Abstract
Acetylcholinesterase plays a key role in cholinergic synaptic transmission by hydrolyzing the neurotransmitter acetylcholine with one of the highest known catalytic rate constants. Hydrolysis occurs in a narrow and deep gorge that contains two sites of ligand binding: A peripheral site, or P-site, near the gorge entrance that contributes to catalytic efficiency both by transiently trapping substrate molecules as they enter the gorge and by allosterically accelerating the transfer of the substrate acyl group to a serine hydroxyl in an acylation site or A-site at the base of the gorge. Thioflavin T is a useful reporter of ligand interactions with the A-site. It binds specifically to the P-site with fluorescence that is enhanced ~1000-fold over that of unbound thioflavin T, and the enhanced fluorescence is quenched 1.5- to 4-fold when another ligand binds to the A-site in a ternary complex. To clarify the structural basis of this advantageous signal change, we here report the X-ray structure of the complex of thioflavin T with Torpedo californica acetylcholinesterase. The two aromatic rings in thioflavin T are coplanar and are packed snugly parallel to the aromatic side chains of Trp279, Tyr334, and Phe330. Overlays of this structure with the crystal structures of Torpedo californica acetylcholinesterase complexes with either edrophonium or m-(N,N,N-trimethylammonio)-2,2,2-trifluoroacetophenone, two small aromatic ligands that bind specifically to the A-site, indicate that the phenyl side chain of Phe330 must rotate to sterically accommodate both thioflavin T and the A-site ligand in the ternary complex. This rotation may allow some relaxation of the strict coplanarity of the aromatic rings in the bound thioflavin T and result in partial quenching of its fluorescence.
Introduction
Acetylcholinesterase (AChE) terminates synaptic transmission at cholinergic synapses by rapid hydrolysis of the neurotransmitter acetylcholine (ACh).1 It has become an important drug target because partial inhibition of AChE results in modest increases in ACh levels that can have therapeutic benefits; thus, AChE inhibitors that penetrate the blood-brain barrier have proved useful in the symptomatic treatment of Alzheimer’s disease.2 However, complete inactivation of AChE, which can occur with organophosphate and carbamate insecticides3 and organophosphate chemical warfare agents,4 leads to toxic accumulation of ACh and failure of cholinergic synaptic transmission, with consequent deterioration of neuromuscular junctions, flaccid muscle paralysis, and seizures in the central nervous system.
Kinetic and thermodynamic studies have revealed that inhibitors can interact with either or both of two binding sites in AChE,5,6 and X-ray crystallography has provided information about the location of these sites.7–10 A narrow gorge, ~20 Å deep, lined with aromatic residues, penetrates nearly to the center of the ~65 kDa catalytic subunits. Near the base of the gorge is the acylation site or A-site. This site includes a catalytic triad consisting of residue Ser200, His440 and Glu327. Similar triads are found in other hydrolases, and they promote acylation and deacylation of the serine residue by the substrate during catalytic turnover. An excellent model of the transition state for acylation by ACh was provided by m-(N,N,N-trimethylammonio)trifluoroacetophenone (TMTFA; see Figure 1 below).
Figure 1.
Chemical structures of AChE ligands discussed in the text.
The crystal structure of the complex of Torpedo californica AChE (TcAChE) with 4-oxo-N,N,N-trimethylpentanaminium (PDB code 2C5F) and with TMTFA (PDB code 1AMN) both indicate a tetrahedral adduct that nearly superimposes on a modeled structure of ACh in the A-site, and reveals that Trp84 in the A-site binds to the trimethylammonium group of ACh as acyl transfer to Ser200 is initiated.9 Crystal structures also showed a binding site near the entrance of the active-site gorge that includes residues Trp279 and Asp72.9–11 Ligands specific for this peripheral site, or P-site, include propidium6 and the fasciculins - three very similar snake venom neurotoxins comprised of 61-amino acid polypeptides.12–14 These P-site ligands are bulky and, to a large extent, lie outside the active-site gorge. Bis-quaternary, as well as long uncharged, ligands that span the 12–15 Å distance between the A- and P-sites,15–17 can have high affinities and have been developed as therapeutic agents.18
One unique advantage of fluorescent ligands that bind selectively to the P-site is their ability to report on molecular interactions occurring at the A-site. The fluorescence of propidium is enhanced nearly 10-fold when it binds to the P-site, and this increase is sufficient to conduct titrations that quantify propidium affinity for the P-site and detect the formation of ternary complexes with propidium bound at the P-site and selective ligands like edrophonium (EDR) (Figure 1) bound at the A-site.6,19,20 Thioflavin T (ThT) (Figure 1) is a fluorophore frequently used to detect amyloid structure in proteins,21 but ligand-binding data indicate that it also binds with high selectivity to the P-site of AChE,22 even though this site shows no indication of the β-structure typical of amyloid.
The fluorescence of ThT is increased ~1000-fold on binding to the P-site of human AChE; on further binding of an A-site ligand, such as EDR or TMTFA, to form a ternary complex, the fluorescence of the bound ThT is quenched 3–4-fold.22 These features make ThT a more sensitive and versatile fluorescent reporter of ligand interactions with AChE than propidium. The quenching of fluorescence in the ternary complex occurs even though there is no spectral overlap, nor any obvious steric overlap (based on an absence of thermodynamic interaction), between the bound ligands.22 This change in fluorescence is perhaps the most direct evidence for conformational interaction between the P- and A-sites that has been obtained. Certain substrates, including acetylthiocholine (ATCh), appear to take functional advantage of this conformational interaction. These substrates can bind to both the P- and the A-sites, as initially indicated by a competition between ATCh and fasciculin for binding to the P-site,23 and confirmed clearly in X-ray structures of several AChE complexes, including those with ACh, choline, ATCh, thiocholine and the nonhydrolysable ACh analogue, 4-oxo-N,N,N-trimethylpentanaminium.24,25 Acylation at the A-site is accelerated when a second substrate molecule is bound at the P-site. Strong evidence supporting this model of substrate activation was obtained from titrations with ThT. These titrations provided thermodynamic estimates of substrate affinities for the A- and P-sites that were in complete agreement with kinetic estimates of these affinities derived from the substrate activation model.26
In view of the sensitivity of ThT fluorescence to the conformation of residues that contact ThT, it is important to obtain three-dimensional structures of binary and ternary complexes of ThT with AChE. Such structures may provide insight into the molecular details of conformational interaction between the P- and A-sites.
Materials and Methods
Materials
Thioflavin T chloride (Sigma) was recrystallized as described.22 TcAChE was purified from the electric organ tissue of T. californica,11,27 and active site concentrations were determined by fluorogenic titration with N-methyl-(7-dimethylcarbamoxy)quinolinium iodide.28 Recombinant domain IV of mouse laminin β2, expressed in 293 cells,29 was a gift from Dr. Takako Sasaki (The Shriners Hospital for Children, Portland, OR).
Crystallographic Analysis
Trigonal P3221TcAChE crystals, in which the active-site gorge is solvent-accessible, were grown by the batch-under-oil method,30 using a Douglas Instruments IMPAX 1–5 robot. In order to obtain the P3221 TcAChE crystals, the protein solution used was a 1:4 (v/v) mixture of TcAChE (13.7 mg/ml) and domain IV of mouse laminin β2 (4.7 mg/ml).29 TcAChE was found to crystallize from this mixture in spacegroup P3221. The batch drops contained 0.25μL of the protein mixture, 0.25μL of Hampton Research Index #85 solution (25% PEG 3350, 0.2 M MgCl2, 0.1 M Tris, pH8.5) and 0.1μL of 1 M MgCl2. Crystals grew to 0.2 × 0.2 × 0.2 mm within 1 week at 18 °C. A supersaturated aqueous solution of ThT in 60% Index #85 was diluted 1:10, and a 0.1μL aliquot was added to a drop containing the crystals (final ThT concentration of 250 μM). X-ray data were collected 5 days after the addition of ThT.
X-ray data to 2.8Å resolution were collected under cryogenic conditions, “in-house” at the Weizmann Institute of Science, on a Rigaku R-AXIS IV++ image plate area detector. Data collection and refinement statistics are shown in Table 1.
Table 1.
Data Collection and Refinement Statistics
| resolution | 2.8 Å |
| wavelength | 1.5418 Å |
| space group | P3221 |
| unit cell | a = b = 139.44 Å, c = 71.42 Å |
| data completeness | 99.1% |
| Rsym | 14.5% |
| I/σ | 24.5 (8.4)a |
| R-factor | 19.6% |
| R-free | 25.1% |
Number in parentheses is value for highest resolution shell 2.8–2.9 Å.
The data were reduced with the XDS program,31 refined with CNS,32 and graphically fitted using COOT.33 Coordinates and structure factors were deposited in the PDB with entry code 2J3Q.
Fluorescence Determinations of the Binding of Thioflavin T to TcAChE
ThT fluorescence was measured with excitation at 450 nm and emission at 490 nm (slits 10 and 20 nm, respectively), in 60 mM NaCl, 40 mM sodium phosphate buffer (pH 7.0) and 0.04% Triton X-100, on a Perkin-Elmer LS-50B luminescence spectrometer thermostatted at 25 °C. Samples were introduced into a Hi-Tech SFA 20 stopped-flow apparatus by mixing equal volumes (300 μl) of AChE and combined inhibitors, and the continuous fluorescence signal (F) was recorded for 3–4 min. Average F values were corrected for inner filter effects34 as described previously.26 Data were analyzed in accordance with Scheme 1, which assumes that AChE (E) can form the indicated binary and ternary complexes with ThT (L) and EDR (I). Ligand bound at the P-site in Scheme 1 is designated by the subscript P, while ligand bound at the A-site has no subscript. Rate equations corresponding to an equilibrium formulation of Scheme 1 were solved in the numerical integration program SCoP (Simulation Resources, Inc., Redlands, CA; version 3.52), and values of F were fitted in a two-step process.22 Initial fitting was conducted in the absence of EDR, with the known total concentrations Etot and Ltot and the known fluorescence intensity coefficient for free ThT (fL) inserted as fixed input parameters, and KLp and the intensity coefficient for thioflavin bound to the P-site (fELp) as the calculated output parameters. This analysis revealed small amounts of an apparent ternary complex involving ThT bound to both the P- and A-sites, so the fitting was extended to obtain KLpL and the intensity coefficient fELpL as additional output parameters. The fitting in principle also depended on KL and the intensity coefficient fEL, but over a reasonable range of values (KL/KLpL > 0.25 and fEL/fELp < 0.2) the values of these parameters were unimportant (<30% change in the output parameters). Greater uncertainty arose because KLpL and fELpL were highly correlated, preventing unique assignments. Consequently, in the second fitting step in the presence of EDR, a reasonable range of fELpL (fELpL/fL = 100–400) and the corresponding fitted value of KLpL (34–5 μM) were examined as fixed input parameters along with Etot, Ltot, Itot and fL, and KLp, KI, KLpI, fELp, fELpI were obtained as fitted outputs.
Scheme 1.
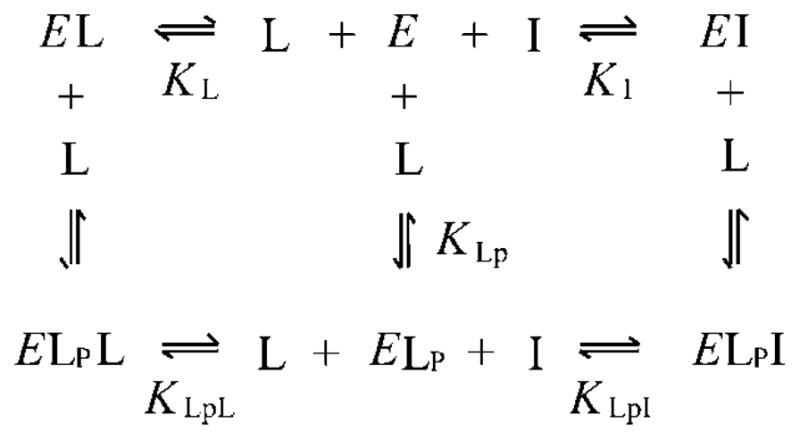
Results
Fluorescence of ThT is Strongly Enhanced in Binary Complexes with TcAChE and Partially Quenched when an A-Site Ligand is Added to Form a Ternary Complex
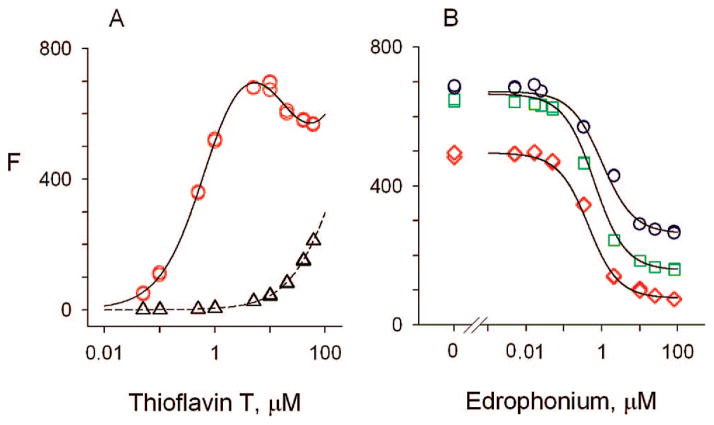
We show in this report that a key residue involved in the binding of ThT to TcAChE is Phe330. Since the corresponding residue in human AChE (hAChE) is a tyrosine (Tyr337), it was important to confirm that ThT complexes involving TcAChE show fluorescence features similar to those involving hAChE. Titration of TcAChE with ThT (Figure 2A) gave the same 1200-fold increase in the relative fluorescence intensity of the bound ligand (fELp/fL) previously observed with hAChE.22 However, at high concentrations of ThT, a decrease in fluorescence occurred that had not been seen with hAChE. We interpreted this decrease to reflect the low-affinity binding of a second molecule of ThT to the A-site of TcAChE (see Scheme 1). As noted in the Introduction, the binding of a ligand to the A-site in the ThT/hAChE complex would be expected to result in partial quenching of the fluorescence of thioflavin T bound at the P-site. The affinity of ThT for the A-site of TcAChE was at least an order of magnitude lower than that for the P-site, and incorporation of this A-site binding did not obscure further thermodynamic analysis with the A-site ligand EDR, but did lower the precision with which some binding parameters could be determined (see Experimental Procedures).
Figure 2.
(A) Fluorescence titrations of TcAChE with ThT. Fluorescence values (F) were measured as outlined in the Methods at the indicated total concentrations of ThT. The fluorescence intensity coefficient for the free ligand (fL) was obtained from the plot in the absence of AChE (△). The fluorescence F in the presence of a fixed concentration of TcAChE (170 nM) (○) was then analyzed as outlined in the Experimental Procedures. Values of KLp = 0.6 ± 0.3 μM and fELp/fL = 1200 ± 300 were obtained. (B) EDR binding decreases the fluorescence of TcAChE-bound ThT. The fluorescence values (F) of mixtures of TcAChE (168 nM), ThT (○, 10 μM; □, 3 μM; ◇, 1 μM), and the indicated concentration of EDR were determined. F values from all three data sets were fitted with the SCoP program simultaneously as outlined in the Experimental Procedures. The fitting gave KLp = 0.7 ± 0.2 μM for ThT; KI = 190 ± 10 nM for EDR; KLpI/KI = 4 ± 1; and fELp/fELpI = 3.4 ± 0.6.
We next examined the effect of increasing EDR concentrations on the fluorescence of bound ThT, and observed a decrease in fluorescence as EDR saturated the A-site (Figure 2B). Fitting of the data to Scheme 1 gave values of KLp for ThT and KI for EDR, and indicated a slight decrease in the affinities of the ligands in the ternary complex relative to the binary complex (KLpI/KI = 4 ± 1). Almost no decrease in these relative affinities had been observed with hAChE (KLpI/KI = 1.12 ± 0.0222). However, the binding of EDR decreased the fluorescence of ThT in the ternary complex (fELp/fELpI) by a factor of 3.4 ± 0.6, comparable to the decrease previously seen with hAChE (2.76 ± 0.02). These data thus confirm that the fluorescence features of ThT complexes involving TcAChE are essentially the same as those involving hAChE.
X-Ray Structure of the ThT/TcAChE Complex
We earlier obtained structures of a number of complexes of TcAChE with A-site ligands,15,35 and with ligands spanning the A- and P-sites,16,36 by diffusing the ligands into the protein crystals in the P3121 trigonal crystal form. In this crystal form, the P-site at the entrance to the active-site gorge is blocked by a segment of a symmetry-related TcAChE molecule. Hence, it was not possible to obtain structures of complexes with bulky P-site ligands using this approach with such crystals. A breakthrough was achieved when it was discovered that crystals of TcAChE could be grown in the presence of gallamine triethiodide, a P-site ligand with relatively low affinity, to yield a P3221 trigonal crystal form in which the entrance to the active-site gorge is more open.37 Since then, several other crystallization additives have been found to yield crystal forms of TcAChE in which the P-site is accessible to bulky ligands.38,39 In the present study, mouse recombinant domain IV of laminin β229 was used as a crystallization additive to obtain the P3221 crystals. The rms deviation of the Cα chain between the P3221 and the P3121 crystal structures is only 0.3Å, indicating a virtually identical overall conformation of the two molecules. However, P-site ligands could be soaked into the P3221 crystal form;37 indeed, a crystal structure at 2.8Å resolution was obtained upon soaking such TcAChE crystals with ThT (Figure 3).
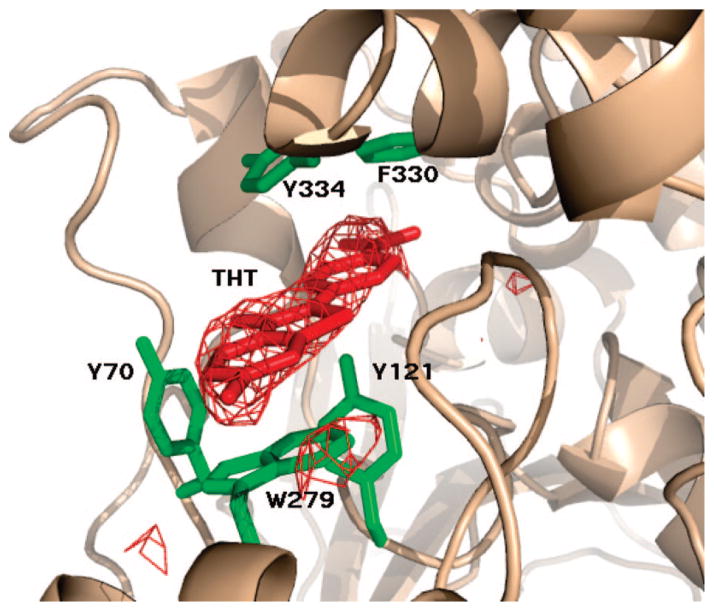
Figure 3.
ThT (red) and its omit electron density map (Fo-Fc drawn at 3σ) in the ThT/TcAChE complex makes short contacts (<3.7Å) with five aromatic residues (green) in the active-site gorge.
The X-ray structure of the ThT/TcAChE complex refined to an R factor of 19.6% and an R-free of 25.1%. Residues 4–536 of TcAChE were traced in the electron density map, as well as one molecule of ThT, 121 water molecules, and 3 N-acetylglucosamine moieties attached to Asn residues 59, 416 and 457. Two bound Mg2+ ions, derived from the mother liquor, are seen in the electron density map. One of these Mg2+ ions is in contact with Glu268Oε1 and Oε2 and with His264Nε2 (2.22, 3.34, and 2.66 Å, respectively); the second with Asp392Oδ2, Asp326Oδ1 and a water molecule (2.35, 2.43, and 2.36 Å, respectively). The positions of the two Mg2+ ions superimpose, respectively, on those of a Mg2+ ion and a Zn2+ ion in a structure of TcAChE crystallized in the presence of MgCl2 (Anne Nicolas, unpublished results).
Figure 3 shows that the planar ThT molecule is lodged in the upper part of the active-site gorge, within the P site, making nonbonding interactions with four of the aromatic side-chains that line the gorge surface, viz. Tyr70, Tyr121, Trp279 and Phe330. Superposition of the ThT/TcAChE structure on that of native TcAChE (PDB code 1EA5) yields rms = 0.33 Å (for 532 Cα atoms), and shows that all the gorge side-chains maintain their native conformation in the complex and that the ThT molecule fills the space within the gorge previously occupied by four water molecules.
More detailed inspection reveals that the benzothiazole ring of ThT is stacked against Trp279, and that its dimethylaminophenyl moiety is nearly coplanar with the phenyl group of Phe330 at a distance of 3.5 Å (Figure 3). Other interactions between ThT and the protein include a 3.2 Å contact from Tyr121OH to the dimethylaminophenyl moiety and 3.4 and 3.7 Å contacts from Tyr70 and Tyr334 to the benzothiazole ring, respectively.
Overlay of the ThT/TcAChE Structure with the Crystal Structures of A-Site Complexes
Since, as mentioned in the Introduction, the A-site ligands, EDR and TMTFA, quench the fluorescence of the ThT/TcAChE complex,22 we overlaid both EDR from the EDR/TcAChE complex15 (Figure 4A) and TMTFA from the TMTFA/TcAChE9 complex9 (not shown) on the ThT/TcAChE crystal structure. From these overlays it is apparent that the ThT/TcAChE structure can sterically accommodate both EDR and TMTFA at the A-site; the aromatic rings of both EDR and TMTFA, in their respective complexes, are nearly coplanar with Trp279/ThT/Phe330 in the ThT/TcAChE complex. However, if one does the reverse, and overlays ThT from the ThT/TcAChE structure on the crystal structures of either EDR/TcAChE (Figure 4B) or TMTFA/TcAChE (not shown), it can be seen that there is a clash of the dimethylaminophenyl moiety of the ThT molecule with the phenyl side-chain of Phe330. The reason for this clash is that in both the EDR/TcAChE and TMTFA/TcAChE structures the phenyl ring is rotated ~115° relative to its orientation in both the native TcAChE and ThT/TcAChE structures.
Figure 4.
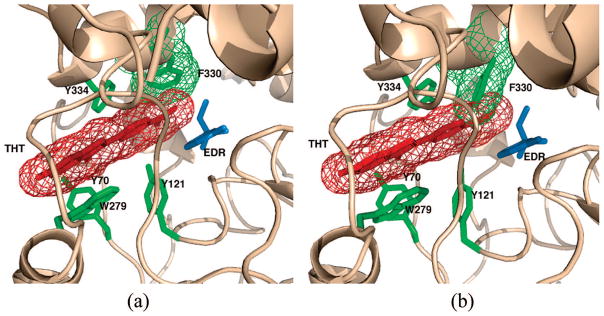
Models of the putative EDR/ThT/TcAChE ternary complex. (a) Crystal structure of the ThT/TcAChE complex, with a model of EDR, taken from the crystal structure of the EDR/TcAChE structure (PDB code 2ACK) superimposed. A π–π stacking system, spanning the P- and A-sites, is formed by ThT (red mesh), EDR (blue stick model), Trp279, Tyr334 (both green stick models) and Phe330 (green mesh). (b) Crystal structure of the EDR/TcAChE complex (PDB code 2ACK), with a model of ThT taken from the ThT/TcAChE crystal structure superimposed. It can be seen that in the conformation that Phe330 (green mesh) adopts in the EDR complex, it clashes severely with the dimethylaminophenyl moiety of the ThT molecule.
Discussion
Crystal structures of TcAChE complexes with A-site ligands,11,15,40 with the P-site ligands fasciculin9,11 and propidium,10 and with ligands which span the two sites,15,36,37 show remarkably few differences in conformation of the entire active-site gorge relative to native TcAChE. These observations indicate that the binding of these ligands does not induce large (and potentially slow) conformational changes in the active site, as might be expected for an enzyme like AChE that is built for unusually high catalytic speed. The structure of the ThT/TcAChE complex also shows few changes from that of native TcAChE, and the basis of the enhanced ThT fluorescence in this complex and its partial quenching in ternary complexes are of interest. The fluorescence of ThT has been shown to depend on solvent viscosity,41 with a relationship previously described for a class of fluorescent dyes called molecular rotors.42,43 These dyes show increased fluorescence when introduced into high-viscosity media due to a decreased torsional relaxation. The X-ray structure of the ThT/TcAChE complex presented here shows the ThT rings system in a coplanar position packing snugly parallel to Trp279, Tyr334 and Phe330. This packing causes a decrease in torsional relaxation and, hence, the increase in fluorescence.
The structures of complexes of TcAChE with bis-quaternary15,36 and other gorge-spanning ligands16,37–39,44,45 confirm that they bridge the A- and P-sites and traverse the region occupied by the bound ThT. In some of these structures the bifunctional ligands display contacts with Trp279 and Phe330 of TcAChE that bear some similarity to those made by ThT. Thus, in the BW284C51/TcAChE complex36 (Figure 1) (PDB code 1E3Q), one quaternary phenylammonium group of the symmetric bifunctional ligand interacts with Trp279, with the two aromatic rings being coplanar, and an allyl group at its other extremity makes a strong hydrophobic interaction with Phe330. The anti-Alzheimer disease drug donepezil (E2020 or Aricept) also spans the A- and P-sites. Its indanone ring stacks against the indole ring of Trp279, and the charged nitrogen of a piperidine ring, separated by one methylene group from the indanone ring, makes a cation-π interaction with the phenyl ring of Phe330.16 In the crystal structure of the complex of TcAChE with the anticancer prodrug CPT-11 (PDB code 1U65), another ligand that spans the A- and P-sites,37 the planar camptothecin moiety superimposes on the planar benzothiazole ring of ThT and stacks against Trp279. The terminal piperidine moiety of CPT-11 occupies the same space as the quaternary ammonium moiety of EDR at the A-site, and the penultimate piperidino ring stacks against the phenyl ring of Phe330. However, in all three of these complexes this phenyl ring rotates away from its position in the ThT/TcAChE complex to allow the ligand to span the A-and P-sites.
The packing of ThT with its rings snugly parallel to Trp279, Tyr334, and Phe330 reveals a precise ligand location that has not been previously observed in X-ray structures of AChEs complexed with other ligands specific for the P-site. In the complex of mouse AChE (mAChE) with the P-site ligand gallamine (Figure 1) (PDB code 1N5M), the aromatic moiety of the ligand, which is the only part of the gallamine molecule seen in the structure, is positioned similarly to ThT, making stacking contacts with both Trp286 and Tyr341 but not Tyr337 (Trp 279, Tyr334, and Phe 330, respectively, in TcAChE).10 In structures of mAChE complexes with two other ligands that bind selectively to the P-site, propidium and decidium, the ligands interacted with Trp286 but were reported to extend outward along the enzyme surface.10 The location and orientation of ThT within the P-site may help to explain why this fluorophore is such a useful reporter for ligand binding at the A-site. The side chain of Phe330 in the ThT/TcAChE complex has the same conformation as in the native 1.8 Å TcAChE X-ray structure (PDB code 1EA5), and is coplanar with the dimethylaminophenyl moiety of ThT. In X-ray structures of the complexes of TcAChE with both EDR and TMTFA, two small quaternary aromatic ligands that bind specifically to the A-site, the χ1 angle of Phe330 is rotated ~115° from its position in native TcAChE.9,15 This rotation is very similar to that observed upon binding of the substrate, acetylthiocholine, or of the product, thiocholine.24,25 As shown in Figure 4B, this rotation produces a clash between Phe330 and the proximal ring of ThT, if the latter occupies the same position as it occupies in the ThT/TcAChE. Hence, the phenyl ring of Phe330 cannot be assumed to occupy the same position in a putative EDR/ThT/ TcAChE ternary complex as in the ThT/TcAChE, and must rotate. This rotation may allow some relaxation of the strict coplanarity of the aromatic rings of the bound ThT and of the enzyme, thus resulting in partial quenching of its fluorescence. Indeed, attempts to obtain crystals of such a ternary complex, as well as of the analogous ternary complex with TMTFA, have been unsuccessful, since crystals of both the EDR/TcAChE and TMTFA/TcAChE complexes lost their diffracting power after ThT had been soaked into them. This implies that, in both cases, formation of the ternary complex produced a conformational change that damaged the crystal lattice. Similarly, it was earlier reported that soaking trigonal crystals (spacegroup P3121) of native TcAChE with a galanthamine derivative that could not be accommodated by the native conformation of the active-site gorge resulted in loss of diffracting power of these otherwise robust crystals.38 Formation of an EDR/ThT/TcAChE or a TMTFA/ThT/TcAChE ternary complex results in a 3–4-fold quenching of bound ThT fluorescence. Another small A-site ligand, 3-(acetamido)-N,N,N-trimethylanilinium, for which a crystal structure in complex with AChE is lacking, also produces strong (viz. 3–4-fold) quenching of ThT fluorescence in the ternary complex,26 while the A-site ligand carbachol quenches ThT fluorescence by only 35%. (T.L. Rosenberry, unpublished data). Carbachol (Figure 1), an ACh analog, probably produces a change in conformation of Phe330 similar to that seen in the crystal structures of the complexes with ATCh and thiocholine,24 which in turn, is similar to those produced by EDR and TMTFA. The smaller quenching produced by carbachol, compared to the much larger quenching produced by EDR and TMTFA, may be due to the fact that it does not participate in a comparable stacking array involving stacking of the ThT with the TcAChE aromatic rings or that it imposes a smaller rotation of the phenyl ring of Phe330 from coplanarity with the dimethylaminophenyl moiety of ThT in the ternary complex.
Acknowledgments
This study was supported by grants from The Israel Science Foundation (to JLS and IS), from the Israel Ministry of Science, Culture, and Sport to the Israel Structural Proteomics Center (ISPC), the European Commission Sixth Framework Research and Technological Development Programme (Grant No.: 031220), the National Institutes of Health (Grant NS-16577 to TLR), the Muscular Dystrophy Association of America (to TLR), the Benziyo Center for Neuroscience (to IS), the Nalvyco Foundation and the Divadol Foundation. We thank Dr. Takako Sasaki for the sample of domain IV of laminin β2, and Michal Cohen and Lilly Toker for the TcAChE preparations used. J.L.S. is the Morton and Gladys Pickman Professor of Structural Biology.
References
- 1.Silman I, Sussman JL. Curr Opin Pharmacol. 2005;5:293–302. doi: 10.1016/j.coph.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Giacobini E. In: Cholinesterase inhibitors: from the Calabar bean to Alzheimer therapy In Cholinesterases and Cholinesterase Inhibitors. Giacobini E, editor. Martin Dunitz; London: 2000. pp. 181–226. [Google Scholar]
- 3.Casida JE, Quistad GB. Annu Rev Entomol. 1998;43:1–16. doi: 10.1146/annurev.ento.43.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Millard CB, Broomfield CA. J Neurochem. 1995;64:1909–1918. doi: 10.1046/j.1471-4159.1995.64051909.x. [DOI] [PubMed] [Google Scholar]
- 5.Changeux JP. Mol Pharmacol. 1966;2:369–392. [PubMed] [Google Scholar]
- 6.Taylor P, Lappi S. Biochemistry. 1975;14:1989–1997. doi: 10.1021/bi00680a029. [DOI] [PubMed] [Google Scholar]
- 7.Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Science. 1991;253:872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- 8.Bourne Y, Taylor P, Marchot P. Cell. 1995;83:503–512. doi: 10.1016/0092-8674(95)90128-0. [DOI] [PubMed] [Google Scholar]
- 9.Harel M, Quinn DM, Nair HK, Silman I, Sussman JL. J Am Chem Soc. 1996;118:2340–2346. [Google Scholar]
- 10.Bourne Y, Taylor P, Radic Z, Marchot P. EMBO J. 2003;22:1–12. doi: 10.1093/emboj/cdg005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL. Nat Struct Biol. 1997;4:57–63. doi: 10.1038/nsb0197-57. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson E, Mbugua PM, Rodriguez-Ithurralde D. J Physiol (Paris) 1984;79:232–240. [PubMed] [Google Scholar]
- 13.Marchot P, Khelif A, Ji YH, Masnuelle P, Bourgis PE. J Biol Chem. 1993;268:12458–12467. [PubMed] [Google Scholar]
- 14.Harel M, Kleywegt GJ, Ravelli RBG, Silman I, Sussman JL. Structure. 1995;3:1355–1366. doi: 10.1016/s0969-2126(01)00273-8. [DOI] [PubMed] [Google Scholar]
- 15.Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL. Proc Natl Acad Sci USA. 1993;90:9031–9035. doi: 10.1073/pnas.90.19.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kryger G, Sillman I, Sussman JL. Structure. 1999;7:297–307. doi: 10.1016/s0969-2126(99)80040-9. [DOI] [PubMed] [Google Scholar]
- 17.Bourne Y, Kolb HC, Radic Z, Sharpless KB, Taylor P, Marchot P. Proc Natl Acad Sci USA. 2004;101:1449–1454. doi: 10.1073/pnas.0308206100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du DM, Carlier PR. Curr Pharm Des. 2004;10:3141–3156. doi: 10.2174/1381612043383412. [DOI] [PubMed] [Google Scholar]
- 19.Berman HA, Decker MM, Nowak MW, Leonard KJ, McCauley M, Baker WM, Taylor P. Mol Pharmacol. 1987;31:610–616. [PubMed] [Google Scholar]
- 20.Barak D, Ordentlich A, Bromberg A, Kronman C, Marcus D, Lazar A, Ariel N, Velan B, Shafferman A. Biochemistry. 1995;34:15444–15452. doi: 10.1021/bi00047a008. [DOI] [PubMed] [Google Scholar]
- 21.LeVine H., III . Quantification of β-sheet amyloid fibril structures with thioflavin T. In: Wetzel R, editor. Methods in Enzymology. Vol. 309. Academic Press; Orlando, FL: 1999. pp. 274–284. [DOI] [PubMed] [Google Scholar]
- 22.De Ferrari GV, Mallender WD, Inestrosa NC, Rosenberry TL. J Biol Chem. 2001;276(2):23282–23287. doi: 10.1074/jbc.M009596200. [DOI] [PubMed] [Google Scholar]
- 23.Szegletes T, Mallender WD, Rosenberry TL. Biochemistry. 1998;37:4206–4216. doi: 10.1021/bi972158a. [DOI] [PubMed] [Google Scholar]
- 24.Colletier JP, Fournier D, Greenblatt HM, Stojan J, Sussman JL, Zaccai G, Silman I, Weik M. EMBO J. 2006;25:2746–2756. doi: 10.1038/sj.emboj.7601175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourne Y, Radic Z, Sulzenbacher G, Kim E, Taylor P, Marchot P. J Biol Chem. 2006;281:29256–29267. doi: 10.1074/jbc.M603018200. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JL, Cusack B, Davies MP, Fauq A, Rosenberry TL. Biochemistry. 2003;42:5438–5452. doi: 10.1021/bi027065u. [DOI] [PubMed] [Google Scholar]
- 27.Sussman JL, Harel M, Frolow F, Varon L, Toker L, Futerman AH, Silman I. J Mol Biol. 1988;203:821–823. doi: 10.1016/0022-2836(88)90213-6. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberry TL, Bernhard SA. Biochemistry. 1971;10:4114–4120. doi: 10.1021/bi00798a016. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki T, Mann K, Miner JH, Miosge N, Timpl R. Eur J Biochem. 2002;269:431–442. doi: 10.1046/j.0014-2956.2001.02663.x. [DOI] [PubMed] [Google Scholar]
- 30.D’Arcy A, MacSweeney A, Stihle M, Haber A. Acta Crystallogr D Biol Crystallogr. 2003;59:396–399. doi: 10.1107/s0907444902022011. [DOI] [PubMed] [Google Scholar]
- 31.Kabsch W. J Appl Crystallogr. 1993;26:795–800. [Google Scholar]
- 32.Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Acta Crystallogr D: Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 33.Emsley P, Cowtan K. Acta Crystallogr D: Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 34.Lakowicz JR. Principles of fluorescence spectroscopy. 2. Kluwer Academic/Plenum; New York: 1999. p. 54. [Google Scholar]
- 35.Koellner G, Kryger G, Millard CB, Silman I, Sussman JL, Steiner T. J Mol Biol. 2000;296:713–735. doi: 10.1006/jmbi.1999.3468. [DOI] [PubMed] [Google Scholar]
- 36.Felder CE, Harel M, Silman I, Sussman JL. Acta Crystallogr D Biol Crystallogr. 2002;58:1765–1771. doi: 10.1107/s0907444902011642. [DOI] [PubMed] [Google Scholar]
- 37.Harel M, Hyatt JL, Brumshtein B, Morton CL, Yoon KJ, Wadkins RM, Silman I, Sussman JL, Potter PM. Mol Pharmacol. 2005;67:1874–1881. doi: 10.1124/mol.104.009944. [DOI] [PubMed] [Google Scholar]
- 38.Greenblatt HM, Guillou C, Guénard D, Argaman A, Botti S, Badet B, Thal C, Silman I, Sussman JL. J Am Chem Soc. 2004;126:15405–15411. doi: 10.1021/ja0466154. [DOI] [PubMed] [Google Scholar]
- 39.Haviv H, Wong DM, Greenblatt HM, Carlier PR, Pang YP, Silman I, Sussman JL. J Am Chem Soc. 2005;127:11029–11036. doi: 10.1021/ja051765f. [DOI] [PubMed] [Google Scholar]
- 40.Dvir H, Wong DM, Harel M, Barril X, Orozco M, Luque FJ, Muñoz-Torrero D, Camps P, Rosenberry TL, Silman I, Sussman JL. Biochemistry. 2002;41:2970–2981. doi: 10.1021/bi011652i. [DOI] [PubMed] [Google Scholar]
- 41.Friedhoff P, Schneider A, Mandelkow EM, Mandelkow E. Biochemistry. 1998;37:10223–10230. doi: 10.1021/bi980537d. [DOI] [PubMed] [Google Scholar]
- 42.Kung CE, Reed JK. Biochemistry. 1986;25:6114–6121. [Google Scholar]
- 43.Loutfy RO, Arnold BA. J Phys Chem. 1982;86:4205–4211. [Google Scholar]
- 44.Wong DM, Greenblatt HM, Dvir H, Carlier PR, Han YF, Pang YP, Silman I, Sussman JL. J Am Chem Soc. 2003;125:363–373. doi: 10.1021/ja021111w. [DOI] [PubMed] [Google Scholar]
- 45.Rydberg EH, Brumshtein B, Greenblatt HM, Wong DM, Shaya D, Williams LD, Carlier PR, Pang YP, Silman I, Sussman JL. J Med Chem. 2006;49:5491–5500. doi: 10.1021/jm060164b. [DOI] [PubMed] [Google Scholar]