Abstract
INTRODUCTION
Guidelines for breast cancer staging exist, but adherence remains unknown. This study evaluates patterns of imaging in early-stage breast cancer usually reserved for advanced disease.
METHODS
Surveillance Epidemiology and End Results data linked to Medicare claims from 1992–2005 were reviewed for stage I/II breast cancer patients. Claims were searched for preoperative performance of CT, PET, and bone scans, and brain MRIs (“advanced imaging”).
RESULTS
There were 67,874 stage I/II breast cancer patients; 18.8% (n=12,740) had preoperative advanced imaging. The proportion of patients having CTs, PET scans and brain MRIs increased from 5.7% to 12.4% (p<0.0001), 0.8% to 3.4% (p<0.0001) and 0.2% to 1.1% (p=0.008), respectively, from 1992–2005. Bone scans declined from 20.1% to 10.7% (p<0.0001). “Breast cancer” (174.x) was the only diagnosis code associated with 62.1% of PET scans, 37.7% of bone scans, 24.2% of CTs, and 5.1% of brain MRIs. ≥1 symptom or metastatic site was suggested for 19.6% of bone scans, 13.0% of CTs, 13.0% of PET scans and 6.2% of brain MRIs. Factors associated (p<0.05) with use of all modalities were urban setting, breast MRI and ultrasound. Breast MRI was the strongest predictor (p<0.0001) of bone scan (OR1.63, 95%CI 1.44–1.86), Brain MRI (OR1.74, 95%CI 1.15–2.63), CT (OR2.42, 95%CI 2.12–2.76), and PET (OR5.71, 95%CI 4.52–7.22).
CONCLUSION
Aside from bone scans, performance of advanced imaging is increasing in early-stage Medicare breast cancer patients, with limited rationale provided by coded diagnoses. In light of existing guidelines and increasing scrutiny about healthcare costs, greater reinforcement of current indications is warranted.
INTRODUCTION
It is estimated that in 2011, there will be 210,000 – 230,480 women in the United States who will be diagnosed with breast cancer.1 Between 2001 and 2007, 60% of patients had disease confined to the breast and 33% had disease spread to regional lymph nodes at diagnosis. Only 5% had distant metastases at their time of diagnosis with the remaining 2% of patients unstaged.2
The indications for imaging in stage I or II breast cancer have been enumerated in the National Comprehensive Cancer Network® Guidelines, including CT, PET and bone scans, and brain MRIs.3 Outside of a complete history and physical examination, a CBC, platelets, liver function tests, and alkaline phosphatase are the only studies recommended. If the patient has abnormal blood work or a specific symptom or sign on examination, staging studies should be performed to investigate further. In an asymptomatic patient, CT of the abdomen and pelvis, PET scan, bone scan, and brain MRI are not felt to be indicated.
While these guidelines are very specific, adherence on a national level is unknown. With healthcare costs on the rise and resources waning, physicians will likely be under greater scrutiny to provide justification for expensive studies. This is particularly likely as metastatic disease is rarely found in patients clinically judged to have early stage breast cancer.4 This study was performed to evaluate patterns of imaging in early stage breast cancer that is usually reserved for advanced stage disease and to discern whether the claims suggest, via the diagnosis codes, that these studies were consistent with national guidelines.
METHODS
Data were derived from Medicare claims linked to the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database with approval of the National Cancer Institute.5 Patients included had breast cancer diagnosed at ≥65 years of age between 1992 and 2005. All patients had cancer-directed surgery and were not enrolled in an HMO, having Medicare parts A and B, to provide a low likelihood of missing data. Patients were excluded if they had American Joint Committee on Cancer Stage 0, III, or IV disease, were of unknown stage, their first physician encounter or therapy date were unknown, or did not have standard therapy on one operative date (i.e. a simultaneous breast and nodal staging procedure), making the preoperative interval indeterminate. Patients having preoperative radiotherapy or chemotherapy, or those having treatment in unknown order were excluded.
Claims were searched for performance of “advanced imaging” in the preoperative interval, defined as computerized tomography (CT), brain magnetic resonance imaging (MRI), bone scans, and positron emission tomography (PET). The start of the preoperative interval was defined by the first physician encounter having a breast-related diagnosis code <1 year before surgery. The end of the preoperative interval was the date on which a breast excision or mastectomy was performed simultaneously with nodal staging. As procedure codes for excisional biopsies and lumpectomies are sometimes used interchangeably, this allowed inference of therapeutic intent from the concurrent nodal procedure. “Early stage” here refers to AJCC Stage I or II.
Claims were searched for the Current Procedural Terminology (CPT) codes and International Classification of Diseases, Revision 9 (ICD-9) procedure codes listed in Table 1. Physician claims were searched first, supplemented by outpatient and inpatient hospital claims. If conflicts arose between CPT codes and ICD-9 procedure codes, whose descriptions are less specific, CPT data were preferentially used. Conflicts between physician and outpatient hospital claims utilized physician claims. ICD-9 diagnosis codes associated with each imaging study were also reviewed.
TABLE 1.
Current Procedural Terminology (CPT) codes and International Classification of Diseases, Revision 9 (ICD-9) procedure codes for which SEER Medicare claims were searched
| Simplified Imaging Modality Description |
CPT Codes | ICD-9-CM Procedure Codes |
|---|---|---|
| Computed Tomography (CT) | 70450, 70460, 70470, 70480–70482, 70486–70488, 70490–70492, 71250, 71260, 71270, 72125–72133, 72192–72194, 73200–73202, 73700–73702, 74150, 74160, 74170, 74176–74178, 76497 | 87.03, 87.41, 87.71, 88.01, 88.38 |
| Positron emission tomography (PET) and PET-CT | 78811–78816, 78890, 78891, 78999, G0235, G0253, G0254 | 92.11, 92.12, 92.18, 92.19 |
| Bone scan | 78300, 78305, 78306, 78315, 78399 | 92.14 |
| Brain MRI | 70551–70553 | 88.91 |
Stage-specific estimates of advanced imaging use by year of diagnosis were determined as the proportion of cases with at least one claim in the pre-operative interval. Trends were evaluated with the Cochran-Armitage test. Multivariable logistic regression was used for inferences about the relationship of advanced imaging use with predictive factors including patient demographics, tumor characteristics, and standard imaging. Predictors were included as categorical variables, and odds ratios reported relative to the reference level. Per NCI privacy requirements, groups involving fewer than 11 patients may not be detailed due to privacy concerns and were listed as <11. Statistical significance was set at p=0.05 (two-sided). Analyses were performed using SAS software, version 9.2 (SAS Institute) and Stata software, release 12 (StataCorp 2011).
RESULTS
Between 1992 and 2005, there were 67,874 SEER-Medicare stage I and II breast cancer patients. Cohort characteristics are listed in Table 2. The majority of patients were female (99.1%), Caucasian (89.5%), and lived in metropolitan areas (84.4%). Ductal histology was most common (86.7%) and most patients had either estrogen or progesterone receptor-positive tumors (73.5%). The patient population was closely divided between stage I (57.7%) and II (42.3%) breast cancer.
TABLE 2.
Advanced imaging, by modality and statistical significance of individual patient characteristics.
| Patient Characteristic | Total Patients |
CT Scan‡ n (%) |
p | PET Scan¶ n (%) |
p | Brain MRI† n (%) |
p | Bone Scan n (%) |
p |
|---|---|---|---|---|---|---|---|---|---|
| Gender | <0.0001 | 0.5758 | 0.9006 | 0.0541 | |||||
| Female | 67,230 | 6324 (9.4) | 701 (1.0) | 496 (0.7) | 9337 (13.9) | ||||
| Male | 644 | 91 (14.1) | <11 | <11 | 110 (17.1) | ||||
| Age | 0.1278 | 0.3864 | 0.0917 | <0.0001 | |||||
| 65–69 yrs | 14,920 | 1430 (9.6) | 160 (1.1) | 97 (0.7) | 2225 (14.9) | ||||
| 70–74 yrs | 19,371 | 1820 (9.4) | 223 (1.2) | 133 (0.7) | 2890 (14.9) | ||||
| 75–79 yrs | 17,701 | 1707 (9.6) | 178 (1.0) | 155 (0.9) | 2491 (14.1) | ||||
| 80–84 yrs | 10,846 | 1023 (9.4) | 102 (0.9) | 77 (0.7) | 1318 (12.2) | ||||
| 85+ yrs | 5,036 | 435 (8.6) | 43 (0.9) | 38 (0.8) | 523 (10.4) | ||||
| Race | <0.0001 | 0.0854 | 0.126 | 0.0009 | |||||
| White | 60,744 | 5561 (9.2) | 624 (1.0) | 437 (0.7) | 8260 (13.6) | ||||
| Black | 3805 | 458 (12.0) | 57 (1.5) | 37 (1.0) | 650 (17.1) | ||||
| Asian | 1384 | 180 (13.0) | 11 (0.8) | <11 | 227 (16.4) | ||||
| Other/Unknown¥ | 1941 | 216 (11.1) | 14 (0.7) | 19 (1.0) | 310 (16.0) | ||||
| Urban/Rural Setting§ | <0.0001 | <0.0001 | 0.0255 | <0.0001 | |||||
| Rural | 1087 | 83 (7.6) | <11 | <11 | 86 (7.9) | ||||
| Less Urban | 5204 | 428 (8.2) | 24 (0.5) | 27 (0.5) | 474 (9.1) | ||||
| Urban | 4270 | 339 (7.9) | 26 (0.6) | 20 (0.5) | 553 (13.0) | ||||
| Metro | 18721 | 1649 (8.8) | 147 (0.8) | 122 (0.7) | 2372 (12.7) | ||||
| Big Metro | 38592 | 3916 (10.1) | 505 (1.3) | 325 (0.8) | 5962 (15.4) | ||||
| Stage | <0.0001 | <0.0001 | 0.3902 | <0.0001 | |||||
| Stage I | 39,157 | 3167 (8.1) | 317 (0.8) | 268 (0.7) | 4616 (11.8) | ||||
| Stage II | 28,717 | 3248 (11.3) | 389 (1.4) | 232 (0.8) | 4831 (16.8) | ||||
| Grade | 0.0813 | 0.0003 | 0.5222 | <0.0001 | |||||
| Well differentiated | 13858 | 1159 (8.4) | 116 (0.8) | 105 (0.8) | 1578 (11.4) | ||||
| Mod. differentiated | 27698 | 2608 (9.4) | 265 (1.0) | 216 (0.8) | 3677 (13.3) | ||||
| Poorly differentiated | 17129 | 1808 (10.6) | 246 (1.4) | 116 (0.7) | 2752 (16.1) | ||||
| Undifferentiated; anaplastic | 1107 | 99 (8.9) | 12 (1.1) | <11 | 131 (11.8) | ||||
| Unknown | 8082 | 741 (9.2) | 67 (0.8) | 55 (0.7) | 1309 (16.2) | ||||
| Receptor Status | 0.3746 | 0.0454 | 0.7992 | 0.0359 | |||||
| Either Positive | 49883 | 4623 (9.3) | 524 (1.1) | 373 (0.7) | 6743 (13.5) | ||||
| Both Negative | 8019 | 849 (10.6) | 109 (1.4) | 60 (0.7) | 1229 (15.3) | ||||
| Unknown | 9972 | 943 (9.5) | 73 (0.7) | 67 (0.7) | 1475 (14.8) | ||||
| Histology | 0.0337 | 0.1377 | 0.0183 | 0.0098 | |||||
| Ductal | 58815 | 5460 (9.3) | 587 (1.0) | 418 (0.7) | 8002 (13.6) | ||||
| Lobular | 7100 | 731 (10.3) | 91 (1.3) | 73 (1.0) | 1137 (16.0) | ||||
| Other/Unknown | 1959 | 224 (11.4) | 28 (1.4) | <11 | 308 (15.7) | ||||
| Breast MRI† | <0.0001 | <0.0001 | 0.0092 | <0.0001 | |||||
| No | 66419 | 6096 (9.2) | 610 (0.9) | 475 (0.7) | 9134 (13.8) | ||||
| Yes | 1455 | 319 (21.9) | 96 (6.6) | 25 (1.7) | 313 (21.5) | ||||
| Mammogram | <0.0001 | 0.4182 | <0.0001 | <0.0001 | |||||
| No | 36203 | 2670 (7.4) | 308 (0.9) | 168 (0.5) | 4531 (12.5) | ||||
| Yes | 31671 | 3745 (11.8) | 398 (1.3) | 332 (1.1) | 4916 (15.5) | ||||
| Ultrasound | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| No | 48747 | 3957 (8.1) | 387 (0.8) | 261 (0.5) | 6677 (13.7) | ||||
| Yes | 19127 | 2458 (12.9) | 319 (1.7) | 239 (1.3) | 2770 (14.5) |
CT= Computerized Tomography;
PET= Positron Emission Tomography;
MRI= Magnetic Resonance Imaging
Includes Hispanic patients as defined by Medicare
Population sizes: rural <2,500; less urban 2,500–19,999; urban 20,000–249,999; metropolitan 250,000–1,000,000; big metropolitan ≥1,000,000
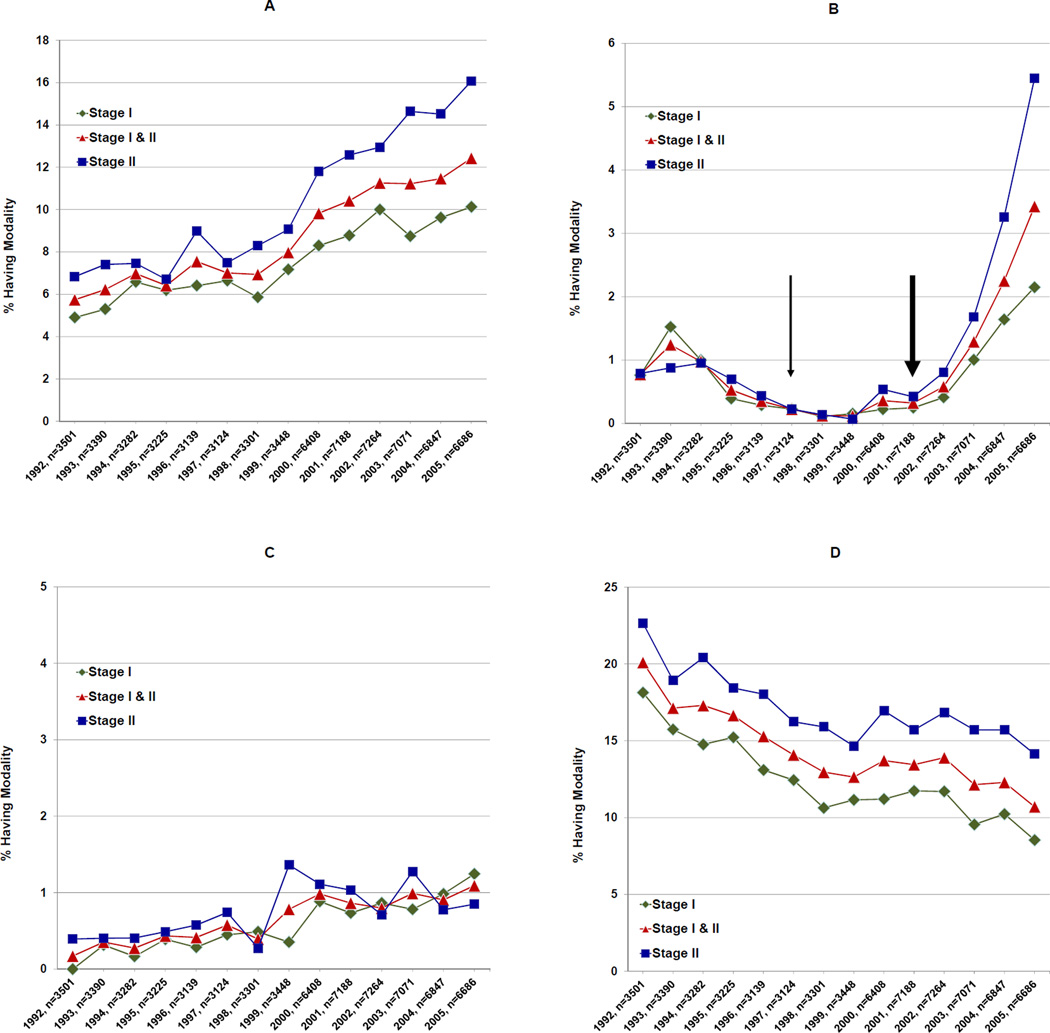
The advanced imaging trends performed in the preoperative interval over the study period demonstrated that 6,415 patients (9.5%) received ≥1 CT scan with patients receiving a CT scan increasing from 5.7% (n=201) in 1992 to 12.4% (n=831) in 2005 (trend, p<0.0001). The total number of patients having ≥1 PET scan was 706 (1.0%), increasing from 0.8% (n=27) to 3.4% (n=229) during the study period (trend, p<0.0001). There were 500 patients (0.7%) having ≥1 brain MRI with the proportion increasing from <11 to 1.1% (n=73) (trend p<0.008). There were 9,446 patients (13.9%) undergoing a bone scan, with those receiving ≥1 bone scan declining from 20.1% (n=704) in 1992 to 10.7% (n=716) in 2005 (trend, p<0.0001) as illustrated in Figure 1.
Figure 1.
Imaging trends from 1992 to 2005. Panels represent: (A) CT Scans, (B) PET Scans, (C) Brain MRIs, and (D) Bone Scans. In all panels, the x-axis represents the year and total number of sample patients having stage I and II breast cancer in that year. For panel (B), the thin arrow represents the year in which PET Scans were FDA approved for use in cancer patients, while the thick arrow represents the year in which PET scans were approved by Medicare for use in breast cancer patients specifically.
Diagnosis codes were reviewed to see if they provided rationale, suggesting that there was guidance offered to the radiologist performing the studies (Table 4). Among 783 claims for PET scans between 1992 and 2005, 62.1% (n=486) had a diagnosis code for “breast cancer” (174.x) as the only code used on the claim. Only 13.0% (n=92) of patients had ≥1 sign or symptom (such as abdominal pain, abnormal physical finding or abnormal lab value) listed on their claim as a reason for the study. There were 10,100 bone scan claims during the period of study. Of those, 37.7% (n=3,808) had “breast cancer” as the only diagnosis code given. Only 19.6% (n=1853) of patients had a sign or symptom consistent with NCCN guidelines (such as bone pain, joint pain or abnormal lab value) listed on the claim. During the study period, there were 12,862 CT scans performed with 24.2% (n=3,106) having “breast cancer” as the only diagnosis code provided. Only 13.0% (n=834) of patients had a diagnosis code supportive of guidelines appended to their CT scan claims. There were 552 brain MRIs performed from 1992 to 2005, and only 5.1% (n=28) had “breast cancer” as the sole diagnosis code given, with 6.2% (n=31) of patients having a neurological sign or symptom indicated on the claim as the reason for the study.
Table 4.
Most frequent diagnosis codes provided for the four advanced imaging modalities, deemed supportive and not supportive of guideline indications. For example, “secondary malignant neoplasm of bone or bone marrow” as an indication for a bone scan describes what the study is looking for (bone metastases), but not why the study is being ordered; i.e. what symptom or sign elicits suspicion for that concern and justification for performance of the study.
| Top 5 Supportive Codes. n represents number of imaging claims (not patient numbers). | |||
|---|---|---|---|
| CT Scan (n=12,860) | PET Scan (n=783) | Brain MRI (n=552) | Bone Scan (n=10,100) |
| 789 Abdominal pain, unspecified site (n=587) |
786.5 Chest pain, unspecified (n=33) |
784 Headache (n=71) |
733.9 Disorder of bone and cartilage, unspecified (n=488) |
| 573.8 Other specified disorders of liver (n=350) |
786.09 Other respiratory abnormalities (n<11) |
780.4 Dizziness (n=44) |
724.2 Lumbago (n=132) |
| 785.6 Enlargement of lymph nodes (n=196) |
786.05 Shortness of breath (n<11) |
436 Acute, but ill defined, cerebrovascular disease (n=32) |
786.5 Chest pain, unspecified (n=130) |
| 793.1 Nonspecific (abnormal) findings on radiological and other examination of lung (n=193) |
786.59 Other chest pain (n<11) |
331.9 Cerebral degeneration, unspecified (n=28) |
722.52 Degeneration of lumbar or lumbosacral intervertebral disc (n=114) |
| 786.5 Chest pain, unspecified (n=191) |
793.1 Nonspecific (abnormal) findings on radiological and other examination of lung (n<11) |
780.2 Syncope and collapse (n=16) |
724.5 Backache, unspecified (n=111) |
| Top 5 Non-Supportive Codes. n represents number of imaging claims (not patient numbers). | |||
|---|---|---|---|
| CT Scan (n=12,860) | PET Scan (n=783) | Brain MRI (n=552) | Bone Scan (n=10,100) |
| 518.89 Other diseases of lung, not elsewhere classified (n=663) |
611.72 Lump or mass in breast (n=23) |
437.1 Other generalized ischemic cerebrovascular disease (n=26) |
V72.5 Radiological examination, not elsewhere classified (n=822) |
| V72.5 Radiological examination, not elsewhere classified (n=615) |
794.31 Nonspecific abnormal electrocardiogram (n=22) |
V72.5 Radiological examination, not elsewhere classified (n=25) |
V10.3 Personal history of malignant neoplasm of breast (n=467) |
| V10.3 Personal history of malignant neoplasm of breast (n=590) |
414.01 Coronary atherosclerosis of native coronary artery (n=17) |
V10.3 Personal history of malignant neoplasm of breast (n=20) |
611.72 Lump or mass in breast (n=453) |
| 611.72 Lump or mass in breast (n=585) |
414 Coronary atherosclerosis of unspecified type of vessel, native or graft (n=14) |
435.9 Unspecified transient cerebral ischemia (n=18) |
198.5 Secondary malignant neoplasm of bone and bone marrow (n=350) |
| 786.6 Swelling, mass, or lump on chest (n=400) |
V10.3 Personal history of malignant neoplasm of breast (n=14) |
348.8 Brain conditions, not elsewhere classified (n=17) |
V71.1 Observation for suspected malignant neoplasm (n=230) |
Several factors significantly predicted advanced imaging use, as listed in Table 2, and three factors predicted the use of all four advanced imaging modalities: urban or rural setting, whether the patient underwent a breast MRI, and whether they had a breast ultrasound. If a patient was from a big metropolitan area (population ≥1,000,000) as versus a rural setting, they were more likely to have advanced imaging. (Table 3) The strongest predictor of preoperative advanced imaging was breast MRI, as illustrated by the odds ratio estimates. If a patient underwent a breast MRI, they were 1.63 times as likely to receive a bone scan, 1.74 times as likely to receive a brain MRI, 2.42 times as likely to have a CT scan and 5.71 times as likely to have a PET scan, when compared with those who did not undergo a breast MRI (Table 3).
TABLE 3.
Odds ratio (OR) estimates and 95% Confidence Intervals (CI) by characteristic
| Characteristic | OR | CT Scan‡ 95% CI |
OR | PET Scan¶ 95% CI |
OR | Brain MRI† 95% CI |
OR | Bone Scan 95% CI |
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Female * | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Male | 1.61 | 1.28–2.02 | 0.78 | 0.32–1.88 | 0.94 | 0.35–2.53 | 1.23 | 1.00–1.51 |
| Age | ||||||||
| 65–69 yrs* | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 70–74 yrs | 1.00 | 0.93–1.08 | 1.14 | 0.92–1.40 | 1.08 | 0.83–1.40 | 1.01 | 0.95–1.07 |
| 75–79 yrs | 1.04 | 0.97–1.12 | 1.01 | 0.82–1.26 | 1.39 | 1.08–1.80 | 0.94 | 0.88–1.00 |
| 80–84 yrs | 1.00 | 0.92–1.09 | 0.94 | 0.73–1.21 | 1.12 | 0.83–1.51 | 0.78 | 0.73–0.84 |
| 85+ yrs | 0.89 | 0.80–1.00 | 0.88 | 0.62–1.23 | 1.20 | 0.82–1.76 | 0.63 | 0.57–0.70 |
| Race | ||||||||
| White | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 1.28 | 1.16–1.42 | 1.28 | 0.97–1.69 | 1.35 | 0.96–1.90 | 1.15 | 1.05–1.25 |
| Asian | 1.46 | 1.25–1.72 | 0.76 | 0.42–1.39 | 0.68 | 0.32–1.43 | 1.19 | 1.03–1.37 |
| Other/Unknown¥ | 1.19 | 1.03–1.38 | 0.65 | 0.38–1.11 | 1.37 | 0.86–2.17 | 1.13 | 1.00–1.28 |
| Urban/Rural Setting§ | ||||||||
| Rural * | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Less Urban | 1.07 | 0.84–1.37 | 1.21 | 0.42–3.50 | 0.92 | 0.38–2.24 | 1.17 | 0.92–1.48 |
| Urban | 1.02 | 0.80–1.32 | 1.65 | 0.57–4.75 | 0.83 | 0.33–2.08 | 1.75 | 1.38–2.22 |
| Metro | 1.13 | 0.89–1.42 | 2.01 | 0.74–5.44 | 1.12 | 0.49–2.55 | 1.71 | 1.37–2.14 |
| Big Metro | 1.26 | 1.01–1.59 | 3.07 | 1.14–8.23 | 1.39 | 0.62–3.14 | 2.10 | 1.68–2.62 |
| Stage | ||||||||
| Stage I * | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Stage II | 1.34 | 1.27–1.42 | 1.48 | 1.27–1.73 | 1.08 | 0.90–1.30 | 1.45 | 1.38–1.52 |
| Grade | ||||||||
| Well Differentiated * | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Mod. Differentiated | 1.07 | 1.00–1.15 | 1.09 | 0.87–1.36 | 1.00 | 0.79–1.27 | 1.13 | 1.06–1.20 |
| Poorly Differentiated | 1.12 | 1.03–1.22 | 1.56 | 1.23–1.98 | 0.83 | 0.62–1.10 | 1.31 | 1.22–1.40 |
| Undifferentiated; anaplastic | 0.97 | 0.78–1.21 | 1.35 | 0.74–2.47 | 0.97 | 0.47–2.02 | 0.97 | 0.80–1.18 |
| Unknown | 1.05 | 0.95–1.16 | 1.01 | 0.74–1.38 | 0.85 | 0.61–1.20 | 1.36 | 1.25–1.48 |
| Receptor Status | ||||||||
| Either Positive* | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Both Negative | 1.05 | 0.97–1.14 | 1.03 | 0.82–1.28 | 1.08 | 0.81–1.44 | 1.02 | 0.95–1.10 |
| Unknown | 1.04 | 0.97–1.12 | 0.74 | 0.57–0.94 | 0.95 | 0.73–1.24 | 1.09 | 1.02–1.15 |
| Histology | ||||||||
| Ductal * | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Lobular | 1.07 | 0.98–1.17 | 1.17 | 0.93–1.49 | 1.39 | 1.07–1.82 | 1.11 | 1.03–1.20 |
| Other/Unknown | 1.18 | 1.02–1.36 | 1.37 | 0.93–2.03 | 0.64 | 0.33–1.25 | 1.08 | 0.95–1.23 |
| Breast MRI† | ||||||||
| No * | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 2.42 | 2.12–2.76 | 5.71 | 4.52–7.22 | 1.74 | 1.15–2.63 | 1.63 | 1.44–1.86 |
| Mammogram | ||||||||
| No * | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 1.45 | 1.37–1.54 | 1.07 | 0.90–1.28 | 1.78 | 1.44–2.19 | 1.30 | 1.24–1.36 |
| Ultrasound | ||||||||
| No * | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 1.30 | 1.23–1.38 | 1.64 | 1.37–1.96 | 1.71 | 1.39–2.09 | 0.88 | 0.84–0.93 |
Indicates referent values;
CT= Computerized Tomography;
PET= Positron Emission Tomography;
MRI= Magnetic Resonance Imaging
Includes Hispanic patients as defined by Medicare
Population sizes: rural <2,500; less urban 2,500–19,999; urban 20,000–249,999; metropolitan 250,000–1,000,000; big metropolitan ≥1,000,000
Histology and mammography were correlated with use of CT scans, bone scans and brain MRIs. Patients were more likely to have advanced imaging if their cancer was of lobular histology and they had a mammogram in the preoperative interval. Among those not having a mammogram in the preoperative interval, an additional 49.4% (n=33,559) patients had a mammogram before the preoperative interval, bringing the total to 96.1%. Stage was significant for use of CT scans, bone scans and PET scans in the preoperative interval, with stage II patients receiving these tests more frequently than stage I patients (Table 2).
Tumor grade and receptor status also predicted two imaging modalities. Patients having poorly differentiated tumors were more likely to receive bone or PET scans than those having well-differentiated tumors. Patients with estrogen and progesterone receptor-negative tumors were also more likely to undergo a bone or PET scan than patients having receptor-positive tumors. Race was a significant predictor of bone scan and CT scan use, with Black or Asian patients having more advanced imaging than Whites (Table 2).
DISCUSSION
We have found that, although the overall rate of preoperative advanced imaging for the early-stage Medicare breast cancer patient is low, aside from bone scans, use is increasing. The percentage of patients receiving CT scans more than doubled from 1992 to 2005, while there was a 4-fold increase in patients receiving PET scans and 5-fold increase in brain MRIs. PET scan use was low prior to its FDA approval in 1997 but began markedly increasing after 2001 when approved by the Medicare Coverage Advisory Committee (Figure 1).6 Conversely, there were half as many patients receiving bone scans in 2005 as in 1992.
Factors predicting advanced imaging use included a higher likelihood in patients from large population centers, which may also reflect the setting of the clinician ordering the study. This is not surprising since larger hospitals and cancer centers are more likely to be located in more populated areas, where concentrated resources and access to more advanced imaging technologies may exist. While one could argue that larger, possibly academic, centers should be the most knowledgeable about current guidelines, easy access to technology and patient demand in these settings may encourage use of these additional tests. Although this should not theoretically enter into decision making, concern about litigation may reinforce such patient demands, especially among surgeons who are frequent targets of malpractice lawsuits7 and radiologists who overestimate their risk of being sued.8
Interestingly, patients who had a breast MRI were more likely to have advanced imaging performed. Although this association existed when adjusting for urban/rural setting, this may support the notion that practitioners having greater access to imaging resources (regardless of urban/rural setting), use it more liberally. Pre-operative breast MRI has been associated with tumor size overestimation and false positive results leading to unnecessary biopsies. Not only does MRI result in downstream tests such as second-look ultrasounds9 and short-term follow up MRIs10, but with an 8–40% incidence of occult lesions seen solely on breast MRI,11 such findings might prompt concern that distant occult lesions exist as well. If a physician has a sufficiently low threshold to order a breast MRI when it may have marginal benefit, the same low threshold may apply to use of advanced imaging. While the specific reasons for the association cannot be determined, imaging overuse is of great importance in light of the increasing proportion of total costs that imaging studies account for in breast cancer patients.20
Patients with lobular histology and those with receptor-negative tumors were more likely to undergo advanced imaging, although not all four modalities. Lobular carcinoma can be more difficult to detect and is often found at a later stage than ductal carcinoma.12 While the survival rates for these are identical, the later presentation may also account for the associated use of advanced imaging. While estrogen receptor-positive tumors are often thought to have a better prognosis, this is controversial13 and tumor phenotype (triple negative, luminal, etc.), not available from this data, may also correlate to imaging use. It must be noted, however, that while these factors were associated with certain modalities, there was little clinical difference, as illustrated by the odds ratios in Table 3. We also noted that, outside of bone scans, age was not a determinant of advanced imaging. This is surprising as the patient ages receiving such studies were distributed well into their 80s, and the benefit conferred in this older population remains unclear.
Stage II patients were more likely to have a bone, CT, or PET scan when compared with Stage I patients. While the likelihood of finding metastatic disease for stage II patients is slightly higher than stage I, it remains low.4,14,15 Gerber et al evaluated tumor size and nodal status for their associations with occult metastases.16 In this study of 1,076 patients, distant metastases were only found with 1.6% and 3.0% of T1 and T2 tumors, respectively. They also found that only 1.9% and 1.8% of patients with N0 and N1 disease, respectively, had distant metastases. In our study, overall stage was used and this data should be applicable to our results. As clinical stage cannot be assessed by SEER (or Medicare) data and only final pathologic stage is recorded, it remains unclear how many patients may have been judged preoperatively as having a more advanced clinical stage until these studies were performed.
When reviewing the diagnosis codes for all four advanced imaging modalities, only a minority of patients had a sign or symptom of metastatic disease indicated. There is little published data on what percentage of early stage breast cancer patients present with signs or symptoms of metastatic disease, but many studies have investigated the utility of advanced imaging. A systematic review by Myers et al evaluated rates of distant metastases found by staging studies in asymptomatic breast cancer patients.14 Bone scans detected skeletal metastases in only 0.5% of women with stage I breast cancer; 2.4% of stage II, and 8.3% of stage III disease. Similarly, they found that routine liver ultrasound detected liver metastases in 0% of stage I, 0.4% of stage II and 2.0% of stage III patients. Chest radiography also detected very few lung metastases, at 0.1%, 0.2% and 1.7% in patients with stage I, II, and III disease, respectively. They also noted false positive rates for these imaging modalities, ranging from 0 to 66%, depending on the study, concluding that routine use of staging studies in stage I and II breast cancer patients is not indicated.
Brennan et al performed a meta-analysis evaluating the prevalence of distant metastases in asymptomatic patients with breast cancer.4 The prevalence of distant metastases in stage I patients ranged from 0 to 5.1% with a median of 0.2%. The prevalence of distant metastases in stage II patients ranged from 0 to 34.3% with a median of 1.2%. Of note, one study reported 34.3% of patients having distant metastases, but this study combined stage IIB and III patients, limiting its relevance here.17 While multiple studies report that advanced imaging has a very low yield in identifying distant metastases, we have found that in the Medicare patient, use of these tests is on the rise with little rationale to support the increase.
As is standard for SEER-Medicare analyses, patients were excluded who were enrolled in an HMO because such patients may not have complete claims information.18 Although the data here are representative of the Medicare population, these trends may not be applicable to either the privately insured or uninsured population. Breast cancer is a diagnosis of older age19 (mean age of diagnosis 61) so the findings herein may represent a significant proportion of breast cancer patients in the US. It must also be recognized that although the diagnosis codes only supported current indications in a minority of claims, this may be the result of poor coding, and not reflect rationales provided on the actual imaging orders, which are not available for review. The majority of claims listed a breast-related issue as the primary diagnosis (e.g. 174.x “malignant breast neoplasm” or V10.3 “personal history of malignant breast neoplasm”) or had a diagnosis that was not cancer related (e.g. V72.5 “Radiological examination, not elsewhere classified” or 401.9 “unspecified essential hypertension”). While all imaging reviewed for this study occurred in the preoperative interval, it is difficult to determine what was performed for staging as versus other medical reasons. We feel that this is still problematic, however, as these codes provide the sole justification for their use and reimbursement.
Unfortunately, the specialty of the physician ordering the studies (as versus performing them) is not available in the SEER Medicare database, as it would be interesting to see whether specialty, work experience, or patient volume correlated to imaging use. While we may not be able to deduce the exact reason for the performance of these studies, it is clear that the amount of imaging being done in Stage I and II breast cancer patients is increasing. This study did not evaluate whether claims were denied, but the intent was to assess what is being ordered, rather than what has been approved or denied by Medicare.
Finally, there may have been a larger number of patients deemed Stage I or II clinically in the preoperative period who underwent imaging, among whom a proportion were found to have metastases. Since only the final pathologic stage is recorded by SEER, these patients would have been solely listed as Stage IV and excluded from this analysis. In that same vein, changing sensitivities of the technology of these imaging modalities may also affect the trends seen here.
The significance of our findings is, in part, related to healthcare costs that have been increasing, especially amongst cancer patients. In a study of Medicare cancer patients, not only was the amount of imaging per patient increasing, but the cost of imaging accounted for an increasing proportion of all cancer costs over time.20 For breast cancer patients specifically, the average total cost per patient increased 4.1% annually, from $23,549 to $33,609 over the course of the study. The imaging costs per patient increased from $840 to $1681, corresponding to an annual increase of 9.9%, more than double that of the total cost. Our data provides one potentially contributing factor for these findings. With progressively fewer healthcare resources per capita, greater justification will be required for tests that have an outcome benefit. Meanwhile, reinforcement and better dissemination of indications for advanced imaging in breast cancer patients should be pursued.
ACKNOWLEDGEMENTS
Supported, in part, by US Public Health Services grant P30 CA006927, by an appropriation from the Commonwealth of Pennsylvania, by American Cancer Society grant #IRG-92-027-17, and by generous private donor support.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
The authors declare no conflicts of interest.
Presented, in part, at the Society of Surgical Oncology 2012 Annual Meeting, March 21–24, Orlando, FL
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2011;61(2):133–134. [Google Scholar]
- 2.National Cancer Institute. Surveillance Epidemiology and End Results Stat Fact Sheets: Breast. [accessed March 12, 2012];2011 Available: http://seer.cancer.gov/statfacts/html/breast.html#survival.
- 3.National Comprehensive Cancer Network. [accessed March 12, 2012];NCCN Guidelines Version 1.2012 Invasive Breast Cancer BINV-1. 2012 Available: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Google Scholar]
- 4.Brennan ME, Houssami N. Evaluation of the evidence on staging imaging for detection of asymptomatic distant metastases in newly diagnosed breast cancer. [Online November 15, 2011];Breast. doi: 10.1016/j.breast.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV, 3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 6.FDG Positron Emission Tomography-Breast Cancer, #CAG-00094A, Decision Memorandum. Washington DC: CMS; 2002. Feb 28, [Google Scholar]
- 7.Zylstra S, D'Orsi CJ, Ricci BA, Halloran EE, Resseguie LJ, Greenwald L, Mondor MC. Defense of breast cancer malpractice claims. Breast J. 2001;7(2):76–90. doi: 10.1046/j.1524-4741.2001.007002076.x. [DOI] [PubMed] [Google Scholar]
- 8.Dick JF, 3rd, Gallagher TH, Brenner RJ, et al. Predictors of radiologists' perceived risk of malpractice lawsuits in breast imaging. AJR Am J Roentgenol. 2009;192(2):327–333. doi: 10.2214/AJR.07.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassett LW, Dhaliwal SG, Eradat J, Khan O, Farria DF, Brenner RJ, Sayre JW. National trends and practices in breast MRI. AJR Am J Roentgenol. 2008;191(2):332–339. doi: 10.2214/AJR.07.3207. [DOI] [PubMed] [Google Scholar]
- 10.Kuhl C, Weigel S, Schrading S, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol. 2010;28(9):1450–1457. doi: 10.1200/JCO.2009.23.0839. [DOI] [PubMed] [Google Scholar]
- 11.Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA Cancer J Clin. 2009;59(5):290–302. doi: 10.3322/caac.20028. [DOI] [PubMed] [Google Scholar]
- 12.Wasif N, Maggard MA, Ko CY, Giuliano AE. Invasive lobular vs. ductal breast cancer: a stage-matched comparison of outcomes. Ann Surg Oncol. 2010;17(7):1862–1869. doi: 10.1245/s10434-010-0953-z. [DOI] [PubMed] [Google Scholar]
- 13.Hilsenbeck SG, Ravdin PM, de Moor CA, Chamness GC, Osborne CK, Clark GM. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res Treat. 1998;52(1–3):227–237. doi: 10.1023/a:1006133418245. [DOI] [PubMed] [Google Scholar]
- 14.Myers RE, Johnston M, Pritchard K, Levine M, Oliver T. Breast Cancer Disease Site Group of the Cancer Care Ontario Practice Guidelines Initiative. Baseline staging tests in primary breast cancer: a practice guideline. CMAJ. 2001;164(10):1439–1444. [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett T, Bowden DJ, Greenberg DC, Brown CH, Wishart GC, Britton PD. Radiological staging in breast cancer: which asymptomatic patients to image and how. Br J Cancer. 2009;101(9):1522–1528. doi: 10.1038/sj.bjc.6605323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerber B, Seitz E, Müller H, Krause A, Reimer T, Kundt G, Friese K. Perioperative screening for metastatic disease is not indicated in patients with primary breast cancer and no clinical signs of tumor spread. Breast Cancer Res Treat. 2003;82(1):29–37. doi: 10.1023/B:BREA.0000003917.05413.ac. [DOI] [PubMed] [Google Scholar]
- 17.Segaert I, Mottaghy F, Ceyssens S, et al. Additional value of PET-CT in staging of clinical stage IIB and III breast cancer. Breast J. 2010;16(6):617–624. doi: 10.1111/j.1524-4741.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 18.Nattinger AB, Schapira MM, Warren JL, Earle CC. Methodological Issues in the Use of Administrative Claims Data to Study Surveillance after Cancer Treatment. Medical Care. 2002;40(8):IV69–IV74. doi: 10.1097/00005650-200208001-00010. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. Surveillance Epidemiology and End Results Stat Fact Sheets: Breast. [accessed March 12, 2012];2011 Available: http://seer.cancer.gov/statfacts/html/breast.html#incidence-mortality.
- 20.Dinan MA, Curtis LH, Hammill BG, Patz EF, Jr, Abernethy AP, Shea AM, Schulman KA. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999–2006. JAMA. 2010;303(16):1625–1631. doi: 10.1001/jama.2010.460. [DOI] [PubMed] [Google Scholar]