosphorylation of Tyr553, which is enough to recruit Dok7, dimerize MuSK and facilitate activation-loop autophosphorylation (Bergamin et al., 2010). Upon kinase activation, Dok7 becomes a substrate of MuSK, with phosphorylation on Tyr396 and Tyr406. Phosphorylated Dok7 recruits the adaptor proteins Crk and Crk-L, which play a role in NMJ formation (Hallock et al., 2010). Thus, Dok7 acts both upstream (as activator) and downstream (as substrate) of MuSK.
Prospects
MuSK is the central component of the postsynaptic signaling complex that coordinates formation and maintenance of NMJs. Impaired MuSK function, caused by either auto-antibodies (myasthenia gravis) or mutations (CMS), can lead to denervation of muscle and muscle weakness. Acetylcholinesterase (AChE) inhibitors, which prevent the breakdown of acetylcholine at the synapse, are the most common treatment of myasthenia patients (Engel, 2007; Skeie et al., 2010). However, patients with MuSK auto-antibodies can exhibit acetylcholine hypersensitivity, and therefore AChE inhibitors must be used with caution (Skeie et al., 2010). Moreover, it has been observed that CMS patients with MuSK or Dok7 mutations do not respond well to AChE inhibitors (Engel, 2007; Maselli et al., 2010; Mihaylova et al., 2009). A possible alternative therapeutic avenue for these disorders would be restoration of MuSK function, either through activating antibodies or small-molecule agonists. The design of such therapeutics would be aided by a more thorough understanding of the molecular mechanisms underlying agrin/LRP4-mediated activation of MuSK.
Similar to myasthenia gravis and CMS, denervation and muscle weakness are common features among patients afflicted with amyotrophic lateral sclerosis (ALS). Even though MuSK has not been directly linked to ALS pathogenesis, ectopic expression of MuSK may stimulate retrograde signaling (muscle to nerve) and improve muscle innervation. Specifically, it was recently shown that three-fold overexpression of MuSK in an ALS mouse model was able to delay the onset of denervation and improve motor function, although there was no change in survival (Perez-Garcia and Burden, 2012). However, this is the first clue that stimulating retrograde signaling via MuSK may be a possible treatment modality for ALS. Interestingly, antibodies have been identified that are capable of inducing MuSK dimerization and activation (Hopf and Hoch, 1998; Xie et al., 1997). Whether the level of MuSK activation induced by these antibodies will be sufficient to prevent muscle denervation warrants an in-depth investigation, including screening for additional activating MuSK antibodies.
Highlights.
MuSK plays a central role in neuromuscular junction formation.
Agrin acts as an allosteric regulator of the LRP4-MuSK interaction.
Dok7 serves as a cytoplasmic activator of MuSK.
Acknowledgments
We thank Dr. Steven Burden for helpful discussion. Previous support is acknowledged from the National Institutes of Health, grant R01 NS053414 (S.R.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arber S, Burden SJ, Harris AJ. Patterning of skeletal muscle. Curr Opin Neurobiol. 2002;12:100–103. doi: 10.1016/s0959-4388(02)00296-9. [DOI] [PubMed] [Google Scholar]
- Bergamin E, Hallock PT, Burden SJ, Hubbard SR. The cytoplasmic adaptor protein Dok7 activates the receptor tyrosine kinase MuSK via dimerization. Mol Cell. 2010;39:100–109. doi: 10.1016/j.molcel.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden SJ. Building the vertebrate neuromuscular synapse. J Neurobiol. 2002;53:501–511. doi: 10.1002/neu.10137. [DOI] [PubMed] [Google Scholar]
- Burden SJ. SnapShot: Neuromuscular Junction. Cell. 2011;144:826–826. e821. doi: 10.1016/j.cell.2011.02.037. [DOI] [PubMed] [Google Scholar]
- Burden SJ, DePalma RL, Gottesman GS. Crosslinking of proteins in acetylcholine receptor-rich membranes: association between the beta-subunit and the 43 kd subsynaptic protein. Cell. 1983;35:687–692. doi: 10.1016/0092-8674(83)90101-0. [DOI] [PubMed] [Google Scholar]
- Chevessier F, Faraut B, Ravel-Chapuis A, Richard P, Gaudon K, Bauche S, Prioleau C, Herbst R, Goillot E, Ioos C, et al. MUSK, a new target for mutations causing congenital myasthenic syndrome. Hum Mol Gen. 2004;13:3229–3240. doi: 10.1093/hmg/ddh333. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Engel AG. The therapy of congenital myasthenic syndromes. Neurotherapeutics. 2007;4:252–257. doi: 10.1016/j.nurt.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AG. Current status of the congenital myasthenic syndromes. Neuromuscul Disord. 2012;22:99–111. doi: 10.1016/j.nmd.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Osta A, Tsokas P, Pollonini G, Landau EM, Blitzer R, Alberini CM. MuSK expressed in the brain mediates cholinergic responses, synaptic plasticity, and memory formation. J Neurosci. 2006;26:7919–7932. doi: 10.1523/JNEUROSCI.1674-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, et al. Agrin acts via a MuSK receptor complex. Cell. 1996;85:513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- Gomez AM, Burden SJ. The extracellular region of Lrp4 is sufficient to mediate neuromuscular synapse formation. Dev Dyn. 2011;240:2626–2633. doi: 10.1002/dvdy.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock PT, Xu CF, Park TJ, Neubert TA, Curran T, Burden SJ. Dok-7 regulates neuromuscular synapse formation by recruiting Crk and Crk-L. Genes Dev. 2010;24:2451–2461. doi: 10.1101/gad.1977710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R, Burden SJ. The juxtamembrane region of MuSK has a critical role in agrin-mediated signaling. EMBO J. 2000;19:67–77. doi: 10.1093/emboj/19.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi O, Hamuro J, Motomura M, Yamanashi Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol. 2011;69:418–422. doi: 10.1002/ana.22312. [DOI] [PubMed] [Google Scholar]
- Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7:365–368. doi: 10.1038/85520. [DOI] [PubMed] [Google Scholar]
- Hopf C, Hoch W. Dimerization of the muscle-specific kinase induces tyrosine phosphorylation of acetylcholine receptors and their aggregation on the surface of myotubes. J Biol Chem. 1998;273:6467–6473. doi: 10.1074/jbc.273.11.6467. [DOI] [PubMed] [Google Scholar]
- Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997;16:5572–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard SR, Wei L, Ellis L, Hendrickson WA. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature. 1994;372:746–754. doi: 10.1038/372746a0. [DOI] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings CG, Dyer SM, Burden SJ. Muscle-specific trk-related receptor with a kringle domain defines a distinct class of receptor tyrosine kinases. Proc Natl Acad Sci USA. 1993;90:2895–2899. doi: 10.1073/pnas.90.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Lefebvre JL, Gordon LR, Granato M. Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor. Neuron. 2009;61:721–733. doi: 10.1016/j.neuron.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135:334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klooster R, Plomp JJ, Huijbers MG, Niks EH, Straasheijm KR, Detmers FJ, Hermans PW, Sleijpen K, Verrips A, Losen M, et al. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain. 2012;135:1081–1101. doi: 10.1093/brain/aws025. [DOI] [PubMed] [Google Scholar]
- Kumar P, Ferns MJ, Meizel S. Identification of agrinSN isoform and muscle-specific receptor tyrosine kinase (MuSK) [corrected] in sperm. Biochem Biophys Res Commun. 2006;342:522–528. doi: 10.1016/j.bbrc.2006.01.161. [DOI] [PubMed] [Google Scholar]
- Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Maselli RA, Arredondo J, Cagney O, Ng JJ, Anderson JA, Williams C, Gerke BJ, Soliven B, Wollmann RL. Mutations in MUSK causing congenital myasthenic syndrome impair MuSK-Dok-7 interaction. Hum Mol Genet. 2010;19:2370–2379. doi: 10.1093/hmg/ddq110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova V, Salih MA, Mukhtar MM, Abuzeid HA, El-Sadig SM, von der Hagen M, Huebner A, Nurnberg G, Abicht A, Muller JS, et al. Refinement of the clinical phenotype in musk-related congenital myasthenic syndromes. Neurology. 2009;73:1926–1928. doi: 10.1212/WNL.0b013e3181c3fce9. [DOI] [PubMed] [Google Scholar]
- Neubig RR, Krodel EK, Boyd ND, Cohen JB. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc Natl Acad Sci USA. 1979;76:690–694. doi: 10.1073/pnas.76.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niks EH, van Leeuwen Y, Leite MI, Dekker FW, Wintzen AR, Wirtz PW, Vincent A, van Tol MJ, Jol-van der Zijde CM, Verschuuren JJ. Clinical fluctuations in MuSK myasthenia gravis are related to antigen-specific IgG4 instead of IgG1. J Neuroimmunol. 2008;195:151–156. doi: 10.1016/j.jneuroim.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Okada K, Inoue A, Okada M, Murata Y, Kakuta S, Jigami T, Kubo S, Shiraishi H, Eguchi K, Motomura M, et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science. 2006;312:1802–1805. doi: 10.1126/science.1127142. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia MJ, Burden SJ. Increasing MuSK Activity Delays Denervation and Improves Motor Function in ALS Mice. Cell Rep. 2012;2:497–502. doi: 10.1016/j.celrep.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevzner A, Schoser B, Peters K, Cosma NC, Karakatsani A, Schalke B, Melms A, Kroger S. Anti-LRP4 autoantibodies in AChR- and MuSK-antibody-negative myasthenia gravis. J Neurol. 2012;259:427–435. doi: 10.1007/s00415-011-6194-7. [DOI] [PubMed] [Google Scholar]
- Skeie GO, Apostolski S, Evoli A, Gilhus NE, Illa I, Harms L, Hilton-Jones D, Melms A, Verschuuren J, Horge HW. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol. 2010;17:893–902. doi: 10.1111/j.1468-1331.2010.03019.x. [DOI] [PubMed] [Google Scholar]
- Stiegler AL, Burden SJ, Hubbard SR. Crystal structure of the agrin-responsive immunoglobulin-like domains 1 and 2 of the receptor tyrosine kinase MuSK. J Mol Biol. 2006;364:424–433. doi: 10.1016/j.jmb.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler AL, Burden SJ, Hubbard SR. Crystal structure of the frizzled-like cysteine-rich domain of the receptor tyrosine kinase MuSK. J Mol Biol. 2009;393:1–9. doi: 10.1016/j.jmb.2009.07.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till JH, Becerra M, Watty W, Lu Y, Ma Y, Neubert TA, Burden SJ, Hubbard SR. Crystal structure of the MuSK tyrosine kinase: insights into receptor autoregulation. Structure. 2002;10:1187–1196. doi: 10.1016/s0969-2126(02)00814-6. [DOI] [PubMed] [Google Scholar]
- Vincent A, McConville J, Farrugia ME, Bowen J, Plested P, Tang T, Evoli A, Matthews I, Sims G, Dalton P, et al. Antibodies in myasthenia gravis and related disorders. Ann NY Acad Sci. 2003;998:324–335. doi: 10.1196/annals.1254.036. [DOI] [PubMed] [Google Scholar]
- Xie MH, Yuan J, Adams C, Gurney A. Direct demonstration of MuSK involvement in acetylcholine receptor clustering through identification of agonist ScFv. Nat Biotech. 1997;15:768–771. doi: 10.1038/nbt0897-768. [DOI] [PubMed] [Google Scholar]
- Zhang B, Liang C, Bates R, Yin Y, Xiong WC, Mei L. Wnt proteins regulate acetylcholine receptor clustering in muscle cells. Mol Brain. 2012;5:7. doi: 10.1186/1756-6606-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60:285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Coldefy AS, Hubbard SR, Burden SJ. Agrin binds to the N-terminal region of Lrp4 protein and stimulates association between Lrp4 and the first immunoglobulin-like domain in muscle-specific kinase (MuSK) J Biol Chem. 2011a;286:40624–40630. doi: 10.1074/jbc.M111.279307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Coldefy AS, Hubbard SR, Burden SJ. Agrin binds to the N-terminal region of Lrp4 protein and stimulates association between Lrp4 and the first immunoglobulin-like domain in muscle-specific kinase (MuSK) J Biol Chem. 2011b;286:40624–40630. doi: 10.1074/jbc.M111.279307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Glass DJ, Yancopoulos GD, Sanes JR. Distinct domains of MuSK mediate its abilities to induce and to associate with postsynaptic specializations. J Cell Biol. 1999;146:1133–1146. doi: 10.1083/jcb.146.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Y, Zhang B, Gu S, Lee K, Zhou J, Yao G, Figueiredo D, Perry K, Mei L, Jin R. Structural basis of agrin-LRP4-MuSK signaling. Genes Dev. 2012;26:247–258. doi: 10.1101/gad.180885.111. [DOI] [PMC free article] [PubMed] [Google Scholar]