Introduction
Exon skipping is one of a number of “steric blocking” applications of oligonucleotides (ONs) and their analogues that in recent years have undergone a renaissance and which have led to new therapeutic opportunities (Kurreck, 2008; Kole et al., 2012; Lightfoot and Hall, 2012). Whereas most standard antisense applications involve cleavage of the RNA by intracellular ribonuclease H (RNase H) or by argonaute2 (Ago2) enzymes, exon skipping by steric blocking merely requires binding of the ON in an antisense orientation to an RNA target and blockage of the important biological function of splicing. In exon skipping, as well as the similar splice-switching activity of exon inclusion, the target is pre-mRNA located in the cell nucleus. Thus, the ON must enter the cell nucleus in sufficient quantity to be in excess over the target pre-mRNA, bind to it strongly, and interfere with the splicing machinery. For drug use, the ON must exhibit additionally a number of other important properties such as good biodistribution to the target organ(s), lack of immune recognition effects, and a good therapeutic window between effective and toxic doses. Further, ON synthesis must be routinely achievable on varying scales. Such simultaneous requirements have taxed the ingenuity of chemists in ON design.
It is necessary in all therapeutic applications of ONs to include modifications to the ON backbone and/or sugar component to protect against nuclease degradation. Interestingly, the very first analogue to find use therapeutically was the phosphorothioate linkage (Matsukura et al., 1987), which is still utilized in most ONs in current clinical trials. Further modifications have followed in subsequent years that have improved stability to nucleases, or even result in the ON being nuclease inert. The best ON analogues both enhance RNA binding strength, compared with an unmodified ON, and reduce nuclease resistance. Increased binding strength has allowed shorter ONs to be used in some cases, thus reducing the chance of binding to an incorrect RNA site through partial sequence complementarity. For example, in the case of ONs containing locked nucleic acids (LNAs), lengths of 12 to 15 are usually used (Lanford et al., 2010; Straarup et al., 2010) and even shorter in the case of all-LNA ONs (Obad et al., 2011). More commonly, lengths of ON analogue synthesized for exon skipping and other steric blocking applications are 14–30 residues.
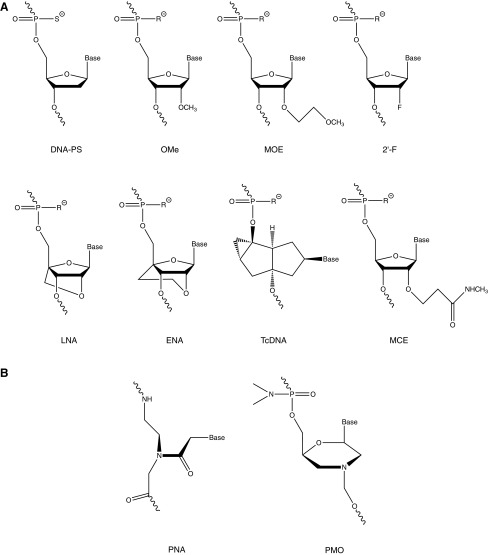
ONs are usually taken up into cells via endocytosis and then they must be released sufficiently from endosomal compartments into the cytosol. The cell nucleus is not thought to be a barrier for ONs once released from endosomes. However, cell entry behavior in culture is not a good predictor of in vivo activity. Thus for a particular exon skipping application, once in vivo delivered, the ON must be able to reach the required cell types. Leading applications for exon skipping and exon inclusion have been neuromuscular diseases such as Duchenne muscular dystrophy (DMD) and spinal muscular atrophy (SMA). In both these cases, as well as in targeting of triplet repeats in myotonic dystrophy, the ON must be able to penetrate muscles of various types. However, it has proved very hard to date to find ONs or derivatives that are, in addition to skeletal muscle, as effective in heart and/or brain. Further, parameters such as biodistribution and organ accumulation, toxicology, and pharmacology in mice or rats must each be assessed, thus requiring at least multimilligram quantities of the ON to be readily obtainable. For such studies in animals and for clinical trials, the ON must be synthesized easily and cost effectively on gram to kilo scale under good manufacturing practice (GMP). Only a very small number of splice switching ON analogues have advanced sufficiently for use in clinical trials (Muntoni and Wood, 2011; Kole et al., 2012; van Deutekom et al., 2013) but some newer analogue types appear promising. The chemistry types that have been under study for exon skipping are depicted in Fig. 1 and the applications for which they have been used are shown in Table 1.
FIG. 1.
Structures of repeating units of analogues of oligonucleotides (ONs) used in exon skipping and related applications. (R=O or S) (A) Charge-negative ONs. (B) Charge-neutral ONs.
Table 1.
Oligonucleotide Types and Their Exon Skipping, Exon Inclusion, and Related Applications
| Oligonucleotide* | Cellular target | ES | EI | Other | Therapeutic application | Reference |
|---|---|---|---|---|---|---|
| Negatively charged ON Analogues | ||||||
| DNA-PS | pre-mRNA | x | DMD | Takeshima et al., 2006 | ||
| OMe | pre-mRNA | x | DMD | Fletcher et al., 2006; Lu et al., 2005; | ||
| x | Goemans et al., 2011; | |||||
| pre-mRNA | x | Dystrophic epidermolysis Bullosa | Goto et al., 2006 | |||
| DMPK-mRNA | x | Myotonic dystrophy type 1 | González-Barrigs et al., 2013; Mulders et al., 2009 | |||
| pre-mRNA | x | Lowering LDL cholesterol | Disterer et al., 2013 | |||
| pre-mRNA | x | SMA | Osman et al., 2012 | |||
| MOE | pre-mRNA | x | SMA | Hua et al., 2010; Passini et al., 2011 | ||
| MOE gapmers | DMPK-mRNA | x | Myotonic dystrophy type 1 | Wheeler et al., 2012 | ||
| LNA | pre-mRNA | x | DMD | Aartsma-Rus et al., 2004 | ||
| pre-mRNA | x | Rheumatoid arthritis | Graziewicz et al., 2008 | |||
| pre-mRNA | x | Chronic inflammatory disease | Yilmaz-Elis et al., 2013 | |||
| TcDNA | pre mRNA | x | DMD | Goyenvalle et al., 2013 | ||
| MCE | pre mRNA | x | DMD | Yamada et al., 2011 | ||
| Charge-neutral ON analogues | ||||||
| PNA | pre-mRNA | x | DMD | Yin et al., 2008a; Yin et al., 2010 | ||
| PMO | pre-mRNA | x | DMD | For reviews see Moulton and Moulton, 2008; | ||
| x | Moulton and Moulton, 2010 | |||||
| pre-mRNA | x | SMA | Porensky et al., 2012; Zhou et al., 2013 | |||
| DMPK-mRNA | x | Myotonic dystrophy type 1 | Wheeler et al., 2009; Koebis et al., 2013 | |||
| Peptide or polymer ON delivery | ||||||
| PEG-PEI with OMe-PS ONs | pre-mRNA | x | DMD | Sirsi et al., 2008; Ferlini et al., 2010; | ||
| x | Bassi et al., 2012 | |||||
| PEG-PEI with PMO | pre-mRNA | x | DMD | Wang et al., 2013 | ||
| Pip2b-PNA | pre-mRNA | x | DMD | Ivanova et al., 2008 | ||
| B/R-peptide-PMO | pre-mRNA | x | DMD | Jearawiriyapaisarn et al., 2008; Wu et al., 2008; | ||
| x | Yin et al., 2008b; Wu et al., 2012 | |||||
| DMPK-mRNA | x | Myotonic dystrophy type 1 | Leger et al., 2013 | |||
| Pip5-PMO, Pip6-PMO | pre-mRNA | x | DMD | Yin et al., 2011; Betts et al., 2012 | ||
| Vivo-morpholinos | pre-mRNA | x | DMD | Wu et al., 2009; Widrick et al., 2011; Yokota et al., 2012; Aoki et al., 2012 | ||
For chemical structures of oligonucleotide (ON) types, see Fig. 1.
DMD, Duchenne muscular dystrophy; DMPK, dystrophia myotonica protein kinase; EI, exon inclusion; ES, exon skipping; LDL, low-density lipoprotein; LNA, locked nucleic acid; MCE, 2′-O-[2-(N-methylcarbamoyl)ethyl]uridine; MOE, 2′-O-methoxyethyl; OMe, 2′-O-methyl; PEG–PEI, polyethylene glycol–poly(ethylene imine); Pip, PMO internalization peptides; PMO, phosphorodiamidate morpholino; PNA, peptide nucleic acids; PS, phosphorothioate; SMA, spinal muscular atrophy; TcDNA, tricycloDNA.
Negatively Charged Oligonucleotide Analogues
A 31-mer oligodeoxynucleotide phosphorothioate (DNA-PS, Fig. 1A) was the first ON analogue to make it to a clinical trial in a single patient for exon skipping in DMD (Takeshima et al., 2006). A disadvantage of DNA-PS is that when bound to RNA it can induce undesirable cleavage of the RNA by cellular RNase H. Very few other types of negatively charged ON analogue that do not induce RNase H cleavage have been reported to be effective in exon skipping or exon inclusion applications (Fig. 1A). In all cases, these ON analogues mimic RNA in their binding character when forming duplex structures with an RNA target, such that binding is tighter than for DNA analogues. The internucleotide linkage is invariably phosphorothioate (PS), which also allows high protein binding in serum and increases circulation time. All of the ON analogues show high resistance to serum and cellular nucleases.
2′-O-methylphosphorothioate
Particularly ubiquitous in exon skipping studies have been 2′-ribose-modified PS ON analogues, the foremost of which is 2′-O-methylphosphorothioate (OMe-PS) (Fig. 1A; Table 1). Exon skipping and functional dystrophin production was demonstrated after intravenous delivery into mdx mice (a standard DMD mouse model) just a few years ago (Lu et al., 2005; Fletcher et al., 2006). OMe-PS quickly became the leading negatively charged ON type in exon skipping, probably because this chemistry is well established for use in vivo and for scale up without onerous license requirements, and OMe-PS ONs appear also to have a relatively good safety profile. Long-term studies have shown that OMe-PS ONs can be administered safely at 200 mg/kg in various DMD mouse models of differing severity for up to 6 months (Tanganyika-de-Winter et al., 2012). An OMe-PS ON (now named Drisapersen) has been taken to clinical trials for exon 51 skipping in DMD patients (Goemans et al., 2011), showing effectiveness in extended treatment in the “6 minute walk test” for ambulatory patients, and phase 3 clinical trial data in Europe are expected imminently. ONs for skipping other exons are also now beginning clinical trials (www.prosensa.eu).
OMe-PS ON effectiveness has been reported for other genetic diseases such as exon skipping in the collagen gene in a rat model of dystrophic Epidermolysis Bullosa (Goto et al., 2006) and also for targeting a triplet repeat sequence in the mRNA of the dystrophia myotonica protein kinase (DMPK) in myotonic dystrophy type 1 (Mulders et al., 2009; González-Barriga et al., 2013). Exon skipping of ApoB (apolipoprotein B) pre-mRNA by OMe-PS ONs has been shown to reduce LDL cholesterol in ApoB transgenic mice (Disterer et al., 2013). In addition, OMe-PS ONs feature prominently in an approach for use of bifunctional RNAs to target the intronic silencer-N1 to cause exon inclusion, to increase survival motor neuron 1 (SMN1) pre-mRNA levels and to reduce disease severity in an animal model of SMA (Osman et al., 2012). This bifunctional steric block approach had first been proposed some years ago for this disease through cell culture studies (Skordis et al., 2003).
2′-O-methoxyethyl oligonucleotides
A 2′-O-methoxyethyl (MOE) ON (Fig. 1A; Table 1) is a 2′-analogue class introduced some years ago by Isis Pharmaceuticals. MOE-PS ONs were assessed as steric blocking agents first in a number of splice-switching models, such as redirection of pre-mRNA splicing of murine interleukin-5 receptor alpha chain (IL5R-α) (Karras et al., 2000), MyD88 (Vickers et al., 2006) and enhanced green fluorescent protein (EGFP) (Sazani et al., 2001). Isis Pharmaceuticals has collaborated with a number of academic groups, for example with the Krainer laboratory for exon inclusion of the SMN2 gene in vivo as a potential approach for treatment of SMA (Hua et al., 2010). MOE-PS appears to be better than OMe-PS (Williams et al., 2009) in this case, since it was reported that an 18-mer MOE-PS ON (ASO-10–27) was more effective after intracerebroventricular (i.c.v.) infusion into adult mice central nervous system (CNS) compared with an overlapping 20-mer OMe-PS ON, and there were fewer unwanted proinflammatory effects (Hua et al., 2010). Intrathecal infusion of ASO-10–27 ameliorated symptoms of severe SMA in cynomolgus monkeys (Passini et al., 2011) and phase 1b/2a clinical trials are now in progress (www.clinicaltrials.gov, ISIS-SMNRx).
MOE gapmers that employ an RNase H antisense mechanism are also effective in vivo for knockdown of toxic RNA and correction of myotonic dystrophy type 1 (Wheeler et al., 2012). Interestingly, the RNase H approach could prove more effective here than targeting of the expanded CUG repeated region in DMPK mRNA (González-Barriga et al., 2013) and further comparisons of the two approaches are to be expected. A recent report suggests that MOE-PS ONs are slightly more effective than OMe-PS ONs in an mdx mouse model of DMD (Yang et al., 2013).
Other negatively charged ON types
It is important to recognize in targeting pre-mRNA splicing that the type of 2′-modification matters when considering mechanism of action. This lesson has been brought home by the startling discovery that a PS ON composed entirely of supposedly steric blocking 2′-deoxy-2′-fluoronucleotides (2′-F, Fig. 1A), became recognized instead by the interleukin enhancer binding factors 2 and 3 (ILF2/3) to direct exon inclusion through activation of the splicing machinery (Rigo et al., 2012). Although in principle a fully 2′-F modified ON could be used in an exon skipping mode if the right pre-mRNA target site is found, 2′-F may perhaps find better use in modulating binding strength of ONs when used in mixmers with other analogue types (Davis et al., 2009).
A well-known analogue type, of which LNA (also known as bridged nucleic acids [BNA]) and ethylene-bridged nucleic acids (ENA) are the archetypes (Fig. 1A), is formed by cyclization of a 2′-alkyl substituent to the 4′-position resulting in “locking” the conformation of the sugar into that similar to that in RNA (Koshkin et al., 1998; Obika et al., 1999; Takagi et al., 2004). Inclusion of just a single LNA or ENA unit within an ON can result in an increase in the melting temperature (Tm) of 5°C or more. Some years ago, an all LNA ON was shown to be effective for exon skipping in cells from an exon 45-deleted DMD patient, but with reduced specificity, probably due to its high Tm with the RNA target (Aartsma-Rus et al., 2004). It is common now to mix LNA with DNA (Elayadi et al., 2002) or with an OMe backbone (Arzumanov et al., 2001) to modulate binding strength and temper possible in vivo toxic effects. In an EGFP splice-switching mouse model, a PS 15-mer 8LNA/7DNA approximately alternating mixmer showed much higher potency in liver, colon, and small intestine than an OMe ON (Roberts et al., 2006). A similar 15-mer LNA/DNA-PS ON was highly effective in splice-switching tumour necrosis factor-α pre-mRNA to induce the soluble form of the protein as a potential therapy for rheumatoid arthritis (Graziewicz et al., 2008). Only a modest increase over an all OMe-PS ON was seen for a 23-residue OMe/LNA-PS mixmer containing five LNA units used for exon-9 skipping of IL-1 receptor accessory protein as a potential strategy for treatment of chronic inflammatory diseases (Yilmaz-Elis et al., 2013). However, it is likely that a substantial survey of such mixmers of various lengths and compositions would be needed for optimization of activity in each indication and to minimize potential hepatotoxicity.
Quite surprisingly, the slightly larger 2′-4′ ringed ENA (Fig. 1A) has not been developed further following an initial report that a 21-mer chimeric ENA/OMe-PS ON showed 40 times the exon skipping activity compared to a 31-mer DNA/PS used originally in exon-19 skipping in myocyte culture from cells derived from a DMD patient (Yagi et al., 2004). Perhaps this is due to restricted availability of ENA. ENA would be predicted to have similar activity to LNA ONs but comparative in vitro and in vivo data are lacking. Other bicyclic derivatives such as 2′,4′-constrained-[S]-2′-O-ethyl (S-cEt) nucleotides have been developed by Isis Pharmaceuticals (Seth et al., 2009) as a rival to LNA, but so far do not seem to have featured in exon skipping or inclusion strategies.
The unusual tricycloDNA analogue (TcDNA, Fig. 1A) was first used in a steric block mode targeting HIV RNA a few years ago (Ivanova et al., 2007). The multistep syntheses required for monomer production hampered development until recently. In an RNase H gapmer mode with a central DNA core, TcDNA has shown better activity against the scavenger receptor B1 mRNA than MOE in the flanks of gapmer ONs in a range of tissue types in systemic treatment of mice (Murray et al., 2012). Now, fully TcDNA ONs are being evaluated for exon skipping and inclusion in models of neuromuscular diseases. For example, a recent conference abstract has suggested substantial levels of exon skipping activity have been obtained in the mdx mouse model of DMD and, more excitingly, restoration of dystrophin in heart muscle was achieved as well as detection of exon skipping in brain (Goyenvalle et al., 2013).
Whilst the use of chemically modified nucleotides that increase binding affinity of an ON for a target RNA (such as LNA or TcDNA) is the most often studied way of searching for steric blocking or splice switching ONs, a novel alternative approach involves attachment at or near the end of (for example) a OMe ON an N,N-diethyl-4-(4-nitronapthalene-1-ylazo)-phenylamine substituent (ZEN) that both increases binding affinity, presumably by an intercalative base interaction, as well as confers additional stability to exonucleases. ZEN-modified OMe ONs (without PS) have proved effective in inhibition of microRNA-122 after cellular transfection and appeared to be less toxic and equally effective as LNA/DNA-PS and LNA/OMe-PS ONs (Lennox et al., 2013). Such novel methods for boosting binding affinity for ON are now likely to be evaluated also in exon skipping and other steric blocking applications.
Although it is likely that a few other analogue types have been evaluated, the only report of a new 2′-analogue being tested in an mdx mouse model of DMD is that of a 30-mer OMe-PS ON in which the 6 uridine residues were substituted by 2′-O-[2-(N-methylcarbamoyl)ethyl]uridine (MCE, Fig. 1A). By intramuscular delivery, this MCE/OMe-PS ON showed a greater level of exon skipping than the parent OMe-PS ON (Yamada et al., 2011).
Charge-Neutral Peptide Nucleic Acids and Phosphorodiamidate Morpholino Oligonucleotides
Charge-neutral ON backbones feature strongly in exon skipping and exon inclusion applications. Peptide nucleic acids (PNA) developed in the laboratory of Nielsen (Egholm et al., 1992) are essentially nucleobase-functionalized derivatives of 2-aminoethylglycine (Fig. 1B). They are synthesized chemically by methods similar to peptide synthesis, and the PNA monomers are readily available commercially. Additional amino acids can be also be added (such as Lys) to maintain good water solubility. Cationic residues in particular help PNA to bind to cells and aid cell uptake. PNA exhibits strong and sequence specific binding to both RNA and DNA targets (Egholm et al., 1993).
A 15-mer PNA-Lys was as effective as a MOE-PS ON when electroporated into BCL1 lymphoma cells in redirecting splicing of the IL5R-α pre-mRNA to give the soluble form (Karras et al., 2001). A PNA-Lys4 18-mer was very effective in a number of organs, such as kidney, liver, heart, and lung, in a splice-switching EGFP mouse model after i.p. injection (Sazani et al., 2002). However, a PNA-Lys4 14-mer was not effective in inducing exon skipping in exon 45-deleted patient myoblasts in the DMD model (Aartsma-Rus et al., 2004), perhaps because this PNA was too short for good binding to this target. By contrast, an electroporated 14-mer PNA-Lys4 was very effective (and a PNA-Lys8 even better) in redirecting splicing in a murine CD40 receptor model in BCL1 cells as well as when incubated at high micromolar concentration in the absence of a transfection agent (Siwkowski et al., 2004). A 20-mer PNA was moderately effective in exon skipping and dystrophin production in the mdx mouse model of DMD following intramuscular injection into the tibialis anterior muscle (Yin et al., 2008a), but a longer 25-mer PNA was more effective under intravenous delivery, and doses up to 100 mg/kg were well tolerated (Yin et al., 2010).
In contrast to PNA, which is not yet available in GMP grade, phosphorodiamidate morpholino oligonucleotide (PMO) first described in the laboratory of Summerton (Summerton and Weller, 1997) (Fig. 1B) has become the uncharged ON analogue of choice for DMD exon skipping and inclusion in muscle and has also been used in many other therapeutic applications (Moulton and Moulton, 2008, 2010). Custom synthesis for researchers is available up to 30-mers and additional Lys residues are not needed because PMO is more water-soluble than PNA. Direct comparisons of PMO and OMe-PS chemistries in exon skipping in the mdx mouse (25-mers targeting exon 23 of the dystrophin pre-mRNA) showed that PMO generated higher exon skipping and dystrophin production by both intramuscular and systemic delivery than OMe-PS (Alter et al., 2006; Fletcher et al., 2006). However, for a range of human exons in humanized DMD mice, the effectiveness of exon skipping for OMe-PS and PMO ONs varied depending on the target exon sequence and on ON length (Heemskerk et al., 2009), suggesting that the mdx mouse exon 23 target is not an ideal comparator for human muscle effectiveness.
PMO has proven to be a remarkably safe drug as demonstrated at the maximum feasible repeated doses over 12 weeks of 320 mg/kg in cynomolgus monkeys (Sazani et al., 2011). However, establishment of good dystrophin production through exon skipping in heart in mdx mice required very high (up to 3000 mg/kg) dosing (Wu et al., 2010). The first systemic delivery results of human clinical trials of a 30-mer PMO (known as Eteplirsen) for exon 51 skipping in DMD patients showed some dystrophin production when delivered intravenously for 12 weeks at up to 20 mg/kg (Cirak et al., 2011). More recent results at 30 and 50 mg/kg have demonstrated higher levels of dystrophin and effectiveness in the 6-minute walk test in extension studies, but the number of patients is small (Mendell et al., 2013). A clinical trial using PMO has also begun in Japan (www.nippon-shinyaku.co.jp/). Further clinical trials are expected. Note that clinically used PMOs have tended to be longer than the corresponding OMe-PS ONs.
A single i.c.v. injection of a PMO ON has been effective in exon inclusion of the SMN1 pre-mRNA in the brains of SMA model mice and prolonged the life of such mice dramatically (Porensky et al., 2012). Systemic delivery into neonatal mice, where the blood-brain barrier is incompletely formed, was also highly effective at extending the life of mice in a severe SMA model (Zhou et al., 2013). Both PMOs target the ISS-N1 enhancer sequence that regulates splicing of intron 7 of the SMN1 gene. For PMO as well as for MOE (Passini et al., 2011), a single ON could prove sufficient to treat all SMA cases, which is not the case for DMD patients.
A PMO targeted to the extended CUG triplet repeat sequence in the mRNA for DMPK has been effective in a transgenic mouse model of myotonic dystrophy type 1 (Wheeler et al., 2009), but this approach has been competitive with MOE gapmers, also targeted at this gene (Wheeler et al., 2012), and it is not clear at this stage which of these approaches will prove the most effective for clinical development. An interesting new method of in vivo delivery of PMO to treat myotonic dystrophy in mice has been reported that involves use of liposomal bubbles and ultrasound that increased the efficiency of exon skipping compared to intravenous injection (Koebis et al., 2013).
Conjugates of Oligonucleotides with Peptides and Other Moieties
In the absence of organ targeting, most ON types tend to accumulate in liver and kidneys. For many other organs, naked ON delivery remains much poorer. Strategies for enhancing delivery include the co-administration of cationic complexing agents (e.g., lipid or polymer) or covalent conjugation with molecules that may enhance cell penetration. Polyethylene glycol–poly(ethylene imine) (PEG–PEI) copolymer complexes with OMe-PS ONs have been shown to enhance effectiveness for exon skipping in mdx mice (Sirsi et al., 2008). Similarly OMe-PS ONs absorbed on ZM2 nanoparticles allowed lower dose restoration of dystrophin in mdx mice by intraperitoneal delivery (Ferlini et al., 2010), which was persistent even after 90 days (Bassi et al., 2012). Further, PEI-modified pluronics have also been shown to enhance delivery of PMO into mdx mice after systemic delivery (Wang et al., 2013).
Most studies by far have involved attachment of cell-penetrating peptides (CPPs) to ONs, however. For negatively charged ONs such as OMe-PS, covalent conjugation with peptides has met with limited success so far in enhancing in vivo delivery. By contrast, significant enhancement of splice switching and exon skipping for conjugates of CPPs has been seen with charge neutral ONs. Such applications of CPPs have been reviewed recently (Lebleu et al., 2008; Said Hassane et al., 2010).
PNA-Lys8 or PNA conjugates to an amphipathic D-Lys-rich peptide or to an Arg/homoArg-rich peptide targeting a splice junction in the PTEN pre-mRNA showed strong inhibition of PTEN expression activity in adipose tissue in mice, but the amphipathic peptide conjugate also showed substantial nephrotoxicity (Wancewicz et al., 2010). In the HeLa pLuc 705 model system, early good splice switching results obtained with PNA conjugates of the CPP R6-penetratin (Abes et al., 2007) later led to the development of the PMO internalization peptides (Pip) series of Arg-rich CPPs and Pip2b-PNA was found to be effective by intramuscular delivery for exon skipping and dystrophin production in the mdx mouse (Ivanova et al., 2008).
However, side-by-side comparisons of CPP-PNA and CPP-PMOs in the mdx mouse model showed that CPP-PMO (now usually called peptide-PMO or P-PMO) had higher activity than CPP-PNA in muscle (Yin et al., 2010). Indeed, conjugation of the AVI Biopharma (Sarepta) Arg-rich “B” or “R” peptide to PMO targeting exon 23 led to dramatic enhancement of exon skipping and dystrophin production over naked PMO in the mdx mouse (Jearawiriyapaisarn et al., 2008; Wu et al., 2008; Yin et al., 2008b). Long-term administration of such P-PMO in mdx mice showed improvements in muscle pathology and function (Wu et al., 2012). In addition, recent papers have reported further Arg-rich Pip peptides in the series, notably Pip5e (Yin et al., 2011) and Pip6a (Betts et al., 2012), which differ from each other only slightly in CPP sequence. The salient feature of Pip-PMOs is their ability to show dystrophin production in heart, albeit less than in skeletal muscle. This class of P-PMO is being developed now for clinical use in DMD.
P-PMO is also effective in the mouse model of DM1 myotonic dystrophy. Here, a 25-mer PMO targeted to the extended CUG repeat sequences was conjugated to either B or R peptide and intravenous delivery led to redistribution of the muscleblind-1 protein and corrections of abnormal downstream splicing events, as well as a reduction in myotonia (Leger et al., 2013). P-PMO application in SMA is also a natural development that is expected.
A non-peptidic octaguanidino dendrimer conjugate of PMO, marketed as “Vivo-morpholinos,” has shown good exon skipping and dystrophin production in the mdx mouse (Wu et al., 2009) and improved muscle function (Widrick et al., 2011). Such Vivo-morpholinos have also been used in a “cocktail” for multiple exon skipping in dystrophic dogs (Yokota et al., 2012). A cocktail of ten Vivo-morpholinos has also been used recently to skip exons 45–55 of dystrophin pre-mRNA in dystrophic mdx52 mice by systemic delivery (Aoki et al., 2012). Spontaneous deletions of this specific region are associated with exceptionally mild phenotypes. It is not clear as yet whether a subset of these PMOs is responsible for producing the large deletion in this specially constructed mouse model of DMD and as to whether this cocktail approach could be applicable to DMD patients.
In principle, ONs can also be conjugated with cell targeting peptides. For example, a conjugate of a OMe-PS ON with a bivalent Arg-Gly-Asp (RGD) peptide that is known to target integrin receptors, enhanced splicing redirection in a reporter luciferase construct in melanoma cells in the absence of a transfection agent (Alam et al., 2008) and a bombesin peptide conjugated to the same OMe-PS ON enhanced splice switching via delivery through G-protein coupled endocytosis in prostate cancer cells (Ming et al., 2010). Surprisingly, however, cell-targeting peptides, for example discovered by phage display, have not yet found significant utility in vivo, although it is known from conference reports that a peptide conjugate of an OMe-PS ON is being considered for development for use in treatment of myotonic dystrophy type 1 (www.prosensa.eu).
Prospects for Novel Chemistries in Exon-Skipping Applications
Although hundreds of promising ON analogues have been described in recent years, very few are being pursued in exon skipping and related applications (see Table 1). In the last 5 years, only TcDNA has shown sufficient promise for development to rival the more standard OMe, MOE, LNA, PNA, and PMO ON varieties (Fig. 1). Clearly further 2′-O-substituted and conformationally restricted 2′-4′-cyclized RNA analogues that have followed on from LNA and ENA, such as S-cEt, would be worth evaluation, particularly in combinations with 2′-F, OMe, or DNA residues. Such analogue types used in steric blocking applications are usually subject to significant intellectual property (IP) barriers and therefore are only investigated for exon skipping applications if it is in the interests of the company holding that IP, but it is likely that such nucleotide derivatives will make it closer to the clinic for some types of steric blocking applications in coming years. In principle, further PNA-like or other charge-neutral ONs, several of which have been published in recent years, may also see development, but none of these appear to be on the immediate horizon.
More likely developments may stem from additional end modifications to ONs, particularly for charge neutral PMO or PNA backbones, in order to modify their pharmacological and/or cellular uptake properties. The key issue here is to ensure that any substituent does not interfere with the ability of the ON to reach its pre-mRNA target and hybridize efficiently. Chemical space is vast and the easy ability to attach moieties of varied chemistry to ONs makes it attractive to pursue such research avenues. For example, there is great scope for the exploration of further synthetic peptides and other derivatives (including amino acid analogues) to tune in vivo parameters of the attached ONs to improve their pharmacology. Indeed, for all ON types, there is a need to redirect ONs away from the kidney and liver (unless these organs are being targeted specifically) and to increase their serum half-life.
Better targeting to specific cell types or organs (such as brain, heart, and muscle) is another generally important goal for all ON types. For example for SMA, intrathecal delivery is currently the only possible method of ON introduction to brain and efforts to find ways to help ONs across the blood–brain barrier following intravenous delivery must remain a priority. Chemical modifications of ONs by known brain penetrating compounds, such as brain-targeting peptides, are worth exploring not only in this disease context but also for many other diseases, such as brain cancers. Improved brain penetration is a goal that is extremely challenging to achieve for macromolecules such as ONs, however. In addition, a key issue for any novel peptide or other chemical moiety as an ON conjugate is the safety margin in its use in vivo. For example, many peptide conjugates show toxic side effects at doses below that of their naked ON counterparts.
In our own laboratory we have developed a method using “Click” chemistry in aqueous solution for labeling P-PMO (and PMO) starting from unfunctionalized, commercially available PMO (Shabanpoor and Gait, 2013). This may aid the tracking and semi-quantification of PMO in vivo and intracellulary. Currently, the only analytical technique available to measure PMO levels in vivo is based on hybridization assays (Schnell et al., 2013). Another method we have developed involves rapid parallel synthesis of sets of CPP conjugates of biocargoes, such as PNA or PMO, for use in cell screening or similar programmes (Deuss et al., 2013). The role of a chemist in the development of therapeutic ON technology may now perhaps be better devoted to exploration of chemical space with regard to what can be attached to commercially available ONs rather than trying to “reinvent the wheel” in discovery of novel ON analogue types.
Acknowledgments
Work in the Gait laboratory is supported by the Medical Research Council (Unit programme U105178803). Liz O'Donovan is supported by a grant from the Association Française contre les Myopathies (AFM, programme grant 14784). We thank Matthew Wood and colleagues (University of Oxford) for continuing fruitful and stimulating collaborations in exon skipping of DMD and other therapeutic applications, and for comments on this manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- AARTSMA-RUS A., KAMAN W.E., BREMMER-BOUT M., JANSON A.A.M., DEN DUNNEN J.T., VAN OMMEN G.B., and VAN DEUTEKOM J.C.T. (2004). Comparative analysis of antisense oligonucleotide analogs for targeted DMD exon 46 skipping in muscle cells. Gene Ther. 11, 1391–1398 [DOI] [PubMed] [Google Scholar]
- ABES S., TURNER J.J., IVANOVA G.D., OWEN D., WILLIAMS D., ARZUMANOV A., CLAIR P., GAIT M.J., and LEBLEU B. (2007). Efficient splicing correction by PNA conjugation to an R6-Penetratin delivery peptide. Nucleic Acid Res. 35, 4495–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALAM M.R., DIXIT V., KANG H., LI Z.-B., CHEN X., TREJO J., FISHER M., and JULIANO R.L. (2008). Intracellular delivery of an anionic antisense oligonucleotide via receptor mediated endocystosis. Nucleic Acids Res. 36, 2764–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALTER J., LOU F., RABINOWITZ A., YIN H., ROSENFELD J., WILTON S.D., PARTRIDGE T.A., and LU Q.L. (2006). Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nature Med. 12, 175–177 [DOI] [PubMed] [Google Scholar]
- AOKI Y., YOKOTA T., NAGATA T., NAKAMURA A., TANIHATA J., SAITO T., DUGUEZ S.M.R., NAGARAJU K., HOFFMAN E.P., PARTRIDGE T., et al. (2012). Bodywide skipping of exons 45–55 in dystrophic mdx52 mice by systemic antisense delivery. Proc. Nat. Acad. Sci. U. S. A. 109, 13763–13768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARZUMANOV A., WALSH A.P., RAJWANSHI V.K., KUMAR R., WENGEL J., and GAIT M.J. (2001). Inhibition of HIV-1 Tat-dependent trans-activation by steric block chimeric 2'-O-methyl/LNA oligoribonucleotides. Biochemistry 40, 14645–14654 [DOI] [PubMed] [Google Scholar]
- BASSI E., FALZARANO S., FABRIS M., GUALANDI F., MERLINI L., VATTEMI D., PERRONE D., MARCHESI E., SABATELLI P., SPARNACCI K, et al. (2012). Persistent dystrophin protein restoration 90 days after a course of intraperitoneally administered naked 2'OMePS AON and ZM2 NP-AON complexes in mdx mice. J. Biomed. Biotechnol. 10.1155/2012/897076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BETTS C., SALEH A.F., ARZUMANOV A.A., HAMMOND S.M., GODFREY C., COURSINDEL T., GAIT M.J., and WOOD M.J.A. (2012). Pip6-PMO, a new generation of peptide-oligonucleotide conjugates with improved cardiac exon skipping activity for DMD treatment. Mol. Ther. Nucl. Acids 1, e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIRAK S., ARECHAVALA-GOMEZA V., GUGLIERI M., FENG L., TORELLI S., ANTHONY K., ABBS S., GARRALDA M.E., BOURKE J., WELLS D.J., et al. (2011). Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open label, phase 2, dose-escalation study. Lancet 378, 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS S., PROPP S., FREIER S.M., JONES L.E., SERRA M.J., KINBERGER G., BHAT B., SWAYZE E.E., BENNETT C.F., and ESAU C. (2009). Potent inhibition of microRNA in vivo without degradation. Nucleic Acids Res. 37, 70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEUSS P., ARZUMANOV A., WILLIAMS D., and GAIT M.J. (2013). Parallel synthesis and splicing redirection activity of cell-penetrating peptide conjugate libraries of a PNA cargo. Org. Biomol. Chem. 11, 7621–7630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISTERER P., AL-SHAWI R., ELLMERICH S., WADDINGTON S.N., OWEN J.S., SIMONS J.P., and KHOO B. (2013). Exon skipping of hepatic ApoB pre-mRNA with splice-switching oligonucleotides reduces LDL cholesterol in vivo. Mol Ther. 21, 602–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGHOLM M., BUCHARDT O., CHRISTENSEN L., BEHRENS C., FREIER S.M., DRIVER D.A., BERG R.H., KIM S.K., NORDEN B., and NIELSEN P. (1993). PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen bonding rules. Nature 365, 566–568 [DOI] [PubMed] [Google Scholar]
- EGHOLM M., BUCHARDT O., NIELSEN P.E., and BERG R.H. (1992). Peptide nucleic acids (PNA). Oligonucleotide analogues with an achiral backbone. J. Amer. Chem. Soc. 114, 1895–1897 [Google Scholar]
- ELAYADI A.N., BRAASCH D.A., and COREY D.R. (2002). Implications of high-affinity hybridization by locked nucleic acid oligomers for inhibition of human telomerase. Biochemistry 41, 9973–9981 [DOI] [PubMed] [Google Scholar]
- FERLINI A., SABATELLI P., FABRIS M., BASSI E., FALZARANO S., VATTEMI D., GUALANDI F., MARALDI N.M., MERLINI L., SPARNACCI K., et al. (2010). Dystrophin restoration in skeletal, heart and skin arrector pili smooth msucle of mdx mice by ZM2 NP-AON complexes. Gene Ther. 17, 432–438 [DOI] [PubMed] [Google Scholar]
- FLETCHER S., HONEYMAN K., FALL A.M., HARDING P.L., JOHNSEN R.D., and WILTON S.D. (2006). Dystrophin expression in the mdx mouse after localised and systemic administration of a morpholino antisense oligonucleotide. J Gene Med. 8, 207–216 [DOI] [PubMed] [Google Scholar]
- GOEMANS N.M., TULINIUS M., VAB DEN AKKER J.T., BURM B.E., EKHART P.F., HEUVELMANS N., HOLLING T., JANSON A.A., PLATENBURG G.J., SIPKENS J.A., et al. (2011). Systemic administration of PRO051 in Duchenne's muscular dystrophy. New Engl. J. Med. 364, 1513–1522 [DOI] [PubMed] [Google Scholar]
- GONZÁLEZ-BARRIGA A., MULDERS S.A.M., VAN DE GIESSEN J., HOOIJER J.D., BIJL S., VAN KESSEL I.D.G., VAN BEERS J., VAN DEUTEKOM J.C., FRANSEN J.A.M., WIERINGA B., et al. (2013). Design and analysis of effects of triplet repeat oligonucleotides in cell models for myotonic dystrophy. Mol. Ther. Nucl. Acids 2, e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTO M., SAWAMURA D., NISHIE W., SAKAI K., MCMILLAN J.R., AKIYAMA M., and SHIMIZU H. (2006). Targeted skipping of a single exon harboring a premature termination codon mutation: implications and potential for gene correction therapy for selective dystrophic epidermolysis bullosa patients. J. Invest. Dermatol. 126, 2614–2620 [DOI] [PubMed] [Google Scholar]
- GOYENVALLE A., BABBS A., AVRIL A., GRIFFITHS G., DUGOVIC B., DAVIES K.E., LEUMANN C., and GARCIA L. (2013). Tricyclo-DNA: a promising chemistry for the synthesis of antisense molecule for splice switching approaches in DMD. Neuromuscul. Disord. 22, 907 [Google Scholar]
- GRAZIEWICZ M.A., TARRANT T.K., BUCKLEY B., ROBERTS J., FULTON L., HANSEN H.F., ØRUM H., KOLE R., and SAZANI P. (2008). An endogenous TNF-alpha antagonist induced by splice-switching oligonucleotides reduces inflammation in hepatitis and arthritis mouse models. Mol. Ther. 16, 1316–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEEMSKERK H.A., DE WINTER C.L., DE KIMPE S.J., VAN KUIK-ROMEIJN P., HEUVELMANS N., PLATENBURG G., VAN OMMEN G.B., VAN DEUTEKOM J.C., and AARTSMA-RUS A. (2009). In vivo comparison of 2'-O-methyl phosphorothioate and morpholino antisense oligonucleotides for Duchenne muscular dystrophy exon skipping. J. Gene Med. 11, 257–266 [DOI] [PubMed] [Google Scholar]
- HUA Y., SAHASHI K., HUNG G., RIGO F., PASSINI M.A., BENNETT C.F., and KRAINER A.R. (2010). Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev. 24, 1634–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVANOVA G.D., ARZUMANOV A., ABES R., YIN H., WOOD M.J.A., LEBLEU B., and GAIT M.J. (2008). Improved cell-penetrating peptide-PNA conjugates for splicing redirection in HeLa cells and exon skipping in mdx mouse muscle. Nucleic Acids Res. 36, 6418–6428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVANOVA G.D., REIGADAS S., ITTIG D., ARZUMANOV A., ANDREOLA M.-L., LEUMANN C., TOULMÉ J.J., and GAIT M.J. (2007). Tricyclo-DNA containing oligonucleotides as steric block inhibitors of Human Immunodeficiency Virus Type 1 Tat-dependent trans-activation and HIV-1 infectivity. Oligonucleotides 17, 54–65 [DOI] [PubMed] [Google Scholar]
- JEARAWIRIYAPAISARN N., MOULTON H.M., BUCKLEY B., ROBERTS J., SAZANI P., FUCHAROEN S., IVERSEN P.L., and KOLE R. (2008). Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice. Mol. Ther. 16, 1624–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARRAS J.G., MAIER M.A., WATT A., and MANOHARAN M. (2001). Peptide nucleic acids are potent modulators of endogenous pre-mRNA splicing of the murine interleukin-5 receptor-a chain. Biochemistry 40, 7853–7859 [DOI] [PubMed] [Google Scholar]
- KARRAS J.G., MCKAY R.A., DEAN N.M., and MONIA B.P. (2000). Deletion of individual exons and induction of soluble murine interleukin-5 receptor-alpha chain expression throygh antisense oligonucleotide-mediated redirection of pre-mRNA splicing. Mol. Pharmacol. 58, 380–387 [DOI] [PubMed] [Google Scholar]
- KOEBIS M., KIYATAKE T., YAMAURA H., NAGANO K., HIGASHIHARA M., SONOO M., HAYASHI Y., NEGISHI Y., ENDO-TAKAHASHI Y., YANAGIHARA D., et al. (2013). Ultrasound-enhanced delivery of morpholino with bubble liposomes ameliorates the myotonia of myotonic dystrophy model mice. Sci. Rep. 3, 10.1038/srep02242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLE R., KRAINER A.R., and ALTMAN S. (2012). RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nature Rev. 11, 125–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSHKIN A.A., SINGH S.K., NIELSEN P., RAJWANSHI V.K., KUMAR R., MELDGAARD M., OLSEN C.E., and WENGEL J. (1998). LNA (locked nucleic acids): synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation, and unprecedented nucleic acid recognition. Tetrahedron 54, 3607–3630 [Google Scholar]
- KURRECK J. (2008). Therapeutic oligonucleotides. (Royal Society of Chemistry, Cambridge: ), pp. 1–343 [Google Scholar]
- LANFORD R.E., HILDEBRANDT-ERIKSEN E.S., PETRI A., PERSSON R., LINDOW M., MUNK M.E., KAUPPINEN S., and ØRUM H. (2010). Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327, 198–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBLEU B., MOULTON H.M., ABES R., IVANOVA G.D., ABES S., STEIN D.A., IVERSEN P.L., ARZUMANOV A., and GAIT M.J. (2008). Cell penetrating peptide conjugates of steric block oligonucleotides. Adv. Drug Deliv. Rev. 60, 517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGER A.J., MOSQUEA L.M., CLAYTON N.P., WU I.-H., WEEDEN T., NELSON C.A., PHILLIPS L., ROBERTS E., PIEPENHAGEN P.A., CHENG S.H., et al. (2013). Systemic delivery of a peptide-linked morpholino oligonucleotide neutraiizes mutant RNA toxicity in a mouse model of myotonic dystrophy. Nucl. Acid Ther. 23, 109–117 [DOI] [PubMed] [Google Scholar]
- LENNOX K.A., OWCZARZY R., THOMAS D.M., WALDER J.A., and BEHLKE M.A. (2013). Improved performance of anti-miRNA oligonucleotides using a novel non-nucleotide modifier. Mol. Ther. Nucl. Acids 2, e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIGHTFOOT H.L., and HALL J. (2012). Target mRNA inhibition by oligonucleotide drugs in man. Nucl. Acids Res. 40, 10585–10595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU Q.L., RABINOWITZ A., CHEN Y.C., YOKOTA T., YIN H.F., ALTER J., JADOON A., BOU-GHARIOS G., and PARTRIDGE T. (2005). Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc. Natl. Acad. Sci. U. S. A. 102, 198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUKURA M., SHINOZUKA K., ZON G., MITSUYA H., REITZ M., COHEN J., and BRODER S. (1987). Phosphorothioate analogs of oligodeoxyribonucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc. Natl. Acad. Sci. U. S. A. 84, 7706–7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDELL J., RODINO-KLAPAC L.R., SAHENK Z., ROUSH K., BIRD L., LOWES L.P., ALFANO L., GOMEZ A.M., LEWIS S., KOTA J., et al. (2013). Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol. 66, 10.1002/ana.23982 [DOI] [PubMed] [Google Scholar]
- MING X., ALAM M.R., FISHER M., YAN Y., CHEN X., and JULIANO R.L. (2010). Intracellular delivery of an antisense oligonucleotide via endocytosis of a G-protein coupled receptor. Nucl. Acids Res. 38, 10.1093/nar/gkq534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOULTON H.M., and MOULTON J.D. (2008). Antisense morpholino oligomers and their peptide conjugates. In: Therapeutic Oligonucleotides. Kurreck J., ed. (Royal Society of Chemistry, Cambridge: ). pp 43–79 [Google Scholar]
- MOULTON H.M., and MOULTON J.D. (2010). Morpholinos and their peptide conjugates: therapeutic promise and challenge for Duchenne muscular dystrophy. Biochim. Biophys. Acta 12, 2296–2303 [DOI] [PubMed] [Google Scholar]
- MULDERS S.A.M., VAN DEN BROECK W.J.A.A., WHEELER T., CROES H.J.E., VAN KUIK-ROMEIJN P., DE KIMPE S.J., FURLING D., PLATENBURG G.J., GOURDON G., THORNTON C.A., et al. (2009). Triplet-repeat oligonucleotide-mediated reversal of RNA toxicity in myotonic dystrophy. Proc. Nat. Acad. Sci. U. S. A. 106, 13915–13920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNTONI F., WOOD M.J.A. (2011). Targeting RNA to treat neuromuscular disease. Nat. Rev. Drug Discov. 10, 621–637 [DOI] [PubMed] [Google Scholar]
- MURRAY S., ITTIG D., KOLLER E., BERDEJA A., CHAPPELL A., PRAKASH T.P., NORRBOM M., SWAYZE E.E., LEUMANN C., and SETH P.P. (2012). TricycloDNA-modified oligo-2'-deoxyribonucleotides reduce scavenger receptor B1 mRNA in hepatic and extra-hepatic tissues: a comparative study of oligonucleotide length, design and chemistry. Nucl. Acids Res. 40, 6135–6143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OBAD S., DOS SANTOS C., PETRI A., HEIDENBLAD M., BROOM O., RUSE C., FU C., LINDOW M., STENVANG J., STRAARUP E., et al. (2011). Silencing of microRNA families by seed targeting tiny LNAs. Nat. Genet. 43, 371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OBIKA S., MORIO K.-I., HARI Y., and IMANISHI T. (1999). Preparation and properties of 2′,5′-linked oligonucleotide analogues containing 3′-O,4′-C-methyleneribonucleosides. Biorg. Med. Chem. Lett. 9, 515–518 [DOI] [PubMed] [Google Scholar]
- OSMAN E.Y., YEN P.-F., and LORSON C.L. (2012). Bifunctional RNAs targeting the intronic splicing silencer N1 increase SMN levels and reduce disease severity in an animal model of spinal muscular atrophy. Mol. Ther. 20, 119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASSINI M.A., BU J., RICHARDS A.M., KINNECOM C., SARDI S.P., STANEK L.M., HUA Y., RIGO F., MATSON J., HUNG G., et al. (2011). Antisense oligonucleotide delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl. Med. 3, 72ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORENSKY P.N., MITRPANT C., MCGOVERN V.L., BEVAN A.K., FOUST K.D., KASPAR B.K., WILTON S.D., and BURGHES A.H.M. (2012). A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mice. Hum. Mol. Genet. 21, 1625–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIGO F., HUA Y., CHUN S.J., PRAKASH T.P., KRAINER A.R., and BENNETT C.F. (2012). Synthetic oligonucleotides recruit ILF2/3 to RNA transcripts to modulate splicing. Nat. Chem. Biol. 8, 555–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS J., PALMA E., SAZANI P., ØRUM H., CHO M., and KOLE R. (2006). Efficient and persistent splice switching by systemically delivered LNA oligonucleotides in mice. Mol. Ther. 14, 471–475 [DOI] [PubMed] [Google Scholar]
- SAID HASSANE F., SALEH A.F., ABES R., GAIT M.J., and LEBLEU B. (2010). Cell penetrating peptides: overview and applications to the delivery of oligonucleotides. Cell. Mol. Life Sci. 67, 715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAZANI P., GEMIGNANI F., KANG S.-H., MAIER M.A., MANOHARAN M., PERSMARK M., BORTNER D., and KOLE R. (2002). Systemically delivered antisense oligomers upregulate gene expression in mouse tissues. Nat. Biotech. 20, 1228–1233 [DOI] [PubMed] [Google Scholar]
- SAZANI P., KANG S.-H., MAIER M.A., WEI C., DILLMAN J., SUMMERTON J., MANOHARAN M., and KOLE R. (2001). Nuclear antisense effects of neutral, anionic and cationic analogs. Nucleic Acids Res. 29, 3965–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAZANI P., VAN NESS K.P., WELLER D.L., POAGE D.W., PALYADA K., and SHREWSBURY S.B. (2011). Repeat-dose toxicology evaluation in cynomolgus monkeys of AVI-4658, a phosphorodiamidate morpholino oligomer (PMO) drug for the treatment of Duchenne muscular dystrophy. Int. J. Toxicol. 30, 313–321 [DOI] [PubMed] [Google Scholar]
- SCHNELL F.J., CRUMLEY S., MOURICH D.V., and IVERSEN P.L. (2013). Development of novel bioanalytical methods to determine the effective concentrations of phosphorodiamidate morpholino oligomers in tissued and cells. Biores. Open Access 2, 61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETH P.P., SIWKOWSKI A.M., ALLERSON C.R., VASQUEZ G., LEE S., PRAKASH T.P., WANCEWICZ E.V., WITCHELL D., and SWAYZE E.E. (2009). Short antisense oligonucleotides with novel 2'-4' conformationally restricted nucleoside analogues show improved potency without increased toxicity in animals. J. Med. Chem. 52, 10–13 [DOI] [PubMed] [Google Scholar]
- SHABANPOOR F., and GAIT M.J. (2013). Development of a general method for labelling peptide-morpholino oligonucleotide conjugates using alkyne-azide click chemistry. Chem. Commun. 49, 10260–10262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRSI S., SCHRAY R.C., GUAN X., LYKENS N.M., WILLIAMS J.H., ERNEY M.L., and LUTZ G.J. (2008). Funtionalized PEG-PEI copolymers complexed to exon-skipping oligonucleotides improve dystrophin expression in mdx mice. Hum. Gene Ther. 19, 795–806 [DOI] [PubMed] [Google Scholar]
- SIWKOWSKI A.M., MALIK L., ESAU C.C., MAIER M.A., WANCEWICZ E.V., ALBERTSHOFER K., MONIA B.P., BENNETT C.F., and ELDRUP A.B. (2004). Identification and functional validation of PNAs that inhibit murine CD40 expression by redirection of splicing. Nucleic Acids Res. 32, 2695–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKORDIS L.A., DUNCKLEY M.G., YUE B., EPERON I.C., and MUNTONI F. (2003). Bifunctional antisense oligonucleotides provide a trans-acting splicing enhancer that stimulates SMN2 gene expression in patient fibroblasts. Proc. Nat. Acad. Sci. U. S. A. 100, 4114–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAARUP E.M., FISKER N., HEDTJÄRN M., LINDHOLM M.W., ROSENBOHM C., AARUP V., HANSEN H.F., ØRUM H., HANSEN J.B.R., and KOCH T. (2010). Short locked nucleic acid antisense oligonucleotides potently reduce apoliprotein B mRNA and serum cholesterol in mice and non-human primates. Nucleic Acids Res., 38, 7100–7111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMMERTON J., and WELLER D. (1997). Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 7, 187–195 [DOI] [PubMed] [Google Scholar]
- TAKAGI M., YAGI M., ISHIBASHI K., TEAKESHIMA Y., SURONO A., MATSUO M., and KOIZUMI M. (2004). Design of 2'-O-Me RNA/ENA chimera oligonucleotides to induce exon skipping in dystrophin pre-mRNA. Nucleic Acids Symp. Series 48, 297–298 [DOI] [PubMed] [Google Scholar]
- TAKESHIMA Y., YAGI M., WADA H., ISHIBASHI K., NISHIYAMA A., KAKUMOTO M., SAKAEDA T., SAURA R., OKUMURA K., and MATSUO M. (2006). Intravenous infusion of an antisense oligonucleotide results in exon skipping in muscle dystrophin mRNA of Duchenne muscular dystrophy. Pediatr. Res. 59, 690–694 [DOI] [PubMed] [Google Scholar]
- TANGANYIKA-DE-WINTER C.L., HEEMSKERK H., KARNAOUKH T.G., VAN PUTTEN M., DE KIMPE S.J., VAN DEUTEKOM J.C., and AARTSMA-RUS A. (2012). Long-term exon skipping studies with 2'-O-methyl phosphorothioate antisense oligonucleotides in dystrophic mouse models. Mol. Ther. Nucl. Acids 1, e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DEUTEKOM J.C., DE KIMPE S.J., and CAMPION G.V. (2013). Antisense oligonucleotides as personalized medicine for Duchenne muscular dystrophy. Drug Discov. Today Ther Strateg. 10.1016/j.ddstr.2013.04.001 [DOI] [Google Scholar]
- VICKERS T.A., ZHANG H., GRAHAM M.J., LEMONIDIS K.M., ZHAO C., and DEAN N.M. (2006). Modification of MyD88 mRNA splicing and inhibition of IL-1 signaling in cell culture and in mice with a 2'-O-methoxyethyl-modified oligonucleotide. J. Immunol. 176, 3652–3661 [DOI] [PubMed] [Google Scholar]
- WANCEWICZ E.V., MAIER M.A., SIWKOWSKI A.M., ALBERTSHOFER K., WINGER T.M., BERDEJA A., GAUS H., VICKERS T.A., BENNETT C.F., MONIA B.P., et al. (2010). Peptide nucleic acids conjugated to short basic peptide show improved pharmacokinetics and antisense activity in adipose tissue. J. Med. Chem. 53, 3919–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG M., WU B., LU P., CLOER C., TUCKER J.D., and LU Q. (2013). Polyethylenimine-modified pluronics (PCMs) improve morpholino oligomer delivery in cell culture and dystrophic mdx mice. Mol. Ther. 21, 210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHEELER T.M., LEGER A.J., PANDEY S.K., MACLEOD A.R., NAKAMORI M., CHENG S.H., WENTWORTH B.M., BENNETT C.F., and THORNTON C.A. (2012). Targeting nuclear RNA for in vivo correction of myotonic dystrophy. Nature 488, 111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHEELER T.M., SOBCZAK K., LUECK J.D., OSBORNE R.J., LIN X., DIRKSEN R.T., and THORNTON C.A. (2009). Reversal of RNA dominance by displacement of protein sequestered on triplet repeat RNA. Science 325, 336–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDRICK J.J., JIANG S., CHOI S.J., KNUTH S.T., and MORCOS P.A. (2011). An octaguanidine-morpholino oligoconjugate improves muscle function of mdx mice. Muscle Nerve 44, 563–570 [DOI] [PubMed] [Google Scholar]
- WILLIAMS J.H., SCHRAY R.C., PATTERSON C.A., AYITEY S.O., TALLENT M.K., and LUTZ G.J. (2009). Oligonucleotide-mediated survival of motor neuron protein expression in CNS improves phenotype in a mouse model of spinal muscular atrophy. J. Neurosci. 29, 7633–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU B., BENRASHID E., MALIK S., ASHAR J., DORAN T.J., and LU Q.L. (2010). Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino. Gene Ther. 17, 132–140 [DOI] [PubMed] [Google Scholar]
- WU B., LI Y.-F., MORCOS P.A., DORAN T.J., LU P., and LU Q.L. (2009). Octa-guanidine morpholino restores dystrophin expression in cardiac and skeletal muscles and ameliorates pathology in dystrophic mdx mice. Mol. Ther. 17, 864–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU B., LU P., CLOER C., SHABAN M., GREWAL S., MILAZI S., SHAH S.N., MOULTON H.M., and LU Q.L. (2012). Long-term rescue of dystrophin expression and improvement in muscle pathology and function in dystrophic mdx mice by peptide-conjugated morpholino. Am. J. Pathol. 181, 392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU B., MOULTON H.M., IVERSEN P.L., JUANG J., LI J., SPURNEY C.F., SALI A., GUERRON A.D., NAGARAJU K., DORAN T., et al. (2008). Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modifies morpholino oligomer. Proc. Natl. Acad. Sci. U. S. A. 105, 14814–14819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAGI M., TAKESHIMA Y., SURONO A., TAKAGI M., KOIZUMI M., and MATSUO M. (2004). Chimeric RNA and 2'-O, 4'-C-ethylene-bridged nucleic acids have stronger activity than phosphorothioate oligodeoxynucleotyides in induction of exon 19 skipping in dystrophin mRNA. Oligonucleotides 14, 33–40 [DOI] [PubMed] [Google Scholar]
- YAMADA T., OKANIWA N., SANEYOSHI H., OHKUBO A., SEIO K., NAGATA T., AOKI Y., TAKEDA S., and SEKINE M. (2011). Synthesis of 2'-O-[2-(N-methylcarbamoyl)ethyl]ribonucleosides using oxa-Michael reaction and chemical and biological properties of oligonucleotide derivatives incoprorating these modified ribonucleosides. J. Org. Chem. 76, 3042–3053 [DOI] [PubMed] [Google Scholar]
- YANG L., NIU H., GAO X., WANG Q., HAN G., CAO L., CAI C., WELLER J., and YIN H. (2013). Effective exon skipping and dystrophin restoration by 2'-O-methoxyethyl antisense oligonucleotide in dystrophin-deficient mice. PloS One 8, e61584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YILMAZ-ELIS A.S., AARTSMA-RUS A., T'HOEN P.A.C., SAFDAR H., BREUKEL C., VAN VLIJMEN B.J.M., VAN DEUTEKOM J.C., DE KIMPE S.J., VAN OMMEN G.B., and VERBEEK J.S. (2013). Inhibition of IL-1 signalling by antisense oligonucleotide-mediated exon skipping of IL-1 receptor accessory protein (IL-1 RAcP). Mol. Ther. Nucleic Acids 2, e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIN H., BETTS C., SALEH A.F., IVANOVA G.D., LEE H., SEOW Y., KIM D., GAIT M.J., and WOOD M.J.A. (2010). Opimization of peptide nucleic acids antisense olignucleotides for local and systemic dystrophin splice correction in the mdx mouse. Mol. Ther. 18, 819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIN H., LU Q., and WOOD M. (2008a). Effective exon skipping and restoration of dystrophin expression by peptide nucleic acid antisense oligonucleotides in mdx mice. Mol. Ther. 16, 38–45 [DOI] [PubMed] [Google Scholar]
- YIN H., MOULTON H.M., SEOW Y., BOYD C., BOUTILIER J., IVERSEN P., and WOOD M.J.A. (2008b). Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum. Mol. Gen. 17, 3909–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIN H., SALEH A.F., BETTS C., CAMELLITI P., SEOW Y., ASHRAF S., ARZUMANOV A., HAMMOND S., MERRITT T., GAIT M.J., et al. (2011). Pip5 transduction peptides direct high efficiency oligonucleotide-mediated dystrophin exon skipping in heart and phenotypic correction in mdx mice. Mol. Ther. 19, 1295–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKOTA T., NAKAMURA A., NAGATA T., SAITO T., KOBAYASHI M., AOKI Y., ECHIGOYA Y., PARTRIDGE T., HOFFMAN E.P., and TAKEDA S. (2012). Extensive and prolonged restoration of dystrophin expression with Vivo-morpholino-mediated multiple exon skipping in dystrophic dogs. Nucl. Acid Ther. 22, 306–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU H., JANGHRA N., MITRPANT C., DICKINSON R.L., ANTHONY K., PRICE L., EPERON I.C., WILTON S.D., MORGAN J., and MUNTONI F. (2013). A novel morpholino oligomer targeting ISSN-1 improves rescue of severe spinal muscular atrophy transgenic mice. Hum. Gene Ther. 24, 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]