Introduction
The most deadly attribute of breast cancer cells is their ability to leave their initial site of growth, travel to discontiguous secondary sites, and proliferate into macroscopic masses. Metastasis involves intrinsic (i.e., genetic) and extrinsic (i.e., microenvironmental signals) factors. In this brief review, we focus on a subset of metastasis-regulatory molecules, termed metastasis suppressors, their mechanisms of action, and when available, early clinical data. The objective is to set the stage for how this class of molecules is becoming recognized for potential prognostic and therapeutic potential. Due to publication space limitations, we cite excellent reviews from which primary literature can be identified. We apologize to authors whose work is not cited.
Metastasis cascade
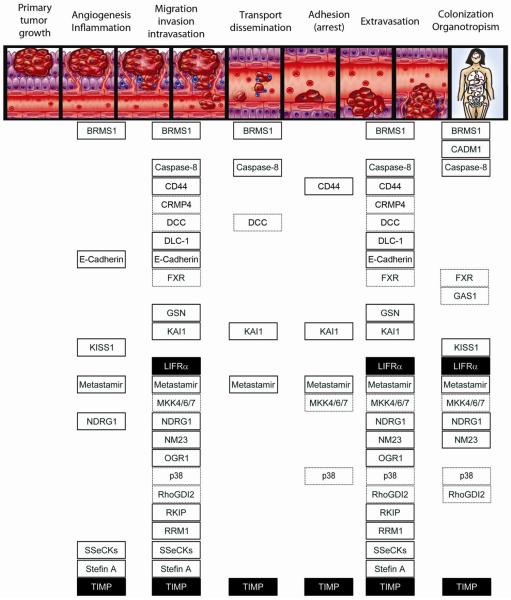
Tumor cells can disseminate via three pathways – the circulatory system, lymphatics, or across body cavities. Hematogenous and lymphatic metastasis begins when tumor cells break away and grow independently from a primary tumor while migrating through the extracellular matrix (ECM). Cells then intravasate and survive the shear stresses of transport, resist anoikis, and evade immune surveillance. Following active and passive transport to distant sites, metastatic cells or emboli containing metastatic cells (both homotypic (comprised solely of tumor cells) or heterotypic (comprised of tumor cells plus another cell (e.g., white blood cell, platelet) or protein (e.g., clot)) may be nonspecifically trapped due to size. Or metastatic cells can specifically adhere to integrins and adhere via other ligand-receptor interactions with endothelial cells in a tissue-specific manner. Responding to chemoattractants, cells extravasate. Successful metastatic cells must then complete the most crucial and selective step, proliferation at secondary sites (also known as colonization). Key steps of the metastatic cascade are depicted schematically in Figure 1.
Figure 1. Hematogenous metastatic cascade and metastasis suppressors' mechanism of action.
Cancer progression and metastasis begin when a subset of tumor cells acquire genomic changes that alter expression of pro and anti-metastatic genes. Tumor cells acquire the ability to migrate and invade surrounding tissues matrices and basement membranes (collectively, the stroma). Blood-borne metastases begin when those invasive cells enter the circulatory system (i.e., intravasation). In the circulation, cells overcome shear forces, anoikis, and immune surveillance as they travel through the vasculature, sometimes forming emboli with platelets, white blood cells and/or red blood cells. Surviving cells arrest at the secondary site through ligand-receptor-based adhesion or physical size constraints. Once attached, cells extravasate and begin to modify the surrounding stroma. If the microenvironment is conducive to growth, the cells begin to multiply, or colonize the secondary tissues. Colonization of the secondary site is the last and most crucial step of the metastatic cascade. While many disseminated cells can seed distant tissue, exceedingly few cells form macroscopic masses. Moreover, certain cancers have predilections to colonize certain organs preferentially (organotropism). For example, the most common sites of breast cancer metastases are bone, lymph nodes, lung, liver and brain.
Metastasis requires completion of every step of this multistep cascade. It was long ago recognized that inhibition of any step rendered a cell non-metastatic. Each metastasis suppressor is listed below the step(s) for which experimental evidence demonstrates inhibition of the metastatic process. Solid black boxes are metastasis suppressors that only have experimental data in breast/mammary cancer models. White boxes with solid black lines are suppressors that have been shown to function in multiple cancer types, including breast. Boxes with dashed black borders are suppressors that have been shown to function in multiple cancer types, excluding breast. Note: In Table 1, some of the metastasis suppressors have reported tumor suppressor activities. For simplicity, tumor suppressor function is not depicted in this figure.
Metastasis Suppressors
A metastasis suppressor is defined by its ability to inhibit metastasis, while having minimal effect on primary tumor growth (defined in this review as <50% growth inhibition) when re-expressed in metastatic tumor cells. This growing family of molecules was first described in the mid-1980's with the discovery of Nm23-H1 by Patricia Steeg. Since then, more than thirty functionally validated metastasis suppressors have been reported with more expected. Readers are cautioned that many publications use the term imprecisely (i.e., We found publications describing >100 “metastasis suppressors” for which no in vivo data to substantiate the claim was presented. Note: in vitro assays are not sufficient to measure metastasis). In this review, we focus solely on molecules for which metastasis and primary tumor growth (preferably orthotopic) have been shown.
As the field of metastasis suppressors has grown, contradictory data have been reported. Some metastasis suppressors function in multiple tumor types, whereas others perform different functions depending upon cell of origin. If a molecule fulfills the criteria as a metastasis suppressor in any model, it is presented here. Discordant data are noted and we encourage readers to weigh the relative strengths and weaknesses in order to make judgments with regard to each molecule's role(s) in breast cancer. Key attributes of the metastasis suppressors are summarized in Table 1 for quick reference. Below we summarize aspects of discovery, functionality, and clinical utility.
Table 1.
Metastasis suppressor proposed mechanisms of action and known clinical correlations
| Metastasis Suppressor |
Aliases | Chromosomal Location |
Cellular Localization1 | Proposed Function(s)2 | Step(s) in metastasis inhibited3 |
Clinical4 and Experimental5 Correlations |
|---|---|---|---|---|---|---|
| BRMS1 | 11q13.1–13.2 | N and C | SIN3-HDAC complexes (Chromatin modeling) Reduce phosphoinositide signaling Restore gap junction communication |
Invasion Transport Colonization |
Breast * + − Melanoma * + NSCLS * + Ovarian * + |
|
| CADM1 | TSLC1 IgSF4 Necl2 Syncam |
11q23 | C | Up-regulates caspase-3, BAX and p21 Cytoskeletal remodeling Down-regulate MMP Cell cycle arrest Apoptosis Invasion |
Colonization | Breast * # + Ovarian * + Pancreatic * # + Colon # Lung # Prostate # ATLL − |
| Caspase-8 | 2q33.1 | C | Induce apoptosis/anoikis Cell cycle arrest Integrin mediated death (IMD) |
Survival Transport Invasion Colonization |
Neuroblastoma * + Breast * # Colon * Medulloblastoma * + Glioma * + Lung * # Prostate # HNSCC − |
|
| CD44 | CDW44 CSPG8 ECMR-III |
11p11.2–13 | M | Binds to hyaluronic acid receptor Cell-cell and cell-matrix adhesion |
Migration | Prostate * + − Breast * + − Osteosarcoma * − Pancreatic − Esophageal − HNSCC − Colon − |
| CRMP4 | DRP-3 ULIP-1 |
5q32 | C and M | Cytoskeletal remodeling | Invasion | Prostate * |
| DCC | CRC18 CRCR1 MRMV1 |
18q21.3 | C | Cytoskeletal remodeling Regulate MAPK signaling Cell cycle arrest Promote apoptosis |
Transport Migration Invasion |
Colon * Breast − Ovarian − Renal − Lung − |
| DLC-1 | STARD12 p122 RhoGAP |
8p22 | C | Cytoskeletal remodeling Rho GTPase activating protein |
Motility Migration Invasion |
Breast * # + − Hepatocellular * NHL # Colon # + − Pancreatic # + − Brain # Gastric # Esophageal # Myeloma # Lung # + − Urothelial − Renal − |
| E-Cadherin | CD324 | 16q22.1 | M | Cell-cell and cell-matrix adhesion | EMT Invasion |
Breast * + AML* + Gastric * + Esophageal * + |
| FXR | NR1H4 | 12q23.1 | N and C | Lipid and glucose metabolism Promote apoptosis | Invasion Colonization |
Liver (HCC)* # + Colon # Breast − Pancreatic − Esophageal − |
| GAS1 | 9q21.3–22 | N and C | Cell cycle arrest Promote apoptosis |
Colonization | Melanoma * + Colorectal * # + Lung # + Gastric # + Glioma # + Bladder # + Menigiomas − |
|
| GSN | ADF AGEL |
9q33 | C | Cytoskeletal remodeling Inhibit EMT |
Migration | Breast * # + − Melanoma * Bladder * # Pancreatic # + Colorectal # Thyroid # Lung # − Prostate − |
| HUNK | MAK-V | 21q22.1 | N and C | Cytoskeletal remodeling | Motility Invasion |
Breast * − |
| KAI1 | CD82 SAR2 TSPAN27 |
11p11.2–13 | M | Bind endothelial DARC (induce apoptosis) EGFR desensitization Up-regulate TIMPs Increase E-cadherin and β-catenin interaction |
Intravasation Transport |
Lung * Prostate * + Esophageal * HNSCC * Bladder * + Pancreatic * + Breast * + Thyroid * Ovarian * + Gastric # + |
| KISS1 | 1q32 | C and S | Ligand for G-protein receptor (KISS1R) Angiogenesis Interact with PGC1α (cell metabolism) |
Colonization Angiogenesis |
Breast * + − Ovarian * + Melanoma * + Pancreatic * + Prostate * + Bladder * + Thyroid * + Endometrial * + Gastric * + Liver (HCC) − |
|
| LIFR | CD118 STWS |
5p13–12 | M and C | Activate hippo signaling | Migration Invasion Colonization |
Breast * # + Liver (HCC) # + |
| LSD1 | KDM1 | 1p36.12 | N | Chromatin remodeling | Invasion | Breast * + Gastroesophageal + Bladder + Colon − Esophageal − NSCLC − Neuroblastoma − |
| MTBP | ACTFS HDMX HDM2 |
12q14.3–15 | N and C | Cell cycle arrest Cytoskeletal remodeling Mitotic progression Chromosome segregation |
Invasion | Breast * Liver (HCC) * Spinal * Osteosarcoma * Lymphoma * Colorectal − Multiple myeloma − B-cell lymphoma − Burkitt's lymphoma − HNSCC + |
| MetastamiR 6a,b 9 19a-3p 31 34a 126 139 145 146a,b 196a2/b 206 335 |
Multiple | N and C | Multiple functions (in general, reducing protein expression of metastasis promoters) |
Migration Invasion EMT Colonization |
Breast * + Colorectal * + Melanoma * + Gastric * + Liver (HCC) * + Bone * + |
|
| MKK4 MKK6 MKK7 P38 |
MAPKK4 MAPKK6 MAPKK7 MAPKK14 |
17p11.2 17q24.3 19p13.3–13.2 6p21.3–21.2 |
C | Stress activated MAPK signaling | Migration Colonization |
Prostate * + Ovarian * + Breast − Endometrial * + Colorectal # + Pancreatic # − Colon # Testicular # Biliary # Gastric − |
| NDRG1 | Drg1, Cap43 Rit42 RTP PROXY-1 |
8q24.3 | C and N | Promote cell differentiation Up-regulate E-cadherin Inhibit TGFβ mediated EMT |
Angiogenesis Invasion Colonization |
Colon * + Prostate * + Breast * + Neuroblastoma # Glioma # Esophageal # Oral squamous cell # Thyroid − Liver (HCC) − NSCLC − |
| Nm23 | NDKB NME1 |
17q21.3 | C and N | Inhibit activation of MAPK pathways Ras signaling Histidine kinase activity NDP kinase activity |
Migration Colonization |
Melanoma * + Breast * + Liver (HCC) * + Colon * + Ovarian * + Neuroblastoma Colorectal − Thyroid − Osteosarcoma − Choriocarcinoma − Gastric − Testicular − |
| OGR1 | GPR68 GPR12A |
14q31 | M | G-protein coupled receptor signaling | Migration | Prostate * + Ovarian * − Breast * − Melanoma * # Pancreatic # Colon − Lung − Liver − Kidney − Laryngeal − |
| RhoGDI2 | ARHGDIB | 12p12.3 | C | Cytoskeletal remodeling Endothelin and Neuromedin U signaling Regulates Rho GTPases |
Migration Colonization |
Bladder * + Colorectal * + − Lung * + Breast − Gastric − Ovarian − |
| RKIP | PFL0955C | 12q24.23 | C | Competitive inhibitor for RAF1-MEK interaction Cytoskeletal remodeling |
Migration Invasion |
Prostate * Breast * + Cervical * Glioma * + Pancreatic * + Gastric * + Renal * + Melanoma * NSCLC + Bladder + |
| RRM1 | RIR1 RR1 |
11p15.5 | C | Increases PTEN expression Decreases FAK phosphorylation Cytoskeletal remodeling |
Motility Invasion |
Breast * Ovarian * Gastric * Bladder * + Lung * Esophageal * Pancreatic + NSCLC + |
| SSeCKs | AKAP12 GRAVIN |
6q24–25 | C | Scaffold for protein kinases Regulate Src, PKC and Rho signaling VEGF secretion |
Angiogenesis Migration |
Prostate * + Ovarian * Breast * |
| Stefin A | CST6 EPM1 STFB |
21q22.3 | C | Cathepsin inhibitor | Angiogenesis Migration Invasion |
Esophageal * Breast * + HNSCC * + |
| TIMP | TIMP1 TIMP2 TIMP3 TIMP4 |
Multiple | C, S and M | Inhibit MMP expression and signaling | Angiogenesis Migration Invasion Transport |
Breast * + Colon * + Thyroid * + Melanoma * + |
Nuclear (N), Cytoplasmic (C), Membrane (M), Secreted (S)
Proposed functions represent biochemical studies ascribing a molecular mechanism of action.
Functions listed are those for which in vivo or in vitro data are published (mostly in vitro correlations) showing metastasis suppressor activity. No attribution is given regarding which step(s) is most relevant to metastasis suppressor activity.
Data are collated from papers published through October 2013. Clinical cohorts vary greatly in size and methodology. Clinical correlations are either positive (+) or negative (−) clinical correlations (i.e., good or bad prognosis, respectively).
Abbreviations: AML, Acute Myeloid Leukemia; ATLL, Adult T cell leukemia/lymphoma; HCC, hepatocellular carcinoma; HNSCC, Head & neck squamous cell carcinoma; NHL, Non-Hodgkin's lymphoma; NSCLC: Non-small cell lung carcinoma.
Summary of experimental data utilizing cell lines and/or animal models representing different histiotypes which show metastasis (*) or tumor (#)suppressor function.
Mechanisms of action are diverse and, in most cases, not yet clear-cut. Nonetheless, metastasis suppressors can be found in every cellular compartment as well as outside of the cell; they inhibit virtually every step in the metastatic cascade, and increasing signs show clinical prognostic and therapeutic relevance [1, 2]. The step(s) of the metastatic cascade impacted by individual metastasis suppressors are depicted in Figure 1.
Non-metastatic 23 (NM23)
Using differential colony hybridization, Nm23 was first identified as the first in-class metastasis suppressor in melanoma cells [3], but the family now includes ten homologs (Nm23-H1 to -H10). Metastasis suppression by homologs other than H1 or H2 has not been clearly demonstrated.
Despite nearly 30 years of investigation, the mechanism(s) of action remain enigmatic. Nm23 has a nucleoside diphosphate kinase activity that does not appear responsible for metastasis suppression. Histidine kinase, phosphorylation of kinase suppressor of Ras (KSR), and exonuclease activities, all contribute to its anti-metastatic actions. Most of the convincing data support these three activities. Still, Nm23-H1 also suppresses ERK activation, down-regulates prune, and interacts with a plethora of cancer- and metastasis-associated proteins. A recent report shows that Nm23-H1 binding to gelsolin; alters gelsolin's actin-severing capacity, thereby reducing motility, invasion, and metastasis. Recent reviews provide critical analyses of the biochemical data [4, 5]. Importantly, many of the reported false-positive protein-protein interactions have been reported and have complicated (i.e., misled) the field.
Clinical studies with Nm23 have been extensive and the preponderance of data reveals an inverse correlation with poor survival and tumor grade in multiple tumor types, including breast cancers. However, studies showing no correlation or positive correlation have been sporadically reported. Recently, the promise of Nm23-H1 as a therapeutic option in treating metastatic cancers in the clinical setting has been gaining a foothold. Treatment with dexamethasone and medroxyprogesterone acetate can restore Nm23-H1 expression and reduce metastases [6, 7]. Gene therapy approaches are also being considered for ovarian cancer.
Breast Metastasis Suppressor 1 (BRMS1)
Although functionality of many metastasis suppressors has been demonstrated in breast cancer models, BRMS1 is the only metastasis suppressor identified deliberately using breast models. BRMS1 has since been found to suppress metastasis in a broad spectrum of cancer types (reviewed in [8]). BRMS1 was discovered using differential display comparing metastatic and metastasis-suppressed hybrids (i.e., complemented with human chromosome 11 via microcell-mediated chromosome transfer (MMCT)). BRMS1 is a member of SIN3 histone deacetylase chromatin remodeling complexes and is the founding member of a family of molecules; the role(s) of other family members in metastasis are uncertain.
The presumptive mechanism of action is, therefore, anticipated to be by down-regulating metastasis promoting and up-regulating expression of metastasis-suppressing genes. Among the key downstream targets are osteopontin, PtdIns(4,5)P2, and metastasis regulatory microRNA. Direct interaction with NFκB, which is often pro-metastatic, is thought to be particularly relevant. miR-146a and -146b are up-regulated by NFκB, which itself is down-regulated by BRMS1 through decreased phosphorylation of IκB and deacetylation of p65, which sensitizes the cells for anoikis. Over-expression of miR-146a or -146b down-regulated epidermal growth factor receptor, suppressed invasion and migration in vitro, and suppressed metastasis in vivo. Interestingly, BRMS1 decreased prometastatic miR-10b, -373, -520c, and increased anti-metastatic miR-146a, -146b, and -335 [9]. The latter data represent one of a number of emerging metastasis suppressor pathways. In clinical studies of melanoma, cytoplasmic BRMS1 expression is associated with positive clinical outcome [10] while cytoplasmic expression was associated with poor clinical outcome in breast cancers [11]. While most studies examining BRMS1 protein have inverse correlations with clinical outcomes, contradictory data exist in others [12].
Using in vitro assays to assess steps in the metastatic cascade, BRMS1 impacts every step at low to moderate levels. BRMS1 functionality is highly dependent upon post-translational stability, subcellular localization, and protein-protein interactions.
Although systematic studies have not yet been done for all metastasis suppressors, few mutations have been reported in advanced human cancers. Like most other metastasis suppressors, BRMS1 expression is regulated by epigenetic mechanisms, such as promoter methylation. Recent analysis of BRMS1 promoter CpG islands in circulating tumor cells shows that methylation predicts expression and corresponds to probability of recurrence with metastasis and survival.
Gelsolin (GSN)
The metastasis suppressor role of GSN was first described in B16-BL6 melanoma [13]. In later studies GSN expression was restored using inhibitors of DNA methylation and lung colonization was inhibited. GSN binds actin and changes actin cytoskeletal architecture based on its ability to sever, cap and induce nucleation of actin filaments. GSN activity is regulated by binding to Ca+2 and PtdIns(4,5)P2. The role of GSN in cancer is ambiguous, functioning as both tumor promoter and suppressor. Many tumor promoting functions of GSN are associated with activation of EGFR, PI3K, and Rac.
Mutations in GSN are linked to metastasis development in patients [14]. Also, the transcription factor ATF3 up-regulates GSN as another means of mediating metastasis suppression. Contrastingly, GSN over-expression increases metastasis in orthotopic mammary tumor models. This effect could be ablated by co-expression with Nm23-H1 (see above).
KAI1/CD82 (kang ai 1)
Initially mapped in rat prostate carcinoma using MMCT of chromosome 11 fragments, KAI1 was validated as a metastasis suppressor in transfectants [15]. Metastasis suppression in a broad spectrum of tumor types in animal models has been subsequently shown. KAI1 is a type III transmembrane glycoprotein and member of the tetraspanin 4 superfamily that is located at the plasma membrane in specific microdomains. KAI1 interacts with integrins, tetraspanins, chemokines, MHC class I and II, CD19, CD21, EGFR, EWI2/PGRL, KITENIN, and PKC and is involved in trafficking and distribution of plasma membrane components (reviewed in [16–18]).
The mechanism of metastasis suppression is not well understood but the following findings in KAI1 expressing cells provide clues regarding mechanism: (i) attenuation of integrin trafficking and ligand-induced dimerization; (ii) redistribution of surface EGFR resulting in fewer lamellipodia, (iii) migration signaling, (iv) increasing E-cadherin and β-catenin interactions, (v) up-regulating tissue inhibitors of metalloproteinase (TIMP), (vi) indirectly reducing urokinase-type plasminogen activators, and (vii) inhibiting p130CAS-Crk complex formation, have all been proposed to regulate cell migration and invasion. KAI1 also increases apoptosis and senescence after tumor cells have migrated to secondary sites by decreasing intracellular β-catenin/Wnt pools and through packaging and secretion in exosomes [19]. KAI1 expression is down-regulated via multiple mechanisms including loss of heterozygosity, promoter methylation and disruption of upstream transcriptional regulators. KAI1 expression is associated with progression and/or positive clinical outcomes in multiple cancer types (Reviewed in [17]).
KISS1
KISS1 was identified by subtractive hybridization and differential display comparing metastasis competent melanoma cells with metastasis suppressed hybrids (created by MMCT with human chromosome 6). Unexpectedly, KISS1 mapped to chromosome 1q32, implicating a regulatory gene on chromosome 6, which was later determined to be CRSP3 (co-factor required for SP1 activity, DRIP130). CRSP3 maps to a hot spot for chromosomal deletions in metastatic melanoma (6q16.3–q23) and up-regulates a thioredoxin interacting protein (TXNIP, vitamin D up-regulated protein 1, VDUP1, thioredoxin binding protein 2) which, in turn, up-regulates KISS1. TCF21, a member of the basic helix-loop-helix (bHLH) family of transcription factors which maps to 6q23–q24 was shown to regulate KISS1 expression in malignant melanomas. Together these molecules implicate complex metastasis suppressor regulatory pathways.
Nascent KISS1 is a secreted proprotein that is proteolytically cleaved by the proprotein convertase furin into kisspeptins (KP). KP54 binds a G-protein coupled receptor, KISS1R (reviewed in [20]) and regulates hypothalamic-pituitary-gonadal axes in puberty and pregnancy. The mechanism of KISS1 metastasis suppression remains unclear, but is presumed to be via stimulation of KISS1R. Complicating this expectation are findings that many cell lines in which KISS1 re-expression results in metastasis suppression do not express KISS1R. The latter observation has compelled the hypothesis of a paracrine cross-talk with tissue-specific cells. Recent data reveal an unexpected interaction of KISS1 with PGC1α, the master regulator of mitochondrial biogenesis. KISS1-expressing cells have increased mitochondrial mass and display a shift from aerobic glycolysis to oxidative phosphorylation (W. Liu and D.R. Welch, accepted pending revisions). The latter data represent a potentially new connection between metabolism and control of metastasis.
Clinical studies with KISS1 have been limited by availability of quality reagents recognizing KISS1 and KP54. Studies measuring mRNA are of dubious value because of required post-translational modifications. Regardless most expression and immunohistochemical (IHC) studies have shown that KISS1 expression is generally correlated with good prognosis, except in hepatocellular carcinoma (HCC).
Interestingly, KISS1 maintains disseminated tumor cells in a dormant state after they have seeded other tissues. Long recognized that blocking any step of the metastatic cascade effectively prevents metastasis, many approaches are limited since steps antecedent to colonization have usually occurred prior to diagnosis. KISS1 affords the opportunity to maintain disseminated cells in a (quasi-) dormant state, potentially rendering metastasis a chronic and controllable aspect of cancer. While dormancy is not a cure, maintenance of tumors in an asymptomatic state would represent a significant advance.
Mitogen Activated Protein Kinase Kinase (MKK4, MKK6, MKK7, p38)
MKK4 was identified as a putative metastasis suppressor in rat prostate cancer by comparing expression profiles following MMCT of a fragment of human chromosome 17, for which loss of function is observed in multiple cancers. MKK4 is one of the stress-activated protein kinases and activates both c-Jun NH2-terminal kinase (JNK), and p38 signaling with further downstream regulation of apoptosis, cell cycle arrest, and immune responses. Cells re-expressing MKK4 can still seed secondary sites and extravasate, but are significantly delayed (until expression is lost) for colonization. The proposed molecular mechanisms of MKK4 metastasis suppression differ between prostate, ovarian and other cancers. MKK7 and JNK activation suppresses prostate cancer metastasis [21], while the MKK6 and p38 signaling are responsible of ovarian cancer metastasis suppression [22].
In clinical samples, MKK4 expression is inversely associated with progression and metastasis in multiple cancers, and phosphorylated MKK4 levels are associated with favorable clinical outcomes in colorectal carcinoma [23]. We emphasize the inclusion of critical signaling controls (i.e., phospho-specific antibodies) that tested enzymatic activities, not simply expression. Measuring kinase activities were critical to infer mechanism.
There are, however, some contradictory data implicating MKK4 as a pro-oncogenic factor. Over-expression in pancreatic and breast cancer cell lines lacking endogenous MKK4 increased proliferation, invasion, and tumor growth in vivo. Corresponding knockdown with siRNA or over-expression of dominant negative forms induce apoptosis, decrease anchorage-independent growth and reduce tumorigenicity in vivo. So, while there are strong data showing stress-activated protein kinases as metastasis suppressors, contextual cues contribute to the biological functions. Much more research will be needed to explore those patterns.
Rho GDP-dissociation factor 2 (RhoGDI2)
RhoGDI2 was identified as a putative metastasis suppressor comparing expression profiles in metastatic and non-metastatic bladder cancer cell lines. RhoGDI2 was one of the most down-regulated genes in the metastatic cells [24, 25]. Restored expression suppressed experimental lung metastasis but did not affect subcutaneous tumor growth.
RhoGDI2 is a guanine nucleotide dissociation inhibitor that binds and inactivates the GTPases involved in the signaling cascades initiated by G-coupled receptors (reviewed in [26, 27]). The molecular mechanism of metastasis suppression is not fully understood, but multiple mechanisms have been implicated. RhoGDI2 re-expression is inversely correlated with neuromedin U and endothelin-1 and both molecules are verified downstream mediators of RhoGDI2 regulated signaling. In preclinical studies, mice treated with endothelin receptor antagonist (Atrasentan) blocked lung metastasis, suggesting that RhoGDI2 regulated genes play a role in bladder cancer metastasis.
RhoGDI2 activity is regulated by post-translational modifications, including phosphorylation by Src. Using phosphomimetic approaches (i.e., Y153R) phosphorylation was determined to be critical for RhoGDI2 suppression [28]. However, GDI activity is inhibited by phosphorylation by protein kinase C, which reduces binding to Rac1 [29]. RhoGDI2 also appears to suppress metastasis by regulating GTPase activity independent of GTPase membrane association [30]. RhoGDI2 lacking the first 55 amino acids (produced by caspase cleavage) no longer inhibits GDP dissociation, but still suppresses metastasis. Therefore, the biochemical mechanism of action is still uncertain.
RhoGDI2 expression inversely correlates with tumor stage and grade in many tumor types; however, RhoGDI2 function is cancer type specific. RhoGDI2 is up-regulated and associated with poor clinical outcomes in breast, gastric, colorectal and ovarian carcinomas. As above, the context in which RhoGDI2 is expressed determines biological activities.
Deleted in Liver Cancer 1 (DLC-1)
DLC-1 was first cloned using subtractive hybridization to identify genes deleted in human hepatocellular carcinomas [31]. It was later identified as a breast cancer metastasis suppressor in microarray comparisons between breast cancer cell lines [32].
DLC-1 is a RhoGAP (GTPase-activating protein) that inhibits/inactivates Rho-dependent signal transduction. DLC-1 over-expression leads to more stable adherens junctions, reduced mobility, fewer actin stress fibers, and focal adhesion structures through cytoskeletal reorganization due to decreased RhoGAP activity. Phosphorylation by PKD, PKC, or Akt decreases RhoGAP activity [33, 34], while phosphorylation by PKA leads to increased dimerization, RhoGAP activity, and tumor/metastasis suppression. Many studies support roles of RhoGAP activity in DLC-1 suppressor function, but DLC-1 can also suppress independently of RhoGAP activity, albeit to a lesser extent.
Interestingly, DLC-1 expression is inversely associated with the expression of pro-metastatic proteins osteopontin and MMP9 in HCC, and positively regulates E-cadherin in prostate cancer. Over-expression decreases in vitro migration and invasion and in vivo breast metastasis to lung and HCC cell lines.
Deletion or promoter methylation of DLC-1 has been described in multiple cancers. Expression correlates with progression and poor clinical outcomes in multiple cancer types, but some data suggest tumor suppressor function as well.
CD44
CD44 is a single-pass transmembrane cell surface glycoprotein. Alternative splicing leads to multiple isoforms, although the predominant form is 85–95kDa (CD44s). CD44 is post-translationally cleaved at both the extracellular and cytoplasmic domains, interacts with a whole host of cancer-associated cellular factors, and is involved in cell-cell and cell-matrix adhesion, cell signaling, survival, growth, stemness and migration (reviewed in [35–38]).
CD44s was investigated as a metastasis suppressor because of its proximity to KAI1 on chromosome 11. CD44 expression was inversely correlated with metastatic potential of prostate cancer cell lines and CD44−/− matings with MMTV-PyMT mice had reduced lung metastasis without changing growth of primary mammary tumors [39]. However, CD44 knockdown with shRNA in human osteosarcoma cells enhanced lung metastasis following intratibial injection.
While there are data supporting CD44 as a metastasis suppressor and as a biomarker associated with positive outcomes, classification as a metastasis suppressor is complicated because of the multiple variant forms which exhibit tissue- and tumor-type specific effects, including tumor promotion. For these reasons, CD44 is a prime example highlighting the importance of understanding post-transcriptional and -translational modifications affecting functions.
Cell Adhesion Molecule 1 (CADM1)
CADM1, an immunoglobulin superfamily adhesion protein was originally described as a tumor suppressor, but was recently shown to be a metastasis suppressor in mouse mammary and laryngeal tumors [40]. CADM1 is described as a germ-line modifier of metastasis susceptibility locus that did not affect primary tumor growth when over-expressed.
Since experimental metastasis is affected, CADM1 most likely inhibits steps downstream of intravasation, possibly colonization. Interestingly, in mice lacking functional T-cell mediated immunity the metastasis suppressive effects of CADM1 were abolished, implying that CADM1 may function via host immune surveillance. This hypothesis is supported by observations that CADM1 interacts with CRTAM a T-cell adhesion protein functioning as a marker for activated CD8+ T cells and NK cells.
CADM1 down-regulation in metastatic breast cancer is mainly due to promoter hypermethylation. Expression levels determine whether CADM1 has tumor or metastasis suppressor activities. Low levels of CADM1 are metastasis suppressive but high levels are tumor suppressing. CADM1 is also regulated post-transcriptionally, in HCC, the pro-metastatic microRNA miR-10b binds the 3'-UTR of CADM1 mRNA and represses its translation.
CADM1 may also play roles in oncogenesis. CADM1 is illustrative of tissue-specific effects, interaction-dependent activities, and the notion that relative expression level might determine function.
MDM2 binding protein (MTBP)
MTBP is a murine double minute (MDM2) interaction partner. MDM2 inhibits TP53 in an ubiquitin-dependent manner [41]. Clues that MTBP may function as a metastasis suppressor were first observed in Mtbp/p53 double heterozygous mice which developed mammary and hepatocellular carcinoma, osteosarcoma, lymphoma and other tumors, but developed 7-fold more metastases than controls. Mechanism data are still being collected, but MTBP interacts with ACTN4 and inhibits F-actin cross-linking leading to decreased cell migration [42]. MTBP also alters mitotic progression and chromosomal segregation [43], which may play important roles in MTBP-dependent tumor progression.
In p53+/MDM2low SCC of the head and neck (HNSCC), decreased MTBP is associated with reduced survival. However, MTBP increases in Burkitt's lymphoma samples that have Myc translocations and the chromosomal region encoding MTBP is often amplified in colorectal cancers and myelomas. MTBP function may be cancer type-specific and needs to be further studied.
Caspase-8
Long studied as a mediator of apoptosis, caspase-8 isoforms also suppress metastasis in multiple tumor types. The linkage between apoptosis and metastasis suggests that caspase-8 selectively sensitizes invasive cells compared to primary tumor cells. To wit caspase-8 mediated apoptosis is independent of the classical death- receptor pathway. Instead, it is through a process termed integrin-mediated death (IMD), which occurs when caspase-8 is recruited and activated by clusters of unligated or inappropriately ligand-bound integrins. Invasive cells are generally more loosely bound to ECM and encounter foreign ECM components they are prone to activating caspase-8 mediated IMD [44]. Since the ECM varies between tissues, the data suggest that caspase-8 may be integral to organotropism of metastases.
The metastasis suppressor function of caspase-8 appears regulated, in part by an inhibitory protein, c-FLIP, whose expression is significantly higher in neoplastic than normal tissues. In endometrial cancers c-FLIP is associated with invasion and lymph node metastasis. Weak expression of caspase-8 is an independent prognostic factor for poor outcome as well. However, there are reports of caspase-8 as a metastasis promoter in HNSCC where high caspase-8 expression correlated with increased metastasis poor and clinical outcomes.
Collapsin response mediator protein 4 (CRMP4)
CRMP4 protein and mRNA are down-regulated in metastatic prostate cancer. CRMP4 is one of a larger family of collapsing proteins. As the only family member to be down regulated in metastatic cancer [45], CRMP4 is of special interest in cancer. If CRMP4 is re-expressed, prostatic carcinoma cells round, extend fewer filopodia and invade less in vitro as well as form fewer metastases in vivo [45]. Why the CRMP4 gene is singled out for down-regulation appears to be due to selective CpG methylation in the promoter, but more detailed molecular and biochemical mechanism of action studies have not been done.
Deleted in Colorectal Cancer (DCC)
DCC was first identified as being deleted in 75% of colon cancers. The cytoplasmic tail of DCC interacts with Src, Fyn, FAK, phosphatidylinositol transferases, ezrin and merlin [46]. Expression of unbound DCC promotes apoptosis, which is presumably the mechanism by which DCC blocks invasion. Re-expression of DCC limits metastatic spread, reportedly by making cells susceptible to hypoxia. MDCK cells cultured in normoxic conditions were unaltered; whereas, DCC+ cells under hypoxic conditions were 20% more likely to undergo apoptosis [46]. To date no clinical studies establishing a role in tumor progression have been published.
Farnesoid X receptor (FXR)
The FXR gene encodes a nuclear receptor superfamily protein with four isoforms. In normal physiology, FXR proteins are required in bile acid, cholesterol, lipid and glucose metabolism; but, dysfunction is associated with atherosclerosis, intestinal bacterial growth, and liver regeneration [47]. FXR is a tumor suppressor gene in HCC and colon cancer, which is consistent with FXR−/− mice spontaneously developing liver and intestinal tumors. There are also reports showing FXR promotes oncogenesis. FXR interacts with NDRG2 [48], which was recently implicated in metastasis regulation. NDRG2 is a direct transcriptional target of FXR. Ectopic expression of FXR in hepatoma cells failed to alter lymph node metastasis, but significantly reduced bone metastasis, suggesting it might alter organotropism of metastasis. FXR expression has been associated with poor clinical outcomes in esophageal cancers [49] and pancreatic cancer [50, 51].
While some clinical studies support a role of FXR as a metastasis suppressor, there are abundant contradictory data showing tumor promotion, correlation with higher grade, and tumor suppression. Additional experiments will be needed to explain these discrepancies; however, it is readily apparent that contextual queues determine function.
Growth arrest-specific 1 (GAS1)
GAS1 was first identified as a metastasis suppressor using a genome-wide shRNA screen in B16 melanoma cells [52]. It is a GPI-anchored membrane protein that inhibits G0→S cell cycle progression and is involved in embryonic development through the regulation of sonic hedgehog (SHH) signaling and induction of apoptosis. GAS1 appears to suppress metastasis by regulating/inducing apoptosis through caspases 3 and 9 after disseminated tumor cells arrive at metastatic sites. GAS1 over-expression also significantly reduces tumorigenicity in glioma, lung and gastric cancers, suggesting tumor suppressor function in some contexts.
GAS1 is in signaling loops with SHH and Wnt, both of which are involved in cancer progression, metastasis, and maintenance of cancer stem cells (reviewed in [53–57]). Therefore, these pathways are thought to be the critical nodes for metastasis suppression. In clinical studies, GAS1 expression is down-regulated in several cancers and is associated with multidrug resistance. Low expression predicts metastasis and recurrence in colorectal cancer, and GAS1 expression is part of an 8-gene biomarker signature proposed for early detection of prostate cancer. GAS1 expression is relatively high in meningiomas, which are rarely malignant or metastasize.
Leukemia Inhibitory Factor Receptor Alpha (LIFRα)
LIFR was first identified as a breast cancer metastasis suppressor using RNASeq to identify genes down-regulated in cell lines that gained metastatic capability following stable expression of miR-9 [58, 59]. Re-expression of LIFR in metastatic cell lines suppressed lung metastasis without significantly affecting orthotopic tumor growth. LIFR suppressed multiple steps throughout the metastatic cascade.
LIFR appears to suppress metastasis through activation of the Hippo signaling pathway that leads to the inactivation of the YAP transcriptional co-activator. LIFR expression is down-regulated in a variety of human cancers and is significantly correlated with poor clinical outcomes, metastasis, and overall survival. A single report suggests that LIFR is a transformation and tumor suppressor in HMLE (human mammary epithelial cells) cells [60].
Raf Kinase Inhibitory Protein (RKIP)
RKIP has been studied in a number of different cancers [61–63] but was first identified as a metastasis suppressor in prostate cancer [64]. RKIP is an inhibitor of the Raf-1 serine-/threonine- kinase, and several potential mechanisms have been posited for RKIP. RKIP positively regulates let-7 miRNA through Myc inhibition of LIN28 transcription by inhibiting the Ras/MEK/ERK pathway. Altering let-7 or LIN28 expression also affected metastasis, which highlights the importance of this pathway in metastasis [61].
Even though the molecular mechanisms of metastasis suppression may vary, RKIP expression has been extensively examined in clinical samples. Low RKIP expression is associated with poor prognosis/survival or metastasis in several cancers [65–69]. Decreased RKIP is associated with high DNA CpG methylation phenotypes. Interestingly, many breast cancer cell lines have increased expression of miR-224, a negative regulator of RKIP [70]. In melanoma, RKIP expression is also inversely correlated with stage and expression of the pro-metastatic factor, MDA-9 [63].
Ribonucleotide reductase subunit M1 (RRM1)
MMCT experiments with chromosome 11 showed inhibition of lung adenocarcinoma growth. A smaller region of 11p, termed COH11A [71] ultimately identified RRM1 as the likely gene responsible for metastasis suppression [72, 73]. Over-expression of RRM1 reduced experimental and spontaneous lung metastasis in different models.
Regarding mechanism, RRM1 is the sole enzyme complex that converts ribonucleotides into deoxyribonucleotides. However, that role has not been clearly demonstrated in regulating metastasis. Instead, knockdown of PTEN reversed RRM1 suppression of invasion and migration, suggesting that RRM1 functions through increased PTEN expression.
RRM1 and PTEN are prognostic markers for disease-free and overall survival in NSCLC, bladder, and pancreatic cancers. However other reports indicate that RRM1 expression is not always clinically advantageous, as it has been found that patients with low RRM1 expression respond better with gemcitabine. Gemcitabine is an analog of deoxycytidine and can directly bind and inactivate RRM1. Interestingly, one of the proposed mechanisms that leads to gemcitabine resistance is over-expression of RRM1, which titres out the effective dose of gemcitabine.
Stefin A
Stefin A, a cysteine protease inhibitor, is a metastasis suppressor in human esophageal SCC and murine mammary carcinomas [74, 75]. Exogenous expression is generally associated with decreased lung metastasis, but there is model-dependent, dose-dependent tumor suppression at superphysiologic expression [74]. Expression in primary tumors is inversely correlated with metastatic potential of clonal variants. Interestingly, Stefin A expression is higher in microscopic metastasis but is not detectable in macroscopic metastases in the 4T1 mammary carcinoma model [75].
Stefin A is thought to function through inhibition of cathepsin B, blocking tumor cells from invading and migrating into the surrounding stroma [75, 76]. Clinical observations that show an inverse relationship between Stefin A and cathepsin B in brain and prostate support this hypothesis. However, correlations between Stefin A expression and clinical outcome vary greatly by tumor type.
N-myc Downstream Regulated Gene 1 (NDRG1)
NDRG1 belongs to a four member family of NDRG genes and promotes differentiation during embryonic development [77, 78]. NDRG1 was first identified as metastasis suppressor gene in colon cancer [79]. Differential display of genes in the primary tumor and metastatic lesions of colon cancer showed NDRG1 expression significantly suppressed metastatic colon cancer compared to the primary tumor. Later, NDRG1 was shown as a metastasis suppressor in prostate and breast cancers. NDRG1 significantly suppressed metastasis in animal models and clinical samples of prostate and breast cancer without significantly affecting the primary tumor growth in prostate cancer. Further the data from both studies showed NDRG1 expression correlated strongly with increased patient survival [80, 81]. NDRG1 is regulated by N-myc, C-myc, p53, HIF-1 and PTEN [82–86].
NDRG1 is mainly localized in the cytoplasm but after DNA damage it accumulates in the nucleus [87]. Localization also appears to be closely associated with metastasis suppressor activity, with membrane localization a poorer prognostic marker than cytoplasm [88]. It appears that NDRG1 is involved in cycling E-cadherin to the plasma membrane stabilizing the interaction between cells and perhaps reversing epithelial-to-mesenchymal transition (EMT). Also, reports show NDRG1 interacts with LRP6 (a Wnt receptor) leading to metastasis suppression [89]. When NDRG1 is proteolytically cleaved loss of metastasis suppressor function occurs.
Although no significant correlation could be made between NDRG1 expression and the histologic grade or primary tumor size in breast cancer, low NDRG1 protein is associated with poor prognosis in several cancers [90–97]. However in several other cancers the reports are ambiguous.
Src-Suppressed protein Kinase C Substrate (SSeCKS)
SSeCKS is an important regulator of cell signaling and cytoskeletal dynamics. It was discovered using subtractive hybridization and was found to be suppressed by oncogenic src, ras, fos, and myc [98, 99]. Re-expression in rat prostatic cancer significantly reduced lung metastasis and only slightly decreased growth of the primary subcutaneous tumors [100].
SSeCKS is phosphorylated by PKC and regulates cytoskeletal architecture. Following mitogenic stimuli, SSeCKS is phosphorylated and translocates to the peri-nuclear membrane where it ultimately allows induction of signaling cascades leading to changes in the cytoskeleton.
SSeCKS is expressed in benign and well differentiated prostate carcinomas, but not in highly aggressive and undifferentiated prostate lesions. High expression has also been correlated with decreased levels of pro-angiogenic factors (e.g., HIF-1, VEGF, FGF-7, angiopoietin, tenascin C, PDGFR and OPN) and concomitant reduction in tumor vascularity [101]. Thus, SSeCKS suppression of metastasis appears to involve multiple mechanisms that impact cell motility, invasion, and angiogenesis.
E-cadherin
E-cadherin was hypothesized to function as a metastasis suppressor when MDCK cells became more invasive when E-cadherin is lost [102, 103]. Invasiveness inversely correlates with E-cadherin expression in multiple cell types and over-expression suppressed invasion [104–106]. E-cadherin is a Ca+2-dependent cell-cell adhesion molecule which forms complexes with catenin family members and regulates association with the cytoskeleton and catenin/WNT signaling pathways.
E-cadherin is down-regulated using many mechanisms in many cancers and occurs during EMT and correlates with metastasis (reviewed in [107–109]). miRNA-9, miRNA-92a and miR-200 down-regulates E-cadherin expression either directly or by inhibiting transcription factors that regulate E-cadherin.
Decreased E-cadherin expression and/or cytoplasmic localization are inversely correlated with favorable clinical outcomes in multiple cancers; however, no correlations were found with various cancers that had metastasized to the brain.
Lysine-Specifc Demethylase 1 (LSD1)
LSD1 is linked with specific high-risk tumors [110] and was first identified as a metastasis suppressor in breast cancer models where over-expression decreased invasion in vitro and suppressed metastasis without affecting the growth of the orthotopic tumor [111]. LSD1 is a histone demethylase and is a component of the CoREST, CtBP, and the nucleosome, remodeling (NuRD) histone deacetylation complexes that repress transcription through chromatin remodeling and epigenetic changes. LSD1 also functions in regulating development, stemness of pluripotent stem cells, and tumorigenesis and cancer progression (reviewed in [110]). Members of these HDAC complexes have been associated with changes that alter cells metastatic potential indicating that the protein composition of the complex alters the functional specificity of the complex and plays an important role during cancer progression [112–114]. LSD1 and/or NuRD complex members have also been shown to be inversely correlated with poor clinical outcomes of gastroesophageal, junction adenocarcinoma, and bladder cancers; however, there is also evidence that LSD1 expression leads to tumorigenesis and poor clinical outcomes.
Hormonally regulated Neu-associated Kinase (HUNK)
HUNK suppresses metastasis in basal-type breast cancers by blocking actin polymerization resulting from inactivation of cofilin-1 and decreasing cell motility and invasion. [115]. HUNK, by phosphorylating cofilin-1, inactivates it, halting actin polymerization. Using two murine mouse models, HUNK expression decreased metastasis and increased survival without affecting primary tumor growth [115]. Contrary to these observations, HUNK promoted metastasis in mammary tumor models [116]. Furthermore, increased HUNK expression was detected in clinical samples of human breast cancer lymph node metastases [116]. This function of HUNK was kinase dependent and resulted from its ability to suppress apoptosis and down-regulate the tumor suppressor p27KIP.
MetastamiR and Non-coding RNA
With the relatively recent discovery that regulatory RNA play roles in cancer, accumulating evidence also suggest that metastamiR (metastasis-regulatory microRNA – there are several metastamiR) play roles in controlling cancer metastasis. At least a dozen miRNA promote or inhibit metastasis in experimental models and that number continues to grow. miR-335, -126, and -206 were the first metastasis suppressing miRNA discovered [117]. miR-146a and -146b suppressed metastasis and, in clinical samples, miR-146a expression is inversely correlated with breast and prostate cancer progression (reviewed in [118]).
MetastamiR are components of complex pathways and are often expressed downstream as well as upstream of pro- or anti-metastatic signals. Some of these pathways represent feed-forward and feedback loops. MetastamiR regulation is context dependent. Further, other regulatory RNA, such as large intergenic non-coding RNA (lincRNA) are being studied that regulate key aspects of metastasis (reviewed in [118]).
Tissue Inhibitor of Metalloproteases (TIMP)
Up-regulation of matrix metalloproteinases (MMP) often occurs to facilitate tumor invasion and metastasis. Under normal conditions, cells regulate MMP through four natural inhibitors, TIMP-1 to -4. The complex roles of MMP and TIMP in virtually every step of cancer progression is well documented, as are examples where TIMP suppress metastasis [119–121]. While self-evident that they would play roles as metastasis suppressors, TIMP functions are clearly dose-and context-dependent.
Conclusions and Perspectives
Breast cancer metastasis is complex, involving both intrinsic and extrinsic factors. In this brief review, we have described the growing class of metastasis suppressors. While mechanisms of action are still largely unclear, some commonalities have begun to emerge. First, all metastasis suppressors sit at the interface between tumor cells and their impact on or response to local microenvironments. Second, functionality of metastasis suppressors is dose- and context-dependent, largely because most of the proteins encoded by metastasis suppressors interact with other proteins that impinge on cellular pathways. Third, as more metastasis suppressors are identified, there appear to be metastasis-regulatory pathways and the pathology of metastasis represents dysfunction of the pathway at one or more steps. But because of the contextual queues, compensatory changes can result in other cellular behavioral changes. Fourth, metastasis suppressor mutations are rare. Instead, expression is down-regulated in advanced cancers.
In this review, the criteria for ascribing a molecule as a metastasis suppressor have been strictly applied – significant inhibition of metastasis in at least one experimental model without >50% inhibition of primary tumor growth. As more data are collected, some of the molecules mentioned will reveal other functions.
While the scope of this review has been to focus on the biological, biochemical and molecular aspects of metastasis suppressors, a brief mention about how metastasis suppressors could be used clinically is warranted. The use of metastasis suppressors has proven useful in some prognostic applications (see above); however, their utility for this purpose is more limited since visualization of gain-of-function or gain-of-expression cellular changes is easier to see [122]. As technologies improve, repair of loss-of-function and loss-of-expression via gene therapy or epigenetic manipulation will become more likely, but has not yet reached its potential. When metastasis suppressors are involved in cell-cell communication (e.g., KISS1), administering the protein could prove useful in a therapeutic context. Despite these limitations, metastasis suppressors still represent prognostic and therapeutic opportunities, but additional data are needed to realize those potentials.
Acknowledgments
Work from the authors' laboratory have been generously supported by grants from: U.S. National Cancer Institute RO1-CA134981 (DRW), Susan G. Komen for the Cure SAC11037 (DRW), National Foundation for Cancer Research-Center for Metastasis Research (DRW) and partial support from the Kansas Bioscience Authority (DRW), RO1-CA87728 (DRW) and P30-CA168524 (DRW). DRW is the Hall Family Foundation Professor of Molecular Medicine and a Kansas Bioscience Authority Eminent Scholar.
Footnotes
Disclosure statement: The authors have no conflicts to declare.
References
- 1.Smith SC, Theodorescu D. Learning therapeutic lessons from metastasis suppressor proteins. Nat Rev Cancer. 2009;9:253–264. doi: 10.1038/nrc2594. DOI 10.1038/nrc2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. DOI 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 3.Steeg PS, Bevilacqua G, Pozzatti R, Liotta LA, Sobel ME. Altered expression of NM23, a gene associated with low tumor metastatic potential, during adenovirus 2 Ela inhibition of experimental metastasis. Cancer Res. 1988;48:6550–6554. [PubMed] [Google Scholar]
- 4.Marino N, Marshall JC, Steeg PS. Protein-protein interactions: a mechanism regulating the anti-metastatic properties of Nm23-H1. Naunyn Schmiedebergs Arch Pharmacol. 2011;384:351–362. doi: 10.1007/s00210-011-0646-6. DOI 10.1007/s00210-011-0646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steeg PS, Horak CE, Miller KD. Clinical-translational approaches to the Nm23-H1 metastasis suppressor. Clin Cancer Res. 2008;14:5006–5012. doi: 10.1158/1078-0432.CCR-08-0238. DOI 10.1158/1078-0432.CCR-08-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouatas T, Halverson D, Steeg PS. Dexamethasone and medroxyprogesterone acetate elevate Nm23-H1 metastasis suppressor gene expression in metastatic human breast carcinoma cells: new uses for old compounds. Clin Cancer Res. 2003;9:3763–3772. [PubMed] [Google Scholar]
- 7.Palmieri D, Halverson DO, Ouatas T, Horak CE, Salerno M, Johnson J, Figg WD, Hollingshead M, Hursting S, Berrigan D, et al. Medroxyprogesterone acetate elevation of Nm23-H1 metastasis suppressor expression in hormone receptor-negative breast cancer. J Natl Cancer Inst. 2005;97:632–642. doi: 10.1093/jnci/dji111. DOI 10.1093/jnci/dji111. [DOI] [PubMed] [Google Scholar]
- 8.Hurst DR, Welch DR. Unraveling the enigmatic complexities of BRMS1-mediated metastasis suppression. FEBS Lett. 2011;585:3185–3190. doi: 10.1016/j.febslet.2011.07.045. DOI 10.1016/j.febslet.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edmonds MD, Hurst DR, Vaidya KS, Stafford LJ, Chen D, Welch DR. Breast cancer metastasis suppressor 1 coordinately regulates metastasis-associated microRNA expression. Int J Cancer. 2009;125:1778–1785. doi: 10.1002/ijc.24616. DOI 10.1002/ijc.24616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slipicevic A, Holm R, Emilsen E, Ree Rosnes AK, Welch DR, Maelandsmo GM, Florenes VA. Cytoplasmic BRMS1 expression in malignant melanoma is associated with increased disease-free survival. BMC Cancer. 2012;12:73. doi: 10.1186/1471-2407-12-73. DOI 10.1186/1471-2407-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frolova N, Edmonds MD, Bodenstine TM, Seitz R, Johnson MR, Feng R, Welch DR, Frost AR. A shift from nuclear to cytoplasmic breast cancer metastasis suppressor 1 expression is associated with highly proliferative estrogen receptor-negative breast cancers. Tumour Biol. 2009;30:148–159. doi: 10.1159/000228908. DOI 10.1159/000228908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Yamashita H, Toyama T, Yamamoto Y, Kawasoe T, Iwase H. Reduced expression of the breast cancer metastasis suppressor 1 mRNA is correlated with poor progress in breast cancer. Clin Cancer Res. 2006;12:6410–6414. doi: 10.1158/1078-0432.CCR-06-1347. DOI 10.1158/1078-0432.CCR-06-1347. [DOI] [PubMed] [Google Scholar]
- 13.Fujita H, Okada F, Hamada J, Hosokawa M, Moriuchi T, Koya RC, Kuzumaki N. Gelsolin functions as a metastasis suppressor in B16-BL6 mouse melanoma cells and requirement of the carboxyl-terminus for its effect. Int J Cancer. 2001;93:773–780. doi: 10.1002/ijc.1413. [DOI] [PubMed] [Google Scholar]
- 14.Baig RM, Mahjabeen I, Sabir M, Masood N, Ali K, Malik FA, Kayani MA. Mutational spectrum of Gelsolin and its down regulation is associated with breast cancer. Disease markers. 2013;34:71–80. doi: 10.3233/DMA-120952. DOI 10.3233/DMA-120952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichikawa T, Ichikawa Y, Dong J, Hawkins AL, Griffin CA, Isaacs WB, Oshimura M, Barrett JC, Isaacs JT. Localization of metastasis suppressor gene(s) for prostatic cancer to the short arm of human chromosome 11. Cancer Res. 1992;52:3486–3490. [PubMed] [Google Scholar]
- 16.Liu WM, Zhang XA. KAI1/CD82, a tumor metastasis suppressor. Cancer letters. 2006;240:183–194. doi: 10.1016/j.canlet.2005.08.018. DOI 10.1016/j.canlet.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Malik FA, Sanders AJ, Jiang WG. KAI-1/CD82, the molecule and clinical implication in cancer and cancer metastasis. Histol Histopathol. 2009;24:519–530. doi: 10.14670/HH-24.519. [DOI] [PubMed] [Google Scholar]
- 18.Tsai YC, Weissman AM. Dissecting the diverse functions of the metastasis suppressor CD82/KAI1. FEBS Lett. 2011;585:3166–3173. doi: 10.1016/j.febslet.2011.08.031. DOI 10.1016/j.febslet.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. DOI 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck BH, Welch DR. The KISS1 metastasis suppressor: a good night kiss for disseminated cancer cells. Eur J Cancer. 2010;46:1283–1289. doi: 10.1016/j.ejca.2010.02.023. DOI 10.1016/j.ejca.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vander Griend DJ, Kocherginsky M, Hickson JA, Stadler WM, Lin A, Rinker-Schaeffer CW. Suppression of metastatic colonization by the context-dependent activation of the c-Jun NH2-terminal kinase kinases JNKK1/MKK4 and MKK7. Cancer Res. 2005;65:10984–10991. doi: 10.1158/0008-5472.CAN-05-2382. DOI 10.1158/0008-5472.CAN-05-2382. [DOI] [PubMed] [Google Scholar]
- 22.Hickson JA, Huo D, Vander Griend DJ, Lin A, Rinker-Schaeffer CW, Yamada SD. The p38 kinases MKK4 and MKK6 suppress metastatic colonization in human ovarian carcinoma. Cancer Res. 2006;66:2264–2270. doi: 10.1158/0008-5472.CAN-05-3676. DOI 10.1158/0008-5472.CAN-05-3676. [DOI] [PubMed] [Google Scholar]
- 23.Huang MJ, Wang PN, Huang J, Zhang XW, Wang L, Liu HL, Wang JP. [Expression and clinicopathological significance of serine257/threonine261 phosphorylated MKK4 in colorectal carcinoma] Zhonghua Yi Xue Za Zhi. 2013;93:746–750. [PubMed] [Google Scholar]
- 24.Seraj MJ, Samant RS, Verderame MF, Welch DR. Functional evidence for a novel human breast carcinoma metastasis suppressor, BRMS1, encoded at chromosome 11q13. Cancer Res. 2000;60:2764–2769. [PubMed] [Google Scholar]
- 25.Gildea JJ, Seraj MJ, Oxford G, Harding MA, Hampton GM, Moskaluk CA, Frierson HF, Conaway MR, Theodorescu D. RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Res. 2002;62:6418–6423. [PubMed] [Google Scholar]
- 26.Griner EM, Theodorescu D. The faces and friends of RhoGDI2. Cancer Metastasis Rev. 2012;31:519–528. doi: 10.1007/s10555-012-9376-6. DOI 10.1007/s10555-012-9376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. DOI 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y, Moissoglu K, Wang H, Wang X, Frierson HF, Schwartz MA, Theodorescu D. Src phosphorylation of RhoGDI2 regulates its metastasis suppressor function. Proc Natl Acad Sci U S A. 2009;106:5807–5812. doi: 10.1073/pnas.0810094106. DOI 10.1073/pnas.0810094106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griner EM, Churchill ME, Brautigan DL, Theodorescu D. PKCalpha phosphorylation of RhoGDI2 at Ser31 disrupts interactions with Rac1 and decreases GDI activity. Oncogene. 2013;32:1010–1017. doi: 10.1038/onc.2012.124. DOI 10.1038/onc.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moissoglu K, McRoberts KS, Meier JA, Theodorescu D, Schwartz MA. Rho GDP dissociation inhibitor 2 suppresses metastasis via unconventional regulation of RhoGTPases. Cancer Res. 2009;69:2838–2844. doi: 10.1158/0008-5472.CAN-08-1397. DOI 10.1158/0008-5472.CAN-08-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan BZ, Miller MJ, Keck CL, Zimonjic DB, Thorgeirsson SS, Popescu NC. Cloning, characterization, and chromosomal localization of a gene frequently deleted in human liver cancer (DLC-1) homologous to rat RhoGAP. Cancer Res. 1998;58:2196–2199. [PubMed] [Google Scholar]
- 32.Goodison S, Yuan J, Sloan D, Kim R, Li C, Popescu NC, Urquidi V. The RhoGAP protein DLC-1 functions as a metastasis suppressor in breast cancer cells. Cancer Res. 2005;65:6042–6053. doi: 10.1158/0008-5472.CAN-04-3043. DOI 10.1158/0008-5472.CAN-04-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko FC, Chan LK, Tung EK, Lowe SW, Ng IO, Yam JW. Akt phosphorylation of deleted in liver cancer 1 abrogates its suppression of liver cancer tumorigenesis and metastasis. Gastroenterology. 2010;139:1397–1407. doi: 10.1053/j.gastro.2010.06.051. DOI 10.1053/j.gastro.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 34.Scholz RP, Regner J, Theil A, Erlmann P, Holeiter G, Jahne R, Schmid S, Hausser A, Olayioye MA. DLC1 interacts with 14-3-3 proteins to inhibit RhoGAP activity and block nucleocytoplasmic shuttling. J Cell Sci. 2009;122:92–102. doi: 10.1242/jcs.036251. DOI 10.1242/jcs.036251. [DOI] [PubMed] [Google Scholar]
- 35.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. DOI 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 36.Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52:189–196. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorne RF, Legg JW, Isacke CM. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J Cell Sci. 2004;117:373–380. doi: 10.1242/jcs.00954. DOI 10.1242/jcs.00954. [DOI] [PubMed] [Google Scholar]
- 38.Louderbough JM, Schroeder JA. Understanding the dual nature of CD44 in breast cancer progression. Mol Cancer Res. 2011;9:1573–1586. doi: 10.1158/1541-7786.MCR-11-0156. DOI 10.1158/1541-7786.MCR-11-0156. [DOI] [PubMed] [Google Scholar]
- 39.Lopez JI, Camenisch TD, Stevens MV, Sands BJ, McDonald J, Schroeder JA. CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res. 2005;65:6755–6763. doi: 10.1158/0008-5472.CAN-05-0863. DOI 10.1158/0008-5472.CAN-05-0863. [DOI] [PubMed] [Google Scholar]
- 40.Faraji F, Pang Y, Walker RC, Nieves Borges R, Yang L, Hunter KW. Cadm1 is a metastasis susceptibility gene that suppresses metastasis by modifying tumor interaction with the cell-mediated immunity. PLoS genetics. 2012;8:e1002926. doi: 10.1371/journal.pgen.1002926. DOI 10.1371/journal.pgen.1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwakuma T, Lozano G. MDM2, an introduction. Mol Cancer Res. 2003;1:993–1000. [PubMed] [Google Scholar]
- 42.Agarwal N, Adhikari AS, Iyer SV, Hekmatdoost K, Welch DR, Iwakuma T. MTBP suppresses cell migration and filopodia formation by inhibiting ACTN4. Oncogene. 2013;32:462–470. doi: 10.1038/onc.2012.69. DOI 10.1038/onc.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agarwal N, Tochigi Y, Adhikari AS, Cui S, Cui Y, Iwakuma T. MTBP plays a crucial role in mitotic progression and chromosome segregation. Cell Death Differ. 2011;18:1208–1219. doi: 10.1038/cdd.2010.189. DOI 10.1038/cdd.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teitz T, Stupack DG, Lahti JM. Halting neuroblastoma metastasis by controlling integrin-mediated death. Cell Cycle. 2006;5:681–685. doi: 10.4161/cc.5.7.2615. DOI 2615 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Gao X, Pang J, Li LY, Liu WP, Di JM, Sun QP, Fang YQ, Liu XP, Pu XY, He D, Li MT, Su ZL, Li BY. Expression profiling identifies new function of collapsin response mediator protein 4 as a metastasis-suppressor in prostate cancer. Oncogene. 2010;29:4555–4566. doi: 10.1038/onc.2010.213. DOI 10.1038/onc.2010.213. [DOI] [PubMed] [Google Scholar]
- 46.Rodrigues S, De Wever O, Bruyneel E, Rooney RJ, Gespach C. Opposing roles of netrin-1 and the dependence receptor DCC in cancer cell invasion, tumor growth and metastasis. Oncogene. 2007;26:5615–5625. doi: 10.1038/sj.onc.1210347. DOI 10.1038/sj.onc.1210347. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Edwards PA. FXR signaling in metabolic disease. FEBS Lett. 2008;582:10–18. doi: 10.1016/j.febslet.2007.11.015. DOI 10.1016/j.febslet.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Deuschle U, Schuler J, Schulz A, Schluter T, Kinzel O, Abel U, Kremoser C. FXR controls the tumor suppressor NDRG2 and FXR agonists reduce liver tumor growth and metastasis in an orthotopic mouse xenograft model. PLoS One. 2012;7:e43044. doi: 10.1371/journal.pone.0043044. DOI 10.1371/journal.pone.0043044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guan B, Li H, Yang Z, Hoque A, Xu X. Inhibition of farnesoid X receptor controls esophageal cancer cell growth in vitro and in nude mouse xenografts. Cancer. 2013;119:1321–1329. doi: 10.1002/cncr.27910. DOI 10.1002/cncr.27910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JY, Lee KT, Lee JK, Lee KH, Jang KT, Heo JS, Choi SH, Kim Y, Rhee JC. Farnesoid X receptor, overexpressed in pancreatic cancer with lymph node metastasis promotes cell migration and invasion. Br J Cancer. 2011;104:1027–1037. doi: 10.1038/bjc.2011.37. DOI 10.1038/bjc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang S, Lee KT, Lee JY, Lee JK, Lee KH, Rhee JC. Inhibition of SCAMP1 suppresses cell migration and invasion in human pancreatic and gallbladder cancer cells. Tumour Biol. 2013;34:2731–2739. doi: 10.1007/s13277-013-0825-9. DOI 10.1007/s13277-013-0825-9. [DOI] [PubMed] [Google Scholar]
- 52.Gobeil S, Zhu X, Doillon CJ, Green MR. A genome-wide shRNA screen identifies GAS1 as a novel melanoma metastasis suppressor gene. Genes Dev. 2008;22:2932–2940. doi: 10.1101/gad.1714608. DOI 10.1101/gad.1714608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinelli DC, Fan CM. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev. 2007;21:1231–1243. doi: 10.1101/gad.1546307. DOI 10.1101/gad.1546307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. DOI 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 55.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. DOI 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 56.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. DOI 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 57.Milla LA, Gonzalez-Ramirez CN, Palma V. Sonic Hedgehog in cancer stem cells: a novel link with autophagy. Biol Res. 2012;45:223–230. doi: 10.4067/S0716-97602012000300004. DOI 10.1590/S0716-97602012000300004. [DOI] [PubMed] [Google Scholar]
- 58.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. DOI 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, Gupta S, Liang H, Lin HK, Hung MC, Ma L. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med. 2012;18:1511–1517. doi: 10.1038/nm.2940. DOI 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iorns E, Ward TM, Dean S, Jegg A, Thomas D, Murugaesu N, Sims D, Mitsopoulos C, Fenwick K, Kozarewa I, et al. Whole genome in vivo RNAi screening identifies the leukemia inhibitory factor receptor as a novel breast tumor suppressor. Breast Cancer Res Treat. 2012;135:79–91. doi: 10.1007/s10549-012-2068-7. DOI 10.1007/s10549-012-2068-7. [DOI] [PubMed] [Google Scholar]
- 61.Dangi-Garimella S, Yun J, Eves EM, Newman M, Erkeland SJ, Hammond SM, Minn AJ, Rosner MR. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 2009;28:347–358. doi: 10.1038/emboj.2008.294. DOI 10.1038/emboj.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinho O, Pinto F, Granja S, Miranda-Goncalves V, Moreira MA, Ribeiro LF, di Loreto C, Rosner MR, Longatto-Filho A, Reis RM. RKIP inhibition in cervical cancer is associated with higher tumor aggressive behavior and resistance to cisplatin therapy. PLoS One. 2013;8:e59104. doi: 10.1371/journal.pone.0059104. DOI 10.1371/journal.pone.0059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das SK, Bhutia SK, Sokhi UK, Azab B, Su ZZ, Boukerche H, Anwar T, Moen EL, Chatterjee D, Pellecchia M, et al. Raf kinase inhibitor RKIP inhibits MDA-9/syntenin-mediated metastasis in melanoma. Cancer Res. 2012;72:6217–6226. doi: 10.1158/0008-5472.CAN-12-0402. DOI 10.1158/0008-5472.CAN-12-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z, Keller ET. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J Natl Cancer Inst. 2003;95:878–889. doi: 10.1093/jnci/95.12.878. [DOI] [PubMed] [Google Scholar]
- 65.Afonso J, Longatto-Filho A, Martinho O, Lobo F, Amaro T, Reis RM, Santos LL. Low RKIP expression associates with poor prognosis in bladder cancer patients. Virchows Arch. 2013;462:445–453. doi: 10.1007/s00428-013-1388-2. DOI 10.1007/s00428-013-1388-2. [DOI] [PubMed] [Google Scholar]
- 66.Song SP, Zhang SB, Li ZH, Zhou YS, Li B, Bian ZW, Liao QD, Zhang YD. Reduced expression of Raf kinase inhibitor protein correlates with poor prognosis in pancreatic cancer. Clin Transl Oncol. 2012;14:848–852. doi: 10.1007/s12094-012-0870-7. DOI 10.1007/s12094-012-0870-7. [DOI] [PubMed] [Google Scholar]
- 67.Yan H, Guoqiang L, Shengxi C, Zhenghao D, Lingjin H. Reduction of Raf Kinase Inhibitor Protein Expression is Associated with Lymph Node Metastasis in Resectable Non-small Cell Lung Cancer. Open Respir Med J. 2012;6:135–138. doi: 10.2174/1874306401206010135. DOI 10.2174/1874306401206010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinho O, Granja S, Jaraquemada T, Caeiro C, Miranda-Goncalves V, Honavar M, Costa P, Damasceno M, Rosner MR, Lopes JM, Reis RM. Downregulation of RKIP is associated with poor outcome and malignant progression in gliomas. PLoS One. 2012;7:e30769. doi: 10.1371/journal.pone.0030769. DOI 10.1371/journal.pone.0030769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moon A, Park JY, Sung JY, Park YK, Kim YW. Reduced expression of Raf-1 kinase inhibitory protein in renal cell carcinoma: a significant prognostic marker. Pathology. 2012;44:534–539. doi: 10.1097/PAT.0b013e32835817e8. DOI 10.1097/PAT.0b013e32835817e8. [DOI] [PubMed] [Google Scholar]
- 70.Huang L, Dai T, Lin X, Zhao X, Chen X, Wang C, Li X, Shen H, Wang X. MicroRNA-224 targets RKIP to control cell invasion and expression of metastasis genes in human breast cancer cells. Biochem Biophys Res Commun. 2012;425:127–133. doi: 10.1016/j.bbrc.2012.07.025. DOI 10.1016/j.bbrc.2012.07.025 S0006-291X(12)01308-3 [pii] [DOI] [PubMed] [Google Scholar]
- 71.Bepler G, O'Briant KC, Kim YC, Schreiber G, Pitterle DM. A 1.4-Mb high-resolution physical map and contig of chromosome segment 11p15.5 and genes in the LOH11A metastasis suppressor region. Genomics. 1999;55:164–175. doi: 10.1006/geno.1998.5659. DOI 10.1006/geno.1998.5659. [DOI] [PubMed] [Google Scholar]
- 72.O'Briant KC, Bepler G. Delineation of the centromeric and telomeric chromosome segment 11p15.5 lung cancer suppressor regions LOH11A and LOH11B. Genes Chromosomes Cancer. 1997;18:111–114. doi: 10.1002/(sici)1098-2264(199702)18:2<111::aid-gcc5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 73.Pitterle DM, Kim YC, Jolicoeur EM, Cao Y, O'Briant KC, Bepler G. Lung cancer and the human gene for ribonucleotide reductase subunit M1 (RRM1) Mamm Genome. 1999;10:916–922. doi: 10.1007/s003359901114. [DOI] [PubMed] [Google Scholar]
- 74.Li W, Ding F, Zhang L, Liu Z, Wu Y, Luo A, Wu M, Wang M, Zhan Q, Liu Z. Overexpression of stefin A in human esophageal squamous cell carcinoma cells inhibits tumor cell growth, angiogenesis, invasion, and metastasis. Clin Cancer Res. 2005;11:8753–8762. doi: 10.1158/1078-0432.CCR-05-0597. DOI 10.1158/1078-0432.CCR-05-0597. [DOI] [PubMed] [Google Scholar]
- 75.Parker BS, Ciocca DR, Bidwell BN, Gago FE, Fanelli MA, George J, Slavin JL, Moller A, Steel R, Pouliot N, et al. Primary tumour expression of the cysteine cathepsin inhibitor Stefin A inhibits distant metastasis in breast cancer. J Pathol. 2008;214:337–346. doi: 10.1002/path.2265. DOI 10.1002/path.2265. [DOI] [PubMed] [Google Scholar]
- 76.Bervar A, Zajc I, Sever N, Katunuma N, Sloane BF, Lah TT. Invasiveness of transformed human breast epithelial cell lines is related to cathepsin B and inhibited by cysteine proteinase inhibitors. Biol Chem. 2003;384:447–455. doi: 10.1515/BC.2003.050. DOI 10.1515/BC.2003.050. [DOI] [PubMed] [Google Scholar]
- 77.Okuda T, Kondoh H. Identification of new genes ndr2 and ndr3 which are related to Ndr1/RTP/Drg1 but show distinct tissue specificity and response to N-myc. Biochem Biophys Res Commun. 1999;266:208–215. doi: 10.1006/bbrc.1999.1780. DOI 10.1006/bbrc.1999.1780. [DOI] [PubMed] [Google Scholar]
- 78.Zhou RH, Kokame K, Tsukamoto Y, Yutani C, Kato H, Miyata T. Characterization of the human NDRG gene family: a newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics. 2001;73:86–97. doi: 10.1006/geno.2000.6496. DOI 10.1006/geno.2000.6496. [DOI] [PubMed] [Google Scholar]
- 79.Guan RJ, Ford HL, Fu Y, Li Y, Shaw LM, Pardee AB. Drg-1 as a differentiation-related, putative metastatic suppressor gene in human colon cancer. Cancer Res. 2000;60:749–755. [PubMed] [Google Scholar]
- 80.Bandyopadhyay S, Pai SK, Gross SC, Hirota S, Hosobe S, Miura K, Saito K, Commes T, Hayashi S, Watabe M, Watabe K. The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res. 2003;63:1731–1736. [PubMed] [Google Scholar]
- 81.Bandyopadhyay S, Pai SK, Hirota S, Hosobe S, Takano Y, Saito K, Piquemal D, Commes T, Watabe M, Gross SC, et al. Role of the putative tumor metastasis suppressor gene Drg-1 in breast cancer progression. Oncogene. 2004;23:5675–5681. doi: 10.1038/sj.onc.1207734. DOI 10.1038/sj.onc.1207734. [DOI] [PubMed] [Google Scholar]
- 82.Bandyopadhyay S, Pai SK, Hirota S, Hosobe S, Tsukada T, Miura K, Takano Y, Saito K, Commes T, Piquemal D, et al. PTEN up-regulates the tumor metastasis suppressor gene Drg-1 in prostate and breast cancer. Cancer Res. 2004;64:7655–7660. doi: 10.1158/0008-5472.CAN-04-1623. DOI 10.1158/0008-5472.CAN-04-1623. [DOI] [PubMed] [Google Scholar]
- 83.Ellen TP, Ke Q, Zhang P, Costa M. NDRG1, a growth and cancer related gene: regulation of gene expression and function in normal and disease states. Carcinogenesis. 2008;29:2–8. doi: 10.1093/carcin/bgm200. DOI 10.1093/carcin/bgm200. [DOI] [PubMed] [Google Scholar]
- 84.Kovacevic Z, Chikhani S, Lui GY, Sivagurunathan S, Richardson DR. The iron-regulated metastasis suppressor NDRG1 targets NEDD4L, PTEN, and SMAD4 and inhibits the PI3K and Ras signaling pathways. Antioxid Redox Signal. 2013;18:874–887. doi: 10.1089/ars.2011.4273. DOI 10.1089/ars.2011.4273. [DOI] [PubMed] [Google Scholar]
- 85.Stein S, Thomas EK, Herzog B, Westfall MD, Rocheleau JV, Jackson RS, 2nd, Wang M, Liang P. NDRG1 is necessary for p53-dependent apoptosis. J Biol Chem. 2004;279:48930–48940. doi: 10.1074/jbc.M400386200. DOI 10.1074/jbc.M400386200. [DOI] [PubMed] [Google Scholar]
- 86.Zhang P, Tchou-Wong KM, Costa M. Egr-1 mediates hypoxia-inducible transcription of the NDRG1 gene through an overlapping Egr-1/Sp1 binding site in the promoter. Cancer Res. 2007;67:9125–9133. doi: 10.1158/0008-5472.CAN-07-1525. DOI 10.1158/0008-5472.CAN-07-1525. [DOI] [PubMed] [Google Scholar]
- 87.Kurdistani SK, Arizti P, Reimer CL, Sugrue MM, Aaronson SA, Lee SW. Inhibition of tumor cell growth by RTP/rit42 and its responsiveness to p53 and DNA damage. Cancer Res. 1998;58:4439–4444. [PubMed] [Google Scholar]
- 88.Song Y, Lv L, Du J, Yue L, Cao L. Correlation of N-myc downstream-regulated gene 1 subcellular localization and lymph node metastases of colorectal neoplasms. Biochem Biophys Res Commun. 2013;439:241–246. doi: 10.1016/j.bbrc.2013.08.049. DOI 10.1016/j.bbrc.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 89.Liu W, Xing F, Iiizumi-Gairani M, Okuda H, Watabe M, Pai SK, Pandey PR, Hirota S, Kobayashi A, Mo YY, et al. N-myc downstream regulated gene 1 modulates Wnt-beta-catenin signalling and pleiotropically suppresses metastasis. EMBO Mol Med. 2012;4:93–108. doi: 10.1002/emmm.201100190. DOI 10.1002/emmm.201100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ando T, Ishiguro H, Kimura M, Mitsui A, Kurehara H, Sugito N, Tomoda K, Mori R, Takashima N, Ogawa R, et al. Decreased expression of NDRG1 is correlated with tumor progression and poor prognosis in patients with esophageal squamous cell carcinoma. Dis Esophagus. 2006;19:454–458. doi: 10.1111/j.1442-2050.2006.00618.x. DOI 10.1111/j.1442-2050.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 91.Dos Santos M, da Cunha Mercante AM, Nunes FD, Leopoldino AM, de Carvalho MB, Gazito D, Lopez RV, Chiappini PB, de Carvalho Neto PB, et al. Prognostic significance of NDRG1 expression in oral and oropharyngeal squamous cell carcinoma. Mol Biol Rep. 2012;39:10157–10165. doi: 10.1007/s11033-012-1889-0. DOI 10.1007/s11033-012-1889-0. [DOI] [PubMed] [Google Scholar]
- 92.Li Q, Chen H. Transcriptional silencing of N-Myc downstream-regulated gene 1 (NDRG1) in metastatic colon cancer cell line SW620. Clin Exp Metastasis. 2011;28:127–135. doi: 10.1007/s10585-010-9366-4. DOI 10.1007/s10585-010-9366-4. [DOI] [PubMed] [Google Scholar]
- 93.Mao Z, Sun J, Feng B, Ma J, Zang L, Dong F, Zhang D, Zheng M. The metastasis suppressor, N-myc downregulated gene 1 (NDRG1), is a prognostic biomarker for human colorectal cancer. PLoS One. 2013;8:e68206. doi: 10.1371/journal.pone.0068206. DOI 10.1371/journal.pone.0068206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maruyama Y, Ono M, Kawahara A, Yokoyama T, Basaki Y, Kage M, Aoyagi S, Kinoshita H, Kuwano M. Tumor growth suppression in pancreatic cancer by a putative metastasis suppressor gene Cap43/NDRG1/Drg-1 through modulation of angiogenesis. Cancer Res. 2006;66:6233–6242. doi: 10.1158/0008-5472.CAN-06-0183. DOI 10.1158/0008-5472.CAN-06-0183. [DOI] [PubMed] [Google Scholar]
- 95.Matsushita K, Uchida K, Saigusa S, Ide S, Hashimoto K, Koike Y, Otake K, Inoue M, Tanaka K, Kusunoki M. Low NDRG1 mRNA expression predicts a poor prognosis in neuroblastoma patients. Pediatr Surg Int. 2013;29:363–368. doi: 10.1007/s00383-012-3248-6. DOI 10.1007/s00383-012-3248-6. [DOI] [PubMed] [Google Scholar]
- 96.Strzelczyk B, Szulc A, Rzepko R, Kitowska A, Skokowski J, Szutowicz A, Pawelczyk T. Identification of high-risk stage II colorectal tumors by combined analysis of the NDRG1 gene expression and the depth of tumor invasion. Annals of surgical oncology. 2009;16:1287–1294. doi: 10.1245/s10434-009-0381-0. DOI 10.1245/s10434-009-0381-0. [DOI] [PubMed] [Google Scholar]
- 97.Sun B, Chu D, Li W, Chu X, Li Y, Wei D, Li H. Decreased expression of NDRG1 in glioma is related to tumor progression and survival of patients. J Neurooncol. 2009;94:213–219. doi: 10.1007/s11060-009-9859-7. DOI 10.1007/s11060-009-9859-7. [DOI] [PubMed] [Google Scholar]
- 98.Lin X, Tombler E, Nelson PJ, Ross M, Gelman IH. A novel src- and ras-suppressed protein kinase C substrate associated with cytoskeletal architecture. J Biol Chem. 1996;271:28430–28438. doi: 10.1074/jbc.271.45.28430. [DOI] [PubMed] [Google Scholar]
- 99.Gelman IH. Suppression of tumor and metastasis progression through the scaffolding functions of SSeCKS/Gravin/AKAP12. Cancer Metastasis Rev. 2012;31:493–500. doi: 10.1007/s10555-012-9360-1. DOI 10.1007/s10555-012-9360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xia W, Unger P, Miller L, Nelson J, Gelman IH. The Src-suppressed C kinase substrate, SSeCKS, is a potential metastasis inhibitor in prostate cancer. Cancer Res. 2001;61:5644–5651. [PubMed] [Google Scholar]
- 101.Su B, Zheng Q, Vaughan MM, Bu Y, Gelman IH. SSeCKS metastasis-suppressing activity in MatLyLu prostate cancer cells correlates with vascular endothelial growth factor inhibition. Cancer Res. 2006;66:5599–5607. doi: 10.1158/0008-5472.CAN-05-4123. [DOI] [PubMed] [Google Scholar]
- 102.Behrens J, Birchmeier W, Goodman SL, Imhof BA. Dissociation of Madin-Darby canine kidney epithelial cells by the monoclonal antibody anti-arc-1: mechanistic aspects and identification of the antigen as a component related to uvomorulin. J Cell Biol. 1985;101:1307–1315. doi: 10.1083/jcb.101.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Behrens J, Mareel MM, Van Roy FM, Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989;108:2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vleminckx K, Vakaet L, Jr., Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 105.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Navarro P, Gomez M, Pizarro A, Gamallo C, Quintanilla M, Cano A. A role for the E-cadherin cell-cell adhesion molecule during tumor progression of mouse epidermal carcinogenesis. J Cell Biol. 1991;115:517–533. doi: 10.1083/jcb.115.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796:293–308. doi: 10.1016/j.bbcan.2009.07.006. DOI 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 108.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. DOI 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 109.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75–90. doi: 10.1016/j.bbcan.2009.03.002. DOI 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 110.Amente S, Lania L, Majello B. The histone LSD1 demethylase in stemness and cancer transcription programs. Biochim Biophys Acta. 2013;1829:981–986. doi: 10.1016/j.bbagrm.2013.05.002. DOI 10.1016/j.bbagrm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 111.Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu W, Liang J, Sun L, Yang X, Shi L, et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138:660–672. doi: 10.1016/j.cell.2009.05.050. DOI 10.1016/j.cell.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 112.Li Q, Shi L, Gui B, Yu W, Wang J, Zhang D, Han X, Yao Z, Shang Y. Binding of the JmjC demethylase JARID1B to LSD1/NuRD suppresses angiogenesis and metastasis in breast cancer cells by repressing chemokine CCL14. Cancer Res. 2011;71:6899–6908. doi: 10.1158/0008-5472.CAN-11-1523. DOI 10.1158/0008-5472.CAN-11-1523. [DOI] [PubMed] [Google Scholar]
- 113.Lin Y, Wu Y, Li J, Dong C, Ye X, Chi YI, Evers BM, Zhou BP. The SNAG domain of Snail1 functions as a molecular hook for recruiting lysine-specific demethylase 1. EMBO J. 2010;29:1803–1816. doi: 10.1038/emboj.2010.63. DOI 10.1038/emboj.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li K, Dias SJ, Rimando AM, Dhar S, Mizuno CS, Penman AD, Lewin JR, Levenson AS. Pterostilbene acts through metastasis-associated protein 1 to inhibit tumor growth, progression and metastasis in prostate cancer. PLoS One. 2013;8:e57542. doi: 10.1371/journal.pone.0057542. DOI 10.1371/journal.pone.0057542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Quintela-Fandino M, Arpaia E, Brenner D, Goh T, Yeung FA, Blaser H, Alexandrova R, Lind EF, Tusche MW, Wakeham A, et al. HUNK suppresses metastasis of basal type breast cancers by disrupting the interaction between PP2A and cofilin-1. Proc Natl Acad Sci U S A. 2010;107:2622–2627. doi: 10.1073/pnas.0914492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wertheim GB, Yang TW, Pan TC, Ramne A, Liu Z, Gardner HP, Dugan KD, Kristel P, Kreike B, van de Vijver MJ, et al. The Snf1-related kinase, Hunk, is essential for mammary tumor metastasis. Proc Natl Acad Sci U S A. 2009;106:15855–15860. doi: 10.1073/pnas.0906993106. DOI 10.1073/pnas.0906993106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. DOI 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hurst DR, Edmonds MD, Welch DR. Metastamir: the field of metastasis-regulatory microRNA is spreading. Cancer Res. 2009;69:7495–7498. doi: 10.1158/0008-5472.CAN-09-2111. DOI 10.1158/0008-5472.CAN-09-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ohta S, Lai EW, Pang AL, Brouwers FM, Chan WY, Eisenhofer G, de Krijger R, Ksinantova L, Breza J, Blazicek P, et al. Downregulation of metastasis suppressor genes in malignant pheochromocytoma. Int J Cancer. 2005;114:139–143. doi: 10.1002/ijc.20670. DOI 10.1002/ijc.20670. [DOI] [PubMed] [Google Scholar]
- 120.Pulukuri SM, Patibandla S, Patel J, Estes N, Rao JS. Epigenetic inactivation of the tissue inhibitor of metalloproteinase-2 (TIMP-2) gene in human prostate tumors. Oncogene. 2007;26:5229–5237. doi: 10.1038/sj.onc.1210329. DOI 10.1038/sj.onc.1210329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 122.Welch DR, Rinker-Schaeffer CW. What defines a useful marker of metastasis in human cancer? J Natl Cancer Inst. 1999;91:1351–1353. doi: 10.1093/jnci/91.16.1351. [DOI] [PubMed] [Google Scholar]