Abstract
Objective
To determine if a single monthly supplement was as effective as a daily maternal supplement in increasing breast milk vitamin D to achieve vitamin D sufficiency in their infants.
Patients and Methods
Forty mothers with exclusively breastfed infants were randomized to receive oral cholecalciferol (vitamin D3) 5000 IU/d for 28 days or 150,000 IU once. Maternal serum, breast milk, and urine were collected on days 0, 1, 3, 7, 14 and 28; infant serum was obtained on days 0 and 28. Enrollment occurred between January 7 and July 29, 2011.
Results
In mothers given daily cholecalciferol, concentrations of serum and breast milk cholecalciferol attained steady levels of 18 and 8 ng/mL, respectively, from day 3 through 28. In mothers given the single dose, serum and breast milk cholecalciferol peaked at 160 and 40 ng/mL, respectively at day 1, before rapidly declining. Maternal milk and serum cholecalciferol concentrations were related (r=0.87). Infant mean serum 25(OH)D (±SD) increased from 17±13 to 39±6 ng/mL in the single dose group and from 16±12 to 39±12 ng/mL in the daily dose group (P=.88). All infants achieved serum 25(OH)D concentrations >20 ng/mL).
Conclusion
Either single dose or daily cholecalciferol supplementation of mothers provided breast milk concentrations that result in vitamin D sufficiency in breastfeeding infants.
INTRODUCTION
Vitamin D is essential for calcium absorption and skeletal growth, and deficiency of vitamin D can cause nutritional rickets. Although considered a historical disease after the advent of vitamin D fortification of foods, rickets persists in the U.S., typically in unsupplemented, exclusively breastfed infants.1-4_enref_1 The U.S. Centers for Disease Control has expressed concern regarding its prevalence.5 Beyond skeletal effects, _enref_5hypovitaminosis D has been associated with infectious, metabolic, neoplastic, and immune disorders.6-8 The prevalence of vitamin D deficiency among infants may be as high as 43-70%, depending on the definition of vitamin D deficiency and the latitude of the population studied.9-11
Vitamin D can be ingested or cutaneously synthesized by ultraviolet (UV) light exposure. Since the American Academy of Pediatrics (AAP) recommends no direct UV exposure during the first six months of life, infants are expected to rely entirely on dietary sources.12 The U.S. FDA requires infant formula be fortified with 40-100 IU vitamin D per 100 kcal, which corresponds to 270-677 IU/L.13 Breastfeeding has many health advantages compared to formula feeding.14The U.S. Healthy People 2010 targeted a goal for 75% of infants to breastfeed for their first six months.15 Breast milk usually contains much less vitamin D than infant formula, with values of 20-80 IU/L.16-18_enref_14_enref_16 Recognizing the high prevalence of vitamin D deficiency in exclusively breastfed infants and the low concentrations of vitamin D in breast milk, the AAP recommends that exclusively breastfed infants receive 400 IU supplemental vitamin D per day._enref_15 However, adherence to this recommendation has been poor, with only 5-36% of exclusively breastfed infants receiving supplemental vitamin D.2,19,20 Poor adherence is the major determinant of vitamin D deficiency in breast fed infants.2_enref_16
Daily and intermittent vitamin D supplementation dosing regimens have been used. High dose monthly regimens in adults and children improve vitamin D status without short-term toxicity.21-25 Lactating mothers supplemented with sufficient doses of oral vitamin D had enriched milk vitamin D concentrations.26-28 The parent compound cholecalciferol (vitamin D3) is the major vitamin D metabolite that crosses from maternal serum into breast milk. The quantity of the downstream metabolites, 25(OH)D and 1,25(OH)2D, in human milk is negligible, because of the avid binding of 25(OH)D to vitamin D binding protein and the low serum concentrations of 1,25(OH)2D.29 Due to the short half-life of cholecalciferol, it may have to be replenished daily to effective.30
We compared the effect of daily versus single dose cholecalciferol supplementation of lactating mothers on breast milk cholecalciferol concentrations and vitamin D status of their infants. We hypothesized that daily supplementation would be superior to monthly supplementation in improving infant vitamin D status, because consistently elevated cholecalciferol concentrations in the mother with daily dosing would sustain improved breast milk vitamin D status.
PATIENTS AND METHODS
Subjects
Healthy, non-pregnant, lactating women ≥18 years of age who were exclusively breastfeeding a single, healthy infant between 1 and 6 months of age were eligible. We recruited via advertisements and letters to post-partum mothers. Exclusion criteria included: 1) travel south of 35° N latitude during or 30 days preceding the study interval; 2) recent or planned indoor tanning; 3) taking medications that affect vitamin D metabolism (i.e. steroids, anticonvulsants, barbiturates) 4) nursing multiple infants, 5) taking >1000 mg elemental calcium supplements, 6) maternal cholecalciferol supplementation >400 IU (dose in prenatal vitamins) or any infant cholecalciferol supplementation, 7) infant weight <1.67 kg (greater than minimal risk for 5 mL blood draw), 8) baseline 25(OH)D concentration >70 ng/mL in mother or infant, 9) baseline hypercalcemia or hyperphosphatemia in mother or infant, 10) history of nephrolithiasis and 11) any serious infant health problems. Prior to this study, we performed a pilot study in 40 non-pregnant, non-lactating women to characterize the pharmacokinetics of daily (5000 IU) and a single dose (150,000 IU) cholecalciferol. We observed no evidence of hypercalcemia or adverse effects over a 28-day interval (unpublished data).
Procedures
Mother-infant pairs were enrolled between January 7 and July 29, 2011, in Rochester, Minnesota (44° N latitude). Vital signs, weight and height/length were recorded for mothers and infants. Maternal and infant blood was collected by venipuncture, women collected their breast milk via a breast pump or self-expression, and maternal urine was collected. Because measurements of vitamin D metabolites do not differ between whole milk and milk whey16, no attempt was made to distinguish foremilk and hind milk collection. Infants were allowed to comfort nurse or administered an oral sucrose solution (Sweet-Ease, Philips Healthcare, Andover, MA) during venipuncture to reduce distress. Serum calcium, phosphorus, and 25(OH)D concentrations in mothers and their infants were measured before enrollment to determine eligibility.
Subject pairs were randomized in blocks of four to maternal administration of either oral cholecalciferol 150,000 IU once or oral cholecalciferol 5000 IU daily for 28 days. The randomization schedule was secured by the research pharmacy, and allocation was concealed until the subject pair was enrolled. One 5000 IU capsule or three 50,000 IU capsules (BioTech Pharmacal, Inc; Fayetteville, AR) were dispensed and ingested under supervision. Based on high performance liquid chromatography analysis of the cholecalciferol content by the manufacturer, the three lots of 5000 IU capsules used in this study contained 5404, 5705, and 5764 IU per capsule. The two lots of 50,000 IU capsules used in this study contained 55,668 and 58,033 IU per capsule. Mothers in the 5000 IU daily group were dispensed their remaining medication, and asked to record adherence in a medication diary. The diary was examined at each visit and collected with the medication container upon study completion. Each participant was instructed to make no dietary changes, avoid additional vitamin D ingestion and utilize sunscreen.
Laboratory measurements
Serum cholecalciferol, 25(OH)D, 1,25(OH)2D, calcium, and phosphorus were measured in mothers on days 0, 1, 3, 7, 14 and 28 and in their infants on days 0 and 28. Maternal urine calcium and creatinine, and breast milk cholecalciferol and 25(OH)D were measured on days 0, 1, 3, 7, 14 and 28. Biochemical analyses were performed on all samples in a single batch to avoid interassay variation. Serum and urine calcium, phosphorus, and creatinine were measured with standard methods. Serum and breast milk vitamin D and its metabolites were measured by isotope-dilution liquid chromatography-tandem mass spectrometry (LC-MS/MS) (ThermoFisher Scientific, Franklin, Massachusetts and Applied Biosystems-MDS Sciex, Foster City, CA).31 All assays for 25(OH)D3 and 25(OH)D2 were standardized against NIST reference material. C3-epi-25(OH)D, a metabolite of uncertain biologic significance, was measured in all children at baseline. Only two subjects had C3-epi-25OHD greater than 20% of total 25(OH)D. Because concentrations of vitamin D2 and its metabolites were very low (≤1.6 ng/mL) or undetectable in all subjects, the absence of subscripts designates cholecalciferol (vitamin D3) and its metabolites. Cholecalciferol was extracted from breast milk using 210 μL of isopropyl alcohol. The extract was injected to the mass spectrometer using online extraction and liquid chromatography. The intra- and inter-assay precisions for cholecalciferol were 8.0% and 6.1% respectively. The recovery and linearity validation parameters were 104% and 100%, respectively. The limit of detection and limit of quantitation were 0.96 ng/mL and 7.0 ng/mL, respectively.
Ethics
The study was approved by the Institutional Review Board of the Mayo Clinic, and all participants provided written informed consent for themselves and their infants.
Statistical Analysis
The primary outcome was infant vitamin D status, as measured by the serum 25(OH)D concentration. Although there is not universal agreement regarding the definition of sufficiency, we considered deficiency as <12 ng/mL (<30 nmol/L), insufficiency as 12-20 ng/mL (30-50 nmol/L), and sufficiency as >20 ng/mL (>50 nmol/L) which is consistent with the Institute of Medicine’s conclusion that levels >20 ng/mL meet the physiologic needs of 97.5% of the healthy population_enref_24.32 We calculated our sample size based on the hypothesis that the single dose group would have fewer days of detectable cholecalciferol in breast milk. Assuming an alpha value of 0.05 and a power of 80%, 17 subjects per group would be sufficient to detect a single standard deviation difference in the number of days of detectable breast milk cholecalciferol. Assuming the standard deviation of the number of days of detectable breast milk cholecalciferol in each group is four, we would be able to detect a difference of four days between groups. Allowing for a 15% dropout rate, we chose a 40 subject pair target.
Seasonal effects were examined by comparing subjects enrolled in January-March and April-July. Data were handled with Excel 2003 (Microsoft Corp., Redmond, WA) and analyzed with JMP 9.0.1 software (SAS Institute, Cary, NC). A paired t-test was used to compare normally-distributed continuous variables with baseline values within subjects. Student’s t-test was utilized to compare continuous variables between the two treatment groups. The nonparametric Wilcoxon test was used to compare variables with unequal variances. Pearson correlation and multiple linear regression analyses were used to identify independent determinants of vitamin D status. P-values <.05 were considered significant.
RESULTS
A total of 42 mother-infant pairs completed the initial study visit; 2 mother-infant pairs were excluded for maternal hyperphosphatemia (5.2 mg/dL) and maternal hypercalcemia (11.0 mg/dL). The remaining 40 pairs were randomized, with 20 per group, and all completed the study.
The two study groups were similar (Table 1). Infant ages ranged from 4 to 28 weeks at enrollment. Mean (±SD) baseline serum 25(OH)D values were 29.0±8.3 ng/mL in mothers (range 10-44) and 16.6±12.5 ng/mL in infants (range 2-55). At baseline, 7 mothers (18%) and 27 infants (68%) had serum 25(OH)D concentrations <20 ng/mL; one mother (3%) and 18 infants(45%) had serum 25(OH)D concentrations <12 ng/mL. Baseline 25(OH)D concentrations in mothers and their infants were positively correlated (r=0.40; P=.01), with infant concentration approximately 60% of maternal concentration. Baseline 25(OH)D concentrations were greater in mothers enrolled in April-July than in January-March (31.2±9.1 ng/mL versus 26.1±6.2 ng/mL; P=.05) and in infants (21.3±12.8 ng/mL versus 10.4±9.1 ng/mL; P=.005). Likewise, baseline serum cholecalciferol concentrations were greater in mothers enrolled in April-July (5.2±5.3 ng/mL) than in January-March (1.6±1.3 ng/mL; P=.004). Mean baseline breast milk cholecalciferol concentrations were below the limit of quantitation of 7 ng/mL. Therefore, mean breast milk cholecalciferol values below the limit of quantitation have been designated as <7 ng/mL in Table 2. Baseline breastmilk cholecalciferol was related to serum cholecalciferol (r=0.38; P=.02). Baseline maternal 25(OH)D values were not related to age (P=.08) or body mass index (P=.39). Infant 25(OH)D values were unrelated to infant age (P=.95), weight (P=.80), or gestational age at birth (P=.52).
Table 1.
Baseline Characteristics of Study Subjects.
| Characteristic | Daily Dose 5000 IU (n=20) |
Single Dose 150,000 IU (n=20) |
|---|---|---|
| Maternal age (years) | 30.3 ± 2.9 | 30.1 ± 4.0 |
| Infant age (weeks) | 13.7 ± 7.3 | 11.0 ± 5.6 |
| Infant gestation at birth (weeks) | 39.9 ± 1.3 | 39.5 ± 0.9 |
| Maternal race (% white) | 95% | 95% |
| Infant gender (% female) | 60% | 60% |
| Maternal weight (kg) | 72.7 ± 10.6 | 67.6 ± 12.1 |
| Maternal height (cm) | 165.6 ± 5.5 | 163.8 ± 4.2 |
| Maternal BMI (kg/m2) | 26.5 ± 4.0 | 25.2 ± 4.7 |
| Infant weight (kg) | 6.0 ± 3.7 | 5.7 ± 1.0 |
| Infant length (cm) | 61.7 ± 3.7 | 60.5 ± 4.3 |
| Maternal serum 25(OH)D (ng/mL) | 28.8 ± 9.2 | 29.3 ± 7.5 |
| Infant serum 25(OH)D (ng/mL) | 16.9 ± 12.9 | 16.4 ± 12.4 |
| Enrollment date | ||
| January-March | 9 (45%) | 8 (40%) |
| April-July | 11 (55%) | 12 (60%) |
Table 2.
Comparison of Metabolic Responses to Daily or Single Dose Vitamin D Supplementation Regimens.
| Vitamin D 5000 IU Daily Dose (N=20) |
Vitamin D 150,000 IU Single Dose (N=20) |
|||
|---|---|---|---|---|
| Concentration | Increment | Concentration | Increment | |
| Maternal Serum | ||||
| Cholecalciferol (ng/mL) | ||||
| Day 0 | 2.6 ± 1.4 | 4.7 ±6.0 | ||
| Day 1 | 10.6 ± 3.8a | 8.0 ± 3.0a | 160.0 ± 38.8a b | 155.2 ± 37.8a b |
| Day 3 | 14.7 ± 3.7a | 12.1 ± 3.0a | 57.9 ± 15.7a b | 53.1 ± 13.5a b |
| Day 7 | 17.2 ± 4.6a | 14.5 ± 4.0a | 17.6 ± 7.6a | 12.9 ± 6.1a |
| Day 14 | 18.5 ± 6.2a | 16.0 ± 5.8a | 9.5 ± 5.1a b | 4.8 ± 3.7a b |
| Day 28 | 18.3 ± 5.2a | 15.7 ± 4.9a | 7.0 ± 6.3a b | 2.1 ± 2.9a b |
| 25(OH)D (ng/mL) | ||||
| Day 0 | 28.8 ± 9.2 | 29.3 ± 7.5 | ||
| Day 1 | 30.7 ± 9.7 | 1.9 ± 3.5a | 43.3 ± 9.7a b | 14.0 ± 3.7a b |
| Day 3 | 31.8 ± 8.9a | 2.7 ± 3.7a | 50.0 ± 10.2a b | 21.2 ± 4.0a b |
| Day 7 | 34.3 ± 8.6a | 5.0 ±3.6a | 47.5 ± 9.6a b | 18.2 ± 4.9a b |
| Day 14 | 38.6 ± 10.3a | 9.7 ± 3.4a | 45.3 ± 9.5a | 16.0 ± 4.7a b |
| Day 28 | 43.9 ± 11.8a | 15.0 ± 5.7a | 41.2 ± 8.9a | 11.9 ± 4.2a |
| 1,25(OH)2D (pg/mL) | ||||
| Day 0 | 52.0 ± 10.7 | 60.4 ± 24.9 | ||
| Day 1 | 58.6 ± 12.0a | 6.2 ± 20.2 | 64.2 ± 17.6 | 6.0 ± 10.2a |
| Day 3 | 58.2 ± 12.2 | −0.3 ± 22.3 | 59.6 ± 16.8 | 7.0 ± 14.0 |
| Day 7 | 56.8 ± 13.2 | 0.2 ± 0.6 | 61.0 ± 9.0 | 2.2 ± 1.2a b |
| Day 14 | 55.0 ± 11.3 | 6.9 ± 21.4 | 67.1 ± 15.2b | 3.9 ± 13.3 |
| Day 28 | 57.5 ± 12.6 | 4.0 ± 25.4 | 65.1 ± 16.9 | 7.0 ± 14.5 |
| Calcium (mg/dL) | ||||
| Day 0 | 9.6 ± 0.3 | 9.6 ± 0.4 | ||
| Day 1 | 9.6 ± 0.4 | −0.01 ± 0.38 | 9.7 ± 0.4 | 0.07 ± 0.37 |
| Day 3 | 9.6 ± 0.4 | 0.04 ± 0.42 | 9.7 ± 0.5 | 0.08 ± 0.36 |
| Day 7 | 9.6 ± 0.5 | 0.04 ± 0.43 | 9.6 ± 0.4 | 0.01 ± 0.33 |
| Day 14 | 9.7 ± 0.4 | 0.13 ± 0.34 | 9.8 ± 0.5 | 0.13 ± 0.32 |
| Day 28 | 9.7 ± 0.4 | 0.19 ± 0.41 | 9.7 ± 0.4 | 0.14 ± 0.39 |
| Phosphorus (mg/dL) | ||||
| Day 0 | 4.1 ± 0.6 | 4.2 ± 0.5 | ||
| Day 1 | 4.2 ± 0.7 | 0.18 ± 0.48 | 4.4 ± 0.5 | 0.17 ± 0.58 |
| Day 3 | 4.5 ± 0.7a | 0.45 ± 0.57a | 4.5 ± 0.5a | 0.27 ± 0.37a |
| Day 7 | 4.3 ± 0.6 | 0.22 ± 0.45a | 4.3 ± 0.5 | 0.11 ± 0.47 |
| Day 14 | 4.3 ± 0.7 | 0.27 ± 0.51a | 4.3 ± 0.6 | 0.10 ± 0.51 |
| Day 28 | 4.3 ± 0.6 | 0.23 ± 0.58 | 4.2 ± 0.4 | 0.0 ± 0.40 |
| Maternal breast milk | ||||
| Cholecalciferol (ng/mL)c | ||||
| Day 0 | <7.0 | <7.0 | ||
| Day 1 | <7.0 | 39.7 ± 16.2a b | ||
| Day 3 | 8.0 ± 3.7a | 24.6 ± 8.9a b | ||
| Day 7 | 7.2 ± 4.8 | 11.2 ± 4.7a | ||
| Day 14 | 8.6 ± 5.4a | <7.0 | ||
| Day 28 | 7.7 ± 3.7a | <7.0 | ||
| Maternal urine calcium/creatinine (mg/g) |
||||
| Day 0 | 72.0 ± 52.6 | 74.6 ± 46.6 | ||
| Day 1 | 72.5 ± 57.2 | 0.5 ± 47.6 | 98.4 ± 65.0 | 23.8 ± 48.9a |
| Day 3 | 77.8 ± 64.0 | 5.8 ± 46.9 | 87.7 ± 77.2 | 13.1 ± 65.0 |
| Day 7 | 72.9 ± 62.8 | −0.6 ± 58.9 | 104.9 ± 71.9a | 30.3 ± 55.8a |
| Day 14 | 94.2 ± 104.5 | 22.2 ± 74.2 | 100.1 ± 44.2a | 25.5 ± 38.9a |
| Day 28 | 106.7 ± 98.4a | 34.7 ± 71.8a | 114.2 ± 70.3a | 39.6 ± 71.5a |
| Infant Serum | ||||
| 25(OH)D (ng/mL) | ||||
| Day 0 | 16.9 ± 12.9 | 16.3 ± 12.4 | ||
| Day 28 | 39.2 ± 6.3a | 22.2 ± 10.6a | 38.7 ± 11.7a | 22.4 ± 5.6a |
| Calcium (mg/dL) | ||||
| Day 0 | 11.0 ± 0.3 | 11.0 ± 0.3 | ||
| Day 28 | 10.9 ± 0.3 | −0.09 ± 0.39 | 10.9 ± 0.3 | −0.05 ± 0.36 |
| Phosphorus (mg/dL) | ||||
| Day 0 | 6.1 ± 0.6 | 6.5 ± 0.5 | ||
| Day 28 | 6.0 ± 0.5 | −0.08 ± 0.41 | 6.2 ± 0.5 | −0.33 ± 0.54a |
p<.05 for comparison with baseline values
p<.05 for comparison with daily dose group
Mean breast milk cholecalciferol values below the limit of quantitation are designated as <7.0 ng/mL
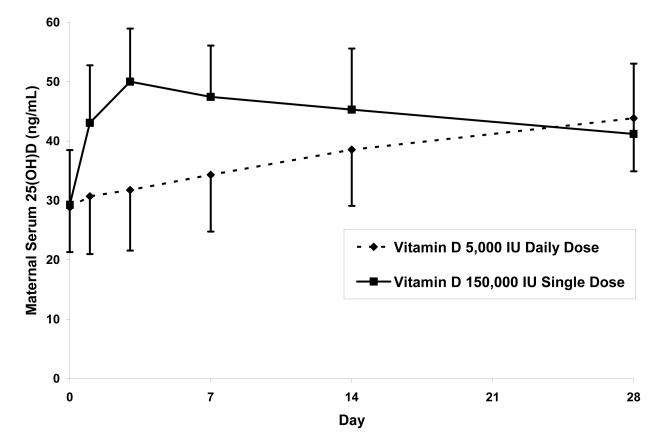
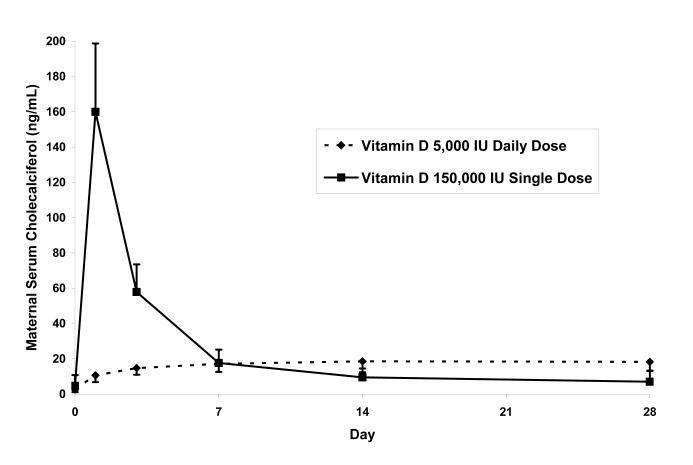
Maternal serum 25(OH)D values increased in both groups from baseline to day 28 (Table 2). The single dose group had significantly greater maternal 25(OH)D concentrations than the daily dose group on days 1, 3, and 7, but not on days 14 and 28 (Figure 1A); the incremental change in 25(OH)D was significantly greater in the single dose group on days 1, 3, 7, and 14 but not on day 28. In the single dose group, maternal 25(OH)D values peaked on day 3, and the maximum value observed in any mother was 72 ng/mL. By day 28 the increase in 25(OH)D between baseline and day 28 was 11.9±4.2 ng/mL in the single dose group and 15.0±5.7 ng/mL in the daily dose group (P=.06). None of the mothers’ serum 25(OH)D concentration remained <20 ng/mL on day 28 (daily dose group 43.9± 11.8, range 22-71ng/mL; single dose group 41.2± 8.9, range 26-60 ng/mL). In the single dose group, maternal serum cholecalciferol concentrations peaked on day 1 and were greater than baseline values in both groups through day 28 (Figure 1B). Breast milk cholecalciferol concentrations mirrored serum concentrations, with peak values approximately 25% of serum values on day one in the single dose group (Figure 1C). Breast milk 25(OH)D was undetectable in all samples. Maternal serum 1,25(OH)2D concentrations remained relatively stable over the 28-day interval (Table 2).
Figure 1.
Maternal Vitamin D Metabolite Values with Two Vitamin D Supplementation Regimens: (A) Serum 25-hydroxyvitamin D, (B) Serum cholecalciferol, and (C) Breast milk cholecalciferol. Error bars represent standard deviation.
By day 28, serum 25(OH)D had a nearly identical increase in the infants of both groups (Figure 2). The increase in infant 25(OH)D concentration was inversely related to the baseline 25(OH)D concentration (r = −0.67; P<.001). By day 28, all infants achieved a serum 25(OH)D concentration >20 ng/mL (range 23-70 ng/mL). The increase of the infant’s 25(OH)D concentration was not related to their mother’s increase of 25(OH)D concentration (r = 0.07; P=.64). Neither the infants’ final value nor the increase of 25(OH)D were related to peak breast milk cholecalciferol values in either group. Assuming that day 14 breast milk cholecalciferol concentration represents an average measure of infant vitamin D intake, we found that infant 25(OH)D values at day 28 were not significantly related to day 14 breast milk cholecalciferol concentrations (r=0.29; P=.07).
Figure 2.
25-hydroxyvitamin D Concentrations in Breast-fed Infants in Response to Two Vitamin D Supplementation Regimens of Their Mothers. Error bars represent standard deviation, the upper error bars are the standard deviation for the 5000 IU/day group and the lower error bars are the standard deviation for the 150,000 IU single dose group.
Adherence to supplementation was 100% in the single dose group and 98% in the daily dose group. No significant changes in serum calcium occurred in either group. The highest serum calcium was 11.1 mg/dL on day 28 in the daily dose group, with a simultaneous urine calcium/creatinine ratio of 48 mg/g. Compared with baseline values, urinary calcium excretion significantly increased by day 28 in the daily dose group and by day 7 in the single dose group, with the incremental change was significant compared to baseline on day 1 as well in the single dose group. Urine calcium/creatinine ratios greater than the upper limit of the accepted reference range (220 mg/g) were observed in four mothers in the daily dose group and in three mothers in the single dose group. The maximum urine calcium/creatinine ratio of 441 mg/g occurred in the daily dose group at day 14. Three mothers had urine calcium/creatinine ratios >220 mg/g on day 28. Urine calcium/creatinine ratios were unrelated to serum 1,25(OH)2D values (r = −0.10). Adverse events during the study included upper respiratory infections (4), diarrhea (3), headache (3), and cellulitis (1) in mothers; and bronchiolitis (1) and altered stool pattern (1) in infants. None of these events was attributed to vitamin D.
DISCUSSION
Contrary to our hypothesis, we found that either daily or single dose cholecalciferol maternal supplementation provides breast milk concentrations that result in infant 25(OH)D concentrations >20 ng/mL over a 28-day period. We observed a mild increase in urinary calcium excretion but no hypercalcemia or adverse symptoms attributable to vitamin D.
We confirmed a high prevalence of poor vitamin D status in unsupplemented, breastfed infants, with two-thirds <20 ng/mL at baseline. Alarmingly, 45% had serum 25(OH)D concentrations <12 ng/mL putting them at risk for nutritional rickets.8 In neighboring Iowa, 70% of exclusively breastfed infants at age 3.5 months had 25(OH)D concentrations <11 ng/mL during winter.11 Although there is no universally agreed upon definition of sufficiency, the Institute of Medicine has concluded that levels >20 ng/mL meet the physiologic needs of 97.5% of the healthy population.32
Our study confirms the benefit of maternal cholecalciferol supplementation on the vitamin D status of breastfed infants. Milk from daily supplemented mothers can provide infants with sufficient vitamin D if the cholecalciferol dose is high enough.26-28,33 In Finland, daily supplementation of lactating mothers with cholecalciferol 2000 IU, but not 1000 IU, improved infant vitamin D status to values similar to those obtained by daily infant supplementation with 400 IU.32 Supplementation of nine lactating mothers with cholecalciferol 6400 IU/day for 6 months resulted in infant 25(OH)D concentrations of 36 ng/mL and 46 ng/mL after one and six months, respectively, similar to values in infants supplemented with 400 IU/day.28 Maternal 25(OH)D concentrations rose from 34 ng/mL at baseline to 47 and 59 ng/mL at one and six months, respectively – slightly higher than values we observed with 5000 IU/day or values obtained in prior 4000 IU/day studies.26,27 No adverse effects were reported, and maternal urinary calcium excretion was similar to that of mothers taking a standard prenatal vitamin (cholecalciferol 400 IU) over six months.
We extend these findings by demonstrating that a single dose of cholecalciferol in lactating mothers is as effective as daily dosing for improving the vitamin D status of their breast fed infants. Currently, adherence to vitamin D supplementation of breastfed infants is poor.20 As a public health measure, intermittent cholecalciferol supplementation could theoretically be administered to lactating mothers during well child visits to improve adherence.34 This supplementation strategy could prove particularly useful in countries where nutritional rickets and maternal vitamin D deficiency is prevalent. However, the optimal interval of intermittent doses needed to ensure adequate cholecalciferol concentrations in mothers’ breast milk to maintain sufficiency in their infants is unknown.
Breast milk cholecalciferol concentrations of 8 ng/mL (the mean 28-day value in the daily dose group) correspond to 320 IU/L (1 microgram=40 IU) and approximate the 400 IU/L infant formula target. Previous reports of “antirachitic activity” of breast milk were attributed to the contribution of both cholecalciferol and 25(OH)D in breast milk.17,35,36 However, using tandem mass spectrometry, we did not detect 25(OH)D in breast milk. Previous studies of breast milk 25(OH)D concentrations have reported values below 1 ng/mL, which was below the lower limit of detection of our assay. Such low concentrations of 25(OH)D are unlikely to have added an important amount of antirachitic activity to the cholecalciferol that was measureable in the breast milk.
The breast milk antirachitic activity of mothers supplemented with 400-800 IU/day has been reported as 33-68 IU/L,37 47-50 IU/L,17 and 38±11 IU/L,26_enref_20 insufficient to meet the needs of exclusively breastfed infants. The antirachitic activity of milk from nine mothers receiving ergocalciferol (vitamin D2) 4000 IU/day for three months increased to 135 IU/L, which is less than half of the value that we observed with 5000 IU/day, possibly related to our use of cholecalciferol (not ergocalciferol).26 Nine lactating mothers supplemented with cholecalciferol 6400 IU daily for six months had mean antirachitic activity in breast milk of 873 IU/L.28 Regulated conversion of cholecalciferol to 25(OH)D likely provides a built-in safety measure. This is consistent with our observation of an inverse relationship between the 25(OH)D increase and the initial 25(OH)D in infants.
During the 28-day study interval, both cholecalciferol dosing regimens appeared safe. However, the mild increase in renal calcium excretion raises concern for an increased risk of nephrolithiasis. The mechanism for the increased calcium excretion is unclear. PTHrP produced by the lactating breast and low estradiol concentrations contribute to mobilization of calcium from bone.29,38 Calcium lost in breast milk also contributes to a negative calcium balance during lactation that is not prevented by calcium supplementation.39 We did not observe a significant increase in 1,25(OH)2D concentrations to suggest that cholecalciferol increased intestinal calcium absorption, and urinary calcium excretion was unrelated to 1,25(OH)2D concentrations. In a large population study, nephrolithiasis was not associated with serum 25(OH)D values.40
In 2011 the Institute of Medicine updated vitamin D supplementation guidelines. Adequate intake for infants less than one year of age was set at 400 IU per day, in line with the AAP recommendations and the goals of supplementation. The recommended dietary allowance for adults, including lactating mothers, was listed at 600 IU daily. The doses of vitamin D used in this study were selected to adequately fortify the mother’s milk to meet the requirement of the breast-fed infant and were not intended for long-term supplementation beyond the period of lactation. The IOM listed a tolerable upper intake level of 4000 IU daily and 10,000 IU daily as the No Observed Adverse Effect Level (NOAEL) with no reports of adverse events for supplementation regimens below this doseage.32
Our study has several limitations. Although we attempted to minimize UV exposure, we cannot exclude endogenous vitamin D production. We did not measure the dietary contribution of vitamin D intake, but this should be small compared with supplement doses. We did not include a control group with no maternal cholecalciferol supplementation, because our aim was to determine if daily supplementation was more advantageous than a single dose. The limited duration of the study does not allow us to determine the effect of continued cholecalciferol supplementation beyond 28 days or to demonstrate long-term safety. Continuous cholecalciferol supplementation with up to 11,000 IU/day produces a stable serum cholecalciferol concentration in approximately 3 weeks.41 However, cholecalciferol supplementation of lactating mothers with 6400 IU/day produced a continued upward trend in breast milk antirachitic activity at 6 months, but the increase in infant 25(OH)D concentrations did not differ from that of infants supplemented with cholecalciferol 300 IU/day.28 Further research is needed to determine how frequently intermittent doses should be administered to prevent breast milk cholecalciferol concentrations falling below levels necessary to maintain infant 25(OH)D.
CONCLUSIONS
In conclusion, either single dose or daily maternal cholecalciferol can provide breast milk concentrations that result in vitamin D sufficiency in their infants. Larger trials demonstrating the safety of cholecalciferol supplementation in lactating mothers need to be conducted before universally adopting this strategy for preventing infant vitamin D deficiency.
ACKNOWLEDGEMENTS
Adam Girtman and Dr. Hemamalini Ketha conducted analysis of breast milk and serum vitamin D metabolites. Donna Rasmussen and Betty Wirt provided study coordination and data collection.
SSO, MEM, PRF, BRL, RJS, SSC, BMG, JMP, ITC and TDT designed research; SSO, ITC and TDT conducted research; SSO, RJS and TDT analyzed data; SSO and TDT wrote the paper; SSO and TDT had primary responsibility for final content. All authors read and approved the final manuscript.
Sources of Support: This research was supported by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Additionally small grants funding from the authors’ institution supported the research.
Abbreviations
- IU
international units
- d
day
- ng
nanogram
- mL
milliliter
- SD
standard deviation
- g
gram
- CDC
Center for Disease Control
- UV
ultraviolet
- AAP
American Academy of Pediatrics
- FDA
Food and Drug Administration
- kcal
kilocalorie
- L
liter
- N
north
- mg
milligrams
- kg
kilogram
- nmol
nanomole
- PTHrP
parathyroid hormone related peptide
Footnotes
Disclaimers: None of the authors have any disclaimers, disclosures or conflicts of interest.
Clinical Trial Registration: clinicaltrials.gov NCT01240265
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sara S. Oberhelman, Department of Family Medicine, Mayo Clinic, Rochester, MN.
Michael E. Meekins, Department of Pharmacy Services, Mayo Clinic, Rochester, MN.
Philip R. Fischer, Department of Pediatric and Adolescent Medicine, Mayo Clinic, Rochester, MN.
Bernard R. Lee, Department of Pharmacy Services, Mayo Clinic, Rochester, MN.
Ravinder J. Singh, Department of Laboratory Medicine, Mayo Clinic, Rochester, MN.
Stephen S. Cha, Department of Health Science Research, Mayo Clinic, Rochester, MN.
Brian M. Gardner, Department of Pharmacy Services, Mayo Clinic, Rochester, MN.
John M. Pettifor, Department of Paediatrics, University of the Witwatersrand, Johannesburg, South Africa.
Ivana T. Croghan, Department of Medicine, Mayo Clinic, Rochester, MN.
Tom D. Thacher, Department of Family Medicine, Mayo Clinic, Rochester, MN.
REFERENCES
- 1.Dawodu A, Agarwal M, Hossain M, Kochiyil J, Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. J Pediatr. 2003;142(2):169–173. doi: 10.1067/mpd.2003.63. [DOI] [PubMed] [Google Scholar]
- 2.Merewood A, Mehta SD, Grossman X, et al. Vitamin D status among 4-month-old infants in New England: a prospective cohort study. J Hum Lact. 2012;28(2):159–166. doi: 10.1177/0890334411434802. [DOI] [PubMed] [Google Scholar]
- 3.Greer FR. 25-Hydroxyvitamin D: functional outcomes in infants and young children. Am J Clin Nutr. 2008;88(2):529S–533S. doi: 10.1093/ajcn/88.2.529S. [DOI] [PubMed] [Google Scholar]
- 4.Thacher TD, Fischer PR, Tebben PJ, et al. Increasing incidence of nutritional rickets: a population based study in Olmsted County, Minnesota. Mayo Clin Proc. 2013;88(2):176–183. doi: 10.1016/j.mayocp.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scanlon KS. Vitamin D Expert Panel Meeting; October 2001; [Accessed September 5, 2012]. http://www.cdc.gov/nccdphp/dnpa/nutrition/pdf/Vitamin_D_Expert_Panel_Meeting.pdf. [Google Scholar]
- 6.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93(6):512–517. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 7.Camargo CA, Jr., Ganmaa D, Frazier AL, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in mongolia. Pediatrics. 2012;130(3):e561–567. doi: 10.1542/peds.2011-3029. [DOI] [PubMed] [Google Scholar]
- 8.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rovner AJ, O’Brien KO. Hypovitaminosis D among healthy children in the United States: a review of the current evidence. Arch Pediatr Adolesc Med. 2008;162(6):513–519. doi: 10.1001/archpedi.162.6.513. [DOI] [PubMed] [Google Scholar]
- 10.Seth A, Marwaha RK, Singla B, et al. Vitamin D nutritional status of exclusively breast fed infants and their mothers. J Pediatr Endocrinol Metab. 2009;22(3):241–246. doi: 10.1515/jpem.2009.22.3.241. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler EE, Hollis BW, Nelson SE, Jeter JM. Vitamin D deficiency in breastfed infants in Iowa. Pediatrics. 2006;118(2):603–610. doi: 10.1542/peds.2006-0108. [DOI] [PubMed] [Google Scholar]
- 12.Ultraviolet light: a hazard to children. American Academy of Pediatrics. Committee on Environmental Health. Pediatrics. 1999;104(2 Pt 1):328–333. [PubMed] [Google Scholar]
- 13.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88(2):558S–564S. doi: 10.1093/ajcn/88.2.558S. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Preventative Services Task Force [Accessed September 5, 2012];Primary care interventions to promote breastfeeding: clinical Summary. AHRQ Publication No 09-05126-EF-3. 2008 Oct; at http://www.uspreventitiveservicestaskforce.org/uspstf2008/breastfeeding/brfeedsum.htm.
- 15.U.S. Department of Health and Human Services . Healthy People 2010. U.S. Government Printing Office; Washington, DC: 2000. [Google Scholar]
- 16.Hollis BW, Roos BA, Draper HH, Lambert PW. Vitamin D and its metabolites in human and bovine milk. J Nutr. 1981;111(7):1240–1248. doi: 10.1093/jn/111.7.1240. [DOI] [PubMed] [Google Scholar]
- 17.Reeve LE, Chesney RW, DeLuca HF. Vitamin D of human milk: identification of biologically active forms. Am J Clin Nutr. 1982;36(1):122–126. doi: 10.1093/ajcn/36.1.122. [DOI] [PubMed] [Google Scholar]
- 18.Dawodu A, Tsang RC. Maternal vitamin D status: effect on milk vitamin D content and vitamin D status of breastfeeding infants. Adv Nutr. 2012;3(3):353–361. doi: 10.3945/an.111.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor JA, Geyer LJ, Feldman KW. Use of supplemental vitamin D among infants breastfed for prolonged periods. Pediatrics. 2010;125(1):105–111. doi: 10.1542/peds.2009-1195. [DOI] [PubMed] [Google Scholar]
- 20.Perrine CG, Sharma AJ, Jefferds ME, Serdula MK, Scanlon KS. Adherence to vitamin D recommendations among US infants. Pediatrics. 2010;125(4):627–632. doi: 10.1542/peds.2009-2571. [DOI] [PubMed] [Google Scholar]
- 21.Pepper KJ, Judd SE, Nanes MS, Tangpricha V. Evaluation of vitamin D repletion regimens to correct vitamin D status in adults. Endocr Pract. 2009;15(2):95–103. doi: 10.4158/EP.15.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binkley N, Gemar D, Engelke J, et al. Evaluation of Ergocalciferol or Cholecalciferol Dosing, 1,600 IU Daily or 50,000 IU Monthly in Older Adults. J Clin Endocrinol Metab. 2011;96(4):981–988. doi: 10.1210/jc.2010-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon CM, Williams AL, Feldman HA, et al. Treatment of hypovitaminosis D in infants and toddlers. J Clin Endocrinol Metab. 2008;93(7):2716–2721. doi: 10.1210/jc.2007-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah BR, Finberg L. Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method. J Pediatr. 1994;125(3):487–490. doi: 10.1016/s0022-3476(05)83303-7. [DOI] [PubMed] [Google Scholar]
- 25.Thacher TD, Fischer PR, Pettifor JM, et al. A comparison of calcium, vitamin D, or both for nutritional rickets in Nigerian children. N Engl J Med. 1999;341(8):563–568. doi: 10.1056/NEJM199908193410803. [DOI] [PubMed] [Google Scholar]
- 26.Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004;80(6 Suppl):1752S–1758S. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- 27.Basile LA, Taylor SN, Wagner CL, Horst RL, Hollis BW. The effect of high-dose vitamin D supplementation on serum vitamin D levels and milk calcium concentration in lactating women and their infants. Breastfeed Med. 2006;1(1):27–35. doi: 10.1089/bfm.2006.1.27. [DOI] [PubMed] [Google Scholar]
- 28.Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed Med. 2006;1(2):59–70. doi: 10.1089/bfm.2006.1.59. [DOI] [PubMed] [Google Scholar]
- 29.Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. Am J Clin Nutr. 2008;88(2):520S–528S. doi: 10.1093/ajcn/88.2.520S. [DOI] [PubMed] [Google Scholar]
- 30.Hollis BW, Wagner CL. The vitamin D requirement during human lactation: the facts and IOM’s ‘utter’ failure. Public Health Nutr. 2011;14(4):748–9. doi: 10.1017/S1368980011000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh RJ. Quantitation of 25-OH-vitamin D (25OHD) using liquid tandem mass spectrometry (LC-MS-MS) Methods Mol Biol. 2010;603:509–517. doi: 10.1007/978-1-60761-459-3_50. [DOI] [PubMed] [Google Scholar]
- 32.Institute of Medicine . Dietary reference intakes for calcium and vitamin D. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 33.Ala-Houhala M, Koskinen T, Terho A, Koivula T, Visakorpi J. Maternal compared with infant vitamin D supplementation. Arch Dis Child. 1986;61(12):1159–1163. doi: 10.1136/adc.61.12.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruk ME, Schwalbe N. The regulation between intermittent dosing and adherence: preliminary insights. Clin Ther. 2006;28(12):1989–1995. doi: 10.1016/j.clinthera.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Hollis BW. Individual quantitation of vitamin D2, vitamin D3, 25-hydroxyvitamin D2, and 25-hydroxyvitamin D3 in human milk. Anal Biochem. 1983;131(1):211–219. doi: 10.1016/0003-2697(83)90157-4. [DOI] [PubMed] [Google Scholar]
- 36.Ala-Houhala M, Koskinen T, Parviainen MT, Visakorpi JK. 25-Hydroxyvitamin D and vitamin D in human milk: effects of supplementation and season. Am J Clin Nutr. 1988;48(4):1057–1060. doi: 10.1093/ajcn/48.4.1057. [DOI] [PubMed] [Google Scholar]
- 37.Hollis BW, Pittard WB, 3rd, Reinhardt TA. Relationships among vitamin D, 25-hydroxyvitamin D, and vitamin D-binding protein concentrations in the plasma and milk of human subjects. J Clin Endocrinol Metab. 1986;62(1):41–44. doi: 10.1210/jcem-62-1-41. [DOI] [PubMed] [Google Scholar]
- 38.Thandrayen K, Pettifor JM. Maternal vitamin D status: implications for the development of infantile nutritional rickets. Endocrinol Metab Clin North Am. 2010;39(2):303–320. doi: 10.1016/j.ecl.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M. The effect of calcium supplementation on bone density during lactation and after weaning. N Engl J Med. 1997;337(8):523–528. doi: 10.1056/NEJM199708213370803. [DOI] [PubMed] [Google Scholar]
- 40.Tang J, McFann KK, Chonchol MB. Association between serum 25-hydroxyvitamin D and nephrolithiasis: the National Health and Nutrition Examination Survey III, 1988-94 [published online ahead of print July 9, 2012] Nephrol Dial Transplant. doi: 10.1093/ndt/gfs297. doi:10.1093/ndt/gfs297. [DOI] [PubMed] [Google Scholar]
- 41.Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87(6):1738–1742. doi: 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]