Abstract
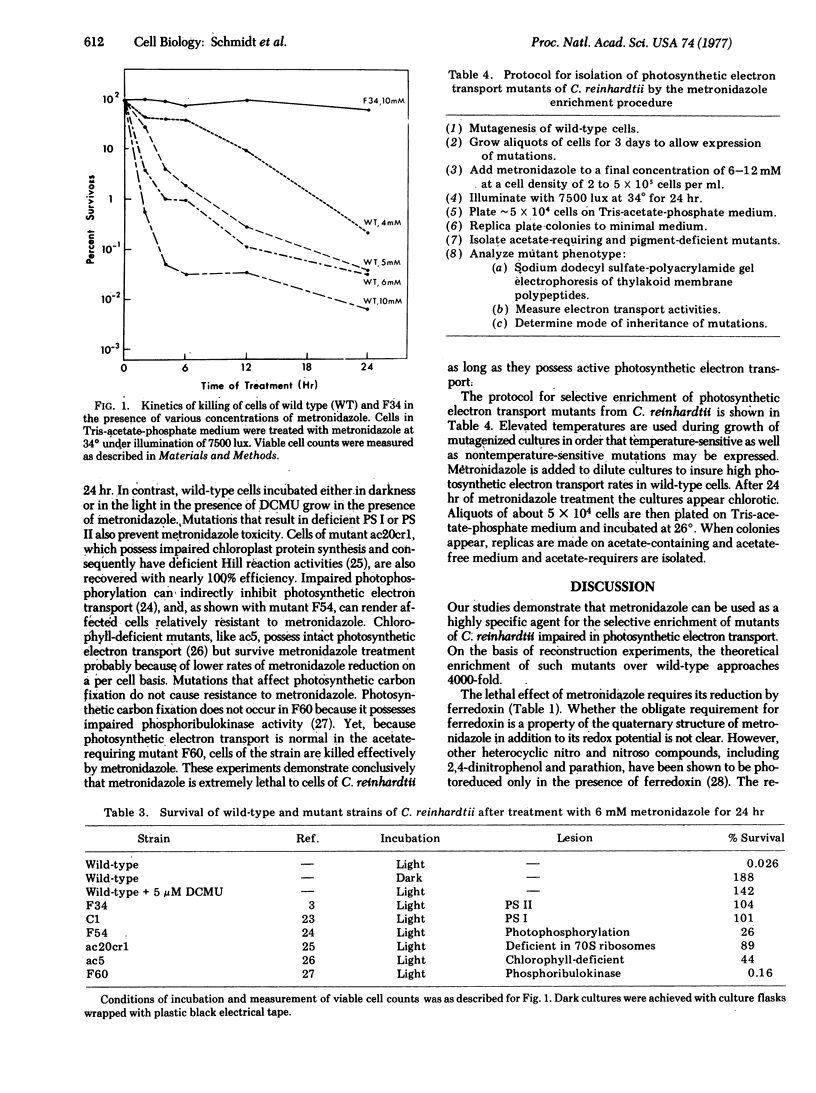
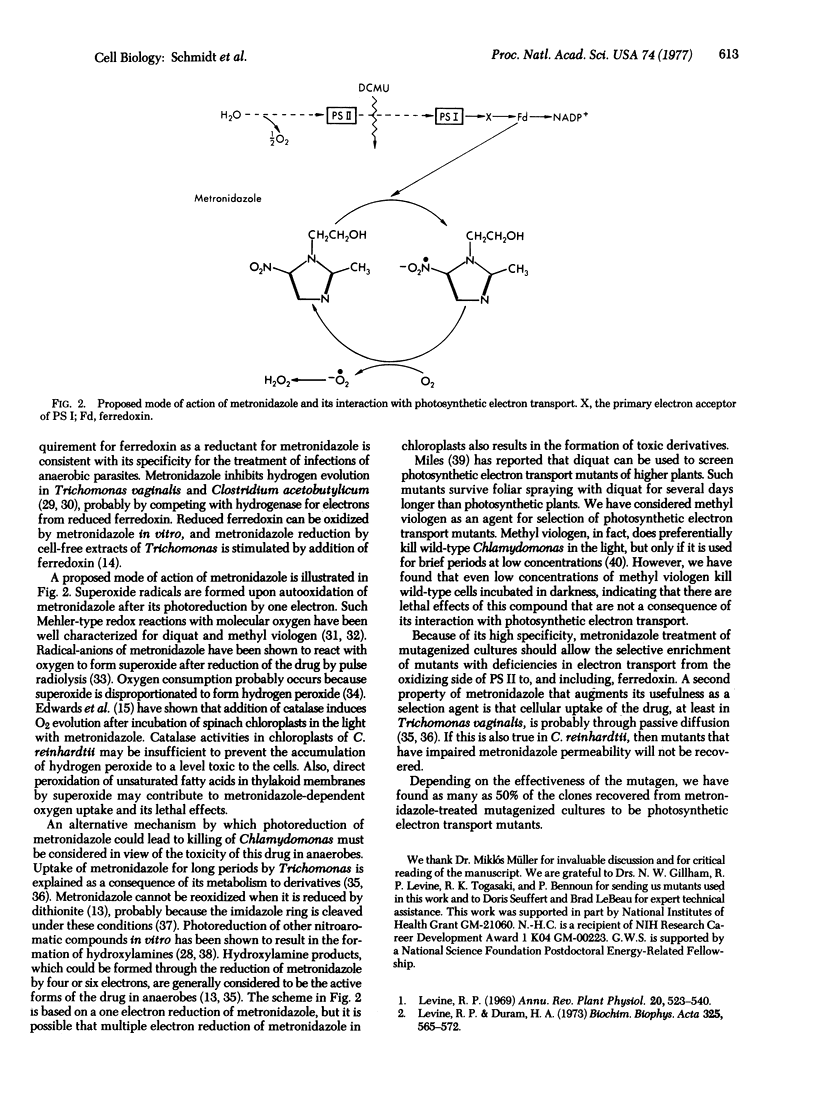
Metronidazole (2-methyl-5-nitroimidazole-l-ethanol) is shown to be effective for the selective enrichment of mutants of Chlamydomonas reinhardtii that posses impaired photosynthetic electron transport. More than 99.9% of wild-type cells are killed when incubated in the presence of 6-10 mM metronidazole for 24 hr under illumination of 7500 lux. Survival of wild-type cells in darkness and of mutants that are blocked at different steps in photosynthetic electron transport is nearly 100% when incubated in the presence of the drug under identical conditions. The toxicity of metronidazole is demonstrated to depend upon its reduction by photosynthetic electron transport. Light-dependent oxygen uptake mediated by metronidazole is shown to require active photosystem l in vitro and in vivo. Ferredoxin is necessary for metronidazole reduction by thylakoid membrane fractions enriched in photosystem l activity. We propose that the toxicity of metronidazole is due to the formation of lethal derivatives of the drug or to the accumulation of hydrogen peroxide, which could occur upon autooxidation of metronidazole reduced by one electron. The results indicate that mutants of C. reinhardtii, and probably other photosynthetic organisms, with any lesion in photosynthetic electron transport from the oxidizing side of photosystem ll to ferredoxin can be isolated by metronidazole treatment of mutagenized cultures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNON D. I., TSUJIMOTO H. Y., MCSWAIN B. D. ROLE OF FERREDOXIN IN PHOTOSYNTHETIC PRODUCTION OF OXYGEN AND PHOSPHORYLATION BY CHLOROPLASTS. Proc Natl Acad Sci U S A. 1964 Jun;51:1274–1282. doi: 10.1073/pnas.51.6.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennoun P., Levine R. P. Detecting mutants that have impaired photosynthesis by their increased level of fluorescence. Plant Physiol. 1967 Sep;42(9):1284–1287. doi: 10.1104/pp.42.9.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Chabot J. F. Chloroplast ribosome deficient mutants in the green alga Chlamydomonas reinhardi and the question of chloroplast ribosome function. J Cell Sci. 1972 Mar;10(2):267–305. doi: 10.1242/jcs.10.2.267. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Matlin K., Bennoun P. A chlorophyll-protein complex lacking in photosystem I mutants of Chlamydomonas reinhardtii. J Cell Biol. 1975 Nov;67(2PT1):361–377. doi: 10.1083/jcb.67.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H. The methyl viologen-catalyzed Mehler reaction and catalase activity in blue-green algae and Chlamydomonas reinhardi. Biochim Biophys Acta. 1971 Sep 7;245(2):277–287. doi: 10.1016/0005-2728(71)90146-0. [DOI] [PubMed] [Google Scholar]
- Edwards D. I., Dye M., Carne H. The selective toxicity of antimicrobial nitroheterocyclic drugs. J Gen Microbiol. 1973 May;76(1):135–145. doi: 10.1099/00221287-76-1-135. [DOI] [PubMed] [Google Scholar]
- Edwards D. I., Mathison G. E. The mode of action of metronidazole against Trichomonas vaginalis. J Gen Microbiol. 1970 Nov;63(3):297–302. doi: 10.1099/00221287-63-3-297. [DOI] [PubMed] [Google Scholar]
- Epel B. L., Neumann J. The mechanism of the oxidation of ascorbate and MN2+ by chloroplasts. The role of the radical superoxide. Biochim Biophys Acta. 1973 Dec 14;325(3):520–529. doi: 10.1016/0005-2728(73)90211-9. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Photosynthetic Electron Transport Chain of Chlamydomonas reinhardi. IV. Purification and Properties of Plastocyanin. Plant Physiol. 1966 Dec;41(10):1637–1642. doi: 10.1104/pp.41.10.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Photosynthetic Electron Transport Chain of Chlamydomonas reinhardi. V. Purification and Properties of Cytochrome 553 and Ferredoxin. Plant Physiol. 1966 Dec;41(10):1643–1647. doi: 10.1104/pp.41.10.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. H., Boynton J. E., Gillham N. W. Chloroplast ribosome biogenesis in Chlamydomonas. Selection and characterization of mutants blocked in ribosome formation. J Cell Biol. 1974 Oct;63(1):160–179. doi: 10.1083/jcb.63.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ings R. M., McFadzean J. A., Ormerod W. E. The mode of action of metronidazole in Trichomonas vaginalis and other micro-organisms. Biochem Pharmacol. 1974 May 1;23(9):1421–1429. doi: 10.1016/0006-2952(74)90362-1. [DOI] [PubMed] [Google Scholar]
- Izawa S., Connolly T. N., Winget G. D., Good N. E. Inhibition and uncoupling of photophosphorylation in chloroplasts. Brookhaven Symp Biol. 1966;19:169–187. [PubMed] [Google Scholar]
- Levine R. P., Duram H. A. The polypeptides of stacked and unstacked Chlamydomonas reinhardi chloroplast membranes and their relation to photsystem II activity. Biochim Biophys Acta. 1973 Dec 14;325(3):565–572. doi: 10.1016/0005-2728(73)90216-8. [DOI] [PubMed] [Google Scholar]
- Lindmark D. G., Müller M. Antitrichomonad action, mutagenicity, and reduction of metronidazole and other nitroimidazoles. Antimicrob Agents Chemother. 1976 Sep;10(3):476–482. doi: 10.1128/aac.10.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles C. D. Selection of diquat resistance photosynthesis mutants from maize. Plant Physiol. 1976 Feb;57(2):284–285. doi: 10.1104/pp.57.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll B., Levine R. P. Characterization of a Photosynthetic Mutant Strain of Chlamydomonas reinhardi Deficient in Phosphoribulokinase Activity. Plant Physiol. 1970 Oct;46(4):576–580. doi: 10.1104/pp.46.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Lindmark D. G. Uptake of metronidazole and its effect on viability in trichomonads and Entamoeba invadens under anaerobic and aerobic conditions. Antimicrob Agents Chemother. 1976 Apr;9(4):696–700. doi: 10.1128/aac.9.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Effect of metronidazole on hydrogen production by Clostridium acetobutylicum. Arch Mikrobiol. 1972;84(3):225–233. doi: 10.1007/BF00425200. [DOI] [PubMed] [Google Scholar]
- Sato V. L., Levine R. P., Neumann J. Photosynthetic phosphorylation in Chlamydomonas reinhardi. Effects of a mutation altering and ATP-synthesizing enzyme. Biochim Biophys Acta. 1971 Dec 7;253(2):437–448. doi: 10.1016/0005-2728(71)90047-8. [DOI] [PubMed] [Google Scholar]
- Shneyour A., Avron M. A Method for Producing, Selecting, and Isolating Photosynthetic Mutants of Euglena gracilis. Plant Physiol. 1975 Jan;55(1):142–144. doi: 10.1104/pp.55.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancliffe T. C., Pirie A. The production of superoxide radicals in reactions of the herbicide diquat. FEBS Lett. 1971 Oct 1;17(2):297–299. doi: 10.1016/0014-5793(71)80168-0. [DOI] [PubMed] [Google Scholar]
- Wardman P., Clarke E. D. Oxygen inhibition of nitroreductase: electron transfer from nitro radical-anions to oxygen. Biochem Biophys Res Commun. 1976 Apr 19;69(4):942–949. doi: 10.1016/0006-291x(76)90464-2. [DOI] [PubMed] [Google Scholar]
- Wessels J. S. Mechanism of the reduction of organic nitro compounds by chloroplasts. Biochim Biophys Acta. 1965 Nov 29;109(2):357–371. doi: 10.1016/0926-6585(65)90163-9. [DOI] [PubMed] [Google Scholar]
- Willson R. L., Cramp W. A., Ings R. M. Metronidazole ('Flagyl'): mechanisms of radiosensitization. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Dec;26(6):557–569. doi: 10.1080/09553007414551591. [DOI] [PubMed] [Google Scholar]



