Abstract
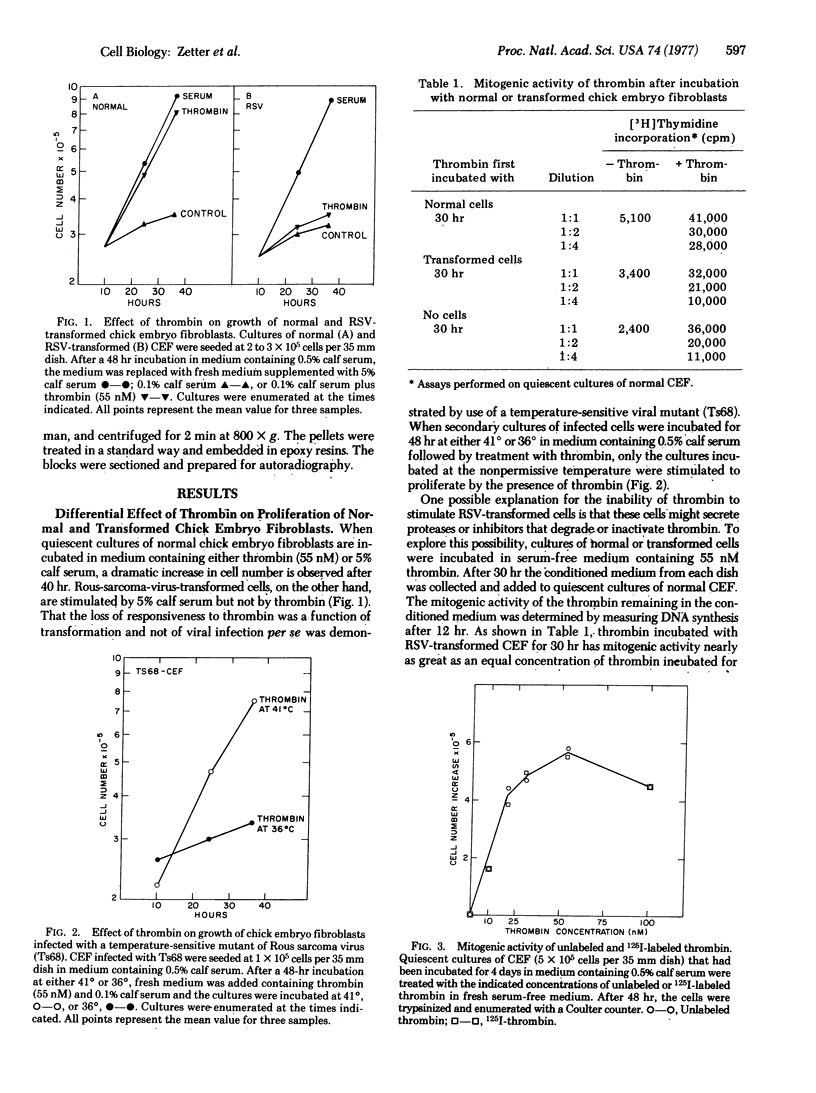
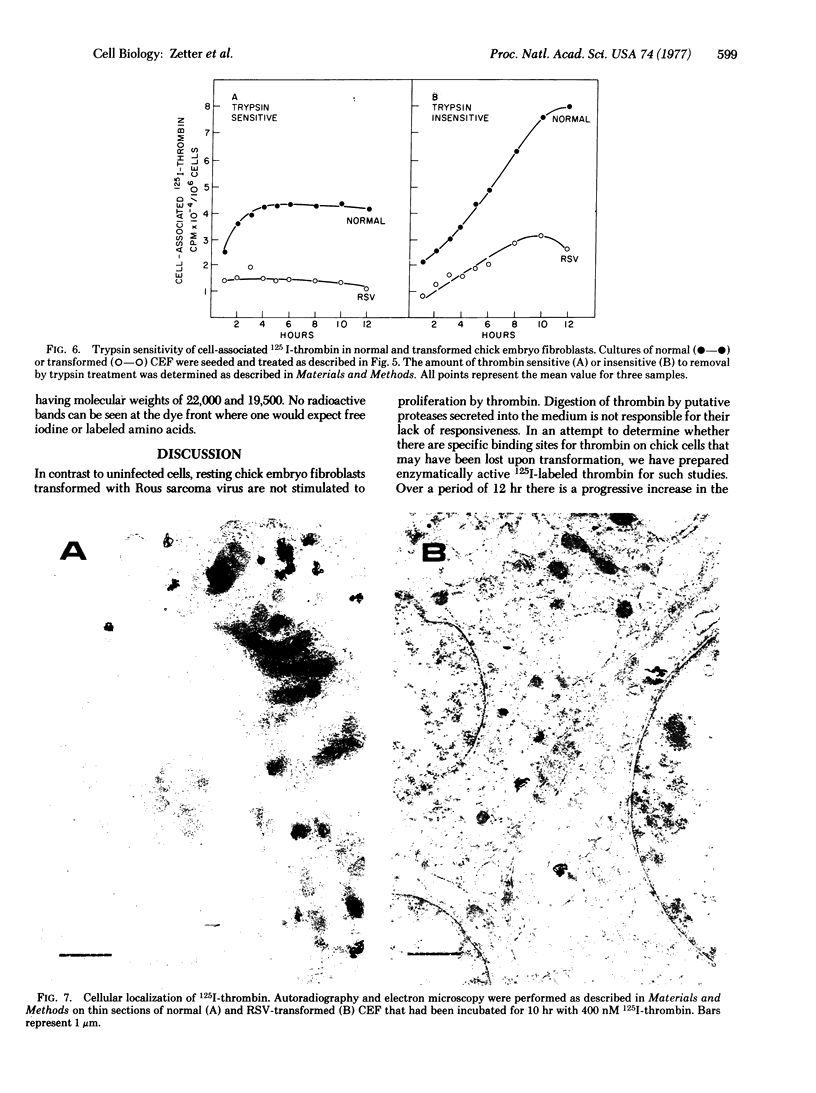
Thrombin stimulates cell proliferation in cultures of normal chick embryo fibroblasts but not in cells transformed with Rous sarcoma virus. Analysis of medium conditioned by Rous-sarcoma-virus-transformed cultures demonstrates that these cells do not secrete molecules that can inhibit or inactivate thrombin. The interaction of thrombin with these cells was investigated with enzymatically active 125I-thrombin. The amount of cell-associated 125I-thrombin was found to be three times greater with normal cells than with transformed cells. In both types of cell, greater than 50% of the total cell-associated 125I-thrombin was found as a component that was not dissociated from the cells by trypsin treatment, an observation suggesting that a significant portion was not on the cell surface. The amount of the trypsin-insensitive fraction increases with time up to 12 hr, whereas the trypsin-sensitive fraction is saturated after 1-4 hr. Autoradiography of thin sections of 125I-thrombin-treated cells observed by electron microscopy reveals that after 10 hr incubation greater than 70% of the label is localized in the cytoplasm of both normal and transformed cells. Autoradiograms of sodium dodecyl sulfate/polyacrylamide slab gels demonstrate that 40% of the intracellular label is the size of native thrombin with the remainder in two large fragments of 22,000 and 19,500 daltons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boquet P., Pappenheimer A. M., Jr Interaction of diphtheria toxin with mammalian cell membranes. J Biol Chem. 1976 Sep 25;251(18):5770–5778. [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Plasminogen-independent fibrinolysis by proteases produced by transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1132–1136. doi: 10.1073/pnas.72.3.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges G. M., Livingston D. C., Franks L. M. The localization of trypsin in cultured mammalian cells. J Cell Sci. 1973 May;12(3):887–902. doi: 10.1242/jcs.12.3.887. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Martin B. M., Wasiewski W. W., Fenton J. W., 2nd, Detwiler T. C. Equilibrium binding of thrombin to platelets. Biochemistry. 1976 Nov 2;15(22):4886–4893. doi: 10.1021/bi00667a021. [DOI] [PubMed] [Google Scholar]
- Mohammed S. F., Whitworth C., Chuang H. Y., Lundblad R. L., Mason R. G. Multiple active forms of thrombin: binding to platelets and effects on platelet function. Proc Natl Acad Sci U S A. 1976 May;73(5):1660–1663. doi: 10.1073/pnas.73.5.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Rubin H. Effects of local cell concentrations upon the growth of chick embryo cells in tissue culture. Exp Cell Res. 1968 Mar;49(3):666–678. doi: 10.1016/0014-4827(68)90213-9. [DOI] [PubMed] [Google Scholar]
- Ryser H. J. Uptake of protein by mammalian cells: an underdeveloped area. The penetration of foreign proteins into mammalian cells can be measured and their functions explored. Science. 1968 Jan 26;159(3813):390–396. doi: 10.1126/science.159.3813.390. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Bo Chen L. The role of surface proteins in cell proliferation as studied with thrombin and other proteases. Proc Natl Acad Sci U S A. 1975 Feb;72(2):413–417. doi: 10.1073/pnas.72.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng N. N., Chen L. B. Thrombin-sensitive surface protein of cultured chick embryo cells. Nature. 1976 Feb 19;259(5544):578–580. doi: 10.1038/259578a0. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Feagler J. R., Majerus P. W. The binding of thrombin to the surface of human platelets. J Biol Chem. 1974 Apr 25;249(8):2646–2651. [PubMed] [Google Scholar]
- Zetter B. R., Chen L. B., Buchanan J. M. Effects of protease treatment on growth, morphology, adhesion, and cell surface proteins of secondary chick embryo fibroblasts. Cell. 1976 Mar;7(3):407–412. doi: 10.1016/0092-8674(76)90170-7. [DOI] [PubMed] [Google Scholar]