Abstract
The sigma-2 receptor has been identified as a biomarker in proliferating tumors. Up to date there is no well-established functional assay for defining sigma-2 agonists and antagonists. Many sigma-2 ligands with diverse structures have been shown to induce cell death in a variety of cancer cells by triggering caspase-dependent and independent apoptosis. Therefore, in the current study, we used the cell viability assay and the caspase-3 activity assay to determine sigma-2 agonists and antagonists. Three classes of sigma-2 ligands developed in our laboratory were evaluated for their potency to induce cell death in two tumor cell lines, mouse breast cancer cell line EMT-6 and human melanoma cell line MDA-MB-435. The data showed that the EC50 values of the sigma-2 ligands using the cell viability assay ranged from 11.4 μM to >200 μM, which were comparable with the EC50 values obtained using the caspase-3 assay. Based on the cytotoxicity of a sigma-2 ligand relative to that of siramesine, a commonly accepted sigma-2 agonist, we have categorized our sigma-2 ligands into agonists, partial agonists, and antagonists. The establishment of functional assays for defining sigma-2 agonists and antagonists will facilitate functional characterization of sigma-2 receptor ligands and sigma-2 receptors.
Keywords: Sigma-2 receptor, agonist, antagonist, caspase-3, cell viability, functional assay
The sigma receptor was originally defined pharmacologically long before its molecular identity was known [1]. It is the specific binding site for a group of compounds, which were later named as sigma ligands. The sigma receptor was once thought to be a subset of the opioid receptor [2], but was subsequently revealed to be a distinct class of receptor system [1]. Radioligand binding studies and biochemical analyses have shown that there are at least two types of sigma receptors, sigma-1 and sigma-2. The sigma-1 receptor gene has been cloned [3–5] from guinea pig, human and rodent origins. The most prominent action of sigma-1 receptors in biological systems is the regulation and modulation of voltage-regulated and ligand-gated ion channels, including Ca2+-, K+-, Na+-, Cl−-, and SK channels, and NMDA and IP3 receptors [6]. The sigma-2 receptor has been identified as a biomarker in proliferating tumors [7, 8]. It regulates cell growth and is an emerging target for cancer diagnosis and therapeutics [9]. Recently, the progesterone receptor membrane component 1 (PGRMC1) protein complex has been identified as the putative sigma-2 receptor binding site [10].
Up to date numerous sigma-2 selective ligands have been developed [9, 11–19]. These ligands were generally characterized by receptor binding assays and determination of agonist/antagonist has been awaiting proper functional assays. Some sigma receptor ligands are referred in the literature as agonists/antagonists based on behavioral studies. For example, haloperidol and pentazocine were called sigma agonists because they have antipsychotic activity and analgesic activity, respectively, in clinical use [20]. BD1047 and BD1063 were called sigma receptor antagonists because they had no effects on their own but attenuated the dystonia produced by DTG and haloperidol in rats [21]. Other sigma-2 ligands were defined as agonists/antagonists using cell-based assays. For example, CB-64D was called a sigma-2 receptor agonist because it elicited calcium release in human neuroblastoma cells [22] and induced cell death in the breast tumor cell line [20]. However, there is no well-established functional assay for defining agonists/antagonists for sigma-2 receptors. This is mainly because the molecular identity of the sigma-2 receptor was unknown until recently, and the mechanism of ligand-receptor interaction is largely unclear.
Sigma-2 receptor-mediated cell death is one of the most active areas in sigma-2 receptor research. Many sigma-2 ligands with diverse structures kill a variety of cancer cells at concentrations in the micromolar range [17, 20, 23–25]. It is suggested that sigma-2 ligands bind to sigma-2 receptors and trigger caspase-independent and caspase-dependent apoptosis. Therefore, in the current study, we propose to use cell viability and caspase-3 activity, a hallmark of apoptosis, as functional assays to define the agonist/antagonist properties of sigma-2 receptor ligands. Three classes of sigma-2 ligands developed in our laboratory were evaluated in these two assays in two tumor cell lines: mouse breast cancer cell line EMT-6 and human melanoma cell line MDA-MB-435. Based on the potency of these ligands to induce cell death in cancer cells relative to the well-accepted sigma-2 agonist siramesine, we were able to categorize the sigma-2 ligands into the traditional terms used to describe intrinsic activity at a receptor: agonists, partial agonists, and antagonists. The establishment of functional assays for defining sigma-2 agonists/antagonists will facilitate the functional characterization of sigma-2 receptor ligands and sigma-2 receptors.
Materials and methods
Receptor binding assays
The sigma-1 and sigma-2 receptor binding affinities of sigma-2 ligands were determined as previously described [26]. Briefly, guinea pig brain (sigma-1 assay) or rat liver (sigma-2 assay) membrane homogenates (~300 μg protein) were diluted with 50 mM Tris-HCl, pH 8.0 and incubated with either ~5 nM [3H](+)-pentazocine (34.9 Ci/mmol; sigma-1 assay) or 1 nM [3H]RHM-1 (80 Ci/mmol; sigma-2 assay) in a total volume of 150 μL in 96 well plates at 25°C. The concentrations of each compound ranged from 0.1 nM to 10 μM. After incubating for 60 min, the reactions were terminated by the addition of 150 μL of cold wash buffer (10 mM Tris-HCl, 150 mM NaCl, pH 7.4) using a 96 channel transfer pipette (Fisher Scientific, Pittsburgh, PA), and the samples harvested and filtered rapidly into a 96 well fiberglass filter plate (Millipore, Billerica, MA) that had been presoaked with 100 μL of 50 mM Tris-HCl, at pH 8.0 for 1 h. Each filter was washed three times with 200 μL of ice-cold wash buffer, and the bound radioactivity quantified using a Wallac 1450 MicroBeta liquid scintillation counter (Perkin Elmer, Boston, MA). Nonspecific binding was determined in the presence of 10 μM cold haloperidol.
Cell culture conditions
EMT-6 mouse breast cancer cells were grown in DMEM containing 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin. MDA-MB-435 human melanoma cells were grown in MEM containing 10% fetal bovine serum, 2 mM L-glutamine, 1 mM sodium pyruvate, 1% nonessential amino acids (Mediatech Inc, Manassas, VA), 2% MEM vitamins (Invitrogen, Carlsbad, CA), 100 units/ml penicillin, and 100 μg/ml streptomycin. Both cell lines were maintained at 37°C in a humidified incubator with a 5% CO2/95% air atmosphere.
MTS cell viability assay
The cytotoxicity of the compounds on EMT-6 and MDA-MB-435 cells was measured by using the CellTiter96 Aqueous One Solution (Promega, Madison, WI), which contains a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS], according to the manufacturer’s protocol. Briefly, cells were plated 5 × 103 cells/well in 96-well plates 24 h prior to treatment with the compounds. Each compound was dissolved in DMSO and serially diluted in culture medium to acquire the desired concentrations. The final concentration of DMSO in the cell culture medium was no more than 1.0%. After a 24 or 48 h treatment with the various compounds, 20μl of the CellTiter 96 AQueous One Solution Reagent was added to each well, and the plate was incubated for 1–2 h at 37°C. The plate was then read at 490 nm in a Victor3 plate reader (PerkinElmer Life and Analytical Sciences, Shelton, CT). Cell viability (%) and cytotoxicity (%) at each concentration of the compound were calculated by formula 1 and 2, respectively:
| (1) |
| (2) |
Where OD490, σ2 is the absorbance at 490 nm for sigma-2 ligand-treated cells, and OD490, control is the absorbance at 490 nm for untreated cells. The EC50, defined as the concentration of the sigma-2 ligand required to inhibit cell proliferation by 50% relative to untreated cells, was determined from the dose-response curves generated using GraFit software, version 5 (Erithacus Software Limited, UK). All compounds were assayed in triplicate, and the EC50 values presented as the mean ± SEM of three independent experiments.
Caspase-3 activation assay
The caspase-3 activity induced by the compounds in EMT-6 and MDA-MB-435 cells was measured using the Apo-ONE® Homogeneous Caspase-3/7 Assay (Promega, Madison, WI). This assay utilizes a profluorescent substrate Z-DEVD-R110 specific for Caspase-3/7 coupled with an optimized cell permeabilization buffer. Cleavage of the peptide sequence DEVD by active caspase-3/7 releases free Rhodamine 110 which when excited at 485nm, becomes intensely fluorescent and can be detected at emission wavelength 535nm. The amount of fluorescent product generated is directly proportional to the caspase-3/7 activity in each sample. The cells were plated 5 × 103 cells/well in 96-well black, clear-bottomed plates 24 h prior to treatment with the compounds. After a 24 h treatment with the various compounds, caspase-3 activity was assessed using the Apo-ONE® Homogeneous Caspase-3/7 Assay. 10ml of buffer was pre-mixed with 100μl of the caspase-3/7 substrate Z-DEVD-R110. 100ul of the substrate-buffer mix was added to each well and the plate was placed on an orbital shaker for 5 min. The plate was then incubated at room temperature in the dark for up to 18 h. The plate was then read at excitation and emission wavelengths 485nm and 535nm, respectively, on a Victor3 plate reader (PerkinElmer Life and Analytical Sciences, Shelton, CT). The EC50, defined as the concentration of the sigma-2 ligand required to elicit the caspase-3 activity by 50% of the maximal caspase-3 activation values, was determined from the dose-response curves generated using GraFit software, version 5 (Erithacus Software Limited, UK). All the compounds were assayed in triplicate, and the EC50 values presented as the mean ± SEM of three independent experiments.
Results
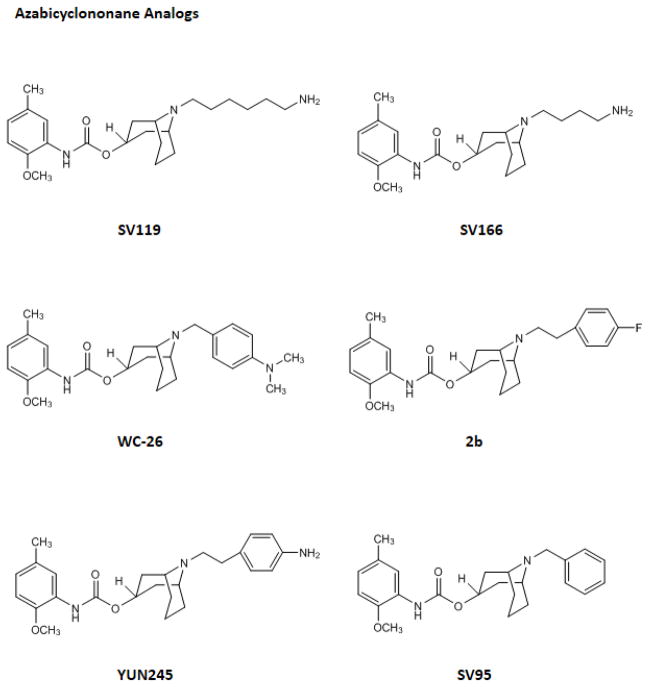
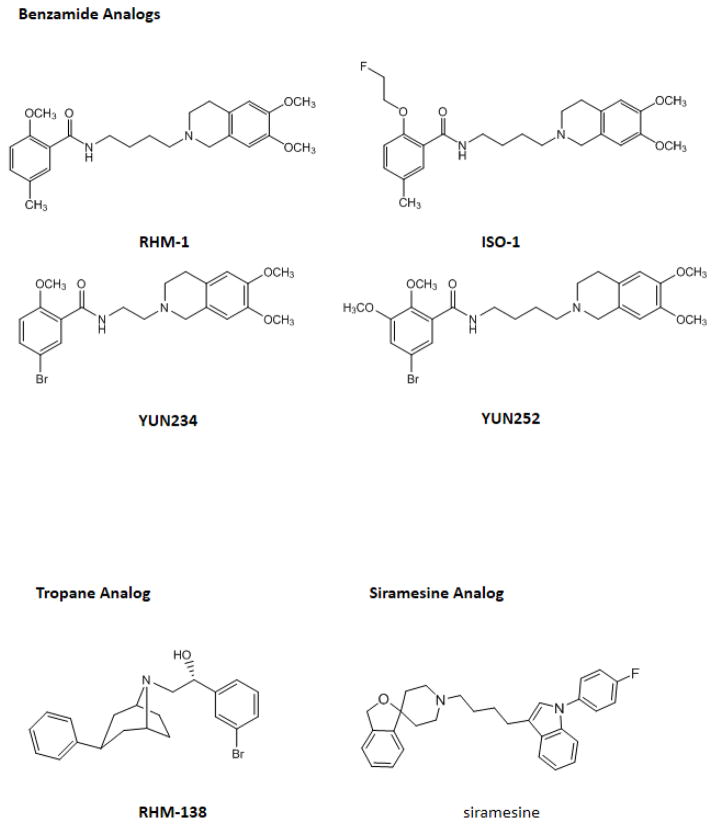
The chemical structures and the sigma receptor binding affinities of the sigma-2 ligands
In the current study, 11 sigma-2 ligands developed in our laboratory including 6 azabicylononane (ABN) analogs, 4 benzamide analogs, and 1 tropane analog, were evaluated for the potency to induce cell death in the cell viability assay and the caspase-3 assay. Siramesine, commonly accepted as a sigma-2 agonist, was also included in these two assays as a control. The structures of these sigma-2 ligands and their affinities for sigma-1 and sigma-2 receptors are published previously and are shown in Fig. 1 and Table 1, respectively. All of the ligands are highly selective for sigma-2 receptors versus sigma-1 receptors. Each class of sigma-2 receptor ligand (ABN, benzamide or tropane analogs) has a distinct chemical structure; however, the sigma-2 binding affinities for all three classes are in general similar.
Fig. 1.
The chemical structures of the sigma-2 ligands
Table 1.
The binding affinities for sigma-1 and sigma-2 receptors
| Compound | Ki for sigma-1 receptors (nM) | Ki for sigma-2 receptors (nM) | References |
|---|---|---|---|
| Azabicyclononane Analogs | |||
| SV119 | 1417.0 | 5.2 | a |
| SV166 | 6292.0 | 28.3 | a |
| WC-26 | 1436.0 | 2.6 | b |
| 2b | 262.0 | 5.9 | c |
| YUN245 | 2250.0 | 5.0 | c |
| SV95 | 92.5 | 3.1 | c |
| Benzamide Analogs | |||
| RHM-1 | 3078.0 | 10.3 | d |
| ISO-1 | 330.0 | 7.0 | e |
| YUN234 | 5484.0 | 12.4 | d |
| YUN252 | 12900.0 | 8.2 | d |
| Tropane Analog | |||
| RHM-138 | 544.0 | 12.3 | f |
| Siramesine Analog | |||
| siramesine | 17.0 | 0.12 | g |
The determination of the potency of sigma-2 ligands to induce cell death in the cell viability assay and the caspase-3 assay
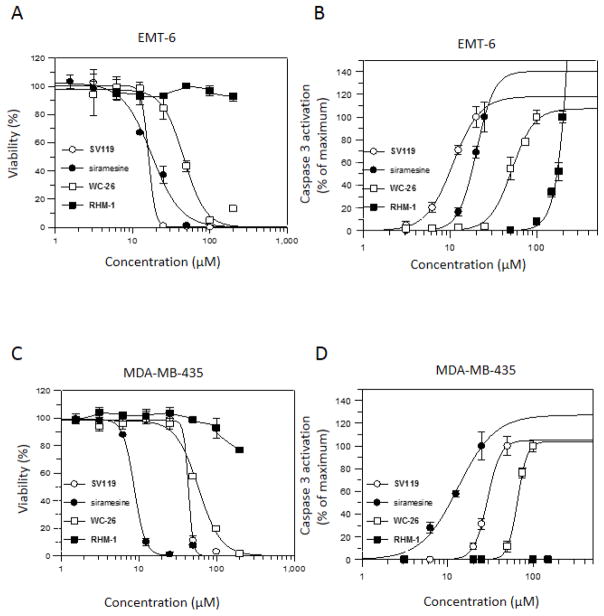
The EC50 values of the sigma-2 ligands were determined in mouse breast cancer cell line EMT-6 and human melanoma cell line MDA-MB-435 using the cell viability assay. The data are shown in Table 2. The dose-response curves for the representative sigma-2 ligands (SV119, WC-26, RHM-1 and siramesine) are shown in Fig. 2A and C. Siramesine exhibited the most potent cytotoxicity with the EC50 values of 5.3 μM and 9.3 μM in EMT-6 and MDA-MB-435, respectively, after 48 hour treatment. The data are consistent with the EC50 values of siramesine in other cell lines reported previously [23, 27].
Table 2.
EC50 of sigma-2 ligands in EMT-6 and MDA-MB-435 cell lines using MTS assay
| EMT-6 | MDA-MB-435 | |||
|---|---|---|---|---|
|
| ||||
| Compound | EC50 (μM, 24h) | EC50 (μM, 48h) | EC50 (μM, 24h) | EC50 (μM, 48h) |
| Azabicyclononane Analogs | ||||
| SV119 | 16.0 ± 1.4 | 11.4 ± 1.7 | 36.7 ± 3.3 | 18.5 ± 1.9 |
| SV166 | 30.0 ± 1.0 | 18.5 ± 2.1 | 34.1 ± 4.4 | 25.0 ± 5.57 |
| WC-26 | 42.5 ± 3.5 | 12.3 ± 1.6 | 49.7 ± 2.5 | 44.9 ± 5.3 |
| 2b | 65.5 ± 7.1 | 29 ± 1.4 | 63.1 ± 5.8 | 33.3 ± 3.26 |
| YUN245 | >200 | 154.5 ± 30.4 | 136.9 ± 4.6 | 101.0 ± 15.1 |
| SV95 | >200 | >200 | >200 | >200 |
| Benzamide Analogs | ||||
| RHM-1 | >200 | >200 | >200 | >200 |
| ISO-1 | >200 | >200 | >200 | >200 |
| YUN234 | >200 | 91 ± 12.7 | >200 | >200 |
| YUN252 | >200 | 137.5 ± 3.5 | >200 | >200 |
| Tropane Analog | ||||
| Siramesine Analog | ||||
| siramesine | 14.9 ± 4.0 | 5.3 ± 1.0 | 9.4 ± 0.4 | 9.3 ± 0.9 |
Fig. 2.
The dose response curves of the sigma-2 ligands in cell viability assay and caspase-3 activation assay. EMT-6 cells (A and B) or MDA-MB-435 cells (C and D) were treated with increasing concentrations of SV119 (○), siramesine (●), WC-26 (□), or RHM-1 (■) for 24 h. The cell viability and caspase-3 activation were then determined by MTS assay (A and C) and caspase-3 assay (B and D), respectively. The bars represent mean ± SD in the representative experiment performed in triplicates.
Azabiocyclonane analogs exhibited different potency of cytotoxicity with EC50 values ranging from 11.4 μM to >200 μM. Benzamide analogs, YUN234, YUN252, RHM-1 and ISO-1, showed little cytotoxicity (Table 2 and Fig. 3). Tropane analog RHM-138 exhibited potent cytotoxicity.
Fig. 3.
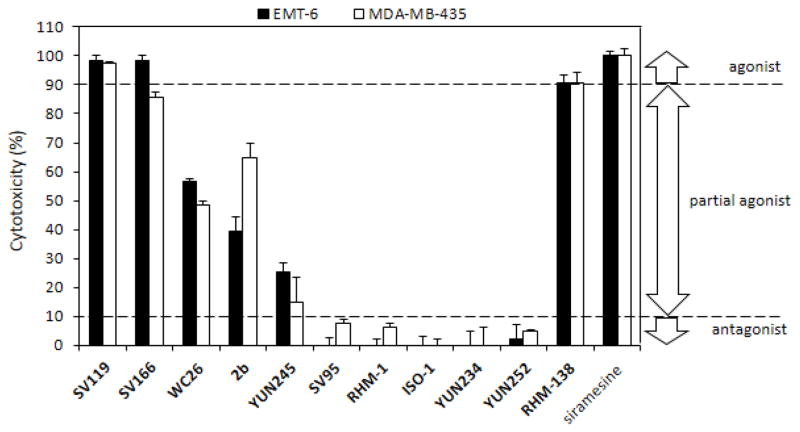
Cytotoxicity of sigma-2 ligands using MTS assay. EMT-6 and MDA-MB-435 cells were treated with 50 μM of sigma-2 ligands for 24h. MTS assay was then performed and cytotoxicity of the sigma-2 ligand was determined using the formula 1 and 2 described in Material and Methods. The bars represent mean ± SD in three independent experiments performed in triplicates.
The EC50 values of the sigma-2 ligands were also determined using the caspase-3 assay in both EMT-6 and MDA-MB-435 cell lines. The data are shown in Table 3. The dose response curves for the representative sigma-2 ligands are shown in Fig. 2B and D. The EC50 values of the sigma-2 ligands determined by the caspase-3 assay are comparable with those determined by the cell viability assay.
Table 3.
EC50 of sigma-2 ligands in EMT-6 and MDA-MB-435 cell lines using caspase-3 activity assay
| EMT-6 | MDA-MB-435 | |
|---|---|---|
|
| ||
| Compound | EC50 (μM, 24h) | EC50 (μM, 24h) |
| Azabicyclononane Analogs | ||
| SV119 | 11.6 ± 3.7 | 35.6 ± 2.97 |
| SV166 | 34.8 ± 4.7 | 30.4 ± 1.0 |
| WC-26 | 51.8 ± 4.4 | 67.4 ± 0.5 |
| 2b | 62.2 ± 3.3 | 75.2 ± 2.1 |
| YUN245 | 175.2 ± 25.5 | 119.4 ± 10.3 |
| SV95 | > 200 | >200 |
| Benzamide Analogs | ||
| RHM-1 | >200 | >200 |
| ISO-1 | >200 | >200 |
| YUN234 | >200 | >200 |
| YUN252 | >200 | >200 |
| Tropane Analog | ||
| RHM-138 | 40.8 ± 4.2 | 52.7 ± 1.2 |
| Siramesine Analog | ||
| siramesine | 15.9 ± 2.7 | 9.9 ± 2.8 |
The proposed criteria for defining sigma-2 agonists and antagonists
We propose to use the following criteria to define agonists and antagonists for the sigma-2 receptor. Siramesine possesses the most potent cytotoxicity and is a commonly accepted agonist. We determined the cytotoxicity of sigma-2 ligands as a percent relative to the cytotoxicity of siramesine (50 μM) using the cell viability assay (Fig. 3). The 50 μM concentration was chosen based on the dose-response curves of sigma-2 ligands (Fig. 2A and C), which showed 100% of cell death induced by siramesine and distinguishable percent of cell death induced by different sigma-2 ligands at this concentration. If the cytotoxicity of a sigma-2 ligand is > 90% relative to the cytotoxicity of siramesine, the sigma-2 ligand is considered an agonist. If the cytotoxicity is between 10–90%, the sigma-2 ligand is considered a partial agonist. If the cytotoxicity is <10%, the sigma-2 ligand is considered an antagonist. Based on these criteria, SV119, SV166, and RHM-138 are agonists, WC-26, 2b and YUN245 are partial agonists, and SV95, RHM-1, ISO-1, YUN234 and YUN252 are antagonists.
Discussion
Determination of sigma-2 agonists/antagonists is important for characterization of sigma-2 ligands for their biological effects. Well-characterized sigma-2 agonists and antagonists will facilitate their investigation as agents for imaging cell proliferation of solid tumors, for chemotherapy, and for studying the biological functions of sigma-2 receptors in cancer and in normal tissues. In the current study, we have proposed to use a cell viability assay and caspase-3 assay to define the functional activity of sigma-2 receptor ligands. Based on the potency of sigma-2 ligands to induce cell death relative to a generally-accepted full agonist (i.e., siramesine), we have been able to categorize the sigma-2 ligands developed in our laboratory into the traditional terms for describing functional activity at receptors: agonists, partial agonists, antagonists.
The ABN analogs showed different potency to induce cytotoxicity and caspase-3 activation (Table 2 and 3). Based on the criteria we have proposed in the current study, the ABN analogs can be considered agonists (SV119 and SV166), partial agonists (WC-26, 2b and YUN245) and antagonists (SV95). The ABN analogs consist of the sigma-2 receptor recognition fragment, the 9-azabicyclo[3.3.1]nonan-3α-yl carbamate moiety, and a sidechain group (Fig. 1). The sigma-2 receptor recognition moiety is mainly responsible for the sigma-2 receptor binding affinity, whereas the sidechain is mainly responsible for the cytotoxicity effect, and hence, intrinsic activity. The difference in the cytotoxicity potency of the ABN analogs is probably due to the difference in the interactions between the sidechain and the sigma-2 receptor/PGRMC1 protein complex, which, in turn, may cause different conformational and functional changes of the sigma-2 receptor/PGRMC1 protein complex.
By using the cell viability assay and the caspase-3 assay we showed that the benzamide analogs did not induce cytotoxicity and caspase-3 activation (Table 2 and 3). Therefore, these compounds are antagonists of the sigma-2 receptor. One possible explanation of the difference in intrinsic activity between the benzamides and ABN is that the two classes of compounds may bind to different regions of the sigma-2 receptor. However, receptor binding assays previously reported by our laboratory showed that a panel of ABN analogs displaced the binding of [3H]RHM-1 to sigma-2 receptors in rat liver membrane homogenates, a standard source of sigma-2 receptors for in vitro binding experiments [26]. Similarly, SV119 blocked the binding of [125I]RHM-4, another benzamide analog, to sigma-2 receptors in Hela cell membrane homogenates [10]. These data confirm that both the benzamide analogs and ABN analogs bind to the same region of the sigma-2 receptor since they have overlapping binding domains. The antagonist properties of the benzamide moieties could be explained by the finding that WC-21, a benzamide-based sigma-2 fluorescent probe structurally-similar to RHM-1 and ISO-1 [10], did not internalize into cells (unpublished observation), whereas SW120, an ABN-based sigma-2 fluorescent probe, rapidly internalized into cells and localized in the mitochondria, endoplasmic reticulum, lysosomes and the plasma membrane [28]. These data suggest that the benzamide analogs bind to receptors residing on the plasma membrane, but do not enter cells via receptor-mediated endocytosis. Consequently, they may not translocate to sigma-2 receptors localized on the mitochondria and endoplasmic reticulum, receptors which may mediate the cytotoxic and caspase-3 activation caused by the sigma-2 agonists.
Alternatively, other mechanisms may be responsible for differentiating between agonists and antagonists of the sigma-2 receptor. Recently, the PGRMC1 protein complex has been identified as the putative sigma-2 receptor binding site [10]. PGRMC1 has been reported to interact with multiple proteins including Insig-1, SCAP, P450 proteins, PAIR-BP1, and epidermal growth factor (EGFR)[29–33]. It is possible that a sigma-2 agonist binds to the PGRMC1 protein complex, changes its conformation, and induces the downstream signaling pathway. For example, PGRMC1 has been reported to bind to EGFR and stabilize the EGFR protein levels, promoting cell growth [30]. A PGRMC1 small molecule ligand AG-205, which inhibits cell growth, destabilized EGFR in tumor cell lines. It is intriguing to know if the sigma-2 agonist acts in a mechanism similar to AG-205. It is possible that sigma-2 agonists bind to the PGRMC1/EGFR complex and destabilize EGFR, leading to cytotoxicity and caspase-3 activation, whereas sigma-2 receptor antagonists have no effect on the PGRMC1/EGFR complex in tumor cells. Further studies are clearly needed to elucidate the biological mechanism of action of sigma-2 receptor agonists and antagonists.
In conclusion, we have proposed to use cell viability assay and caspase-3 assay as functional assays to define the functional activity of sigma-2 receptor ligands. By normalizing the activity of signa-2 receptor ligands to the activity of the generally-accepted sigma-2 agonist, siramesine, in the cell viability assay, it is possible to characterize sigma-2 receptor ligands as either agonists, partial agonists or antagonists at this receptor. These assays are simple and straightforward, and can be used as routine assays to characterize the functional activity of sigma-2 receptor ligands. The biological mechanisms responsible for the agonist and antagonist properties of sigma-2 receptor ligands requires further investigation.
Acknowledgments
This study was supported by CA 102869 and U.S. Army Medical Research and Material Command under DAMD 17-01-1-0446.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsumoto RR. Sigma receptors: Historical perspective and background. Springer Science; New York, NY 10013: 2007. [Google Scholar]
- 2.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. The Journal of pharmacology and experimental therapeutics. 1976;197:517–532. [PubMed] [Google Scholar]
- 3.Mei J, Pasternak GW. Molecular cloning and pharmacological characterization of the rat sigma1 receptor. Biochemical pharmacology. 2001;62:349–355. doi: 10.1016/s0006-2952(01)00666-9. [DOI] [PubMed] [Google Scholar]
- 4.Seth P, Fei YJ, Li HW, Huang W, Leibach FH, Ganapathy V. Cloning and functional characterization of a sigma receptor from rat brain. Journal of neurochemistry. 1998;70:922–931. doi: 10.1046/j.1471-4159.1998.70030922.x. [DOI] [PubMed] [Google Scholar]
- 5.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacology & therapeutics. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler KT, Wang LM, Wallen CA, Childers SR, Cline JM, Keng PC, Mach RH. Sigma-2 receptors as a biomarker of proliferation in solid tumours. British journal of cancer. 2000;82:1223–1232. doi: 10.1054/bjoc.1999.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mach RH, Smith CR, al-Nabulsi I, Whirrett BR, Childers SR, Wheeler KT. Sigma 2 receptors as potential biomarkers of proliferation in breast cancer. Cancer research. 1997;57:156–161. [PubMed] [Google Scholar]
- 9.Mach RH, Zeng C, Hawkins WG. The sigma Receptor: A Novel Protein for the Imaging and Treatment of Cancer. Journal of medicinal chemistry. 2013 doi: 10.1021/jm301545c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Zeng C, Chu W, Pan F, Rothfuss JM, Zhang F, Tu Z, Zhou D, Zeng D, Vangveravong S, Johnston F, Spitzer D, Chang KC, Hotchkiss RS, Hawkins WG, Wheeler KT, Mach RH. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nature communications. 2011;2:380. doi: 10.1038/ncomms1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowen WD, Bertha CM, Vilner BJ, Rice KC. CB-64D and CB-184: ligands with high sigma-2 receptor affinity and subtype selectivity. European journal of pharmacology. 1995;278:257–260. doi: 10.1016/0014-2999(95)00176-l. [DOI] [PubMed] [Google Scholar]
- 12.Mach RH, Wu L, West T, Whirrett BR, Childers SR. The analgesic tropane analogue (+/−)-SM 21 has a high affinity for sigma-2 receptors. Life Sci. 1999;64:PL131–137. doi: 10.1016/s0024-3205(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 13.Mach RH, Yang B, Wu L, Kuhner RJ, Whirrett BR, West T. Synthesis and sigma receptor binding affinities of 8-azabicyclo [3.2.1]octan-3α-yl and 9-azabicyclo [3.3.1]nonan-3α-yl phenylcarbamates. Medicinal Chemistry Research. 2001;10:339–355. [Google Scholar]
- 14.Vangveravong S, Xu J, Zeng C, Mach RH. Synthesis of N-substituted 9-azabicyclo [3.3.1]nonan-3alpha-yl carbamate analogs as sigma2 receptor ligands. Bioorganic & medicinal chemistry. 2006;14:6988–6997. doi: 10.1016/j.bmc.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Perregaard J, Moltzen EK, Meier E, Sanchez C. Sigma ligands with subnanomolar affinity and preference for the sigma 2 binding site. 1. 3-(omega-aminoalkyl)-1H-indoles. Journal of medicinal chemistry. 1995;38:1998–2008. doi: 10.1021/jm00011a019. [DOI] [PubMed] [Google Scholar]
- 16.Mach RH, Huang Y, Freeman RA, Wu L, Vangveravong S, Luedtke RR. Conformationally-flexible benzamide analogues as dopamine D3 and sigma 2 receptor ligands. Bioorganic & medicinal chemistry letters. 2004;14:195–202. doi: 10.1016/j.bmcl.2003.09.083. [DOI] [PubMed] [Google Scholar]
- 17.Berardi F, Abate C, Ferorelli S, Uricchio V, Colabufo NA, Niso M, Perrone R. Exploring the importance of piperazine N-atoms for sigma(2) receptor affinity and activity in a series of analogs of 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28) Journal of medicinal chemistry. 2009;52:7817–7828. doi: 10.1021/jm9007505. [DOI] [PubMed] [Google Scholar]
- 18.Abate C, Perrone R, Berardi F. Classes of sigma2 (sigma2) receptor ligands: structure affinity relationship (SAfiR) studies and antiproliferative activity. Current pharmaceutical design. 2012;18:938–949. doi: 10.2174/138161212799436485. [DOI] [PubMed] [Google Scholar]
- 19.Fan KH, Lever JR, Lever SZ. Effect of structural modification in the amine portion of substituted aminobutyl-benzamides as ligands for binding sigma1 and sigma2 receptors. Bioorganic & medicinal chemistry. 2011;19:1852–1859. doi: 10.1016/j.bmc.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford KW, Bowen WD. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer research. 2002;62:313–322. [PubMed] [Google Scholar]
- 21.Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. European journal of pharmacology. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- 22.Vilner BJ, Bowen WD. Modulation of cellular calcium by sigma-2 receptors: release from intracellular stores in human SK-N-SH neuroblastoma cells. The Journal of pharmacology and experimental therapeutics. 2000;292:900–911. [PubMed] [Google Scholar]
- 23.Ostenfeld MS, Fehrenbacher N, Hoyer-Hansen M, Thomsen C, Farkas T, Jaattela M. Effective tumor cell death by sigma-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress. Cancer research. 2005;65:8975–8983. doi: 10.1158/0008-5472.CAN-05-0269. [DOI] [PubMed] [Google Scholar]
- 24.Zeng C, Rothfuss J, Zhang J, Chu W, Vangveravong S, Tu Z, Pan F, Chang KC, Hotchkiss R, Mach RH. Sigma-2 ligands induce tumour cell death by multiple signalling pathways. British journal of cancer. 2012;106:693–701. doi: 10.1038/bjc.2011.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashiwagi H, McDunn JE, Simon PO, Jr, Goedegebuure PS, Xu J, Jones L, Chang K, Johnston F, Trinkaus K, Hotchkiss RS, Mach RH, Hawkins WG. Selective sigma-2 ligands preferentially bind to pancreatic adenocarcinomas: applications in diagnostic imaging and therapy. Molecular cancer. 2007;6:48. doi: 10.1186/1476-4598-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Tu Z, Jones LA, Vangveravong S, Wheeler KT, Mach RH. [3H]N-[4-(3,4-dihydro-6,7-dimethoxyisoquinolin-2(1H)-yl)butyl]-2-methoxy-5-methyl benzamide: a novel sigma-2 receptor probe. European journal of pharmacology. 2005;525:8–17. doi: 10.1016/j.ejphar.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 27.Spirkoski J, Melo FR, Grujic M, Calounova G, Lundequist A, Wernersson S, Pejler G. Mast cell apoptosis induced by siramesine, a sigma-2 receptor agonist. Biochemical pharmacology. 2012;84:1671–1680. doi: 10.1016/j.bcp.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 28.Zeng C, Vangveravong S, Jones LA, Hyrc K, Chang KC, Xu J, Rothfuss JM, Goldberg MP, Hotchkiss RS, Mach RH. Characterization and evaluation of two novel fluorescent sigma-2 receptor ligands as proliferation probes. Molecular imaging. 2011;10:420–433. [PMC free article] [PubMed] [Google Scholar]
- 29.Cahill MA. Progesterone receptor membrane component 1: an integrative review. The Journal of steroid biochemistry and molecular biology. 2007;105:16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed IS, Rohe HJ, Twist KE, Craven RJ. Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity. The Journal of biological chemistry. 2010;285:24775–24782. doi: 10.1074/jbc.M110.134585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suchanek M, Radzikowska A, Thiele C. Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. Nature methods. 2005;2:261–267. doi: 10.1038/nmeth752. [DOI] [PubMed] [Google Scholar]
- 32.Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone’s antiapoptotic action. Endocrinology. 2006;147:3133–3140. doi: 10.1210/en.2006-0114. [DOI] [PubMed] [Google Scholar]
- 33.Hughes AL, Powell DW, Bard M, Eckstein J, Barbuch R, Link AJ, Espenshade PJ. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell metabolism. 2007;5:143–149. doi: 10.1016/j.cmet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Chu W, Xu J, Zhou D, Zhang F, Jones LA, Wheeler KT, Mach RH. New N-substituted 9-azabicyclo[3.3.1]nonan-3alpha-yl phenylcarbamate analogs as sigma2 receptor ligands: synthesis, in vitro characterization, and evaluation as PET imaging and chemosensitization agents. Bioorganic & medicinal chemistry. 2009;17:1222–1231. doi: 10.1016/j.bmc.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mach RH, Vangveravong S, Huang Y, Yang B, Blair JB, Wu L. Synthesis and sigma receptor binding affinities of 9-azabicyclo[3.3.1]nonan-3 alpha-yl phenylcarbamate analogs as sigma-2 receptor ligands. Med Chem Res. 2003;11:380–398. [Google Scholar]
- 36.Tu Z, Xu J, Jones LA, Li S, Dumstorff C, Vangveravong S, Chen DL, Wheeler KT, Welch MJ, Mach RH. Fluorine-18-labeled benzamide analogues for imaging the sigma2 receptor status of solid tumors with positron emission tomography. Journal of medicinal chemistry. 2007;50:3194–3204. doi: 10.1021/jm0614883. [DOI] [PubMed] [Google Scholar]