Abstract
Sulfhydryl groups on protein Cys residues undergo an array of oxidative reactions and modifications, giving rise to a virtual redox zip code with physiological and pathophysiological relevance for modulation of protein structure and functions. While over two decades of studies have established NO-dependent S-nitrosylation as ubiquitous and fundamental for the regulation of diverse protein activities, proteomic methods for studying H2S-dependent S-sulfhydration have only recently been described and now suggest that this is also an abundant modification with potential for global physiological importance. Notably, protein S-sulfhydration and S-nitrosylation bear striking similarities in terms of their chemical and biological determinants, as well as reversal of these modifications via group-transfer to glutathione, followed by the removal from glutathione by enzymes that have apparently evolved to selectively catalyze denitrosylation and desulfhydration. Here we review determinants of protein and low-molecular-weight thiol S-sulfhydration/desulfhydration, similarities with S-nitrosylation/denitrosylation, and methods that are being employed to investigate and quantify these gasotransmitter-mediated cell signaling systems.
Keywords: Nitric oxide, hydrogen sulfide, persulfide, cysteine, glutathione, sulfhydryl, sulfhydration, Biotin Switch, SNOSID
Like NO, H2S is an endogenous cell signaling molecule which, with CO, contributes to a family of diffusible gasotransmitters that can engage in short-lived covalent reactions with proteins and thereby modulate protein structure and function. While decades of research have established the generality and broad physiological importance of protein S-nitrosylation as a cell-regulatory system, the potential ubiquity of H2S addition to Cys thiol in proteins (aka S-sulfhydration, or persulfide (-SSH) formation) has only recently been appreciated – this was accomplished using an adaptation of the biotin-switch method [1], relative to the method previously established for proteome-wide SNO-site detection – considered below. Protein-SSH formation has now been implicated in serving a fundamental role in cell signaling by H2S, based on the observed diversity of proteins that apparently undergo endogenous S-sulfhydration, abundance of endogenous protein persulfides and demonstrations of perturbed functions for some detected S-sulfhydrated proteins (for review, see [2]).
Protein S-nitrosylation is a major effector of NO bioactivities and has been likened to phosphorylation in terms of both its ubiquity and pervasive role in mammalian cell biology [3–5]. However, since protein S-nitrosylation is chemically labile and labeling of the NO group is much less tractable than labeling of phosphate, identification of endogenous nitrosylation sites has been a significant analytical challenge. A breakthrough advance in addressing this challenge was the Biotin Switch method, described in 2001 by Jaffrey et al. – this method provided for the replacement of labile NO on protein Cys residues with a stable biotin moiety, marking the site and also allowing for subsequent streptavidin affinity capture of what were previously SNO-proteins for identification by either western blotting or MS/MS [6, 7]. The SNOSID (SNO Site IDentification) method [8, 9] and a related adaptation by Ischiripoulos and colleagues [10] allowed for extension of the Biotin Switch method to the analysis of peptides, in lieu of intact proteins, thereby enabling proteome-wide recognition of the specific Cys residues that undergo reversible S-nitrosylation. In a more recent adaptation, solid-phase organomercury was used to replace solution-phase biotinylation for the tagging of SNO-proteins and peptides, providing a methodological simplification that affords increased sensitivity and expanded discovery of endogenous SNO-proteins and modification sites [11–13].
A modified Biotin Switch method for proteomic detection of protein sulfhydration
Prompted by their unexpected finding that the control arm in the Biotin Switch assay for protein S-nitrosylation identified numerous mouse brain proteins, irrespective of whether neuronal NOS was expressed or not, Snyder and colleagues raised the possibility that these were, in fact, protein persulfides and that a modified Biotin Switch method can be adapted for selective detection of protein S-sulfhydration. Accordingly, a modification of the original Biotin-Switch method was established and shown to detect and identify endogenous protein S-sulfydration sites in cells and tissues [1]. In the first step of this modified procedure, methyl methane sulfonate (MMTS) was used to block free protein thiols, with the intent of sparing protein persulfides, i.e., sites of H2S-mediated sulfhydration. In the second step, reaction with a thiol-specific biotinylating agent, biotin-HPDP (N-[(6-biotinamido) hexyl]-3′-(2′-pyridyldithio) propionamide) was used to selectively tag (MMTS-unreactive) persulfide sites with biotin, allowing for subsequent capture and identification of these sites using a streptividin-based affinity technology. Biotin-conjugated proteins were detected after resolution on SDS/PAGE and visualized by blotting with a streptavidin-coupled reagent as well as after capture on streptavidin beads for subsequent site specification using LC-MS/MS. Applying this approach to studies of murine liver and cells in culture, Snyder and colleagues revealed that endogenous protein sulfhydration was completely eliminated by CSE gene deletion in mouse liver and following knockdown of CSE in HEK293 cells – these findings confirmed that protein S-sulfhydration (or biotin dependent labeling of thiol) in these tissues has an absolute dependence on Cys-derived H2S production [1]. In further accord with the view that the observed protein persulfides derive from the reaction of H2S (or a derived species) with protein thiols, signal intensities were shown to increase following exposure of cells/tissues to NaHS, a chemical donor of H2S. This investigation identified a total of 39 sulfhydrated proteins using MS [1] and it is notable that most of these proteins had been previously identified as S-nitrosoproteins in studies employing the conventional Biotin Switch method. Importantly, protein S-sulfhydration has been implicated to function as an endogenous modulator of cell functions, including ion channel flux [14, 15], suppression of apoptosis [16] and cellular senescence [17], modulation of the endoplasmic reticulum stress response [18] and enhancing various enzyme activities, including the E3-ubiquitin ligase activity of parkin [19] and glycolytic activity of GAPDH [1]
Protein S-Sulfhydration in mammalian systems and its relation to S-Nitrosylation
For a posttranslational modification to function in a cell signaling capacity, tight regulation of the addition and removal of the modification is essential. Of course, protein phosphorylation is known to be precisely controlled by the reciprocal activities of selective kinase and phosphatase families that serve to add and remove phosphate groups, respectively, from protein Ser, Thr and Tyr residues. A variation on this theme is appreciated for protein S-nitrosylation, which is thought to predominantly arise from the selective solution-phase addition of NOS-derived species to protein thiols and removal (denitrosylation) as a consequence of oxidative reactions and via transnitrosylation to GSH, yielding GSNO and protein Cys-SH as co-products. The accumulated GSNO is then susceptible to enzymatic denitrosylation, catalyzed by a GSNO reductase (GSNOR) that was discovered by Stamler and colleagues [20] and indirectly functions to control SNO-protein levels due to an equilibrium existing between NO on Cys-sulfur in proteins and GSH. The importance of GSNOR for control of SNO-protein levels was established by gene knockout studies in mice, demonstrating markedly increased tissue levels of both SNO-proteins and GSNO [21, 22]. Furthermore, GSNOR was shown to have profound biological consequences that have been attributed to excessive SNO-protein accumulation, including enhanced susceptibility to septic shock lethality [22], hepatocellular carcinoma [23] and impaired capacity for DNA repair [24].
Current evidence suggests that protein S-sulfhydration adheres closely to the generally acknowledged paradigm for S-nitrosylation. Indeed, analogous to the three mammalian NOS enzyme isoforms, three distinct enzymes have been recognized with the capability to reduce Cys-sulfur to yield H2S. These H2S-producing enzymes - cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE) and mercaptopyruvate sufurtransferase (MST) - all display differential and distinct tissue distributions and contribute to abundant cellular accumulation of sulfhydration on both proteins and low-molecular-weight thiols, yielding GSSH and protein persulfides as products. Again similar to denitrosylation of GSNO, desulfydration of GSSH is mediated by a distinct enzyme (a mitochondrial persulfide dioxygenase enzyme, termed ETHE1) that has likely evolved for indirect control of protein S-sulfhydration levels, owing to a presumably rapid equilibration between levels of GSSH and protein persulfides. Figure 1 provides a schematic representation of the enzymes and mechanisms considered to be involved in H2S biosynthesis and S-sulfhydration/desulfhydration of proteins and low-molecular-weight thiols. Notably, we suggest that cellular levels of protein S-sulfhydration are maintained in tight equilibrium with levels of GSSH, which are in turn dependent on the balance of solution-phase synthesis and degradation, where degradation (i.e., desulfhydration) is at least partially enzymatic, occurring in the mitochondrial inner matrix via a three-enzyme pathway that ultimately yields GSH, sulfite and thiosulfate as reaction products. While pure recombinant human ETHE1 was recently characterized and found to use GSSH as a sole substrate for S-desulhydration [25], we have synthesized an array of small molecule persulfides with potential biological relevance (including cysteine persulfide, homocystein persulfide and γ-glutamyl-cysteine persulfide) and found that GSSH is the only significant substrate of ETHE1 (unpublished).
Figure 1.
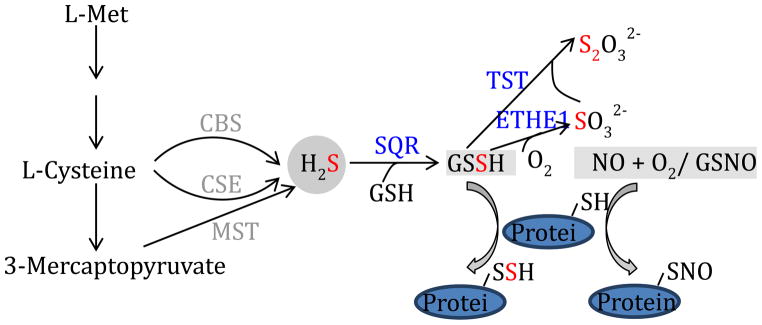
H2S biosynthesis and degradation, along with a comparison of pathways that contribute to protein S-sulfhydration and S-nitrosylation. The schematic depicts enzymatic pathways that are responsible for endogenous H2S production, formation of low-molecular-weight and proteinaceous persulfides and the mitochondrial pathway for persulfide degradation. Notably, in addition to endogenous mammalian enzymes, sulfate-reducing bacteria (SRB) of the colon are major contributors to H2S generation in vivo. Abbreviations: CSE, cystathionine γ-lyase; CBS, cystathionine β-synthase ; MST, mercaptopyruvate sulfurtransferase; SQR, sulfide quinone reductase; ETHE1, persulfide dioxygenase; TST, thiosulfate sulfur transferase (a.k.a. rhodanese).
Cysteine is the biochemical precursor to H2S and low-molecular-weight persulfides
Methionine is the sole essential source of sulfur in mammalian systems and can serve as a precursor for the synthesis of cysteine and all other sulfur-containing molecules. Notably, cysteine provide sulfur for the synthesis of key sulfur-containing molecules that function as important enzyme cofactors, including coenzyme A, biotin, lipoic acid, thiamine, S-adenosylmethionine, molybdopterin and iron-sulfur clusters in enzyme active-sites [26]. It is notable that the sulfur in Cys can undergo two alternative metabolic fates that are relevant to S-sulfhydration in cells/tissues - these are enzymatic reduction to H2S (via the actions of CSE, CBS and MST, as depicted in Fig. 1) and oxidation to a sulfonic acid (via the action of cysteine dioxygenase). Cellular determinants of this critical sulfur-fate decision – thiol reduction or oxidation - are poorly defined as yet and key control mechanisms await definition. However, the decision to produce H2S can have important biochemical consequences for cellular activities and may additionally contribute to pathophysiology under conditions of H2S excess, i.e., when synthesis outstrips the capacity for detoxification of H2S via enzymatic oxidation to yield relatively unreactive sulfite and thiosulfate (catalyzed by the SQR/ETHE1/TST enzyme pathway in the mitochondrial matrix, see Fig. 1).
Relation of protein S-sulfhydration to S-nitrosylation
Interestingly, many of the protein sites reported to undergo endogenous S-nitrosylation have also been found to undergo S-sulfydration. For example, unbiased analyses have regularly found Cys150 in GAPDH to be endogenously S-nitrosylated in mammalian tissue extracts [10, 27, 28] and this site has also been found to be >25% sulfhydrated by Snyder and colleagues [1]. Although close proximity of GAPDH to NOS provides one explanation for preferential S-nitrosylation, susceptibility to both S-nitrosylation and S-sulfhydration may be rooted in shared chemical properties of protein thiols that serve to promote both modifications. Notably, H2S-derived SH− preferentially adds to protein thiolate anion (S−), rather than protein-SH, and accordingly, should preferentially targeted protein Cys residues with low pKa values, i.e. where S− predominates in the physiological pH range. Notably, S-nitrosylation chemistry also occurs preferentially on S− and therefore should preferentially target the same population of (low pKa) protein-Cys residues. Thus, based solely on chemical considerations (i.e., structural constraints aside), one would predict a shared specificity for S-nitrosylation and S-sulfhydration, where both modifications would preferentially occur on low pKa Cys residues in proteins.
Persulfide generation by H2S is likely to be mediated largely by SH−, formed by the dissociation into H+ and SH−. Given that the pKa of H2S is ≈6.98 at 25ºC, dissociation to SH− should be approximately 2/3 complete at a physiological pH of 7.4. One mechanism for sulfhydration chemistry involves the preferential addition of SH− to a sulfenic (S-OH) group, rather than thiolate (S−) in proteins. Since protein thiolate is a strong nucleophile, it readily reacts with H2O2 to yield the corresponding sulfenic group that, in turn, reacts with SH− to yield the corresponding persulfide. A highly-efficient mechanism for H2S-mediated protein S-sulfhydration, at least in vitro, has also been shown to occur via the addition of sulfane-sulfur from a small molecule polysulfide (i.e., GSSH) rather than from SH− as the primary thiol adduct [29]. Alternatively, a sulfenic acid moiety can form a disulfide bond with another thiol group (inter- or intra-molecular, via dehydration of neighboring SOH- and SH- moieties), followed by SH− addition across the nascent disulfide bond - this addition yields persulfide and thiol as co-products [30]. Since protein S-nitrosylation can promote intramolecular disulfide bond formation (because NO serves as a good leaving group, e.g., best appreciated for disulfide formation involving vicinal protein thiols), an initial protein S-nitrosylation event can potentially promote the formation of a more enduring sulfhydration reaction. This inferred capability of S-nitrosylation to serve as a driver of S-sulfhydration offers another explanation for why both modifications may be found on a similar subset of protein-Cys residues. Also, in accord with knowledge of intermolecular transnitrosylation reactions as a vehicle for NOmigration between thiols, accumulation of GSSH is likely to redistribute sulfane sulfur to preferential Cys-SH acceptors and thus contribute significantly to persulfide trafficking among proteins via transsulfhydration.
A method for quantification of H2S, GSSH and GSH in cells and tissues
H2S, GSSH and GSH are labile metabolites, posing a challenge to accurate quantification. However, levels in biological samples can be confidently determined after homogenization in monobromobimane (MBB) solution, which provides for rapid formation of stable bimane adducts that are readily quantified using liquid chromatography coupled to mass spectrometry (LC-MS/MS). Due to the inherent lability of H2S and GSSH prior to formation of cognate MBB reaction products, standard solutions must be freshly made and cell/tissue extracts must be carefully prepared and handled, as described below.
Preparation of cell/tissue extracts for analysis
Freshly scrape-harvested cells and excised animal tissues are added to 2 mL Eppendorf tubes containing 1 mL of deoxygenated 2 mM monobrombimane (MBB) in CH3OH:H2O (80:20) at 4ºC and incubated for 2 h, followed by an additional 2 h at room temperature. The water used for MBB solution preparation should contain 0.1 mM diethylenetriamine pentaacetic acid (DTPA) to chelate metal ions that might otherwise promote sulfide/thiol oxidation, precluding MBB-adduct formation and detection. This initial incubation is followed by tissue disruption using stainless steel beads in a TissueLyser (Qiagen) and an additional 20 min at 4ºC, then 30 min at room temperature. Samples are then centrifuged and the resulting supernatants are analyzed by LC-MS or LC-MS/MS as described below for the presence of predicted MBB-derivatized species that reflect reaction products with H2S, GSSH and GSH (as monoisotopic sulfide-dibimane, 414.1362 Da; GSS-bimane, 529.1301 Da and GS-bimane, 497.1580 Da, respectively). Cell/tissue pellets from extracts are analyzed for protein content and this information is used to normalize observed bimane adduct levels to protein content. For protein quantification, 200 μL of 0.2 M NaOH is added to each cell/tissue pellet, followed by incubation at 95º C for 30 min. Protein content in the NaOH extract is determined using the Bradford assay (Bio-Rad), relative to bovine serum albumin standards (0–1.5 mg/mL). For plasma analysis of H2S, GSSH and GSH (rather than cells/tissue), 20 μL of rapidly isolated plasma is added directly to deoxygenated 3 mM MBB in CH3OH containing 0.1 mM DTPA, followed by incubation at room temperature for 30 min. Further processing of plasma samples is as described above for cells/tissues.
Synthesis and quantification of GSSH standard
Pure GSSH as a reference standard should be freshly made and is prepared by reaction of GSH with dispersed elemental sulfur. Depending on conditions, this reaction can yield a series of sulfane sulfur-containing species with the formula GSSnH (where “n” typically equals 1–4 and relative product yields are influenced by GSH and sulfur concentrations, GSH:S stoichiometries, pH value, solvent, temperature and reaction time). Because the GSSnH product is unstable under these synthetic conditions, MBB is used to trap the persulfide and polysulfide products for analysis by Q-TOF LC-MS/MS and assessment of GSSH, GSS2H, GSS3H, and GSS4H abundance. We found that high purity GSSH (i.e., devoid of higher polysulfides) can be obtained by vortexing 10 mM GSH with 2–3 mg elemental sulfur at room temperature for 90s at pH 7.4. In principal, persulfide groups can be conveniently quantified spectrophotmetrically based on a broad absorbance at 330–350 nm, however the absorption coefficient of this species is relatively low making this quantification approach suboptimal. For greater sensitivity and precision, a cyanolysis procedure can be employed for GSSH quantification, as previously described [31]. Briefly, 0.1 mL of 0.5 M KCN is added to the GSSH standard, followed by incubation for 40 min at 55ºC. This is followed by the addition of 20 L of 38% formaldehyde and 0.2 mL of 0.6 M iron(III) nitrate prepared in 13% nitric acid. This reaction yields a red solution, with max at 460 nm for quantifying the ferric-thiocyanate product of GSSH vs. a standard curve prepared using pure potassium thiocyanate. Reactions describing the formation of GSSH and detection as Fe(SCN)2+ are as follows:
GSH + S8 → GSSH + GSS2H + GSS3H +……
GSSnH + nCN− → GSH + nSCN− (where n 0)
Fe3+ + SCN−→ Fe(SCN)2+ (quantified at 460 nm)
Preparation of MBB-adduct standards for H2S, GSSH, and GSH quantification by LC-MS or LC-MS/MS
Standard solutions in a 20 L volume of NaHS (1–150 M), GSSH (2–200 M) and GSH (20–1000 M) containing 0.1 mM DTPA are added to 80 L of N2-saturated 3 mM MBB in CH3OH. Standards are incubated in the dark at room temperature for 30 min, followed by centrifugation. A 20 L aliquot of each MBB-adduct standard is diluted to 100 L with 50% CH3OH:H2O and cognate ion counts are quantified by LC-MS.
Quantification of thiol/sulfide-bimane reaction products may be performed using either LC-MS or LC-MS/MS. We routinely perform this analysis using reverse phase (RP) C18 chromatographic separation (2.1×100 mm, 1.8 mm particle size) and an Agilent 6538 UHD high resolution Accurate Mass Q-TOF mass spectrometer (Agilent Technologies, Santa Clara, CA) with dual-spray electrospray ionization. The LC system consists of a binary pump, on-line degasser, thermostated dual 54-well plate autosampler and a thermostated column compartment. A rapid resolution cartridge (Eclipse XDB-C8, Agilent technologies) is placed in front of the RP column to prolong column lifetime and an isocratic pump is used to deliver an internal reference mass solution (ions m/z 121.0509 and 922.0093) to the second ESI source for continuous mass calibration during sample analysis. LC parameters are set as follows: 1–4 L injection volume, 0.4 mL/min mobile phase flow rate, 25º C column temperature and 4–6º C autosampler temperature. The mobile phase consists of 1 mM ammonium formate and 0.1% formic acid in H2O (solvent A) and 0.1% formic acid in CH3CN (solvent B). The elution gradient is as follows: 0–2 min, 1% B; 2–20 min, 50% B; 20.1–29 min, 98% B; 29.1–35 min, 1% B. Both positive and negative mass spectra are acquired in 2 GHz (extended dynamic range) mode with 1.41 spectra/sec sampled over a mass/charge range of 50–1000 Daltons. The TOF capillary voltage is set to 4000 V for positive ions and 3500 V for negative ions with the fragmenter set to 175 V. The nebulizer pressure is 35 psi and the nitrogen drying gas is 250º C, delivered at a flow rate of 12 L/min. Data are saved in centroid mode using Agilent MassHunter data acquisition software.
Important assay considerations
MBB and its derivatives are light-sensitive, so all sample processing, reactions and product analyses should be conducted in the dark.
All solvents, including water and CH3OH, should be LC-MS grade purity. To avoid the potential oxidation of analytes due to metal ion contamination of solutions, 0.1 mM DTPA (for metal chelation) is added to the water used for sample processing.
To minimize the potential for autooxidation of H2S, GSSH, and GSH, it is optimal for a low oxygen environment to be maintained throughout the reaction (e.g. a hypoxic chamber or nitrogen glove box).
GSSH is unstable at pH <5.5 (unpublished data), so solutions should be freshly prepared and kept at pH ≥ 7.
How abundant is S-sulfhydration in mammalian biology?
Applying the above method, we sought to address this question. By comparison with S-nitrosylation, the answer is very. While S-NO concentrations in tissues and biofluids have been measured in the low nM - μM range, S-SH levels have been suggest to be of much greater magnitude, in line with the concentration of disulfides [1]. Quantification of both thiols and persulfides in mouse plasma and tissues has been performed using the MBB method and LC-MS/MS analysis as detailed above. Remarkably, these measurements reveal that GSSH is present in mouse plasma at ≈10% of GSH levels (≈1 μM and 7 μM, respectively; see Fig. 2). In tissues such as brain and liver, GSSH achieves still greater overall levels (estimated at 0.2 – 0.5 nmol/mg protein, ≈2–4% of GSH). This analysis also found free H2S levels in murine plasma and tissues to be approximately equal to that of GSSH. Compelling studies using mice that lack either CSE or CBS have shown that CSE (but not CBS) is the predominant source of H2S in liver and peripheral tissues [32], whereas CBS may to be the primary source of H2S in brain. The abundant quantities of GSSH and H2S that we observe in mouse tissues would predictably be consistent with marked protein sulfhydration and contribute significantly to transsulfuration reactions.
Figure 2.

Concentrations of H2S, GSSH, and GSH measured in murine plasma, liver and brain were quantified as detailed in the text. Indicated values are mean determinations +/− SD (n = 3).
Perspectives
By comparison with protein S-nitrosylation, our current understanding of the role of S-sulfhydration as a protein signaling modality is still in its infancy. Studies that seek to identify and quantify labile molecules (such as those containing S-SH and S-NO bonds) in complex biological milieu are inherently problematic. However, the emergence of new methods for proteomic analysis and quantitation of endogenous S-sulfhydration suggests that rapid advances and new insights are on the horizon.
HIGHLIGHTS.
We describe the finding in mammalian cells/tissues of abundant sulfhydrated proteins and glutathione persulfide (GSSH) that contribute to cell signaling by endogenous H2S. Analogous to SNO-protein levels, maintained in equilibrium with S-nitrosoglutathione (GSNO) via transnitrosylation, and the recognition of a GSNO reductase that indirectly governs SNO-protein levels, GSSH engages in transsulfuration reactions and its levels are modulated by selective desulfhydration via ETHE1, a mitochondrial persulfide dioxygenase that converts GSSH to glutathione and sulfite, at the expense of O2. We now recognize desulfhydration of GSSH as a fundamental determinant of H2S-mediated S-sulfhydration, cell signaling and toxicity. This review considers global similarities in systems that have apparently evolved for both protein S-sulfhydration/desulfhydration and S-nitrosylation/denitrosylation. Additionally, an MS-based method is described for quantifying small molecule persulfides in cells/tissues and a modified Biotin Switch method is considered for identifying protein persulfides in complex biological systems.
Highlights.
Abundant glutathione persulfide (GSSH) and sulfhydrated proteins are found in mammalian cells.
Protein and small molecule S-sulfhydration contribute to cell signaling by endogenous H2S.
An LC/MS-based method is described for quantifying small molecule persulfides in cells/tissues.
Similar systems evolved in mammalian cells for protein-SNO and -SSH addition and removal.
ETHE1 is a persulfide oxidase, selective for GSSH, that indirectly governs protein-SSH levels.
Acknowledgments
This research was supported by NIH grants R37 HL87062 and PO1 HD67244 to SSG as well as training grant T32 HL094284 for support of CL and T32 CA062948 for support of AK.
ABBREVIATIONS
- GSNO
S-nitrosoglutathione
- GSSH
glutathione persulfide
- SH
thiol
- MMTS
methyl methane thiosulfonate
- Biotin-HPDP
(N-[(6-biotinamido) hexyl]-3′-(2′-pyridyldithio) propionamide)
- carboxy-PTIO
2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide
- CSE
cystathionine γ-lyase
- CBS
cystathionine β-synthase
- MST
mercaptopyruvate sulfurtransferase
- SQR
sulfide quinone reductase
- ETHE1
persulfide dioxygenase
- TST
thiosulfate sulfur transferase (a.k.a. rhodanese)
- SRB
sulfate reducing bacteria
- MS/MS
tandem mass spectrometry
Footnotes
No Author has any Conflict of Interest to Disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Mustafa AK, et al. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2(96):ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura H. Antioxid Redox Signal. 2013. Production and Physiological Effects of Hydrogen Sulfide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hess DT, Stamler JS. Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem. 2012;287(7):4411–8. doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess DT, et al. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6(2):150–66. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 5.Lane P, Hao G, Gross SS. S-nitrosylation is emerging as a specific and fundamental posttranslational protein modification: head-to-head comparison with O-phosphorylation. Sci STKE. 2001;2001(86):RE1. doi: 10.1126/stke.2001.86.re1. [DOI] [PubMed] [Google Scholar]
- 6.Jaffrey SR, et al. Protein s-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3(2):193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 7.Jaffrey SR. The biotin switch method for the detection of S-nitrosylated proteins. SciSTKE. 2001:PL1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 8.Gang Hao BD, Shi Lei, Campagne Fabien, Gross Steven S. SNOSID, a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc Natl Acad Sci USA. 2006;103:1012–1017. doi: 10.1073/pnas.0508412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derakhshan B, Wille PC, Gross SS. Unbiased identification of cysteine S-nitrosylation sites on proteins. Nat Protoc. 2007;2(7):1685–91. doi: 10.1038/nprot.2007.210. [DOI] [PubMed] [Google Scholar]
- 10.Greco TM, et al. Identification of S-nitrosylation motifs by site-specific mapping of the S-nitrosocysteine proteome in human vascular smooth muscle cells. Proc Natl Acad Sci U S A. 2006;103(19):7420–5. doi: 10.1073/pnas.0600729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doulias PT, et al. Structural profiling of endogenous S-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein S-nitrosylation. Proc Natl Acad Sci U S A. 2010;107(39):16958–63. doi: 10.1073/pnas.1008036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doulias PT, et al. Mass spectrometry-based identification of S-nitrosocysteine in vivo using organic mercury assisted enrichment. Methods. 2012 doi: 10.1016/j.ymeth.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doulias PT, et al. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci Signal. 2013;6(256):rs1. doi: 10.1126/scisignal.2003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gade AR, Kang M, Akbarali HI. Hydrogen sulfide as an allosteric modulator of ATP-sensitive potassium channels in colonic inflammation. Mol Pharmacol. 2013;83(1):294–306. doi: 10.1124/mol.112.081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munaron L, et al. Hydrogen sulfide as a regulator of calcium channels. Cell Calcium. 2013;53(2):77–84. doi: 10.1016/j.ceca.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Sen N, et al. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol Cell. 2012;45(1):13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang G, et al. Hydrogen Sulfide Protects Against Cellular Senescence via S-Sulfhydration of Keap1 and Activation of Nrf2. Antioxid Redox Signal. 2013;18(15):1906–19. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan N, et al. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal. 2011;4(203):ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandiver MS, et al. Sulfhydration mediates neuroprotective actions of parkin. Nat Commun. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, et al. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410(6827):490–4. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 21.Que LG, et al. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308(5728):1618–21. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116(4):617–28. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 23.Tang CH, et al. Hepatocarcinogenesis Driven by GSNOR Deficiency Is Prevented by iNOS Inhibition. Cancer Res. 2013;73(9):2897–904. doi: 10.1158/0008-5472.CAN-12-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei W, et al. S-nitrosylation from GSNOR deficiency impairs DNA repair and promotes hepatocarcinogenesis. Sci Transl Med. 2010;2(19):19ra13. doi: 10.1126/scitranslmed.3000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabil O, Banerjee R. Characterization of patient mutations in human persulfide dioxygenase (ETHE1) involved in H2S catabolism. J Biol Chem. 2012;287(53):44561–7. doi: 10.1074/jbc.M112.407411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller EG. Trafficking in persulfides: delivering sulfur in biosynthetic pathways. Nat Chem Biol. 2006;2(4):185–94. doi: 10.1038/nchembio779. [DOI] [PubMed] [Google Scholar]
- 27.Hara MR, et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7(7):665–74. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 28.Hao G, et al. SNOSID, a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc Natl Acad Sci U S A. 2006;103(4):1012–7. doi: 10.1073/pnas.0508412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greiner R, et al. Antioxid Redox Signal. 2013. Polysulfides link H2S to protein thiol oxidation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan J, Carroll KS. Persulfide Reactivity in the Detection of Protein S-Sulfhydration. ACS Chem Biol. 2013 doi: 10.1021/cb4001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood JL. Sulfane sulfur. Methods Enzymol. 1987;143:25–9. doi: 10.1016/0076-6879(87)43009-7. [DOI] [PubMed] [Google Scholar]
- 32.Yang G, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322(5901):587–90. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]


